NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Phagocytosis is important for a wide diversity of organisms. From simple unicellular organisms that use phagocytosis to eat, to complex metazoans in which phagocytic cells represent an essential branch of the immune system. Evolution has armed cells with a fantastic repertoire of molecules that serve to bring about this complex event regardless of the organism or specific molecules concerned. However, all phagocytic processes are driven by a finely controlled rearrangement of the actin cytoskeleton where calcium (Ca2+) signals play important roles. Ca2+ plays many roles in cytoskeletal changes by affecting the actions of a number of contractile proteins, as well as being a cofactor for the activation of a number of intracellular signaling proteins, known to play important roles during phagocytosis. In the mammalian immune system, the requirement of Ca2+ for the initial steps in phagocytosis, and the subsequent phagosome maturation, can be quite different depending on the type of cell and on the type of receptor that is driving phagocytosis.
Introduction
Phagocytosis, the internalization of large particles by cells, was first described by the Russian scientist Elie Metchnikoff in the late eighteen hundreds, nearly 120 year ago.1 Metchnikoff first described how “amoeboid” cells moved, within a transparent starfish larvae, towards an inserted rose thorn and engulfed the thorn. Metchnikoff named this process “phagocytosis”. Today, phagocytosis is used to define the cellular engulfment of particles larger than 0.5 μm in diameter.
Phagocytosis is usually associated with the function of the immune system in relation to the elimination of invading microorganisms, foreign particles, and the elimination of infected or dying cells.2 However, this process is not restricted to the function of immune cells or indeed to the function of mammalian cells, but developed early in evolution and is present in Dictyostelium, nematodes and insect haemocytes.3,4
Phagocytosis is important for a wide diversity of organisms. From simple unicellular organisms that use phagocytosis to obtain their next meal, to complex metazoans in which phagocytic cells represent an essential branch of the immune system, evolution has armed cells with a fantastic repertoire of molecules that serve to bring about this complex event. Regardless of the organism or specific molecules concerned, however, all phagocytic processes are driven by a finely controlled rearrangement of the actin cytoskeleton. Calcium plays many roles in cytoskeletal changes by affecting the actions of a number of contractile proteins. It has been shown that calcium can affect cytoskeletal changes by stimulating myosin contractility,5 activation of actin filament severing,6 inhibition of actin crosslinking by α-actinin,7 or the 34kDa actin-crosslinking protein,8 binding to annexins,9 calmodulin,10 or other low molecular weight calcium-binding proteins including CBP1-4,11 calpain,12 or other potential targets.
In mammals, several cell types are capable of phagocytosis, but their phagocytic activity is very varied. This fact is reflected by dividing the cells that are capable of phagocytosis into: professional phagocytes; paraprofessional phagocytes; and nonprofessional phagocytes, this terminology refers to cells with high, medium or low phagocytic activity respectively.13 Because of the large variety and complexity of phagocytic cells, the scope of this chapter will be limited to the professional phagocytes.
Professional phagocytes encompass mainly neutrophils and cells of the monocytic/macrophage lineage, sentinels of the immune system that hunt and destroy senescent, apoptotic or otherwise defective host cells, pollutant particles and, perhaps most importantly, foreign, potentially pathogenic organisms.13,14 The unique ability of phagocytic leukocytes to efficiently internalize a variety of targets is attributable to the expression of an array of specialized phagocytic receptors. Supporting this notion, it has been shown that the phagocytic capacity of nonprofessional phagocytes, such as Chinese hamster ovary or COS cells, is greatly increased by the heterologous expression of specialized phagocytic receptors, such as Fcγ receptors (FcγRs), that are normally found in neutrophils or macrophages.15
In mammalian cells, phagocytosis is a receptor-mediated and actin-dependent process. Macrophages and neutrophils eliminate invading pathogens by first ingesting them into a plasma membrane-derived vacuole, named phagosome. The resulting phagosomes undergo a series of fission and fusion events that modify the composition of their limiting membrane and of their contents, by a process termed phagosomal maturation, which empowers the vacuole with a host of degradative properties central to the destruction of the invading pathogen.16 However, phagocytosis can also have unwanted effects for the host in that certain pathogens, such as Mycobacterium Tuberculosis, take advantage of the phagocytic machinery to gain access to the cell interior, without being destroyed by the phagocytic machinery, and become intracellular pathogens.17,18
Receptors Involved in Phagocytosis
Phagocytosis is initiated by the interaction of surface receptors with their cognate ligand. Ligands can be endogenous components of the particle, exemplified by lipopolysaccharides of bacteria and phosphatidylserine in apoptotic cells.14,18 Internalization triggered by endogenous ligands of the particle is known as nonopsonic. The immune system is equipped with a variety of receptors that recognize nonopsonic ligands, including CD14 that binds to lipopolysaccharides, as well as receptors that recognize specifically phosphatidylserine, mannose or fucose residues.19 Alternatively, phagocytic ligands can be classified as opsonins, which are host-derived proteins that coat the surface of a particle. The best characterized opsonins are the complement fragment C3b, iC3b and IgG antibodies. C3b and iC3B bind relatively nonspecifically to the surface of foreign particles, whereas IgG molecules attach to the phagocytic target by recognizing specific surface epitopes.14 C3b or iC3b-opsonized particles are recognized by complement receptors members of the integrin superfamily, while IgG-opsonized particles engage FcγRs.15 In any case, receptor engagement leads to internalization of the particle into a phagosome by a complex sequence of events that require kinase activation, alterations in phospholipid metabolism, remodeling of the actin cytoskeleton and acceleration of membrane traffic.3,14 These processes that are usually associated with increase in intracellular calcium.
