NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
One important aspect of myogenic differentiation in Drosophila melanogaster, as in many other organisms, is the generation of multinucleate muscle fibers through the fusion of myoblasts. This process cannot be initiated until the myoblasts have differentiated to a point at which they become competent to fuse and express genes associated with the fusion process. The myoblasts must then identify and adhere to their fusion partners, a process that is seeded by the founder cell and involves its recruitment of fusion-competent myoblasts. Cell adhesion molecules that are members of the Immunoglobulin Superfamily mediate recognition between these two populations of myoblasts. In subsequent events, intracellular proteins that are essential for the fusion process are recruited to the plasma membrane, where they likely contribute to reorganization of the cytoskeleton through activation of small GTPases. Morphological changes associated with fusion include recruitment of electron dense vesicles and formation of fusion plaques at points of cell-cell contact. Molecules such as Antisocial/Rolling pebbles, Myoblast City and Loner, among others, function at these stages. While little is known at present about the actual molecules or process by which the lipid bilayers break down, it seems likely that known proteins essential for fusion will provide an entry into their identification. This chapter provides a review of the literature describing these genes and a discussion of their proposed roles in the pathway.
Introduction
In organisms ranging from Drosophila to mammals, the musculature is comprised of an elaborate array of distinct fibers that are generated by the fusion of committed myoblasts. As discussed elsewhere in this volume, the muscle fibers differ from each other in features that include location, pattern of innervation, site of attachment, and size. These unique features are controlled by information contained within a muscle specific myoblast—the founder cell. Founder cells initiate fusion with surrounding “naïve” fusion-competent myoblasts, which in turn, take on the identity and features of the original founder cell. The final size of the muscle fiber is then attained through multiple rounds of fusion of the developing syncitia with additional fusion-competent myoblasts. The smallest muscles of the embryo will be formed by fusion of as few as 3-5 cells, whereas larger muscles require approximately 30-35 cells.
Several distinct events occur in the developing myoblasts to achieve this ultimate end. After the myoblasts become specified as either founder cells or fusion-competent myoblasts, these cells must identify and adhere to their fusion partners. Recognition is specific and directional, in that fusion does not appear to occur between two equivalent myoblasts.1 Instead, a founder cell initiates the process by recruitment of a fusion-competent myoblast. Both cell specific and nonmuscle specific proteins contribute to this process, and genetic studies in Drosophila embryos have identified a number of key players in recent years. These studies begin to define the genes and steps necessary for the recognition and adhesion between myoblasts and, perhaps, the fusion of two lipid bilayers into one.
Ultrastructure of the Adherent Myoblast Interface
In a detailed morphological analysis of fusing myoblasts at the level of the electron microscope, Doberstein et al2 described a series of intracellular events that accompany fusion and are shown diagrammatically in Figure 1. The first obvious change is the accumulation of clusters of electron dense vesicles on the cytoplasmic sides of opposed plasma membranes of two associated myoblasts. These vesicles, which have not been observed in other cell types, will eventually align with one another across the intervening membranes to form “paired vesicles”.2 While founder cells and fusion-competent myoblasts were not identified in this ultrastructural analysis, we infer that the vesicles appear in both cell types as well as in the developing myotube and associated fusion-competent cells.
After alignment, the vesicles are thought to resolve into electron dense plaques, termed “prefusion complexes”, that are observed on the cytoplasmic sides of the corresponding plasma membranes. These plaques are reminiscent of structures identified in fusing vertebrate myoblasts that are also thought to result from the fusion of electron dense vesicles to the plasma membrane.3 As vesicles are being recruited and plaques form at sites of cell-cell contact, the myoblasts elongate to maximize contact points. Multiple pores are then observed in the fusing membranes, adjacent to the electron dense fusion plaques. This morphology suggests that fusion occurs at multiple sites,2 similar to that seen previously in the Lepidopteran Antheraea polyphemus.4 Hence it does not appear that myoblast fusion in Drosophila utilizes a simple zipper mechanism in which it originates from a single site. The fusion process is completed as the membrane vesiculates and is removed. In an effort to advance our understanding of genes associated with these morphological changes, Doberstein et al2 carried out similar studies on mutant embryos in which myoblast fusion does not occur to determine the point at which fusion was arrested. The results of these studies are discussed at appropriate places below and indicated in the schematic in Figure 1.
