NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Summary
The epithelial lining of luminal organs such as the gastrointestinal and respiratory tract forms a regulated, selectively permeable barrier between luminal contents and the underlying tissue compartments. Paracellular permeability across epithelial and endothelial cells is in large part regulated by an apical intercellular junction also referred to as the tight junction (TJ). The tight junction and its subjacent adherens junction (AJ) constitute the apical junctional complex (AJC). The AJC is composed of a multiprotein complex, which affiliates with the underlying apical perijunctional F-actin ring. Such AJC association with the perijunctional F-actin ring is vital for maintaining its structure and function in health. Stimuli such as nutrients, internal signaling molecules and cytokines influence the apical F-actin organization and also modulate the AJC structure and paracellular permeability. Here we review some of the key stimuli that influence F-actin organization, AJC structure and paracellular permeability.
Introduction
The tight junction (TJ) is the apical most intercellular junction in epithelial and endothelial cells and in association with the subjacent adherens junction (AJ) it constitutes the apical junctional complex (AJC). TJs form regulated, selectively permeable barriers between two distinct compartments.1,2 Thus, for example in the intestinal and respiratory tract, TJs interface luminal contents and underlying tissue compartments. TJs do not just represent static structural elements but they are dynamically regulated to control paracellular solute and ion transport in diverse physiologic states. In part such regulation in health and dysregulation in disease occurs secondary to signaling events influencing the underlying perijunctional actin cytoskeleton.3,4
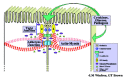
Columnar epithelial cells have a prominent perijunctional actin-myosin II ring that encircles the apical pole of polarized cells. This perijunctional filamentous actin ring is readily visualized by fluorescence labeling of filamentous actin and by electron microscopy. In fact the major interface of this ring with the lateral membrane occurs just below the TJ in the AJ. However, actin filaments project from this ring and interface with specific sites of membrane kisses in TJs that represent regions where membranes from apposing cells come into close apposition (fig. 1).5 Thus, it is logical to envision regulation of TJ structure and paracellular solute transport by factors that influence lateral tension within the perijunctional actin-myosin II ring.6-8
Our knowledge of TJ protein composition and regulation of paracellular transport is rapidly expanding. It is clearly evident that transmembrane proteins in TJs such as occludin, claudin(s) and junction adhesion molecule (JAM)1 affiliate with cytoplasmic plaque proteins that in turn have been implicated in the association of the TJ protein complex with the actin cytoskeleton.9-12 Prototypes of such actin associating proteins are the zonula occludens proteins (ZO-1, ZO-2 and ZO3). Cingulin also interacts with the zonula occludens proteins and enterocyte myosin heavy chain.13 The overall organization of TJ associated proteins is illustrated in Figure 1. Structure and functional properties of these proteins are detailed in other chapters of this issue.
Current knowledge of TJs is consistent with a view that they are specialized membrane microdomains14-16 that might function as molecular platforms involved in actin organization (Rho-GTPases, EFA6), cell signaling (c-src and c-yes), membrane trafficking (VAP-33, Rab3b, Rab13, Rab 8, Sec6, and Sec8), and cell polarity (Par3 and Par6).17-20 Thus TJs represent very dynamic platforms that regulate paracellular movement of ions and solutes in many physiologic and pathologic states. Diverse stimuli that influence TJ function and its subjacent actin cytoskeleton are discussed below.