In mammals, binding of immunoglobulins (Igs) to foreign particles (opsonization) leads to the prompt clearance of those particles from the organism. The conserved Fc-domains of the Igs are recognized by Fc receptors present on professional phagocytes, such as macrophages and neutrophils. An opsonized particle binds to Fc-receptors and is rapidly internalized by an actin-dependent extension of the plasma membrane around the opsonized particle. This process is accompanied by the production of proinflammatory and toxic molecules, such as the production of superoxide and the release of cytokines from the phagocytic cell.20 The major Ig opsonin is IgG, which binds to the corresponding Fc-gamma-receptors (FcγRs), although IgA and IgE also have cognate Fc receptors (FcαRs and FcεRs, respectively) that are involved in phagocytosis.21,22
A range of FcγRs exist.23 FcγRI, FcγRIIA, and FcγRIIIA can all support phagocytosis.24-26 FcγRIIB negatively regulates phagocytosis;27 while FcγRIIIB is able to initiate calcium signaling and actin polymerization, but its role in phagocytosis remains unclear.28-30 Fc receptor-mediated phagocytosis is fully reviewed in.31
Another group of receptors that mediate phagocytosis by professional phagocytes is the complement receptors. Complement-receptor-mediated phagocytosis is morphologically distinct from that mediated by FcRs, although both processes require actin polymerization. Complement-opsonized particles get internalized, with minimal membrane disturbance and this does not usually lead to an inflammatory response or the generation of superoxide. The complement system is evolutionarily much older than adaptive immunity and is present even in simple organisms such as sea urchins,32 and yet still represents an important part of the innate immune system in higher organisms, including humans.33 In higher vertebrates the complement system is composed of at least 30 proteins, which are activated by enzymatic cascades, by exposure to microbial macromolecules or by binding to antibodies (primarily IgM or IgG) bound to the surface of a pathogen. One of the molecules produced following the complement cascade is C3b. This molecule can bind to molecules on microbial surfaces, where C3b acts like an opsonin, and is recognized by the complement receptor 1 (CR1 also known as CD35). C3b can be further modified by plasma factors H and I, which convert it to iC3b. iC3b is a very potent opsonin that can be recognized by the complement receptor 3, CR3, also known as Mac-1, CD11b/CD18 or αMβ2 integrin; iC3b can also be recognized by complement receptor 4, CR4, also known as CD11c/CD18 or αxβ2 integrin. The complement receptors CR1, CR3 and CR4, are expressed on macrophages and neutrophils and are capable of mediating phagocytosis.34 CR3 has been the most widely studied of the complement receptors and is capable of binding to several ligands through different recognition sites.35 However, the phagocytosis of iC3b-opsonised particles by CR3 can only proceed efficiently if the phagocytes are first activated, either by proinflammatory cytokines/chemokines, or by binding to the extracellular matrix.36,37 It is believed that the preactivation triggers a conformational change in the complement receptor,38 possibly through phosphorylation of the β subunit.39 This triggers clustering of the receptor;40 a precondition for particle binding, which allows the phagocytosis to occur.41
A Variety of Nonprofessional Phagocytic Receptors
It is becoming apparent that a growing number of cell-surface receptors can mediate phagocytic uptake of particles. These include noncomplement-receptor integrins such as α5β1 and αvβ3, which mediate uptake of particles coated with fibronectin,42 lectins such as the mannose receptor,43 the lipopolysaccharide (LPS) receptor CD14,44 and the diverse scavenger receptor group.45 Internalization by these receptors appears, at least in some cases, to be morphologically dynamic, as in the case of Fc receptors, but in contrast to uptake through CR3. The membrane is extended around the attached particle, and there is transient ruffling in surrounding areas of the cell.46 However, uptake does not trigger inflammation,47 and might actively suppress it.48,49 Recently, there has been a resurgence of interest in these receptors in an attempt to understand the removal of apoptotic cells.45 In metazoans development is accompanied by massive apoptosis (e.g., during limb formation), and these ‘corpses’ are engulfed both by professional phagocytes and by neighboring cells that act as ‘nonprofessional’ phagocytes.50 The recent report of a receptor for phosphatidylserine,51 a membrane phospholipid exposed externally on apoptotic cells, is likely to stimulate rapid progress in this field. Additionally, Caenorhabditis elegans appears to use a common mechanism to engulf apoptotic and necrotic cell corpses,52 whether this is also true for ‘higher’ organisms remains to be seen. In common with FcR- and complement-receptor-mediated phagocytosis, phagocytosis mediated by this diverse group of receptors is also actin dependent,45 and many of the downstream components are the same as those lying downstream of CR3 or FcγRs. Nevertheless, the events immediately following receptor-ligand interaction remain largely unknown. Some of these receptors might act only to tether particles, and then utilize accessory receptors to deliver the phagocytic signal.50 This would explain the ability of some receptors (such as CD14) to induce inflammatory responses when binding to one ligand (LPS) but not another.44 Some of these receptors can signal through tyrosine kinases, and uptake is, at least in some cases, phosphorylation dependent.53 In this regard it is particularly intriguing that the newly cloned ced-1 gene from C. elegans (which encodes a transmembrane receptor that is essential for the uptake of apoptotic cells) contains an intracellular YXXL motif.54 This sequence is also found in the ITAMs of mammalian FcRs, where it mediates interactions with downstream signaling elements.
Phagocytosis has been known to be an actin-dependent process since 1977, when it was reported that cytochalasin B, a toxin that blocks actin polymerization, inhibited the uptake of IgG-coated erythrocytes by mouse macrophages.55 Although originally proposed to be a unique feature of Fc-receptor mediated phagocytosis, remodeling of the actin cytoskeleton is now known to be required for phagocytosis through other types of receptor as well. However, one key characteristic for all receptor-triggered actin remodeling is the sensitivity to changes in intracellular calcium.
Calcium the Ubiquitous Second Messenger
Cytosolic Ca2+ is a ubiquitous intracellular signal, pivotal in many signal transduction pathways, controlling a wide range and diversity of cellular activities ranging from proliferation and differentiation to cell death.56 Resting cells have a Ca2+ concentration around 100 nM, this amount of intracellular Ca2+ is not sufficient to trigger substantial cellular activities, however, when cells are stimulated the amount of intracellular Ca2+ can rise very quickly reaching up to 1 μM, and Ca2+ triggered cellular activities occur.56 It is well established that the increase of cytosolic Ca2+ can be temporally and spatially very complex. This is due to the fact that different cells respond differently to a particular stimulus, and indeed different stimuli triggered a particular cell in different ways. Thus, the Ca2+ signals can of a single burst and very transient or long-lasting and oscillatory, and can happen in a localized microenvironment or can be triggered as a wide-spread event.57
Cells generate their Ca2+ signals by using both internal and external sources of Ca2+. Internally Ca2+ is stored in specialized compartments such as the endoplasmic reticulum (ER), sarcoplasmic reticulum (SR), or on smaller compartments called calciosomes which are thought to be present in many cell types.58 Endosomes and phagosomes also store calcium.59 Ca2+ signals are controlled by the generation of intracellular second messengers binding to specific receptors/channels. There are several intracellular second messengers known to increase cytosolic Ca2+, these include: inositol 1,4,5-trisphosphate (IP3); cyclic adenosine 5'-diphosphoribose (cADPR); nitric oxide (NO); hydrogen peroxide (H2O2); superoxide (O2-); nicotinic acid adenine dinucleotide phosphate (NAADP); diacylglycerol (DAG); arachidonic acid (AA); phosphatidic acid (PA); sphingosine; sphingosine-1-phosphate (S1P); and Ca2+ itself.57 Of these intracellular second messengers, some act on intracellular Ca2+ channels found on internal compartments for Ca2+ release, some act on Ca2+ entry channels found on the plasma membrane, while some may act on both release and entry.56,57 Due to the specificity of these Ca2+ triggering messengers we can say that there must be several different types of intracellular Ca2+ release channels, however, only IP3 receptors and ryanodine receptors (RYRs) have been well characterized. Therefore, Ca2+-mobilizing second messengers, generated when cell-surface receptors are stimulated, determine whether Ca2+ channels can be activated. Thus, generated IP3 can diffuse in the cell's cytoplasm to engage the IP3 receptors and release Ca2+ from the ER.56 The activity of the RYRs is modulated by the generation of cADPR,58 NAADP acts on a separate-uncharacterized channel.59 Exactly how sphingosine-1-phosphate causes Ca2+ release from intracellular stores is still unclear. Until recently, the best candidate for the sphingosine-1-phosphate receptor was a protein known as ‘sphingolipid Ca2+-release mediating protein of endoplasmic reticulum’ (SCaMPER).60 It had been proposed that this protein formed a widely occurring channel responsive to sphingosine-1-phosphate and sphingosylphosphorylcholine. A recent reinvestigation of SCaMPER found that there was little correlation between its intracellular location and that of known intracellular Ca2+ stores.61 Furthermore, expression of SCaMPER was found not to confer sensitivity to sphingolipids, nor to affect Ca2+ homeostasis, but could lead to cell death.