Myoblast Recognition and Adhesion
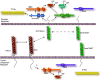
Prior to myoblast fusion in the Drosophila embryo, fusion-competent myoblasts must identify, migrate to, and adhere to the founder cell with which they will eventually fuse. Genetic studies in Drosophila have identified three members of the Immunoglobulin Superfamily (IgSF) that are essential for myoblast recognition and adhesion (reviewed in refs. 5-8; shown in fig. 2 and/or fig. 3). These include sticks and stones (sns),9 dumfounded/kin of irreC (duf/kirre)10 and irregular-chiasm-C/roughest (irreC/rst).6 The hibris (hbs) gene, which encodes a fourth IgSF member in Drosophila myoblasts, appears to regulate the fusion process.5,11,12
Sticks and Stones (sns)
The sns locus was identified on the basis of its mutant phenotype in embryos, in which there is a complete absence of multinucleate muscle fibers and a correspondingly large number of unfused Myosin-expressing myoblasts (fig. 2).9 The unfused fusion-competent myoblasts do not extend filopodia towards the founder cells, suggesting that recognition of these cells does not occur in the absence of sns. Consistent with a role at the cell surface, sns is predicted to encode a cell adhesion molecule with 8 extracellular immunoglobulin-like (Ig-like) domains, a single fibronectin type-III (FN-III) domain, a transmembrane region, and a cytoplasmic region. The sns transcript and protein are expressed exclusively in the fusion-competent cells of the somatic (fig. 3)9 and visceral13,14 musculature, and no expression is observed in the founder cells. sns protein is evident just prior to fusion and decreases rapidly as fusion is completed. Both biochemical and wholemount embryo analyses have indicated that it localizes to the plasma membrane of myoblasts prior to fusion.9 sns shares homology with human Nephrin, which has been implicated in Congenital Nephrotic Syndrome,15,16 Drosophila Hbs11,12 and C. elegans SYG-2.61 Of note, genetic and molecular studies have revealed that mutations in the sns locus9 and EMS-induced mutants in the rolling stone (rost),17,18 locus are actually allelic, and map to the genetic position of sns.9,19 This finding is particularly relevant because the EMS-induced rost15 mutation, which was examined at the ultrastructural level by Doberstein,2 actually represents a mutation in sns. Interestingly, it appears that fusion is arrested at a fairly late stage in these mutant embryos, since electron dense plaques accumulate but the plasma membrane does not breakdown. However, since the sequence lesion in the rost15 has not yet been identified, it may be a hypomorphic allele in which the sns protein is partially functional. Support for this possibility is provided by the mutant phenotype of rost15 alleles, in which myoblast fusion does not appear to be completely blocked.18
The membrane localization of SNS in combination with its identification as a cell adhesion molecule suggest the possibility that SNS may mediate recognition and/or adhesion between founder cells and fusion-competent myoblasts. On closer examination of the sns mutant phenotype, it is also apparent that the fusion-competent myoblasts are unable to migrate toward founder myoblasts. This myoblast behavior is in contrast to that observed in other fusion mutant backgrounds, such as myoblast city (mbc) and blown fuse (blow),20 in which myoblasts have clearly associated with each other but fusion does not proceed (Fig. 2). Thus, SNS seems to act as a receptor on the surface of fusion-competent myoblasts that responds to an attractant generated by the founder cells, inducing migration via activation of an intracellular signal transduction cascade. Consistent with this possibility, the cytoplasmic region of SNS contains potential phosphorylation sites, proline rich regions, and stretches of homology with related proteins that may mediate interaction with cytoplasmic proteins. The presence of these motifs may indicate that it directs intracellular events that regulate myoblast migration, recognition and/or adhesion. Consistent with this hypothesis, the transmembrane and cytodomains are essential for SNS function (Banerji and Abmayr, unpublished). Moreover, candidate extracellular ligands for SNS that serve as attractants for fusion-competent cells have been identified, 6,10 and are discussed below.
Dumbfounded/Kin of Irregular Chiasm C (Duf/Kirre) and Kin of Irregular Chiasm C/Roughest (Irrec/Rst)
Complementing expression of SNS in the fusion-competent myoblasts is the expression of two other IgSF members in the founder cells. One gene, dumbfounded/kin of irreC (duf/kirre), was identified through its association with the founder cell specific enhancer trap line rP298,10 which directs expression of β-galactosidase specifically in the founder cells.21 It is predicted to encode a single pass membrane spanning protein with 5 extracellular Ig-like domains and a cytoplasmic tail.10 Early in development, expression of the duf/kirre transcript is observed at low levels in the developing mesoderm. This broad expression becomes restricted to a limited number of cells in the embryo from which the founder cells arise (as depicted in Fig. 3). It is not expressed in the fusion-competent cells. Like sns, the duf/kirre transcript remains detectable in muscle precursors as long as they are fusing, but its level drops quickly after fusion is completed.
A second member of the IgSF, irreC/rst, is located 127 kilobases (kb) away from duf/kirre. The irreC/rst gene was identified on the basis of defects in axonal projections in the adult brain.22 The encoded protein is 45% similar to Duf/Kirre, and includes 5 extracellular Ig-like domains, a transmembrane region and a highly homologous cytoplasmic tail.6,22,23 Interestingly, both Duf/Kirre and IrreC/RST share homology with Neph1 which interacts with Nephrin in the kidney,62 and SYG-1 which is the binding partner of SYG-2 in C.elegans.61,63
Like the duf/kirre transcript, IrreC/RST protein is detected in regions of the embryo where the founder cells and fusion-competent myoblasts arise. In contrast to Duf/Kirre, IrreC/RST is not restricted to the founder cells and is expressed in at least some fusion-competent myoblasts (as depicted in Fig. 3).6 While its role in these fusion-competent myoblasts remains unclear, IrreC/RST clearly serves a function redundant with that of Duf/Kirre in the founder cells. Specifically, examination of embryos bearing a deficiency that removes both genes revealed a complete absence of differentiated muscle fibers and large number of unfused Myosin-expressing myoblasts (Fig. 2).10 However, expression of either duf/kirre or irreC/rst in the mesoderm was sufficient to rescue this defect.6,10
Duf/Kirre and IrreC/RST both appear to act as attractants for fusion-competent myoblasts, since expression of either protein in the embryonic ectoderm is sufficient to target migration of these cells.6,10 Of much interest is the question of whether Duf/Kirre functions, in part, as a secreted form that sets up a concentration gradient recognized by the migrating fusion-competent cells. Consistent with this possibility, a cleaved form of Duf/Kirre is detected in transfected S2 cells,24 and a truncated ectodomain is detected in the culture media (Galletta and Abmayr, unpublished data). However, the fusion-competent myoblasts may also be capable of extending long processes that scan for, and contact, the founder cell.