Modulation of Myosin Light Chain-Phosphorylation in the Perijunctional F-Actin Ring Influences TJ Function
Circumferential contraction and therefore tension in the apical perijunctional actin-myosin ring regulates solute transport across the paracellular space.21-25 Such modifications in the perijunctional actin-myosin ring are achieved by the phosphorylation of the myosin light chain (MLC) of myosin II by MLC kinase (MLCK), which in turn acts on bipolar F-actin fibers in the perijunctional F-actin ring. Increased epithelial permeability in MLCK-dependent fashion has been demonstrated in model intestinal epithelial cell lines exposed to enteropathogenic Escherichia coli, transmigrating polymorphonuclear leukocytes or cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α).26-28 In addition, physiologic agonists have also been demonstrated to influence paracellular transport by modifications in the perijunctional actin-myosin ring. A classic scenario involves the uptake of glucose in the intestinal tract.29 Early ultrastructural examination of the intestinal mucosa has demonstrated that intrajunctional dilatations and condensation of the perijunctional cytoskeleton occur with Na+-glucose cotransport induced increase in permeability.22,30,31 Such modifications in the actin cytoskeleton support the concept of cytoskeletal regulation of paracellular permeability. In fact, subsequent studies linked the activation of enterocyte Na+-glucose cotransporter with phosphorylation of MLC.32 Na+-glucose cotransport induces cytoplasmic alkalinization that is dependent on the activation of the brush border Na+/H+ exchanger isoform NHE3.33 Inhibition of the NHE3 exchanger reduces MLC phosphorylation that is associated with an increase in transepithelial resistance (TER) to passive ion flow.33 It has therefore been proposed that NHE3 activation may be a critical component of the signaling pathway for Na+-glucose cotransport-dependent TJ regulation. Further evidence supporting the role of MLC phosphorylation in TJ regulation comes from studies using epithelial cells transfected with truncated MLCK gene construct lacking the inhibitory domain required for kinase regulation.34 Expression of this construct in model epithelial cell lines induced an increase in myosin regulatory light chain phosphorylation and an increase in paracellular permeability.27,34 Upstream regulation of the MLCK by protein kinase C has been proposed as a mechanism regulating the TJ permeability.35
Other physiological agonists influencing the cytoskeleton and junctional transport through modulation of the actin cytoskeleton include responses to histamine36 and lysophosphatidic acid37 that induce phosphorylation of MLCs. Ethanol and low concentration of extracellular calcium increase activity of MLCK and disrupt the TJ protein complex by influencing ZO-1 and occludin organization in the AJC.38,39 Agonists that influence TJ permeability in rat hepatocytes include angiotensin II, vasopressin and epinephrine.40
Modulation of Barrier Function by Rho GTPases
The Rho family of small GTPases, comprising Rho, Rac and Cdc42, are believed to play an important role in regulating and maintaining the perijunctional actin ring, TJ structure/function, and assembly of polarized epithelial cells.41-46 Rho function is modulated by a set of regulatory proteins and is activated through GDP-GTP exchange, which is promoted by guanine nucleotide exchange factors (GEF) and is inactivated through GTPase-activating proteins.47,48 Rho guanine nucleotide dissociation inhibitors mediate stabilization of inactive GDP-bound form of Rho.49 Conformational changes then allow the GTPases to interact with multiple effector molecules involved in actin cytoskeletal control50,51 Rho activity cycles are rapidly reversible, and are terminated upon hydrolysis of GTP by GTPase-activating proteins.
Several insights have been gained, based on the use of diverse pharmacological and molecular tools that interfere with function of the Rho family of GTPases. We have previously shown that inactivation of Rho GTPases (with Clostridium botulinum C3 transferase and Clostridium difficile toxins A and B) is associated with a compromised barrier function in T84 intestinal epithelial cells manifested both as functional decrease in TER, increase in paracellular flux of labeled dextran (3kDa) and a structural redistribution of ZO-1 and occludin away from the lateral plasma membrane. This effect was associated with reorganization of perijunctional F-actin.45 Moreover, we demonstrated, that disassembly of TJs induced by Clostridium difficile toxins A and B reduced the hyperphosphorylated occludin species and ZO-1 in “raft-like” membrane microdomains.14 The downstream effector of Rho referred to as Rho kinase (ROCK) has also been documented to regulate TJ function.52 However, ROCK inhibition induces profound reorganization of the apical F-actin cytoskeleton without influencing TJ protein distribution in the lateral membrane. These findings imply that ROCK mediated effects on TJ function are primarily due to its influence on the apical actin cytoskeleton in epithelial cells. These observations were further supported by transfection studies, in which a dominant negative mutant of ROCK induced loss of the apical F-actin—rich brush border and a reduction in the apical perijunctional F-actin ring without influencing occludin localization. Studies using transfected epithelial cell lines expressing dominant negative mutants of Rho GTPases demonstrated increase in paracellular permeability without influencing the TJ protein organization.53 In addition to the above downstream effector, an upstream GEF of the Dbl family of proto-oncogenes that activates Rho has been shown to associate with TJs.54 A link between the TJ cytoplasmic plaque protein ZO-3 and RhoA related signaling has been proposed.17 These studies reported that transfection of the amino terminal half of ZO-3 (NZO-3) in MDCK cells resulted in decreased RhoA GTPase activity and a change in cellular F-actin organization. The authors proposed a model whereby altered interactions between ZO-3 and an AJ protein, p120 catenin in NZO-3 expressing cells influences RhoA GTPase activity.