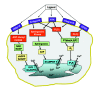
A range of Ca2+ influx channels have been established for some time. In addition to their activation by some of the messengers listed above, Ca2+ influx channels are activated by stimuli including membrane depolarization, stretch, noxious stimuli, extracellular agonists and depletion of intracellular stores.56,57 Recent studies have expanded the numbers of Ca2+-increasing messengers and channels yet further. In addition, it is becoming evident that different Ca2+ signaling pathways can interact to control the source and characteristics of cytosolic Ca2+ signals. 56,57 Depletion of intracellular Ca2+ stores by activation of IP3 or ryanodine receptors switches on a Ca2+ influx pathway through storeoperated channels (SOCs).62 The mechanism underlying SOC activation, and the identity of the channels involved, is unclear. It was thought that SOCs provide the main route for Ca2+ entry into nonelectrically excitable cells. However, accumulating evidence suggests that intracellular messengers can activate Ca2+ influx during physiological stimulation. When IP3 is produced from phosphoinositide hydrolysis, there is a concomitant production of diacylglycerol (DAG). Unlike the water soluble IP3, DAG stays in the plane of the plasma membrane where it can activate protein kinase C (PKC) or be metabolized in various ways. PKC and DAG have both been shown to cause Ca2+ influx distinct from SOC.63 Furthermore, other messengers resulting from DAG metabolism, including arachidonic acid and leukotrienes, activate nonSOC (NSOC) Ca2+ influx.64 At present, the best molecular candidates for SOC and NSOC channels are the TRP proteins (so-called because of their homology with the transient receptor potential protein that underlies phototransduction in Drosophila). The TRP superfamily has been subdivided into multiple subfamilies on the basis of sequence similarity.65 In the case of SOC, much attention has been focused on the canonical TRP (TRPC) subfamily. Despite considerable effort, it is unclear exactly which of the seven TRPC isoforms are the molecular constituents of endogenous SOCs. It was recently suggested that a channel belonging to the TRPV subfamily, CaT1 (TRPV6), could be a candidate for a form of SOC known as ICRAC (Ca2+-release activated current).66 However, the correlation between CaT1 and ICRAC has been disputed.67 (fig. 1) shows a general reprensetation of receptor-triggered calcium release from internal stores.
What Couples Phagocytic-receptor Activation to Rise on Intracellular Ca2+
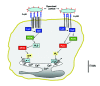
Ca2+ is a key second messenger in leukocyte activation. It mediates, at least in part, activation of the respiratory burst and secretion of microbicidal granule constituents.68,69 As in other systems, the resting cytosolic free Ca2+ concentration (intracellular Ca2+) hovers in the 100nM range, but is acutely elevated upon the engagement of phagocytic receptors.70,71 Release of Ca2+ stored in the endoplasmic reticulum and opening of storeoperated channels are largely responsible for this elevation.
It has been realized recently that organelles other than the endoplasmic reticulum can contribute to the elevation of intracellular Ca2+. Ca2+ is now thought to be released also by early and late endosomes, lysosomes and the yeast vacuole.72,73 Along the same lines, it is entirely conceivable that Ca2+ trapped in the lumen of forming phagosomes, or accumulated afterwards by plasmalemmal Ca2+ pumps, may be released at critical stages of the maturation sequence. Indeed, preliminary evidence to this effect has been presented.74 Consistent with this model, a localized periphagosomal increase in intracellular Ca2+ has been recorded,70 although this was attributed to the preferential distribution of endoplasmic reticulum in the immediate vicinity of phagosomes.
The release of Ca2+ from internal stores, following receptor engagement in immune cells, is triggered by phospholipid-derived second messengers. Inositol-1,4,5-trisphosphage (IP3) is the best characterized second messenger responsible for triggering Ca2+ release from internal stores.75 However, Fc-receptor-triggered Ca2+ release from internal stores in neutrophils, mast cells and monocytes has also been shown to be IP3 independent.76-81 Indirect evidence suggests that L-plasmin, an actin-binding protein, phosphorylated in response to phagocytosis, might participate in the IP3-independent Ca2+ increase mediated by FcγRIIA in neutrophils.82 Furthermore, it has been shown that the second messenger sphingosine-1-phosphate is the actual trigger responsible for the release of Ca2+ from internal stores stimulated by FcγRI aggregation in monocytes or FcεRI aggregation in mast cells.77-81 However, when monocytes are differentiated to a more macrophage phenotype, FcγRI triggers PLCγ activation and Ca2+ signals that are IP3-dependent.78 Of interest, is has recently been reported that in human mast cells, FcεRI triggers a dual calcium response.81 Thus, mast cells appear to concurrently utilize different messengers; in this case IP3 and sphingosine-1-phosphate to generate the Ca2+ signals that underlie the synthesis and release of inflammatory mediators.81 Essentially, the FcεRI, antigen receptor, on these cells trigger multiple signaling pathways. One of these is phosphoinositide hydrolysis, leading to IP3 production. Another is the stimulation of phospholipase D, which hydrolyses phosphatidylcholine into phosphatidic acid and choline. It has been suggested that phosphatidic acid can activate a kinase that phosphorylates sphingosine into sphingosine-1-phosphate.83 The dual activation of these pathways leads to a Ca2+ signal with a rapid peak (sphingosine-1-phosphate dependent) and a sustained plateau (IP3 dependent).
