NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Recent data demonstrate that estrogens and their receptors play an important role in modulating cholangiocyte proliferation. Rat cholangiocytes, in fact, express estrogen receptors (ER)-α and -β subtypes, which are overexpressed in cholangiocytes proliferating after bile duct ligation (BDL) in the rat, in association with enlarged bile duct mass and with enhanced estradiol serum levels. Estrogen antagonists (tamoxifen, ICI 182, 780) impair cholangiocyte proliferation, during BDL, and activate Fas mediated apoptosis. In addition, ovariectomy depresses cholangiocyte proliferation during BDL an effect reversed by estrogen replacement treatment. Definitive evidences on the role of estrogens as modulators of cholangiocyte proliferation derive by studies in vitro showing how 17-beta-estradiol induces cholangiocyte proliferation. As far as the intracellular pathways are concerned, we demonstrate that estrogens induce proliferation by activating the Src/Shc/ERK pathway in cholangiocytes suggesting a possible synergistic effect with growth factors. In conclusion, our data indicate that estrogens are important modulators of cholangiocyte proliferation and this open new interesting perspectives for the physiopathology and treatment of cholangiopathies, a group of chronic cholestatic liver diseases preferentially affecting the female sex.
Introduction
Cholangiocytes are the epithelial cells which line the intrahepatic bile ducts and which participate in the process of bile formation through the manipulation of native hepato-canalicular bile.1 Cholangiocytes are the preferential target of damage in a group of chronic cholestatic liver diseases called cholangiopathies and recently classified as vanishing bile duct syndromes.1-4 These diseases, which include primary biliary cirrhosis and primary sclerosing cholangitis, share common pathological features including the damage and disappearance of interlobular bile ducts, the proliferation of residual ducts and intralobular cholestasis.1-4 The progressive disappearance of intrahepatic bile ducts leads to a severe ductopenic condition in terminal stages. In the early stage of the disease, the disappearance of bile ducts is compensated by the proliferation of residual ducts.1-4 Thus, the course of these diseases is characterized by a balance between damage (loss) of bile ducts and compensatory proliferation of the residual ducts. In the terminal decompensated stage, the inefficacy of proliferation to balance for the loss of intrahepatic bile ducts leads to the clinical manifestations of overt cholestasis.1-4 Therefore, the design of a therapeutic strategy aimed at supporting an efficacious cholangiocyte proliferation could delay the progression to ductopenia and this represents a challenge for the future. For these reasons mechanisms and agents involved in the modulation of cholangiocyte proliferation were extensively investigated, in the last years, especially at the experimental level. To this latter regard, the experimental model of the common bile duct ligated (BDL) rat was very helpful since the selective cholangiocyte proliferation typical of BDL rats induce a marked increase in the mass of intrahepatic bile ducts and cholangiocytes which arrive to represent more than 30% of parenchymal hepatic cells (normally less than 2%).5 Thanks to recent studies, we now know that cholangiocyte proliferation is regulated by several growth factors, hormones, neuropeptides and by bile salts (BS). As far as hormones are concerned, acetylcholine and activators of adenylate cyclase (i.e., forskolin) induce cholangiocyte proliferation while somatostatin and gastrin display inhibitory effects.1,2
Estrogens are important inducers of growth and differentiation in target cells expressing estrogen receptors (ER).6 In the liver, ER are expressed in hepatocytes where estrogens regulate the process of growth and regeneration.6 After partial hepatectomy for example, the expression of ER in hepatocytes increases in association with their translocation to the nucleus where they induce DNA synthesis and favor the restoration of a normal liver mass.6-10 It is also known that estrogens are involved in the liver growth of neonates and that their chronic administration, for pharmacological purposes, in adults results in an enlargement of liver mass.6-10 Sex steroids may also influence the development and course of several liver diseases. Several studies, in fact, have addressed the relationship between ER expression, estrogen metabolism and biliary excretion and the development and course of chronic liver diseases.6-10