Recent studies reported, that not only inactivation, but also activation of the Rho family of proteins enhances paracellular permeability.43,53,55 Using an elegant inducible transfection system in MDCK cells, convincing evidence for an involvement of RhoA, Rac1 and Cdc42 in regulation of epithelial barrier function has been obtained.53,55,56 In this system, induction of dominant-active RhoA, Rac1 and Cdc42 activity was correlated with inability of epithelial cells to develop high TER. Moreover, increased paracellular permeability to molecules of different sizes was accompanied by redistribution of occludin and ZO-1 from the lateral membrane as well as modifications in junctional associated F-actin cytoskeleton. Using the same system, we found that activation as well as inactivation of RhoA, Rac1 or Cdc42 induced time-dependent disruptions in epithelial gate function and distinct morphological alterations in apical and basal F-actin pools. Constitutive activation of Rho A and Cdc42 induced redistribution of occludin, ZO-1, claudin-1, claudin-2 and JAM-1 from the lateral membrane. Constitutively active Rac1 on the other hand primarily influenced claudin-1 and -2 organization in TJs. These structural alterations were accompanied by changes in the biochemical properties of the TJ proteins.97 Interestingly, an increased activation of RhoA has been described in biopsies of patients with Crohn's disease indicating that RhoA may be involved in the cascade that leads to impaired barrier function in these patients.57 Reported effects of the activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor (CNF)-1 on epithelial TER have to date been diverse. While one report suggests a lack of CNF-1 effect on TER,58 two other reports using intestinal epithelial cell lines T84 and Caco2 document CNF-1 induced an increase in paracellular permeability.43,59 In the latter study, increased paracellular permeability was associated with significant redistribution of the TJ proteins occludin, ZO-1, claudin-1 and JAM-1 following basolateral exposure of epithelial cells to CNF- 1. In parallel, CNF-1 incubation resulted in decreased apical F-actin that was accompanied by formation of prominent basal F-actin cables.43 Thus, increased activation of Rho appears to disrupt the continuity between adjacent F-actin pools in microvilli, perijunctional ring and the terminal web that in turn could destabilize the “scaffold” of the TJ protein complex.43
Interestingly, CNF-1 treatment induced internalization of TJ proteins into endosomal/ caveolar-like membranous structures, evidenced by colocalization of TJ proteins with caveolin-1 by immunogold electron-microscopy.43 By immunolabeling and confocal microscopy TJ proteins were observed to colocalize with internalized early and recycling endosomal markers (EEA-1, Rab-11). This provides novel evidence that increased activation of Rho-GTPases induces internalization of TJ proteins into endosomal structures. Interestingly, dominant-active Rac1 and Cdc42 have been shown to affect endocytic trafficking in epithelial cells.55,60,61 The colocalization of TJ proteins with markers of recycling endosomes also suggests that recycling of TJ proteins back to the lateral membrane could occur, thereby providing a route for the rapid reestablishment of barrier function during the recovery phase following injury and internalization of TJ proteins. Given that inactivation as well as activation of Rho-GTPases adversely affects epithelial barrier function, it is likely that a delicate balance of Rho activity/quiescence is required for the maintenance of the optimal epithelial/endothelial barrier function.
Modulation of Barrier Function by Cytokines
Many cytokines have been shown to influence epithelial TJ function and the actin cytoskeleton both in vivo and in vitro. The cytokines IL-1, IL-4, IL-10, IL-13, TNF-α, and IFN-γ have all been shown to regulate TJs of both epithelia and endothelia.62-64 In addition, IL-1β influences TJ permeability through an effect on the claudin family of transmembrane proteins thought to be important in maintaining junctional integrity in astrocytes.65 A complete review of all the cytokines shown to modulate epithelial barrier function is beyond the scope of this article. Our review therefore has focused on the influence of few select cytokines on TJ structure/function and its adjoining actin cytoskeleton.
Interferon-gamma
IFN-γ is a 20- to 25-kDa glycoprotein released by activated T cells and natural killer cells in inflammatory states. In vitro models have been extensively used to examine the influence of this pro-inflammatory cytokine on intercellular junctions of epithelial cells. The initial studies addressing the influence of this cytokine on TJs utilized model epithelial cell line, T84.63,66,67 These studies demonstrated that IFN-γ induced a time-dependent increase in paracellular permeability that was accompanied by disorganization of apical F-actin and loss of ZO-1 from TJs.63,68 These morphological effects were associated with a change in the differential detergent solubility profiles of ZO-1 and ZO-2. The investigators did not observe IFN-γ induced change of phosphorylation status of these proteins. This was unexpected as phosphorylation status of TJ proteins is considered to modulate assembly of the TJ protein complex.69 Using the model T84 intestinal epithelial cell line, we have recently reported a IFN-γ-induced disruption of epithelial gate and fence function that was associated with differential internalization of TJ transmembrane proteins occludin, JAM-1, Claudin-1 and —4.70 We have also observed a concomitant reorganization of apical F-actin (our unpublished results). In contrast ZO-1 maintained its localization in the TJs. It is intriguing to speculate why ZO-1 localization and expression levels in our study were only slightly affected by IFN-γ. ZO-1 is a key TJ cytoplasmic plaque protein that provides a scaffold upon which other proteins can be assembled.71,72 We hypothesized that ZO-1 maintains its localization to provide this scaffold for efficient reassembly of TJ proteins upon cytokine withdrawal, which would be required for rapid and critical reestablishment of epithelial barrier function. Recent results from our laboratory support a IFN-γ induced internalization of TJ proteins into endosomal structures and this event is in part mediated by restructuring of the apical actin cytoskeleton (unpublished observations) (fig. 2). A similar mechanism of TJ protein endocytosis has been observed following depletion of extracellular calcium and disassembly of TJs.73 Thus, the actin cytoskeleton appears to be essential in not only maintaining a functioning TJ but is also required for the regulated disassembly and reassembly of the TJ.