Downstream Events Triggered by Ca2+ Following Phagocytic-receptor Activation
One of the first reported signals to be observed in response to phagocytic-receptor activation is an increase in intracellular Ca2+ concentration.84 In neutrophils, this calcium pulse has been reported to be required for FcγR-mediated phagocytosis by one group,85 but not by another.86 In contrast, CR3-mediated phagocytosis in neutrophils appears to be independent of changes in intracellular Ca2+ concentration, at least in the initial stages of phagocytosis.86,87 However, following the internalization of IgG-opsonized particles by neutrophils, Ca2+ appears to trigger actin depolymerization at phagosomes,88 a step that may be necessary for phagosome-lysosome fusion.89 In macrophages, there is a degree of controversy on the role of Ca2+ during phagocytosis; some groups report that neither phagocytosis nor phagosome-lysosome fusion are calcium-dependent,90,91 proposed to reflect the involvement of different phagocytic receptors;92 whereas, in other studies it has been shown that during phagocytosis by macrophages, there is a rise in Ca2+ concentration in the cytoplasm surrounding the phagocytic cup,93 it is believed that this rise in intracellular Ca2+ is directly triggered by receptor activation during phagocytosis, and that it is important at least for phagosome maturation.93,94,95 However, some studies show that this increase in intracellular Ca2+ may be caused by the exit of calcium from the phagosome through Ca2+ channels, rather than by Ca2+ release from intracellular stores.94 In this case the reduction of Ca2+ concentration in the phagosome seems to be important for phagosome maturation.94 Independently of its origin, Ca2+ seems to be important for triggering depolymerazition around the phagosomes.95
How Might Intracellular Ca2+ Control Actin Depolymerization During Phagocytosis?
One possible model is that a local rise in intracellular Ca2+ concentration activates gelsolin. Gelsolin caps the barbed (fast growing) end of actin filaments, preventing filament elongation, and can also sever filaments in a Ca2+-dependent manner.96,97 Gelsolin localizes to nascent phagosomes in macrophages.97 Furthermore, neutrophils from gelsolin knockout (Gsn-/-) mice have a profound defect in Fcγ R-mediated phagocytosis.98 However, the Ca2+-dependent depo- lymerization of actin filaments from around particles after internalization is normal in Gsn-/- neutrophils, 98 which suggest that intracellular Ca2+ plays a role wider than simply activating gelsolin. However, there are other powerful pathways, important for phagocytosis, that require Ca2+ signals to be activated, such as is the case with the protein Kinase C family of serine/threonine kinases.
The PKC family of serine/threonine kinases, are activated by the phospholipase product diacylglycerol (DAG), by Ca2+, and by pharmacological agents such as phorbol esters. PKCα localizes to macrophage phagosomes during FcγR-, CR3- and mannose-receptor-mediated phagocytosis.99,100,101 Complement-receptor-mediated phagocytosis, both of iC3b and β-glucan-opsonized particles, appears to require PKC activity.53,99 In contrast, conflicting results have been obtained for its involvement in FcγR-mediated phagocytosis.99,101-103 In addition to the PKCα isoform, PKCβ,104 PKCγ,105 PKCδ and PKCε,101 have all been shown to localize to the phagosome membrane during FcγR-mediated phagocytosis. The isoforms recruited may depend on the differentiation state of the cells and/or the exact FcγR involved,105 or different isoforms controlling different aspects of phagocytosis.101 PKC has a range of downstream targets that are implicated in phagocytosis. For example, plekstrin, the major PKC phosphorylation target in platelets, is expressed in macrophages and recruited to the phagosome membrane during FcγR-mediated phagocytosis,106 although its role there is unknown. More is known about MARCKS and MacMARCKS, two other PKC targets implicated in phagocytosis. MARCKS (myristoylated alanine-rich C kinase substrate) and the closely related MacMARCKS,107 are actin-filament crosslinking proteins that can also link actin filaments to the membrane.108 MARCKS localizes to phagosomes,99,100 and becomes phosphorylated during zymosan phagocytosis.100 However, macrophages derived from MARCKS-/- mice show normal rates of FcγR- and CR3-mediated phagocytosis and only a minor reduction in the uptake of zymosan particles.109 MacMARCKS also localizes to phagosomes.110,111 Mutations in MacMARCKS were reported to block zymosan phagocytosis (a mannose-receptor-mediated event) by one group,111 but MacMARCKS-/- macrophages do not show phagocytic defects, a discrepancy that might be attributable to the use of different cell lines.110
Role of Ca2+ On the Maturation of Phagosomes During Phagocytosis?
Release of Ca2+ from intracellular stores could have significant implications for membrane fusion, in that a localized amount of high intracellular Ca2+ may form in the immediate vicinity of a phagosome, promoting and targeting fusion with cognate vesicles. In fact, a number of studies implicate intraorganellar Ca2+ in the homotypic fusion of early endosomes and yeast vacuoles, and in late-endosome—lysosome heterotypic fusion.72,73,112 In the endocytic pathway, the effects of the intracellular Ca2+ released locally are thought to be mediated by calmodulin,72,73,112 which it is proposed acts downstream of the Rab GTPases and SNARE complex by promoting bilayer coalescence.73,113
While the evidence implicating Ca2+ in the endocytic pathway is well reported, the role of Ca2+ in phagosome maturation is far from clear. There is at least one convincing report that discounts a role for intracellular Ca2+ in phagolysosome biogenesis in macrophages.114 By contrast, preventing changes in intracellular Ca2+ was shown to impair phagosome—lysosome fusion in neutrophils and macrophages.115 Similar observations were also obtained with engineered phagocytes expressing FcγRIIA.116 Moreover, clamping intracellular Ca2+ prevented efficient killing of Staphylococcus by neutrophils, suggesting that phagosome maturation was defective.117
However, how intracellular Ca2+ may regulate phagosome maturation is still not well understood. One possibility is that intracellular Ca2+ induces disassembly of the actin coating the surface of the phagosome, permitting access to incoming vesicles.95 Of interest, it has been reported that phagosome-endosome fusion is impaired when periphagosomal actin is stabilized.118 Interestingly, retention of an actin coat around Mycobacterium-containing phagosomes by inhibition of Ca2+ is consistent with the presence of coronin, an actin-binding protein, in Mycobacterium-containing phagosomes.119 Nonetheless, exceptions have been reported: in macrophages, actin assembly and disassembly appeared to be normal when Ca2+ was clamped at a very low concentration.120 Alternatively, Ca2+ may regulate fusion by a more direct approach, through annexins, calmodulin and/or Ca2+/calmodulin-dependent protein kinase 73,112,121-123 Calmodulin and the calmodulin-dependent kinase may in turn regulate tethering or docking factors such as EEA1 and syntaxin 13, and/or regulate bilayer fusion between phagosomes and endo/lysosomes.73,113
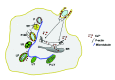
Figure 3 is a diagram illustrating phagosome maturation to phagolysosome and the potential role for Ca2+ in the process.