Based on their mutant phenotypes, Duf/Kirre, IrreC/RST and SNS all play roles in the recognition of founder cells by fusion-competent cells. In the absence of sns, the fusion-competent cells remain round and do not appear to extend filopodia. In contrast, the fusion-competent cells of embryos lacking duf/kirre and irreC/rst extend filopodia, but these projections are randomly oriented rather than directed toward the founder cells.10 This behavior is consistent with a model in which the Duf/Kirre and IrreC/RST attractants may be ligands for the SNS receptor on the surface of fusion-competent cells, as depicted in Figure 3. Consistent with a role in adhesion of founder cell: fusion-competent cell recognition, these proteins are capable of mediating adhesive events in cultured cells. Though the biological relevance remains unclear, aggregation experiments in S2 cells indicate that Duf/Kirre and IrreC/RST mediate homotypic aggregation12,64 (also Chakravarti, Galletta and Abmayr, unpublished). More importantly, cells expressing SNS adhere to cells expressing Duf/Kirre or IrreC/RST, while expression of SNS alone is not sufficient to induce cell aggregation12 (also Chakravarti, Galletta and Abmayr, unpublished). It remains to be determined whether the interaction of SNS-expressing cells with cells expressing either Duf/Kirre or IrreC/RST reflects a direct molecular interaction between these molecules. However, the above cell interactions in conjunction with the restricted patterns of expression of these molecules appears to satisfy the need for directional fusion machinery in the embryo.
Hibris (hbs)
A fourth IgSF member that is expressed in the developing musculature, and plays a role in myoblast fusion, is encoded by hibris (hbs).11,12 Hbs was identified in a screen for transcripts that were differentially expressed in founder cells versus fusion-competent cells11 and, simultaneously, through database searches for members of the IgSF in Drosophila.12 Hbs is predicted to have 8 or 9 Ig-like domains, a FN-III domain, a transmembrane spanning region and a cytoplasmic region. Like SNS, the cytoplasmic region contains potential phosphorylation sites, tyrosine residues that are conserved with SNS and/or Nephrin, and a candidate PEST sequence. Hbs is 48% identical and 63% similar to SNS and 29% identical and 44% similar to human Nephrin.11 It is expressed earlier in development than SNS, and can be seen in precursors to tissues other than muscle that include the trachea and nervous system. Like SNS, Hbs expression becomes restricted to myoblasts by embryonic Stage 12. This expression includes a large subset of the fusion-competent cells but does not include the founder cells (Fig. 3).11,12 In cells that express both proteins, SNS and Hbs co-localize at discrete points on the cell surface.11
These features suggest that Hbs and SNS, like Duf/Kirre and IrreC/RST,23 might have arisen from a single gene and serve redundant functions. However, loss-of-function alleles have revealed that hbs plays a quite different role in myoblast fusion. Specifically, it is not essential for viability, and mutants survive to become semi-fertile adults. These flies do display a rough-eyed phenotype, consistent with hbs expression in imaginal discs11,12 hbs mutant embryos do, however, exhibit a modest increase in the number of unfused myoblasts, and may have missing or smaller muscles. However, these defects are not sufficient to impair survival. Like the embryonic loss-of-function phenotype, targeted expression of Hbs in the developing mesoderm causes muscle loss and an increase in the number of unfused myoblasts. This effect is mediated through the cytoplasmic domain, since expression of this domain alone mimics the overexpression phenotype11,12 and is not observed upon ectopic expression of either a secreted or membrane bound extracellular domain. Interestingly, the mild myoblast fusion defect of hbs mutant embryos is dominantly suppressed by loss of one copy of sns. Conversely, sns mutations enhance the phenotype seen by overexpression of Hbs in the mesoderm. Thus, an antagonistic interaction occurs between hbs and sns mutants, as depicted in Figure 3.11 Artero et al have proposed three models by which hbs and sns could antagonize one another during myoblast fusion. (1) SNS and Hbs could compete for the same extracellular ligand. This model is reasonable if Duf/ Kirre is the ligand for SNS, since Hbs-expressing cells interact heterotypically with Duf/Kirreexpressing cells in culture.12 However, the lack of myoblast fusion defects in embryos expressing the extracellular domain of Hbs argues against this hypothesis. (2) Hbs and SNS may combine to form a “negative” receptor. In this scenario the Hbs/SNS coreceptor may respond differently to ligand than the “positive” SNS receptor. (3) Hbs and SNS may converge on an intracellular downstream target that plays a role in regulating fusion. It must be noted that all of these models accommodate Hbs as a nonessential regulator of SNS function. However, further study will be necessary to understand the exact role of Hbs in myoblast fusion.