An inflammatory response is regulated by a complex array of inhibitory and stimulatory cytokines, and thus it is likely that effects produced by IFN-γ are modulated by other cytokines. Several studies using different epithelial cell lines have shown, that TNF-α, another pro-inflammatory cytokine, can act synergistically with IFN-γ to increase paracellular permeability, 27,70,74,75 most likely due to TNF-α-induced up-regulation of the IFN-γ receptor. Coyne et al74 demonstrated in human epithelial airway cells, that combined treatment of TNF-α and IFN-γ induced profound effects on TJ barrier function, which could be blocked by inhibitors of protein kinase C. These studies emphasized the importance of the link between the actin cytoskeleton and TJs in regulation of barrier function both in the baseline state and following exposure to pro-inflammatory cytokines such as IFN-γ and TNF-α. In contrast to TNF-α, TGFβ or IL-10 have a negative influence on the IFN-γ induced changes in paracellular permeability.76,77
The above-described effects of IFN-γTNF-α on TJs, although complex, might have pathophysiological relevance because an increase in paracellular permeability across intestinal epithelial cells has been observed in patients with inflammatory bowel diseases (IBD).78 Since enhanced paracellular permeability across intestinal epithelium also occurs in first-degree relatives of patients with Crohn's disease, altered TJ permeability may be a contributing factor in this process,79,80 whereas disturbed barrier function in patients with ulcerative colitis is more likely secondary to the array of inflammatory signals that characterize this state.79,80 In this regard it is important, that redistribution of AJC proteins has been observed in tissues from patients with active IBD.81,82
Tumor Necrosis Factor-alpha
TNF-α is a 17-kDa proinflammatory cytokine produced mainly by mononuclear cells, and it influences barrier function of some epithelial cells. A biphasic response of TNF-α on TER has been reported in a porcine renal epithelial cell line, LLC-PK1.83 In this study, an initial fall in TER and increased paracellular permeability was followed by an increase in TER. The latter phase correlated with decreased relative anion selectivity of TJs. A role of tyrosine kinase and protein kinase A in mediating such effects of TNF-α on this cell type were proposed. In contrast to the findings above, TNF-α induced a fall in TER without the subsequent rebound in the intestinal epithelial cell lines HT-29 and Caco2. The decrease in TER was associated with increased paracellular permeability to mannitol in HT-29 monolayers but not in the Caco2 cells. In the latter, fall in TER was associated with increased paracellular permeability to Na+ and Cl-, implying altered charge but not size selectivity in TJs. The only morphological correlate in the above studies was a decrease in the number of TJ strand complexity by freeze-fracture EM in HT29 cells.84
TNF-a has however been implicated in modulating claudin-1 expression and ZO-1 organization in TJs.85 Studies in T84 cells did not report an effect of TNF-α alone on barrier function,66,70,86 indicating that TNF-α can exert different effects on barrier function depending on the target epithelium. In contrast to epithelial cells in pulmonary endothelial monolayers, TNF-a can induce an increase in permeability by influencing the actin cytoskeleton. In such endothelial cells, TNF-α significantly increased MLC phosphorylation, formation of prominent stress fiber and paracellular gaps.87
Hepatocyte Growth Factor
(HGF) HGF is a 103 kDa disulfide-linked, heterodimeric protein, that is produced chiefly by mesenchyme-derived cells and influences epithelial permeability in a paracrine fashion via ligation with its receptor, c-met. It was previously documented that analogous to IFN-γ, HGF induced a delayed decrease in TER of T84 epithelial monolayers over a period of 48 h.88 Structural studies to analyze the influence of HGF on intercellular junctions have not yielded unifying results. Depending on the origin of epithelial cells, variable effects of HGF on protein organization in the TJ versus its subjacent AJ have been proposed. Our studies in T84 epithelial cells have suggested an initial effect of HGF on apical F-actin organization that is accompanied by alterations in epithelial paracellular permeability (our unpublished observation). Such observations further emphasize the importance of apical F-actin structures in regulating paracellular permeability. In renal MDCK cells, HGF has been documented to influence AJC assembly.89 HGF-induced inhibition of junction assembly was associated with an increase in the Triton X-100 insoluble pool of E-cadherin90 and plakoglobin89 without influencing their total concentration thereby suggesting a cytokine induced change in their cytoskeletal association.