Conclusion
Despite the great amount of data acquired since Metchnikoff's initial description of the phagocytic process, still we remain with enormous gaps in our understanding of phagocytosis, and can only now realize the complexity of the process and the many issues that need to be addressed. Notable among these are the determinants or selectivity that dictates particle uptake and couples this process with the progressive fusion and elements of the endocytic pathway. It is important to notice the role of Ca2+ and the cytoskeleton in the uptake and the process of phagosome maturation. Although in the past few years the amount of data on the role of Ca2+ on phagocytosis has exponentially increased, there is still some controversy about the role(s) played by Ca2+ during phagocytosis: the relevance of Ca2+ for phagocytosis is challenged by the fact that some studies have shown that some receptors may not utilize Ca2+ for the initial stages of phagocytosis, such as FcγRI-mediated phagocytosis; however, other receptors such as FcγRII-mediate phagocytosis in a Ca2+-dependent manner. Despite the controversy, a role for Ca2+ is becoming well established in endosome-phagosome fusion, phagosome maturation, and in the general actin-cytoskeleton remodeling during phagocytosis. Thus, whereas Ca2+ may not be a prerequisite for the initial particle uptake for some receptors, it is becoming clearer that Ca2+ plays a major role in phagosome maturation and potentially in antigen presentation.
The Ca2+ signaling field is moving forward in many areas: we now have a clearer picture of how different messengers and channels generate Ca2+ signals, and their roles in different cellular processes. These advances are coupled with the significant progress made in identifying molecules that are potentially involved in phagocytic uptake, and phagosome maturation. An important challenge for the future is to discover how these mechanisms relate to each other, and how Ca2+ signals triggered by different receptor-systems may regulate the process of phagocytosis.
References
- 1.
- Metchnikoff E. Sur la lutte des cellules de l'organisme contre l'invasion des microbes. Ann Inst Pasteur. 1887;1:321–345.
- 2.
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. [PubMed: 10358769]
- 3.
- Cardelli J. Phagocytosis and micropinocytosis in Dictyostelium: Phosphoinositidebased processes, biochemically distinct. Traffick. 2001;2:311–320. [PubMed: 11350627]
- 4.
- Franc NC, White K, Ezekowitz RA. Phagocytosis and development: Back to the future. Curr Opin Immunol. 1999;11:47–52. [PubMed: 10047544]
- 5.
- Tan Z, Boss WF. Association of phosphatidylinositol kinase, phosphatidylinositol monophosphate kinase, and diacylglycerol kinase with the cytoskeleton and F-actin fractions of carrot (Daucus carota L.) cells grown in suspension culture. Plant Physiol. 1992;100:2116–2120. [PMC free article: PMC1075917] [PubMed: 16653250]
- 6.
- Yamamoto K, Pardee JD, Reidler J. et al. Mechanism of interaction of Dictyostelium severin with actin filaments. J Cell Biol. 1982;95:711–719. [PMC free article: PMC2112927] [PubMed: 6897549]
- 7.
- Witke W, Hofmann A, Koppel B. et al. The Ca2+-binding domains in nonmuscle type alpha-actinin: Biochemical and genetic analysis. J Cell Biol. 1993;121:599–606. [PMC free article: PMC2119564] [PubMed: 8486739]
- 8.
- Fechheimer M, Taylor DL. Isolation and characterization of a 30,000-dalton calcium-sensitive actin cross-linking protein from Dictyostelium discoideum. J Biol Chem. 1984;259:4514–4520. [PubMed: 6707016]
- 9.
- Doring V, Veretout F, Albrecht R. et al. The in vivo role of annexin VII (synexin)-characterization of an annexin VII deficient Dictyostelium mutant indicates an involvement in calcium-regulated processes. J Cell Sci. 1995;108:2065–2076. [PubMed: 7657724]
- 10.
- Zhu Q, Liu T, Clarke M. Calmodulin and the contractile vacuole complex in mitotic cells of Dictyostelium discoideum. J Cell Sci. 1993;104:1119–1127. [PubMed: 8314896]
- 11.
- Dharamsi A, Tessarolo D, Coukell B. et al. CBP1 associates with the Dictyostelium cytoskeleton and is important for normal cell aggregation under certain developmental conditions. Exp Cell Res. 2000;258:298–309. [PubMed: 10896781]
- 12.
- Huttenlocher A, Palecek SP, Lu Q. et al. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. [PubMed: 9407041]
- 13.
- Rabinovitch M. Professional and nonprofessional phagocytes: An introduction. Trends Cell Biol. 1995;5:85–87. [PubMed: 14732160]
- 14.
- Kwiatkowska K, Sobota A. Signaling pathways in phagocytosis. BioEssays. 1999;21:422–431. [PubMed: 10376013]
- 15.
- Indik ZK, Park JG, Hunter S. et al. The molecular dissection of Fcγ receptor mediated phagocytosis. Blood. 1995;86:4389–4399. [PubMed: 8541526]
- 16.
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed: 9787133]
- 17.
- Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J Exp Med. 2000;191:287–302. [PMC free article: PMC2195750] [PubMed: 10637273]
- 18.
- Ofek I, Goldhar J, Keisari Y. et al. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. [PubMed: 8561460]
- 19.
- Schutt C. Fighting infection: The role of lipopolysaccharide binding proteins CD14 and LBP. Pathobiology. 1999;67:227–229. [PubMed: 10725789]
- 20.
- In: RoittIM ed.The basis of immunology Essential ImmunologyOxford: Blackwell Scientific,1994 .
- 21.
- van EgmondMH, Hanneke van Vuuren AJ, van de Winkel JG. The human Fc receptor for IgA. Immunol Lett. (FcαRI CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis;1999;68:83–87. [PubMed: 10397160]
- 22.
- Yokota A, Yukawa K, Yamamoto A. et al. Two forms of the lowaffinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: Identification of the critical cytoplasmic domains. Proc Nat Acad Sci USA. 1992;89:5030–5034. [PMC free article: PMC49222] [PubMed: 1534410]
- 23.
- Sanchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. J Leuk Biol. 1998;63:521–533. [PubMed: 9581795]
- 24.
- Indik ZK, Hunter S, Huang MM. et al. The high affinity Fc gamma receptor (CD64) induces phagocytosis in the absence of its cytoplasmic domain: The gamma subunit of Fc gamma RIIIA imparts phagocytic function to Fc gamma RI. Exp Hematol. 1994;22:599–606. [PubMed: 7516890]
- 25.