Intracellular Events Associated with Myoblast Fusion
Following recognition and adhesion between myoblasts, cytoplasmic machinery to direct migration and membrane fusion must be in place. One enticing possibility is that the cell surface molecules involved in recognition and adhesion may themselves initiate intracellular signaling events via their cytoplasmic tails, and play a role in recruiting muscle specific machinery to the sites of fusion. A second possibility is that myoblast fusion will require more broadly-expressed proteins for reorganization of the cytoskeleton that underlies the fusing membranes. Lastly, cytoplasmic events may bring about morphological changes associated with migration, perhaps also requiring cytoskeletal rearrangement. It remains to be determined whether the intracellular events directing migration and fusion involve the same molecules or converge on a common pathway. Proteins that may function in these processes and/or interact with IgSF members have been identified and are discussed below.
The Antisocial/Rolling Pebbles (Ants/Rols)-Myoblast City(MBC)-Drosophila Rac1(Drac1) Associated Pathway
Mutant myoblast city (mbc) embryos are characterized by the absence of multinucleate muscle fibers and presence of large numbers of unfused myoblasts (Fig. 2).25 The morphology of these mutant embryos differs from that seen in embryos lacking either sns or duf/kirre and irreC/rst in that the unfused fusion-competent cells migrate to, and cluster around, the founder cells.20 Thus, MBC appears to be required for fusion but not necessarily migration. At the ultrastructural level, the number of prefusion complexes seen in these embryos was significantly reduced, suggesting that it might play a role in vesicle accumulation.2 The mbc locus encodes a cytoplasmic protein with extensive homology to C. elegans Ced-526 and human Dock18027 All three of these proteins contain src homology 3 (SH3) domains at their N-terminus, multiple proline-rich, putative SH3 binding domains at their C-terminus, and additional blocks of homology that may be associated with guanine nucleotide exchange activity (see below). MBC is expressed in a wide variety of tissues in the developing embryo.28 Consequently, the defects seen in mbc mutant embryos are not limited to myoblast fusion, and include incomplete dorsal closure of the epidermis, abnormal fasciculation of the ventral nerve cord neurons, and severely impaired migration of border cells in the adult ovary.28-30 Orthologs of mbc are involved in diverse processes that include cell engulfment, cell migration, epithelial morphogenesis and oncogenic transformation.26,27 While these processes appear diverse at first glance, they have in common the potential reorganization of the cytoskeleton. In fact, mbc mutant embryos exhibit perturbations in the actin cytoskeleton along the leading edge of the migrating epidermis during dorsal closure.28
Numerous studies have suggested that the small GTPase Rac1 is a major target of the MBC/ Dock-180/Ced-5 family (Fig. 3),30-35 and is associated with these cytoskeletal changes. Rac1 is a member of the Rho family of small GTPases, which are often associated with regulation of changes in the cytoskeleton. In vertebrate cell culture systems, two groups have recently demonstrated that a conserved region of Dock 180, termed the “Docker” domain or “Dock Homology Region-2”(DHR-2),36,37 functions as an unconventional guanine nucleotide exchange factor (GEF) for Rac1. Expression of the DHR-2 region increases the GTP loading of Rac1 in 293-T cells36 and appears to be dependent on coexpression of the ELMO1 protein. ELMO1 is the vertebrate ortholog of C. elegans Ced-12 which, like Ced-5, was identified on the basis of a defect in the engulfment of cell corpses.26,31,38-40 These data provide compelling evidence that Dock180 and Ced-5 function through Rac1. In the Drosophila musculature, expression of dominant negative or constitutively active forms of DRac1 in the mesoderm causes defects in myoblast fusion,1 presumably by interfering with critical cytoskeletal events. Ultrastructural analysis of fusing myoblasts in embryos expressing constitutively active DRac1 revealed the presence of prefusion complexes, electron dense vesicles and isolated fusion pores. The plasma membranes of the myoblasts are in close apposition, but do not appear to vesiculate.2 For these reasons, constitutively active Rac1 is thought to block myoblast fusion at a relatively late step. Recently, double mutant loss-of-function alleles of Drac1 and Drac2 have been shown to exhibit significant defects in myoblast fusion,42 as anticipated from analysis of embryos expressing the above constructs. In addition, Drac1 and mbc have been linked genetically in the eye, in which loss of one copy of mbc suppresses the overexpression phenotype of Drac1.30
A biochemical link between MBC, and associated DRac1, and the IgSF members discussed earlier is provided by the Antisocial (Ants) /Rolling pebbles (Rols) protein (Fig. 3). Ants/Rols was identified in three independent screens for genes affecting embryonic muscle development. 24,43,44 Myoblast fusion does not occur in ants/rols mutant embryos. Ultrastructural analysis of these mutant embryos revealed small syncitia containing 2-4 nuclei.44 However fusion did not proceed further, and neither prefusion complexes nor electron dense plaques were observed. The predicted Ants/Rols protein contains several domains with the potential to mediate protein-protein interaction, including a RING finger, 9 ankyrin repeats, 3 tetratricopeptide repeats (TPRs) and a coiled-coil region.24,43,44 In the embryo, Ants/Rols is expressed in the founder myoblasts at a time consistent with myoblast fusion. Moreover, Duf/Kirre and Ants/Rols are present on the myoblast membrane, and colocalize at points of cell:cell contact.43 Immunoprecipitations from cotransfected S2 cells has demonstrated that it can interact biochemically with the N-terminal region of MBC and with Duf/Kirre.24 In addition, the mbc locus was identified as a dominant enhancer of the irreC/rst eye phenotype, suggesting that MBC interacts with IrreC/RST.45 Together, these data support a model in which MBC is recruited by Duf/Kirre to discrete points on the membrane via Ants/Rols, leading to localized changes in the cytoskeleton through DRac1 (Fig. 3).