Other Cytokines
TGFβ1 has been reported to enhance barrier function in human enterocytes and to promote intestinal epithelial restitution.91 Moreover, TGFβ1 curtails the effects of barrier reducing cytokines such as IFN-γ, Il-4 and —10.76,92 In contrast, TGFβ1 has been shown to prevent glucocorticoid stimulated TJ formation and to reduce TER in 31EG4 polarized murine mammary epithelial cells.93,94 Such effects were accompanied by redistribution of ZO-1 from the lateral membrane and restructuring of perijunctional F-actin.93 Subsequently, it has been demonstrated that glucocorticoid induced downregulation of RhoA was required for its regulation of TJ and actin cytoskeletal organization.95
Exposure of the epidermal A431 cell line to epidermal growth factor (EGF) promotes TJ assembly. EGF facilitates restructuring of apical F-actin and induces phosphorylation of TJ cytoplasmic plaque proteins ZO-1 and ZO-2.96 Other cytokines such as interleukin (IL)-1, IL-4, IL-13, TGF-alpha, insulin-like growth factor (IGF)-I and -II, PDGF and vascular endothelial growth factor (VEGF) have been documented to decrease the barrier properties of epithelial cells. Mechanisms ranging from redistribution of TJ proteins to alterations in the actin cytoskeletal organization have been proposed to mediate the cytokine-induced effects on epithelial barrier function.
References
- 1.
- Diamond JM. Twenty-first Bowditch lecture. The epithelial junction: Bridge, gate, and fence. Physiologist. 1977;20(1):10–18. [PubMed: 16304]
- 2.
- van MeerG, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5(7):1455–1464. [PMC free article: PMC1166965] [PubMed: 3743548]
- 3.
- Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. Faseb J. 1995;9(10):910–918. [PubMed: 7615160]
- 4.
- Gumbiner BM. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. [PubMed: 8608588]
- 5.
- Staehelin LA. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. [PubMed: 4611943]
- 6.
- Madara JL, Moore R, Carlson S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol. 1987;253(6 Pt 1):C854–861. [PubMed: 3425707]
- 7.
- Madara JL, Parkos C, Colgan S. et al. The movement of solutes and cells across tight junctions. Ann NY Acad Sci. 1992;664:47–60. [PubMed: 1456668]
- 8.
- Pitelka DR, Taggart BN. Mechanical tension induces lateral movement of intramembrane components of the tight junction: Studies on mouse mammary cells in culture. J Cell Biol. 1983;96(3):606–612. [PMC free article: PMC2112411] [PubMed: 6682108]
- 9.
- Furuse M, Hirase T, Itoh M. et al. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–1788. [PMC free article: PMC2290891] [PubMed: 8276896]
- 10.
- Furuse M, Itoh M, Hirase T. et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127(6 Pt 1):1617–1626. [PMC free article: PMC2120300] [PubMed: 7798316]
- 11.
- Fanning AS, Jameson BJ, Jesaitis LA. et al. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273(45):29745–29753. [PubMed: 9792688]
- 12.
- Liu Y, Nusrat A, Schnell FJ. et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–2374. [PubMed: 10852816]
- 13.
- Citi S, Cordenonsi M. Tight junction proteins. Biochim Biophys Acta. 1998;1448(1):1–11. [PubMed: 9824655]
- 14.
- Nusrat A, von Eichel-StreiberC, Turner JR. et al. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69(3):1329–1336. [PMC free article: PMC98024] [PubMed: 11179295]
- 15.
- Nusrat A, Parkos CA, Verkade P. et al. Tight junctions are membrane microdomains. J Cell Sci. 2000;113(Pt 10):1771–1781. [PubMed: 10769208]
- 16.
- Harhaj NS, Barber AJ, Antonetti DA. Platelet-derived growth factor mediates tight junction redistribution and increases permeability in MDCK cells. J Cell Physiol. 2002;193(3):349–364. [PubMed: 12384987]
- 17.
- Wittchen ES, Haskins J, Stevenson BR. NZO-3 expression causes global changes to actin cytoskeleton in Madin-Darby canine kidney cells: Linking a tight junction protein to Rho GTPases. Mol Biol Cell. 2003;14(5):1757–1768. [PMC free article: PMC165074] [PubMed: 12802052]
- 18.