- Tuijnman WB, Capel PJ, van de Winkel JG. Human low affinity IgG receptor Fc gamma RIIa (CD32) introduced into mouse fibroblasts mediates phagocytosis of sensitized erythrocytes. Blood. 1992;79:1651–1656. [PubMed: 1532752]
- 26.
- Park JG, Isaacs RE, Chien P. et al. In the absence of other Fc receptors, Fc gamma RIIIA transmits a phagocytic signal that requires the cytoplasmic domain of its gamma subunit. J Clin Invest. 1993;92:1967–1973. [PMC free article: PMC288363] [PubMed: 8408649]
- 27.
- Indik ZK, Pan XQ, Huang MM. et al. Insertion of cytoplasmic tyrosine sequences into the nonphagocytic receptor Fc gamma RIIB establishes phagocytic function. Blood. 1994;83:2072–2080. [PubMed: 8161778]
- 28.
- Kimberly RP, Ahlstrom JW, Click ME. et al. The glycosyl phosphatidylinositol-linked Fc gamma RIIIPMN mediates transmembrane signaling events distinct from Fc gamma RII. J Exp Med. 1990;171:1239–1255. [PMC free article: PMC2187837] [PubMed: 2139101]
- 29.
- Salmon JE, Brogle EJ, Edberg JC. et al. Fcgamma receptor III induces actin polymerization in human neutrophils and primes phagocytosis mediated by Fcgamma receptor II. J Immunol. 1991;146:997–1004. [PubMed: 1824853]
- 30.
- Chuang FY, Sassaroli M, Unkeless JC. Convergence of Fc-gamma receptor IIA and Fc gamma receptor IIIB signaling pathways in human neutrophils. J Immunol. 2000;164:350–360. [PubMed: 10605030]
- 31.
- Garcia-Garcia E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leuk Biol. 2002;72:1092–1108. [PubMed: 12488490]
- 32.
- Smith LC, Azumi K, Nonaka M. Complement systems in invertebrates. The ancient alternative and lectin pathways. Immunopharmacology. 1999;42:107–120. [PubMed: 10408372]
- 33.
- Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. [PubMed: 9597136]
- 34.
- Brown EJ. Complement receptors and phagocytosis. Curr Opin Immunol. 1991;3:76–82. [PubMed: 1675856]
- 35.
- Diamond MS, Garcia-Aguilar J, Bickford J. et al. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. [PMC free article: PMC2200080] [PubMed: 7679388]
- 36.
- Brown EJ. The role of extracellular matrix proteins in the control of phagocytosis. J Leuk Biol. 1986;39:579–591. [PubMed: 3517208]
- 37.
- Pommier CG, Inada S, Fries LF. et al. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983;157:1844–1854. [PMC free article: PMC2187060] [PubMed: 6854210]
- 38.
- Oxvig C, Lu C, Springer TA. Conformational changes in tertiary structure near the ligand binding site of an integrin I domain. Proc Nat Acad Sci USA. 1999;96:2215–2220. [PMC free article: PMC26763] [PubMed: 10051621]
- 39.
- Chatila TA, Geha RS, Arnaout MA. Constitutive and stimulus-induced phosphorylation of CD11/ CD18 leukocyte adhesion molecules. J Cell Biol. 1989;109:3435–3444. [PMC free article: PMC2115914] [PubMed: 2574726]
- 40.
- Detmers PA, Wright SD, Olsen E. et al. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987;105:1137–1145. [PMC free article: PMC2114803] [PubMed: 2958480]
- 41.
- Allen LA, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40. [PubMed: 8729444]
- 42.
- Blystone SD, Graham IL, Lindberg FP. et al. Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J Cell Biol. 1994;127:1129–1137. [PMC free article: PMC2200054] [PubMed: 7525603]
- 43.
- Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. [PubMed: 9523111]
- 44.
- Devitt A, Moffatt OD, Raykundalia C. et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. [PubMed: 9548256]
- 45.
- Platt N, da SilvaRP, Gordon S. Recognizing death: The phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. [PubMed: 9728398]
- 46.
- Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and nonprofessional phagocytes. Curr Biol. 2000;10:857–860. [PubMed: 10899007]
- 47.
- Meagher LC, Savill JS, Baker A. et al. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leuk Biol. 1992;52:269–273. [PubMed: 1522386]
- 48.
- Fadok VA, Bratton DL, Konowal A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. [PMC free article: PMC508637] [PubMed: 9466984]
- 49.
- Voll RE, Herrmann M, Roth EA. et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. [PubMed: 9389474]
- 50.
- Savill J. Apoptosis. Phagocytic docking without shocking. Nature. 1998;392:442–443. [PubMed: 9548247]
- 51.
- Fadok VA, Bratton DL, Rose DM. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. [PubMed: 10811223]
- 52.
- Chung S, Gumienny TL, Hengartner MO. et al. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nature Cell Biol. 2000;2:931–937. [PubMed: 11146658]
- 53.
- Roubey RA, Ross GD, Merrill JT. et al. Staurosporine inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (CD11b/CD18). J Immunol. 1991;146:3557–3562. [PubMed: 1673986]
- 54.
- Zhou Z, Hartwleg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. [PubMed: 11163239]
- 55.
- Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol. 1977;6:797–807. [PubMed: 561436]
- 56.
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. [PubMed: 11413485]
- 57.
- Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: More messengers, more channels, more complexity. Curr Biol. 2002;12:R563–R565. [PubMed: 12194839]
- 58.
- Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca2+ signals. J Cell Sci. 2001;114:2213–2222. [PubMed: 11493661]
- 59.
- Pryor PR, Mullock BM, Bright NA. et al. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–1062. [PMC free article: PMC2174832] [PubMed: 10831609]
- 60.
- Mao C, Kim SH, Almenoff JS. et al. Molecular cloning and characterization of SCaMPER, a sphingoid Ca2+ release-mediating protein from endoplasmic reticulum. Proc Natl Acad Sci USA. 1996;93:1993–1996. [PMC free article: PMC39897] [PubMed: 8700873]
- 61.
- Schnurbus R, De Pietri Tnelli D, Grohovas F. et al. Reevaluation of primary structure, topology and localization of SCaMPER, a putative intracellular Ca2+ channel activated by sphingosylphosphocholine. Biochem J. 2002;362:183–189. [PMC free article: PMC1222375] [PubMed: 11829755]
- 62.
- Berridge MD. Capacitative calcium entry. Biochem J. 1995;312:1–11. [PMC free article: PMC1136219] [PubMed: 7492298]
- 63.