The Loner-Drosophila ADP Ribosylation Factor 6 (Darf6) Associated Pathway
The loner locus was identified in a genetic screen for embryos defective in myoblast fusion.46 In loner mutant embryos, the fusion-competent myoblasts appear to recognize and extend filopodia toward the founder cells but do not fuse into syncitia. Expression of Loner protein is restricted to founder cells (Fig. 3), where it is localized in punctate foci that, in some cases, include Ants/Rols.46 Interestingly, it loses this characteristic localization and becomes more diffuse within the cytoplasm of embryos lacking duf/kirre and irreC/rst. It is localized to discrete points in the cytoplasm of transfected S2 cells, but gets recruited to the membrane at sites of cell contact in the presence of Duf/Kirre. Loner localization is not affected in ants/rols mutant embryos and vice versa, suggesting that they function independently even though they can colocalize.
The Loner protein appears to function as a GEF, and is present in three different isoforms. All of these contain a Sec7 domain, a pleckstrin homology (PH) domain, a coiled coil domain and an IQ-motif. Sec7 domains are often directly associated with GEF activity toward ADP ribosylation factors (ARF), while PH domains enhance the activity of GEFs. Analysis of Loner deletion mutants, assayed by their ability to rescue the mutant phenotype, revealed that the Sec7 and PH domains are essential.46 In light of its homology to Sec7, Chen et al hypothesized that Loner functions as a GEF for the small GTPase dARF6, and demonstrated that the Sec7 domain was sufficient to cause GDP/GTP exchange in GDP release assays. Consistent with this association between Loner and dARF6, expression of a dominant negative form of dARF6T27N in founder cells disrupts the fusion process.46 ARF6 is known to act through membrane lipid modifications,47 and enhances Rac mediated actin cytoskeleton remodeling.48 Interestingly, Loner may also associate with DRac1, since DRac1 localization to discrete points in founder cells is lost in loner mutant embryos.46 Thus, Loner may provide an alternative to the MBC associated pathway for regulating DRac1. It may also function in an independent pathway to regulate cytoskeletal rearrangements and/or lipid modifications that are critical in the fusion process.
Other Genes with Roles in Myoblast Fusion
Several genes have been identified that appear to be involved in myoblast fusion on the basis of a mutant or overexpression phenotype, though their specific biochemical role remains unclear. The blown fuse (blow) locus was identified in a screen for lethal mutations defective in guidance of motoneurons.2 However blow is essential for myoblast fusion (Fig. 2), and the neuronal defect is an indirect consequence of the lack of somatic muscles. The blow transcript is restricted to myoblasts, and appears just prior to fusion. The predicted Blow protein, which contains a putative PH domain, appears to reside in the cytoplasm (Fig. 3).2 In blow mutant embryos, electron dense vesicles accumulate in prefusion complexes, but electron dense plaques are not observed. Assuming that plaques form from an association between vesicles and the plasma membrane, it is possible that Blow is involved in this step.
Two genes that have not been fully characterized also contribute to myoblast fusion. The singles bar gene appears to encode a hydrophobic protein that is essential for myoblast fusion,49 though the fusion-competent myoblasts migrate to the founder cells.10 This mutant phenotype suggests that singles bar functions after cell migration and adhesion, possibly in membrane associated events critical during the fusion process. Embryos mutant for the bona fide rolling stone (rost) locus, originally identified through a P-element line with insertions in both the rost and sns loci,9,19 have defects in myoblast fusion.17,18 The transcript associated with this locus is expressed in the mesoderm, and a promoter-lacZ reporter that includes 400 bp upstream of the ATG is expressed in mature muscles.17 The predicted Rost protein is hydrophobic and may span the membrane up to 7 times. It is enriched in biochemical preparations of partially-purified membranes,17 supporting its association with the membrane. While the true rost loss-of-function phenotype remains unclear, embryos expressing antisense rost do exhibit a block in myoblast fusion.17
D-Titin, a protein of approximately 2.0 MDa, that is composed primarily of Ig-like, FN-III and PEVK domains,50,51 serves as an elastic scaffold for both muscle sarcomeres and for chromosomes. 51-53 It is expressed at a high level in myoblasts prior to fusion and appears to accumulate on the myoblast surface, often at points of contact between myoblast and myotube.50,51 Interestingly, recruitment of D-Titin to points of cell-cell contact in founder myoblasts is dependent on the Ants/Rols protein discussed above (Fig. 3).43 Analysis of D-Titin mutant embryos has shown that it is essential for the integrity of the sarcomere, but also plays a role in myoblast fusion.50,51 Thus, it is possible that D-Titin is playing a role in either directly or indirectly organizing the actin cytoskeleton. The involvement of a muscle structural protein in the myoblast fusion process is not unique to Titin. Recent studies from the Bernstein lab54 have shown that Paramyosin plays a role in myoblast fusion and accumulates at discrete points between fusing myoblasts. Lastly, a biochemical screen for proteins that interact with MBC led to the identification of dCrk, a cytoplasmic adapter protein with one SH2 domain and two SH3 domains.60 Mutants in the dCrk locus have not been described. However, expression of a membrane-targeted myristylated form of dCrk in the musculature severely perturbs myoblast fusion (Galletta and Abmayr, unpublished data).