- van der Wouden JM, Maier O, van ISC. et al. Membrane dynamics and the regulation of epithelial cell polarity. Int Rev Cytol. 2003;226:127–164. [PubMed: 12921237]
- 19.
- Meyer TN, Hunt J, Schwesinger C. et al. Galpha12 regulates epithelial cell junctions through Src tyrosine kinases. Am J Physiol Cell Physiol. 2003;285(5):C1281–1293. [PubMed: 12890651]
- 20.
- Luton F, Klein S, Chauvin JP. et al. EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement. Mol Biol Cell. 2003 [PMC free article: PMC363093] [PubMed: 14668475]
- 21.
- Madara JL. Tight junction dynamics: Is paracellular transport regulated? Cell. 1988;53(4):497–498. [PubMed: 3286009]
- 22.
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–164. [PubMed: 3430571]
- 23.
- Madara JL, Stafford J, Dharmsathaphorn K. et al. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92(5 Pt 1):1133–1145. [PubMed: 3557010]
- 24.
- Turner JR, Madara JL. Physiological regulation of intestinal epithelial tight junctions as a consequence of Na(+)-coupled nutrient transport. Gastroenterology. 1995;109(4):1391–1396. [PubMed: 7557112]
- 25.
- Hecht G, Pothoulakis C, LaMont JT. et al. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82(5):1516–1524. [PMC free article: PMC442717] [PubMed: 3141478]
- 26.
- Yuhan R, Koutsouris A, Savkovic SD. et al. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113(6):1873–1882. [PubMed: 9394726]
- 27.
- Zolotarevsky Y, Hecht G, Koutsouris A. et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in vitro models of intestinal disease. Gastroenterology. 2002;123(1):163–172. [PubMed: 12105845]
- 28.
- Edens HA, Levi BP, Jaye DL. et al. Neutrophil transepithelial migration: Evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. J Immunol. 2002;169(1):476–486. [PubMed: 12077279]
- 29.
- Madara JL. Sodium-glucose cotransport and epithelial permeability. Gastroenterology. 1994;107(1):319–320. [PubMed: 8020682]
- 30.
- Pappenheimer JR. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J Membr Biol. 1987;100(2):137–148. [PubMed: 3430570]
- 31.
- Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100(2):123–136. [PubMed: 3430569]
- 32.
- Turner JR, Rill BK, Carlson SL. et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273(4 Pt 1):C1378–1385. [PubMed: 9357784]
- 33.
- Turner JR, Black ED, Ward J. et al. Transepithelial resistance can be regulated by the intestinal brush-border Na(+)/H(+) exchanger NHE3. Am J Physiol Cell Physiol. 2000;279(6):C1918–1924. [PubMed: 11078707]
- 34.
- Hecht G, Pestic L, Nikcevic G. et al. Expression of the catalytic domain of myosin light chain kinase increases paracellular permeability. Am J Physiol. 1996;271(5 Pt 1):C1678–1684. [PubMed: 8944652]
- 35.
- Turner JR, Angle JM, Black ED. et al. PKC-dependent regulation of transepithelial resistance: Roles of MLC and MLC kinase. Am J Physiol. 1999;277(3 Pt 1):C554–562. [PubMed: 10484342]
- 36.
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267(3 Pt 1):L223–241. [PubMed: 7943249]
- 37.
- van Nieuw Amerongen GP, Vermeer MA, van HinsberghVW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000;20(12):E127–133. [PubMed: 11116077]
- 38.
- Ma TY, Nguyen D, Bui V. et al. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276(4 Pt 1):G965–974. [PubMed: 10198341]
- 39.
- Ma TY, Tran D, Hoa N. et al. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: Role of cytoskeletal involvement. Microsc Res Tech. 2000;51(2):156–168. [PubMed: 11054866]
- 40.
- Lowe PJ, Miyai K, Steinbach JH. et al. Hormonal regulation of hepatocyte tight junctional permeability. Am J Physiol. 1988;255(4 Pt 1):G454–461. [PubMed: 2845804]
- 41.
- Braga VM, Machesky LM, Hall A. et al. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137(6):1421–1431. [PMC free article: PMC2132529] [PubMed: 9182672]
- 42.
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. [PubMed: 9438836]
- 43.
- Hopkins AM, Walsh SV, Verkade P. et al. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116(Pt 4):725–742. [PubMed: 12538773]
- 44.
- Nobes CD, Hall A. Rho, rac and cdc42 GTPases: Regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23(3):456–459. [PubMed: 8566347]
- 45.
- Nusrat A, Giry M, Turner JR. et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92(23):10629–10633. [PMC free article: PMC40665] [PubMed: 7479854]
- 46.