- Cancela JM, van CoppenolleF, Galione A. et al. Transformation of local Ca2+ spikes to global Ca2+ transients: The combinatioral roles of multiple Ca2+ releasing messengers. EMBO J. 2002;21:909–919. [PMC free article: PMC125894] [PubMed: 11867519]
- 64.
- Mingen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J Biol Chem. 2001;276:35676–35683. [PubMed: 11470795]
- 65.
- Montell C. Physiology, phylogeny and functions of the TRP superfamily of cation channels. Science's STKE. 2001 http//stkesciencemagorg/cgi/content/full/OC_sigtrans 2001/90/re1 [PubMed: 11752662]
- 66.
- Yue LX, Peng JB, Hediger MA. et al. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 2001;410:705–709. [PubMed: 11287959]
- 67.
- Voets T, Prenen J, Fleig A. et al. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J Biol Chem. 2001;276:47767–47770. [PubMed: 11687570]
- 68.
- Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukocyte Biol. 1996;59:613–622. [PubMed: 8656045]
- 69.
- Brumell JH, Volchuk A, Sengelov H. et al. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol. 1995;155:5750–5759. [PubMed: 7499863]
- 70.
- Floto RA, Mahaut-Smith MP, Somasundaram B. et al. IgG-induced Ca2+ oscillations in differentiated U937 cells; a study using laser scanning confocal microscopy and coloaded Fluo-3 and Fura-red fluorescent probes. Cell Calcium. 1995;18:377–389. [PubMed: 8581966]
- 71.
- Mandeville JT, Maxfield FR. Calcium and signal transduction in granulocytes. Curr Opin Hematol. 1996;3:63–70. [PubMed: 9372053]
- 72.
- Pryor PR, Mullock BM, Bright NA. et al. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–1062. [PMC free article: PMC2174832] [PubMed: 10831609]
- 73.
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. [PubMed: 9859992]
- 74.
- Lundqvist-Gustafsson H, Gustafsson M, Dahlgren C. Dynamic Ca2+ changes in neutrophil phagosomes. A source for intracellular Ca2+ during phagolysosome formation? Cell Calcium. 2000;27:353–362. [PubMed: 11013465]
- 75.
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. [PubMed: 8381210]
- 76.
- Rosales C, Brown EJ. Signal transduction by neutrophil immunoglobulin G Fc receptors: Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5,-trisphosphate. J Biol Chem. 1992;267:5265–5271. [PubMed: 1531982]
- 77.
- Choi OH, Kim JH, Kinet J-P. Calcium mobilization via sphingosine kinase in signaling by the FcεRI antigen receptor. Nature. 1996;380:634–636. [PubMed: 8602265]
- 78.
- Melendez AJ, Floto RA, Cameron AJ. et al. A molecular switch changes the signalling pathway used by FcγRI antibody receptor to mobilise calcium. Curr Biol. 1998;8:210–221. [PubMed: 9501983]
- 79.
- Melendez A, Floto RA, Gillooly DJ. et al. FcγRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J Biol Chem. 1998;273:9393–9402. [PubMed: 9545263]
- 80.
- Melendez AJ, Bruetschy L, Floto RA. et al. Functional coupling of FcγRI to nicotinamide dinucleotide phosphate (reduced form) oxidative burst and immune complex trafficking requires the activation of phospholipase D1. Blood. 2001;98:3421–3428. [PubMed: 11719383]
- 81.
- Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. [PubMed: 11856736]
- 82.
- Rosales C, Jones SL, McCourt D. et al. Bromophenacyl bromide binding to the actin-bundling protein l-plastin inhibits inositol trisphosphate-independent increase in Ca2+ in human neutrophils. Proc Natl Acad Sci USA. 1994;91:3534–3538. [PMC free article: PMC43614] [PubMed: 8170942]
- 83.
- Olivera A, Rosenthal J, Spiegel S. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem. 1996;60:529–537. [PubMed: 8707892]
- 84.
- Young JD, Ko SS, Cohn ZA. The increase in intracellular free calcium associated with IgG gamma 2b/gamma 1 Fc receptor-ligand interactions: Role in phagocytosis. Proc Nat Acad Sci USA. 1984;81:5430–5434. [PMC free article: PMC391718] [PubMed: 6236462]
- 85.
- Kobayashi K, Takahashi K, Nagasawa S. The role of tyrosine phosphorylation and Ca2+ accumulation in Fc gamma-receptormediated phagocytosis of human neutrophils. J Biochem (Tokyo). 1995;117:1156–1161. [PubMed: 7490254]
- 86.
- Della BiancaV, Grzeskowiak M, Rossi F. Studies on molecular regulation of phagocytosis and activation of the NADPH oxidase in neutrophils. IgG- and C3bmediated ingestion and associated respiratory burst independent of phospholipid turnover and Ca2+ transients. J Immunol. 1990;144:1411–1417. [PubMed: 2105997]
- 87.
- Lew DP, Andersson T, Hed J. et al. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985;315:509–511. [PubMed: 3158824]
- 88.
- Bengtsson T, Jaconi ME, Gustafson M. et al. Actin dynamics in human neutrophils during adhesion and phagocytosis is controlled by changes in intracellular free calcium. Eur J Cell Biol. 1993;62:49–58. [PubMed: 8269978]
- 89.
- Jaconi ME, Lew DP, Carpentier JL. et al. Cytosolic free calcium elevation mediates the phagosome lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. [PMC free article: PMC2200167] [PubMed: 2110568]
- 90.
- Greenberg S, el KhouryJ, di Virgilio F. et al. Ca2+-independent F-actin assembly and disassembly during Fc receptor-mediated phagocytosis in mouse macrophages. J Cell Biol. 1991;113:757–767. [PMC free article: PMC2288985] [PubMed: 2026648]
- 91.
- Zimmerli S, Majeed M, Gustavsson M. et al. Phagosome-lysosome fusion is a calcium-independent event in macrophages. J Cell Biol. 1996;132:49–61. [PMC free article: PMC2120694] [PubMed: 8567729]
- 92.
- Edberg JC, Lin CT, Lau D. et al. The Ca2+ dependence of human Fc gamma receptor-initiated phagocytosis. J Biol Chem. 1995;270:22301–22307. [PubMed: 7673212]
- 93.
- Stendahl O, Krause KH, Krischer J. et al. Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science. 1994;265:1439–1441. [PubMed: 8073285]
- 94.
- Sawer DW, Sullivan JA, Mandell GL. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985;230:663–666. [PubMed: 4048951]
- 95.
- Bengtsson T, Jaconi ME, Gustafson M. et al. Actin dynamics in human neutrophils during adhesion and phagocytosis is controlled by changes in intracellular free calcium. Eur J Cell Biol. 1993;62:49–58. [PubMed: 8269978]
- 96.