Although almost nothing is known on the expression of ER in the intrahepatic biliary epithelium or on the role of estrogens in the pathophysiology of the intrahepatic biliary tree, the relationship between estrogens and cholangiopathies has been often taken into consideration. Estrogens and their metabolites, in fact, have been hypothesized to have a pathogenic role in these diseases, which preferentially affect the female sex.11 Furthermore, marked alterations of estrogen hepatic metabolism occurs in cholestasis which is one of the hallmarks of cholangiopathies, including the decreased hepatic levels of P450-dependent microsomal enzymes with a consequent enhanced estradiol serum level.12 In addition, indirect evidence suggests the presence of endocrine dysfunctions in primary biliary cirrhosis (PBC), which is the most common acquired cholangiopathy.13 These dysfunctions include an increased incidence of menstrual disturbance and hysterectomy and high incidence of osteoporosis in post-menopausal women, which is a sign of estrogenic functional deficiency.11-13
Estrogens Modulate Cholangiocyte Proliferation: Experimental Evidences
We have recently investigated the occurrence of ER-α and -β subtypes in the rat intrahepatic biliary epithelium and the effect and mechanisms of estrogen in the modulation of cholangiocyte proliferation.14 By both immunohistochemistry and western blot, we found that rat cholangiocytes express both ER-α and -β subtypes while hepatocytes only express ER-α.14 Quantitative immunoblotting revealed that cholangiocyte proliferation after BDL is associated with a marked increase in the expression of ER and especially the ER-β while, hepatocytes which do not proliferate after BDL, display a ten-fold decrease of ER-α protein expression (fig. 1). Seeing that estradiol accumulates in the serum during BDL, we speculated that the enhanced estradiol serum levels contribute in modulating cholangiocyte proliferation during experimental cholestasis probably by potentiating the effects of growth factors. The role of estrogens in modulating cholangiocyte proliferation was further confirmed by experiments showing that, when BDL rats were treated with tamoxifen or the pure ER antagonist, ICI 182,780, the intrahepatic bile duct mass was markedly decreased in comparison with control BDL rats. This was caused by both impaired cholangiocyte proliferation, as demonstrated by the decreased PCNA (proliferating cellular nuclear antigen) immunohistochemical expression, and enhanced apoptotic cell death of proliferating cholangiocytes, demonstrated by the increased TUNEL positivity. Interestingly, TUNEL positivity is associated with enhanced Fas expression and this suggests that the overexpression of Fas occurs as a primary event in the activation of apoptotic cascade by ER antagonists. In breast cancer and hepatocellular carcinoma, tamoxifen induces apoptosis by multiple mechanisms including the blocking of the mitogenic effect of estrogens and induction of apoptosis-related genes.15 To this latter regard, the Fas receptor/Fas ligand pathway, the best characterized model of apoptosis, plays a crucial role in tamoxifen induced apoptosis in hepatocellular carcinoma and in cholangiocarcinoma cell lines.16-20 In the biliary epithelium, the Fas antigen was demonstrated by immunohistochemistry in primary biliary cirrhosis and primary sclerosing cholangitis,19 where the dysregulation of apoptosis could play an important role in the pathogenesis and progression of the disease. The most likely explanation of our findings is that when the mitogenic effects of estrogens are blocked by their antagonists, cholangiocyte proliferation is impaired and apoptosis is activated by a Fas-dependent mechanism. However, other mechanisms of apoptosis activation especially for tamoxifen cannot be excluded.