The genes described above likely do not represent the entire complement of molecules in the founder cells and fusion-competent myoblasts that are required for fusion. Efforts to identify additional genes have included traditional genetic approaches as well as targeted molecular approaches, with a variety of results. For example, the utilization of green fluorescent protein (GFP) to mark muscle cells dramatically increased the feasibility of phenotypic screens, and Chen et al are likely to have identified loss-of-function alleles of other critical loci. A microarray approach was carried out by Furlong et al,55 and utilized twist deficient embryos to identify loci preferentially expressed in the mesoderm55 This screen resulted in the isolation of gleeful (gfl), independently identified by two other groups and termed lameduck (lmd) and myoblasts incompetent (minc).56,57 The lmd/minc/gfl gene, is involved in specification of the fusion-competent myoblasts. Many other genes identified in this screen remain excellent candidates, and simply await functional analysis. These include kinases, actin binding proteins, Calcium binding molecules and small GTPases. Identification of transcripts differentially expressed in the founder cells versus the fusion-competent myoblasts has been mentioned earlier in the context of the discovery of the hbs locus. This screen has yielded other potential players in the fusion process, 58 including cytoplasmic factors such as kinases or proteins that can mediate protein:protein interactions. The general usefulness of this approach in identifying functionally important genes will require further characterization of these candidate proteins. Finally, a genome-wide protein-interaction map was generated, and has identified proteins that could be components of the myoblast fusion pathway on the basis of their ability to interact with molecules described earlier in this chapter.59 The relevance of the proteins identified in this screen also await functional analysis. Nevertheless, the large number of candidates identified in these screens has the potential for significant impact on the field of myoblast fusion.
Conclusion
In the last decade, remarkable advances have been made in our understanding of Drosophila myogenesis in general. Similarly, knowledge of the molecules through which the embryonic myoblasts migrate, recognize and fuse to each other did not exist a decade ago. Many advances have been made possible by new technologies that pair molecular biology with genetics or cell biology, and include in situ hybridization, confocal microscopy, large-scale genome wide genetic screens, and the availability of the D. melanogaster genome sequence to name a few. Nevertheless, there is much more to be learned. Among the issues to be investigated are the downstream pathways associated with binding of adhesion molecules at the cell surface, the molecular components of the fusion associated vesicles, details of the fusion process itself, and the mechanism through which muscle size is determined. Hopefully the rate of progress will lead to significant advances in our understanding of these processes in another decade.
References
- 1.
- Baylies MK, Bate M, Ruiz-Gomez M. Myogenesis: A view from Drosophila. Cell. 1998;93(6):921–927. [PubMed: 9635422]
- 2.
- Doberstein SK, Fetter RD, Mehta AY, Goodman CS. Genetic analysis of myoblast fusion: Blown fuse is required for progression beyond the prefusion complex. J Cell Biol. 1997;136(6):1249–1261. [PMC free article: PMC2132517] [PubMed: 9087441]
- 3.
- Rash JE, Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol. 1973;30:166–186. [PubMed: 4735364]
- 4.
- Stocker RF. Electron microscopic observations on the fusion of myoblasts in Antheraea polyphemus (Lepidoptera). Experientia. 1974;30(8):896–898. [PubMed: 4409428]
- 5.
- Dworak HA, Sink H. Myoblast fusion in Drosophila. Bioessays. 2002;24(7):591–601. [PubMed: 12111720]
- 6.
- Strunkelnberg M, Bonengel B, Moda LM. et al. rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development. 2001;128(21):4229–4239. [PubMed: 11684659]
- 7.
- Taylor MV. Muscle development: Molecules of myoblast fusion. Curr Biol. 2000;10(17):R646–648. [PubMed: 10996092]
- 8.
- Taylor MV. Muscle differentiation: How two cells become one. Curr Biol. 2002;12(6):R224–228. [PubMed: 11909553]
- 9.
- Bour BA, Chakravarti M, West JM. et al. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 2000;14(12):1498–1511. [PMC free article: PMC316690] [PubMed: 10859168]
- 10.
- Ruiz-Gomez M, Coutts N, Price A. et al. Drosophila dumbfounded: A myoblast attractant essential for fusion. Cell. 2000;102(2):189–198. [PubMed: 10943839]
- 11.
- Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by ras and notch signaling. Development. 2001;128(21):4251–4264. [PubMed: 11684661]
- 12.