- Harris HE, Weeds AG. Plasma gelsolin caps and severs actin filaments. FEBS Lett. 1984;177:184–188. [PubMed: 6094243]
- 97.
- Yin HL, Albrecht JH, Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981;91:901–906. [PMC free article: PMC2112796] [PubMed: 6276414]
- 98.
- Serrander L, Skarman P, Rasmussen B. et al. Selective inhibition of IgG-mediated phagocytosis in gelsolin-deficient murine neutrophils. J Immunol. 2000;165:2451–2457. [PubMed: 10946270]
- 99.
- Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. [PMC free article: PMC2192718] [PubMed: 8760816]
- 100.
- Allen LH, Aderem A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med. 1995;182:829–840. [PMC free article: PMC2192156] [PubMed: 7650489]
- 101.
- Larsen EC, DiGennaro JA, Saito N. et al. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J Immunol. 2000;165:2809–2817. [PubMed: 10946313]
- 102.
- Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. [PMC free article: PMC2190886] [PubMed: 7678851]
- 103.
- Zheleznyak A, Brown EJ. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation. Evidence for protein kinase C translocation to phagosomes. J Biol Chem. 1992;267:12042–12048. [PubMed: 1376316]
- 104.
- Dekker LV, Leitges M, Altschuler G. et al. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem J. 2000;347:285–289. [PMC free article: PMC1220958] [PubMed: 10727429]
- 105.
- Melendez AJ, Harnett MM, Allen JM. Differentiation dependent switch in protein kinase C isoenzyme activation by FcgammaRI, the human high-affinity receptor for immunoglobulin G. Immunology. 1999;96:457–464. [PMC free article: PMC2326762] [PubMed: 10233728]
- 106.
- Brumell JH, Howard JC, Craig K. et al. Expression of the protein kinase C substrate pleckstrin in macrophages: Association with phagosomal membranes. J Immunol. 1999;163:3388–3395. [PubMed: 10477609]
- 107.
- Li J, Aderem A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell. 1992;70:791–801. [PubMed: 1516135]
- 108.
- Hartwig JH, Thelen M, Rosen A. et al. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calciumcalmodulin. Nature. 1992;356:618–622. [PubMed: 1560845]
- 109.
- Carballo E, Pitterle DM, Stumpo DJ. et al. Phagocytic and macropinocytic activity in MARCKS-deficient macrophages and fibroblasts. Am J Physiol. 1999;277:C163–173. [PubMed: 10409119]
- 110.
- Underhill DM, Chen J, Allen LA. et al. MacMARCKS is not essential for phagocytosis in macrophages. J Biol Chem. 1998;273:33619–33623. [PubMed: 9837946]
- 111.
- Zhu Z, Bao Z, Li J. MacMARCKS mutation blocks macrophage phagocytosis of zymosan. J Biol Chem. 1995;270:17652–17655. [PubMed: 7629059]
- 112.
- Colombo MI, Beron W, Stahl PD. Calmodulin regulates endosome fusion. J Biol Chem. 1997;272:7707–7712. [PubMed: 9065429]
- 113.
- Wickner W, Haas A. Yeast homotypic vacuole fusion: A window on organelle trafficking mechanisms. Annu Rev Biochem. 2000;69:247–275. [PubMed: 10966459]
- 114.
- Zimmerli S, Majeed M, Gustavsson M. et al. Phagosome-lysosome fusion is a calcium-independent event in macrophages. J Cell Biol. 1996;132:49–61. [PMC free article: PMC2120694] [PubMed: 8567729]
- 115.
- Jaconi ME, Lew DP, Carpentier JL. et al. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555– 1564. [PMC free article: PMC2200167] [PubMed: 2110568]
- 116.
- Downey GP, Botelho RJ, Butler JR. et al. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcγRIIA receptors. J Biol Chem. 1999;274:28436–28444. [PubMed: 10497205]
- 117.
- Wilsson A, Lundqvist H, Gustafsson M. et al. Killing of phagocytosed Staphylococcus aureus by human neutrophils requires intracellular free calcium. J Leuk Biol. 1996;59:902–907. [PubMed: 8691076]
- 118.
- Jahraus A, Egeberg M, Hinner B. et al. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell. 2001;12:155–170. [PMC free article: PMC30575] [PubMed: 11160830]
- 119.
- Ferrari G, Langen H, Naito M. et al. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. [PubMed: 10338208]
- 120.
- Greenberg S, el Khoury J, di Virgilio F. et al. Ca2+-independent F-actin assembly and disassembly during Fc receptor-mediated phagocytosis in mouse macrophages. J Cell Biol. 1991;113:757–767. [PMC free article: PMC2288985] [PubMed: 2026648]
- 121.
- Ernst JD. Annexin III translocates to the periphagosomal region when neutrophils ingest opsonized yeast. J Immunol. 1991;146:3110–3114. [PubMed: 1826705]
- 122.
- Majeed M, Perskvist N, Ernst JD. et al. Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain of Mycobacterium tuberculosis in human neutrophils. Microb Pathog. 1998;24:309–320. [PubMed: 9600863]
- 123.
- Malik ZA, Iyer SS, Kusner DJ. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: Contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J Immunol. 2001;166:3392–3401. [PubMed: 11207296]
- Introduction
- Receptors Involved in Phagocytosis
- A Variety of Nonprofessional Phagocytic Receptors
- Calcium the Ubiquitous Second Messenger
- What Couples Phagocytic-receptor Activation to Rise on Intracellular Ca2+
- Downstream Events Triggered by Ca2+ Following Phagocytic-receptor Activation
- How Might Intracellular Ca2+ Control Actin Depolymerization During Phagocytosis?
- Role of Ca2+ On the Maturation of Phagosomes During Phagocytosis?
- Conclusion
- References
- Calcium Signaling During Phagocytosis - Madame Curie Bioscience DatabaseCalcium Signaling During Phagocytosis - Madame Curie Bioscience Database
- Regulation of Cell Adhesion Responses by Abl Family Kinases - Madame Curie Biosc...Regulation of Cell Adhesion Responses by Abl Family Kinases - Madame Curie Bioscience Database
- X Chromosome Inactivation and Embryonic Stem Cells - Madame Curie Bioscience Dat...X Chromosome Inactivation and Embryonic Stem Cells - Madame Curie Bioscience Database
- NOTCH SIGNALING AND THE DEVELOPING INNER EAR - Madame Curie Bioscience DatabaseNOTCH SIGNALING AND THE DEVELOPING INNER EAR - Madame Curie Bioscience Database
- Restriction-Modification Systems as Mobile Epigenetic Elements - Madame Curie Bi...Restriction-Modification Systems as Mobile Epigenetic Elements - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...