Definitive data in support of the positive modulatory effect of estrogens on cholangiocyte proliferation come from in vitro experiments showing that both PCNA protein expression and 3H-thymidine incorporation into DNA of isolated rat cholangiocytes were significantly increased by 10 nM 17β-estradiol and that these effects were individually blocked by two distinct ER antagonists (tamoxifen, ICI 182,780).14
Role of Endogenous Estrogens in the Modulation of Cholangiocyte Proliferation
To further investigate the role of endogenous estrogens on modulating cholangiocyte proliferation during experimental cholestasis, we evaluated the effects of ovariectomy (OVX) and estrogen replacement treatment on the bile duct mass of BDL rats.21 OVX rats were submitted to BDL for three weeks and the bile duct mass was compared with control BDL rats submitted to sham-OVX and with BDL-OVX rats treated with exogenous administration of 17-β estradiol (estrogen replacement treatment). We found that OVX induced a significant reduction of bile duct mass in BDL rats. The reduction of bile duct mass induced by OVX was associated with a decreased expression of ER-α (2.5 fold) and mainly ER-β (35 fold). PCNA expression in cholangiocytes was impaired by OVX indicating depression of proliferation, whereas TUNEL and Fas positivity were markedly enhanced indicating activation of Fas-mediated apoptosis. Administration of 17-β estradiol during BDL in OVX rats induced a normalization of bile duct mass, ER expression, cholangiocyte proliferation (PCNA) and apoptosis (Fas and TUNEL) in comparison with untreated BDL rats. These findings further support the role of endogenous estrogens in sustaining the enhanced proliferative activities of cholangiocyte in cholestasis (fig. 1). On the basis of these data, the hypothesis of an estrogenic functional deficiency in chronic cholestatic liver diseases should merit careful attention.
Intracellular Signaling Pathways Activated by Estrogens in Cholangiocytes
The signaling pathways triggering cholangiocyte proliferation in BDL and those activated by estrogens are unknown. In general, estrogens may trigger the proliferation of target cells expressing ER by acting trough a direct (genomic) pathway, where the activated receptor directly induces in the nucleus the transcriptional machinery or, alternatively, by an indirect (non genomic) pathway where a cascade of protein-protein interactions with different transcription factors is activated.22-25 In the uterus, for example, estrogens activate a series of phosphorylation events in the Ras/Raf/MAPKinases signaling cascade.26 This signaling cascade, typically activated by growth factors acting through tyrosine kinase receptors, involve the recruitment of the steroid receptor-coactivator (Src) and adapter protein Shc (Src-homology/collagen protein) which act upstream to the mitogen-activated protein (MAP) kinase isoforms ERK1/2 (extracellular signal-regulated kinase).22-25,27 On this basis, a cross-talk between estrogens and growth factors, including IGF1 (insulin like growth factor) may result in a synergistic growth stimulation.28 To elucidate, the intracellular signaling pathways activated by estrogen during stimulation of cholangiocyte proliferation, we performed a series of in vivo and in vitro experiments in BDL rats and isolated cholangiocytes. We found that cholangiocyte proliferation induced by BDL is associated with enhanced protein expression of total and phosphorylated ERK1/2 and of the adapter protein Shc in cholangiocytes as demonstrated by both immunohistochemistry in liver sections and western blot in cholangiocytes isolated from BDL rats. In addition, inhibition of cholangiocyte proliferation through administration of ER antagonists, tamoxifen and ICI 182,780, during 3 weeks of BDL, induced a decrease in ERK and Shc protein expression. These in vivo experiments suggests that when cholangiocyte proliferate during experimental cholestasis, an activation of the adapter protein Shc and ERK system occurs and this is modulated by estrogens given the inhibitory effects of their antagonists, tamoxifen and ICI 182, 780. To direct evaluate the role of estrogens, cholangiocyte proliferation was induced in vitro by incubation with 17β-estradiol. In this experimental conditions, enhanced protein expression (western-blot) of p-ERK1/2, Src and Shc was found. Very importantly, 17β-estradiol-induced cholangiocyte proliferation, in vitro, was inhibited by the ER antagonist, ICI 182,780, and by inhibitors of MEK and Src. Taken together, these findings indicate that the stimulatory effects of estrogens on cholangiocyte proliferation involve the activation of the Src/Shc/ERK signalling cascade. Previous reports showed that lipopolysaccharides induce cholangiocyte proliferation via a IL-6-mediated activation of ERK1/229 and that in cholangiocarcinoma, blockade of ERK1/2, as in other tumors, suppresses cell growth.30 These studies and our recent findings suggest that the ERK system plays a key role as intracellular pathway modulating cholangiocyte proliferation.