- Ridley AJ, Hall A. Distinct patterns of actin organization regulated by the small GTP-binding proteins Rac and Rho. Cold Spring Harb Symp Quant Biol. 1992;57:661–671. [PubMed: 1339704]
- 47.
- Chuang TH, Xu X, Knaus UG. et al. GDP dissociation inhibitor prevents intrinsic and GTPase activating protein-stimulated GTP hydrolysis by the Rac GTP-binding protein. J Biol Chem. 1993;268(2):775–778. [PubMed: 8419353]
- 48.
- Fukumoto Y, Kaibuchi K, Hori Y. et al. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5(9):1321–1328. [PubMed: 2120668]
- 49.
- Hall A. G proteins and small GTPases: Distant relatives keep in touch. Science. 1998;280(5372):2074–2075. [PubMed: 9669963]
- 50.
- Aspenstrom P. The Rho GTPases have multiple effects on the actin cytoskeleton. Exp Cell Res. 1999;246(1):20–25. [PubMed: 9882511]
- 51.
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249(4969):635–640. [PubMed: 2116664]
- 52.
- Walsh SV, Hopkins AM, Chen J. et al. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology. 2001;121(3):566–579. [PubMed: 11522741]
- 53.
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142(1):101–115. [PMC free article: PMC2133025] [PubMed: 9660866]
- 54.
- Benais-Pont G, Punn A, Flores-Maldonado C. et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol. 2003;160(5):729–740. [PMC free article: PMC2173357] [PubMed: 12604587]
- 55.
- Rojas R, Ruiz WG, Leung SM. et al. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2001;12(8):2257–2274. [PMC free article: PMC58593] [PubMed: 11514615]
- 56.
- Wojciak-Stothard B, Potempa S, Eichholtz T. et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(Pt 7):1343–1355. [PubMed: 11257000]
- 57.
- Segain JP, Raingeard de la Bletiere D, Sauzeau V. et al. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: Evidence in Crohn's disease and experimental colitis. Gastroenterology. 2003;124(5):1180–1187. [PubMed: 12730857]
- 58.
- Hofman P, Flatau G, Selva E. et al. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect Immun. 1998;66(6):2494–2500. [PMC free article: PMC108229] [PubMed: 9596707]
- 59.
- Gerhard R, Schmidt G, Hofmann F. et al. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco2 cells. Infect Immun. 1998;66(11):5125–5131. [PMC free article: PMC108639] [PubMed: 9784513]
- 60.
- Jou TS, Leung SM, Fung LM. et al. Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1. Mol Biol Cell. 2000;11(1):287–304. [PMC free article: PMC14775] [PubMed: 10637309]
- 61.
- Leung SM, Rojas R, Maples C. et al. Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA. Mol Biol Cell. 1999;10(12):4369–4384. [PMC free article: PMC25764] [PubMed: 10588664]
- 62.
- Oshima T, Laroux FS, Coe LL. et al. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61(1):130–143. [PubMed: 11162203]
- 63.
- Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276(5 Pt 1):G1279–1288. [PubMed: 10330020]
- 64.
- Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281(6):C2029–2038. [PubMed: 11698262]
- 65.
- Duffy HS, John GR, Lee SC. et al. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20(23):RC114. [PMC free article: PMC6773083] [PubMed: 11090614]
- 66.
- Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83(2):724–727. [PMC free article: PMC303735] [PubMed: 2492310]
- 67.
- Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150(6):2356–2363. [PubMed: 8450217]
- 68.
- Sugi K, Musch MW, Field M. et al. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120(6):1393–1403. [PubMed: 11313309]
- 69.
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276(5 Pt 2):F737–750. [PubMed: 10330056]
- 70.
- Bruewer M, Luegering A, Kucharzik T. et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171(11):6164–6172. [PubMed: 14634132]
- 71.
- Itoh M, Furuse M, Morita K. et al. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147(6):1351–1363. [PMC free article: PMC2168087] [PubMed: 10601346]
- 72.
- Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274(49):35179–35185. [PubMed: 10575001]
- 73.
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15(1):176–188. [PMC free article: PMC307538] [PubMed: 14528017]
- 74.
- Coyne CB, Vanhook MK, Gambling TM. et al. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13(9):3218–3234. [PMC free article: PMC124154] [PubMed: 12221127]
- 75.
- Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45(2):191–198. [PMC free article: PMC1727614] [PubMed: 10403730]
- 76.
- Planchon SM, Martins CA, Guerrant RL. et al. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153(12):5730–5739. [PubMed: 7989770]
- 77.
- Madsen KL, Lewis SA, Tavernini MM. et al. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113(1):151–159. [PubMed: 9207273]
- 78.