- Dworak HA, Charles MA, Pellerano LB. et al. Characterization of Drosophila hibris, a gene related to human nephrin. Development. 2001;128(21):4265–4276. [PubMed: 11684662]
- 13.
- San MartinB, Bate M. Hindgut visceral mesoderm requires an ectodermal template for normal development in Drosophila. Development. 2001;128(2):233–242. [PubMed: 11124118]
- 14.
- Klapper R, Stute C, Schomaker O. et al. The formation of syncytia within the visceral musculature of the Drosophila midgut is dependent on duf, sns and mbc. Mech Dev. 2002;110(1-2):85–96. [PubMed: 11744371]
- 15.
- Kestila M, Lenkkeri U, Mannikko M. et al. Positionally cloned gene for a novel glomerular protein-Nephrin-is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:572–582. [PubMed: 9660941]
- 16.
- Lenkkeri U, Mannikko M, McCready P. et al. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet. 1999;64:51–61. [PMC free article: PMC1377702] [PubMed: 9915943]
- 17.
- Paululat A, Goubeaud A, Damm C. et al. The mesodermal expression of rolling stone (rost) is essential for myoblast fusion in Drosophila and encodes a potential transmembrane protein. J Cell Biol. 1997;138:337–348. [PMC free article: PMC2138187] [PubMed: 9230076]
- 18.
- Paululat A, Burchard S, Renkawitz-Pohl R. Fusion from myoblasts to myotubes is dependent on the rolling stone gene (rost) of Drosophila. Development. 1995;121:2611–2620. [PubMed: 7671823]
- 19.
- Paululat A, Holz A, Renkawitz-Pohl R. Essential genes for myoblast fusion in Drosophila embryogenesis. Mech Dev. 1999;83(1-2):17–26. [PubMed: 10507836]
- 20.
- Abmayr SM, Balagopalan L, Galletta BJ. et al. Cell and molecular biology of myoblast fusion. Int Rev Cytol. 2003;225:33–89. [PubMed: 12696590]
- 21.
- Nose A, Isshiki T, Takeichi M. Regional specification of muscle progenitors in Drosophila: The role of the msh homeobox gene. Development. 1998;125:215–223. [PubMed: 9486795]
- 22.
- Ramos RG, Igloi GL, Lichte B. et al. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 1993;7(12B):2533–2547. [PubMed: 7503814]
- 23.
- Strunkelnberg M, de CouetHG, Hertenstein A. et al. Interspecies comparison of a gene pair with partially redundant function: The rst and kirre genes in D. virilis and D. melanogaster. J Mol Evol. 2003;56(2):187–197. [PubMed: 12574866]
- 24.
- Chen EH, Olson EN. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev Cell. 2001;1(5):705–715. [PubMed: 11709190]
- 25.
- Rushton E, Drysdale R, Abmayr SM. et al. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. [PubMed: 7635046]
- 26.
- Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392(6675):501–504. [PubMed: 9548255]
- 27.
- Hasegawa H, Kiyokawa E, Tanaka S. et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol. 1996;16(4):1770–1776. [PMC free article: PMC231163] [PubMed: 8657152]
- 28.
- Erickson MRS, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure and cytoskeletal organization. J Cell Biol. 1997;138(3):589–603. [PMC free article: PMC2141626] [PubMed: 9245788]
- 29.
- Duchek P, Somogyi K, Jekely G. et al. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107(1):17–26. [PubMed: 11595182]
- 30.
- Nolan KM, Barrett K, Lu Y. et al. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. [PMC free article: PMC317223] [PubMed: 9808621]
- 31.
- Gumienny TL, Brugnera E, Tosello-Trampont AC. et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107(1):27–41. [PubMed: 11595183]
- 32.
- Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2(12):899–905. [PubMed: 11146654]
- 33.
- Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in C. elegans. Nat Cell Biol. 2000;2(3):131–136. [PubMed: 10707082]
- 34.
- Kiyokawa E, Hashimoto Y, Kobayashi S. et al. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12(21):3331–3336. [PMC free article: PMC317231] [PubMed: 9808620]
- 35.
- Kiyokawa E, Hashimoto Y, Kurata T. et al. Evidence that DOCK180 up-regulates signals from the CrkII-p130Cas complex. J Biol Chem. 1998;273:24479–24484. [PubMed: 9733740]
- 36.
- Cote JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115(Pt 24):4901–4913. [PubMed: 12432077]
- 37.
- Brugnera E, Haney L, Grimsley C. et al. Unconventional Rac-GEF activity is mediated through the dock180-ELMO complex. Nat Cell Biol. 2002;4(8):574–582. [PubMed: 12134158]
- 38.
- Zhou Z, Caron E, Hartwieg E. et al. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell. 2001;1(4):477–489. [PubMed: 11703939]
- 39.
- Wu YC, Tsai MC, Cheng LC. et al. C. elegans CED-12 acts in the conserved crkII/DOCK180/ Rac pathway to control cell migration and cell corpse engulfment. Dev Cell. 2001;1(4):491–502. [PubMed: 11703940]
- 40.
- Chung S, Gumienny TL, Hengartner MO. et al. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol. 2000;2(12):931–937. [PubMed: 11146658]
- 41.