Our findings obtained in cholangiocytes which express both ER-α and -β are consistent with studies performed in other estrogen-responsive tissues where hormone-dependent transcriptional activation of ER and other nuclear receptors involves assembly of a coactivation complex which includes various cofactors such as the steroid receptor-coactivator, Src.22-25 Very recently, it has been shown that sex steroids may induce assembly of a ternary complex constituted of the androgen receptor, ER (-α or -β) and Src. The complex triggers activation of the pathway, S-phase entry and cell proliferation.22-25,31 Furthermore, induction of the c-fos gene,32 neuroprotection33 and vasorelaxation34 are other estrogenic effects, which seems to be mediated by this intracellular pathway. In the same studies, the estrogen antagonist, ICI 182,780, prevented complex assembly and pathway activation induced not only by estradiol but also by androgen. In transfected Cos cells,24 either ER-α or ER-β, once occupied by the agonist, are able to activate the Src/Shc/ERK cascade. The association of Src with ER-α or ER-β was recently analyzed in details24 by using glutathione S-transferase (GST) fusion protein. It was shown24 that ER-α or ER-β associates with the SH2 domain of Src and on this basis, it was proposed that the interaction of SH2 domain of Src with one or both the two ER leads to a less constrained conformation of Src and this could be responsible for a stronger stimulation of Src activity. In some cells such as MCF-7 cells, estrogen action is mediated by ER-α, in other cells such as LNCaP cells by ER-β and similar results have been obtained in Cos cells cotransfected with wild-type androgen receptor, ER-α or ER-β, thus suggesting a general role of these events.24 In cholangiocytes, it is likely that both ER-α and ER-β are involved by forming heterodimers. With regard to Shc, it has been shown in different cell types that estradiol signaling to Erk-1/-2 required phosphorylation of tyrosine 317 of this adapter protein.25 Thus, we showed that the ER/Src/Shc/ERK pathway is activated by estrogens and is important in sustaining cholangiocyte proliferation (fig. 2). Since this pathway is typically activated by growth factors, our findings could suggest that estrogens may synergistically potentiate the effect of putative growth factors activated in the course of cholestasis. Preliminary experiments indicate that cholangiocytes produces insulin like growth factor 1 and express the receptors for this factor. On the basis of these findings, new potential perspectives in the pharmacological management of cholangiocyte proliferation in human pathologies are opened. ER agonists or MAPkinase inducers could be proposed to avoid evolution of cholangiopathies toward the terminal ductopenic stage while, on the contrary, inhibitors of MAP kinases or coactivators (Src, Shc) could be of potential utility in depressing cholangiocyte proliferation in neoplastic (cholangiocarcinoma) or displastic disorders (polycystic liver disease).
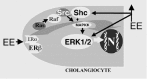
Figure 2
Intracellular pathways mediating estrogen-induced cholangiocyte proliferation. Abbreviations: EE= estrogens; ER= estrogen receptors; ERK= extracellular regulated kinases, Src= steroid receptor-coactivator; Shc= Src-homology/ collagen protein.
Conclusions
In conclusion, our recent findings gave further support to the role of endogenous estrogens as modulators of cholangiocyte proliferation in the course of cholestasis. In our hypothesis, estrogens act by synergizing the effects of growth factors in sustaining the proliferative machinery and depressing apoptosis. This may have a number of clinical implications for diseases involving the biliary epithelium, where cholangiocyte proliferation is a typical hallmark influencing disease progression. We speculate that an estrogenic deficiency could exist in the terminal, ductopenic stage of these diseases as a basis of the inefficacy of cholangiocyte proliferation to balance for the loss of intrahepatic bile ducts. In this hypothesis, the study of estrogen receptors and the related intracellular signaling in different stages of cholangiopathies is demanding.