- Tsukada Y, Nakamura T, Iimura M. et al. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97(11):2820–2828. [PubMed: 12425554]
- 79.
- Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology. 2000;119(6):1740–1744. [PubMed: 11113095]
- 80.
- Soderholm JD, Olaison G, Lindberg E. et al. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease: An inherited defect in mucosal defence? Gut. 1999;44(1):96–100. [PMC free article: PMC1760070] [PubMed: 9862833]
- 81.
- Kucharzik T, Walsh SV, Chen J. et al. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159(6):2001–2009. [PMC free article: PMC1850599] [PubMed: 11733350]
- 82.
- Gassler N, Rohr C, Schneider A. et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G216–228. [PubMed: 11408275]
- 83.
- Peralta SolerA, Mullin JM, Knudsen KA. et al. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am J Physiol. 1996;270(5 Pt 2):F869–879. [PubMed: 8928850]
- 84.
- Schmitz H, Fromm M, Bentzel CJ. et al. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112(Pt 1):137–146. [PubMed: 9841910]
- 85.
- Poritz LS, Garver KI, Tilberg AF. et al. Tumor necrosis factor alpha disrupts tight junction assembly( 1,2). J Surg Res. 2004;116(1):14–18. [PubMed: 14732344]
- 86.
- Colgan SP, Parkos CA, Matthews JB. et al. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994;267(2 Pt 1):C402–410. [PubMed: 8074176]
- 87.
- Petrache I, Verin AD, Crow MT. et al. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1168–1178. [PubMed: 11350795]
- 88.
- Nusrat A, Parkos CA, Bacarra AE. et al. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest. 1994;93(5):2056–2065. [PMC free article: PMC294323] [PubMed: 8182137]
- 89.
- Pasdar M, Li Z, Marreli M. et al. Inhibition of junction assembly in cultured epithelial cells by hepatocyte growth factor/scatter factor is concomitant with increased stability and altered phosphorylation of the soluble junctional molecules. Cell Growth Differ. 1997;8(4):451–462. [PubMed: 9101091]
- 90.
- Balkovetz DF, Sambandam V. Dynamics of E-cadherin and gamma-catenin complexes during dedifferentiation of polarized MDCK cells. Kidney Int. 1999;56(3):910–921. [PubMed: 10469359]
- 91.
- Howe K, Gauldie J, McKay DM. TGF-beta effects on epithelial ion transport and barrier: Reduced Cl- secretion blocked by a p38 MAPK inhibitor. Am J Physiol Cell Physiol. 2002;283(6):C1667–1674. [PubMed: 12388073]
- 92.
- Di LeoV, Yang PC, Berin MC. et al. Factors regulating the effect of IL-4 on intestinal epithelial barrier function. Int Arch Allergy Immunol. 2002;129(3):219–227. [PubMed: 12444319]
- 93.
- Woo PL, Cha HH, Singer KL. et al. Antagonistic regulation of tight junction dynamics by glucocorticoids and transforming growth factor-beta in mouse mammary epithelial cells. J Biol Chem. 1996;271(1):404–412. [PubMed: 8550596]
- 94.
- Guan Y, Woo PL, Rubenstein NM. et al. Transforming growth factor-alpha abrogates the glucocorticoid stimulation of tight junction formation and reverses the steroid-induced down-regulation of fascin in rat mammary epithelial tumor cells by a Ras-dependent pathway. Exp Cell Res. 2002;273(1):1–11. [PubMed: 11795941]
- 95.
- Rubenstein NM, Guan Y, Woo PL. et al. Glucocorticoid down-regulation of RhoA is required for the steroid-induced organization of the junctional complex and tight junction formation in rat mammary epithelial tumor cells. J Biol Chem. 2003;278(12):10353–10360. [PubMed: 12525486]
- 96.
- Van ItallieCM, Balda MS, Anderson JM. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J Cell Sci. 1995;108(Pt 4):1735–1742. [PubMed: 7542259]
- 97.
- Bruewer M, Hopkins AM, Hobert ME. et al. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol. 2004;287(2):C327–35. [PubMed: 15044152]
- Regulation of Paracellular Transport across Tight Junctions by the Actin Cytoske...Regulation of Paracellular Transport across Tight Junctions by the Actin Cytoskeleton - Madame Curie Bioscience Database
- Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity - Ma...Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity - Madame Curie Bioscience Database
- The Role of Maspin in Tumor Progression and Normal Development - Madame Curie Bi...The Role of Maspin in Tumor Progression and Normal Development - Madame Curie Bioscience Database
- Rapid Evolution of Sex-related Genes: Sexual Conflict or Sex-specific Adaptation...Rapid Evolution of Sex-related Genes: Sexual Conflict or Sex-specific Adaptations? - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...