- Luo L, Liao YJ, Jan LY. et al. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. [PubMed: 7958857]
- 42.
- Hakeda-Suzuki S, Ng J, Tzu J. et al. Rac function and regulation during Drosophila development. Nature. 2002;416(6879):438–442. [PubMed: 11919634]
- 43.
- Menon SD, Chia W. Drosophila rolling pebbles: A multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast attractant dumbfounded. Dev Cell. 2001;1(5):691–703. [PubMed: 11709189]
- 44.
- Rau A, Buttgereit D, Holz A. et al. rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development. 2001;128(24):5061–5073. [PubMed: 11748142]
- 45.
- Tanenbaum SB, Gorski SM, Rusconi JC. et al. A screen for dominant modifiers of the irreC-rst cell death phenotype in the developing Drosophila retina. Genetics. 2000;156(1):205–217. [PMC free article: PMC1461222] [PubMed: 10978286]
- 46.
- Chen EH, Pryce BA, Tzeng JA. et al. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114(6):751–762. [PubMed: 14505574]
- 47.
- Donaldson JG. Multiple roles for Arf6: Sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278(43):41573–41576. [PubMed: 12912991]
- 48.
- Donaldson JG. Myoblasts fuse when loner meets ARF6. Dev Cell. 2003;5(4):527–528. [PubMed: 14536050]
- 49.
- Maeland AD, Bloor JW, Brown NH. Characterization of singles bar a new mutant in Drosophila melanogaster required for muscle development. Mol Cell Biol. 1996;7:39a.
- 50.
- Zhang Y, Featherstone D, Davis W. et al. Drosophila D-titin is required for myoblast fusion and skeletal muscle striation. J Cell Sci. 2000;113(Pt 17):3103–3115. [PubMed: 10934048]
- 51.
- Machado C, Andrew DJ. D-Titin: A giant protein with dual roles in chromosomes and muscles. J Cell Biol. 2000;151(3):639–652. [PMC free article: PMC2185597] [PubMed: 11062264]
- 52.
- Gregorio CC, Granzier H, Sorimachi H. et al. Muscle assembly: A titanic achievement? Curr Opin Cell Biol. 1999;11(1):18–25. [PubMed: 10047523]
- 53.
- Trinick J, Tskhovrebova L. Titin: Aa molecular control freak. Trends Cell Biol. 1999;9(10):377–380. [PubMed: 10481174]
- 54.
- Liu H, Mardahl-Dumesnil M, Sweeney ST. et al. Drosophila paramyosin is important for myoblast fusion and essential for myofibril formation. J Cell Biol. 2003;160(6):899–908. [PMC free article: PMC2173770] [PubMed: 12642615]
- 55.
- Furlong EE, Andersen EC, Null B. et al. Patterns of gene expression during Drosophila mesoderm development. Science. 2001;293(5535):1629–1633. [PubMed: 11486054]
- 56.
- Duan H, Skeath JB, Nguyen HT. Drosophila Lame duck, a novel member of the gli superfamily, acts as a key regulator of myogenesis by controlling fusion-competent myoblast development. Development. 2001;128(22):4489–4500. [PubMed: 11714674]
- 57.
- Ruiz-Gomez M, Coutts N, Suster ML. et al. myoblasts incompetent encodes a zinc finger transcription factor required to specify fusion-competent myoblasts in Drosophila. Development. 2002;129(1):133–141. [PubMed: 11782407]
- 58.
- Artero R, Furlong EE, Beckett K. et al. Notch and Ras signaling pathway effector genes expressed in fusion-competent and founder cells during Drosophila myogenesis. Development. 2003;130(25):6257–6272. [PubMed: 14602676]
- 59.
- Giot L, Bader JS, Brouwer C. et al. A protein interaction map of Drosophila melanogaster. Science. 2003 [PubMed: 14605208]
- 60.
- Galletta BJ, Niu XP, Erickson MR. et al. Identification of a Drosophila homologue to vertebrate Crk by interaction with MBC. Gene. 1999;228(1-2):243–52. [PubMed: 10072777]
- 61.
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116(6):869–81. [PubMed: 15035988]
- 62.
- Donoviel DB, Freed DD, Vogel H. et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21(14):4829–36. [PMC free article: PMC87176] [PubMed: 11416156]
- 63.
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112(5):619–30. [PubMed: 12628183]
- 64.
- Schneider T, Reiter C, Eule E. et al. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15(2):259–71. [PubMed: 7646884]
- Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion - Madame Curie Bi...Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion - Madame Curie Bioscience Database
- Development of the Larval Somatic Musculature - Madame Curie Bioscience DatabaseDevelopment of the Larval Somatic Musculature - Madame Curie Bioscience Database
- Tissue Engineering Constructs and Commercialization - Madame Curie Bioscience Da...Tissue Engineering Constructs and Commercialization - Madame Curie Bioscience Database
- Electrophysiological Simulation of Developmental Changes in Action Potentials of...Electrophysiological Simulation of Developmental Changes in Action Potentials of Cardiomyocytes - Madame Curie Bioscience Database
- A Guide to Modeling Reaction-Diffusion of Molecules with the E-Cell System - Mad...A Guide to Modeling Reaction-Diffusion of Molecules with the E-Cell System - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...