Acknowledgments
Portions of the studies outlined in this chapter were supported by a grant award from Scott & White Hospital and Texas A&M University, by an NIH grant DK58411 and by VA Merit Award to Dr. Alpini.
References
- 1.
- Alvaro D. Biliary epithelium: a new chapter in cell biology. It J Gastroenterol Hepatology. 1999;31:78–83. [PubMed: 10091109]
- 2.
- Alvaro D, Gigliozzi A, Attili AF. Regulation and deregulation of cholangiocyte proliferation. J Hepatol. 2000;33:333–340. [PubMed: 10952254]
- 3.
- Desmet V, Roskams T, Van Eyken. Ductular reaction in the liver. Path Res Pract. 1995;191:513–524. [PubMed: 7479372]
- 4.
- Desmet V, Roskams T, Van Eyken P. et al. Histopathology of vanishing bile duct diseases. Advanc Clin Pathol. 1998;2:87–99. [PubMed: 10358336]
- 5.
- Alpini G, Lenzi R, Sarkozi L. et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. [PMC free article: PMC329605] [PubMed: 2448343]
- 6.
- Eagon PK, Porter LE, Francavilla A. et al. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. [PubMed: 3885401]
- 7.
- Eagon PK, Elm MS, Epley MJ. et al. Sex steroid metabolism and receptor status in hepatic hyperplasia and cancer in rats. Gastroenterology. 1996;110:1199–1207. [PubMed: 8613010]
- 8.
- Fisher B, Gunduz N, Saffer EAS. et al. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Research. 1984;44:2410–2415. [PubMed: 6722782]
- 9.
- Blum A, Cannon RO. Effects of oestrogens and selective oestrogen receptor modulators on serum lipoproteins and vascular function. Curr Opin Lipidol. 1998;9:575–86. [PubMed: 9868594]
- 10.
- Francavilla A, Polimeno L, DiLeo A. et al. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology. 1989;9:614–620. [PMC free article: PMC2987643] [PubMed: 2784403]
- 11.
- Joplin R, Neuberger JM. Antigen expression in bile duct epithelia in cholestatic liver diseaseIn: Manns MO, Boyer JL, Jansen PLM, Reichen J, eds.Cholestatic liver diseases Falk Symposium 102Dordrecht/Boston/London: Kluwer Academic Publ.,1998226–238.
- 12.
- Chen J, Robertson G, Field J. et al. Effect of bile duct ligation on hepatic expression of female-specific CYP2C12 in male and female rats. Hepatology. 1998;28:624–630. [PubMed: 9731550]
- 13.
- Floreani A, Titta M, Plebani M. et al. Sex hormone changes in post-menopausal women with primary biliary cirrhosis (PBC) and with cryptogenic chronic liver disease. Clin Exp Obstet Gynecol. 1991;18:229–34. [PubMed: 1838720]
- 14.
- Alvaro D, Alpini G, Onori P. et al. Estrogens Stimulate Proliferation of Intrahepatic Biliary Epithelium in Rats. Gastroenterology. 2000;119:1681–1691. [PubMed: 11113090]
- 15.
- Jordan VC. Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Research and Treatment. 1994;31:41–52. [PubMed: 7981455]
- 16.
- Pan G, Vickers SM, Pickens A. et al. Apoptosis and tumorigenesis in human cholangiocarcinoma cells. Involvement of Fas/APO-1 and calmodulin. Am J Pathol. 1999;155:193–203. [PMC free article: PMC1866679] [PubMed: 10393851]
- 17.
- Patel T, Roberts LR, Jones BA. et al. Dysregulation of apoptosis as a mechanism of liver disease: an overview. Semin Liver Dis. 1998;18:105–114. [PubMed: 9606808]
- 18.
- Sampson LK, Vickers SM, Ying W. et al. Tamoxifen-mediated growth inhibition of human cholangiocarcinoma. Cancer Res. 1997;57:1743–1749. [PubMed: 9135018]
- 19.
- Kuroki T, Seki S, Kawakita N. et al. Expression of antigens related to apoptosis and cell proliferation in chronic nonsuppurative destructive cholangitis and biliary cirrhosis. Virchows Arch. 1996;429:119–129. [PubMed: 8917713]
- 20.
- Yano H, Fukuda K, Haramaki M. et al. Expression of Fas and anti-Fas-mediated apoptosis in human hepatocellular carcinoma cell lines. J Hepatol. 1996;25:454–464. [PubMed: 8912144]
- 21.
- Alvaro D, Alpini G, Onori P. et al. Effect of ovariectomy on the proliferative capacity of intrahepatic rat cholangiocytes. Gastroenterology. 2002;36:336–344. [PubMed: 12105861]
- 22.
- Migliaccio A, Di DomenicoM, Castoria G. et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–300. [PMC free article: PMC450032] [PubMed: 8635462]
- 23.
- Tremblay A, Giguere V. Contribution of steroid receptor coactivator-1 and CREB binding protein in ligand-independent activity of estrogen receptor beta. J Steroid Biochem Mol Biol. 2001;77:19–27. [PubMed: 11358671]
- 24.
- Migliaccio A, Castoria G, Di Domenico M. et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5410. [PMC free article: PMC314017] [PubMed: 11032808]
- 25.
- Filardo EJ, Quinn JA, Bland KI. et al. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. [PubMed: 11043579]
- 26.
- Ryzycky AL. Effects of 17 beta-estradiol and progesterone on mitogen-activated protein kinase expression and activity in rat uterine smooth muscle. Eur J Pharmacol. 1996;300:247–54. [PubMed: 8739215]
- 27.
- Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. [PubMed: 11730323]
- 28.
- Adesanya OO, Zhou J, Samathanam C. et al. Insulin-like growth factor 1 is required for G2 progression in the estradiol-induced mitotic cycle. Proc Natl Acad Sci USA. 1999;96:3287–91. [PMC free article: PMC15934] [PubMed: 10077676]
- 29.
- Park J, Gores GJ, Patel T. Lipopolysaccharides induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology. 1999;29:1037–1043. [PubMed: 10094943]
- 30.
- Sebolt-Leopold JS, Dudley DT, Herrera R. et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nature Medicine. 1999;5:810–816. [PubMed: 10395327]
- 31.
- Lee HW, Eghbali-Webb M. Estrogen enhances proliferative capacity of cardiac fibroblasts by estrogen receptor- and mitogen-activated protein kinase-dependent pathways. J Mol Cell Cardiol. 1998;30:1359–1368. [PubMed: 9710804]
- 32.
- Watters JJ, Campbell JS, Cunningham MJ. et al. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signaling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. [PubMed: 9275096]
- 33.
- Singer CA, Figueroa-Masot XA, Batchelor RH. et al. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. [PMC free article: PMC6786088] [PubMed: 10087060]
- 34.
- Chen Z, Yuhanna IS, Galcheva-Garcova Z. et al. Estrogen receptor mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. [PMC free article: PMC407904] [PubMed: 9927501]
- Estrogen Regulation of Cholangiocyte Proliferation - Madame Curie Bioscience Dat...Estrogen Regulation of Cholangiocyte Proliferation - Madame Curie Bioscience Database
- Cytokines in the Treatment and Prevention of Autoimmune Responses - Madame Curie...Cytokines in the Treatment and Prevention of Autoimmune Responses - Madame Curie Bioscience Database
- Metal-ion Transporters— From Yeast to Human Diseases - Madame Curie Bioscience D...Metal-ion Transporters— From Yeast to Human Diseases - Madame Curie Bioscience Database
- Class II Fusion Proteins - Madame Curie Bioscience DatabaseClass II Fusion Proteins - Madame Curie Bioscience Database
- Expression of Hox Genes in the Nervous System of Vertebrates - Madame Curie Bios...Expression of Hox Genes in the Nervous System of Vertebrates - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...

