NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The larval somatic musculature of Drosophila is arranged in a highly stereotyped pattern of 30 muscle fibers per hemisegment. Each muscle possesses a distinctive set of properties: size, shape, orientation, attachments to the epidermis and specific innervation. These qualities make each myofiber a unique element within the pattern. In this chapter we review what is known to date about how a muscle acquires its particular identity. We discuss the mechanisms that underlie the specification of muscle founder cells and fusion-competent myoblasts and how both types of myoblasts contribute to the final pattern of each larval muscle. While a body of evidence supports a crucial role for founder cells in conferring particular muscle identities, the function of fusion-competent myoblasts during muscle morphogenesis remains an open question for future investigation in the field.
Introduction
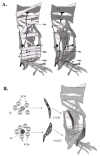
During Drosophila embryogenesis, mesodermal cells undergo a stereotypical series of movements, cell fate decisions and morphological changes to create a complex pattern of thirty larval muscles per abdominal hemisegment (fig. 1A). Each muscle consists of a single syncytium, which is created by the fusion of neighboring myoblasts. While all muscles share general properties, such as the contractile proteins and neurotransmitter receptors, each muscle in the pattern is a unique structure that can be identified by its orientation, size, shape, epidermal attachments and motoneuron innervation. The acquisition of these muscle specific properties during myogenesis depends upon the prior specification of a special class of myoblasts called founder cells (fig. 1B). Muscle founder cells were originally identified morphologically,1 and through the specific expression of the homeodomain protein Slouch (S59) in subsets of these cells.2 Each larval muscle is prefigured by a single founder cell, which seeds muscle formation by fusing with surrounding fusion-competent myoblasts. The number of fusion events is variable (3-25), depending on the specific muscle.3 Analysis of embryos carrying mutations in genes required for the fusion process, such as myoblast city, supports the idea that each founder cell contains the “identity” information for an individual muscle: when fusion is blocked, founder cells make mononucleated muscles that are correctly innervated and properly orientated. These muscles, however, are much smaller and often are unable to attach to tendon cells. Under these conditions, fusion-competent myoblasts remain undifferentiated, although they can express general muscle-markers such as the contractile protein Myosin.4 Thus, it has been proposed that each founder cell contains the unique information required to direct the morphogenesis of a specific muscle.
The purpose of this chapter is to review the molecules and mechanisms underlying the “birth” of the founder cell and fusion-competent myoblasts. We start post-gastrulation after mesodermal cells have completed their dorsal migration over the ectoderm and are being allocated to specific mesodermal cell fates, such as the somatic muscles or heart, with a view to the particular signals and transcriptional regulators key for this process. We then focus on the actual “birthing” process of founder cells, which are specified as sibling pairs from the division of progenitor cells. We highlight the dynamic interplay between several signal transduction pathways and mesoderm-specific transcriptional regulators required for progenitor specification as well as the molecules known to be involved in conferring a unique founder cell fate. Lastly, we consider the information content of the muscle founder cell and suggest how this information is mobilized to form a specific muscle.
Allocation of Somatic Mesoderm-Mesodermal Subdivision
Transplantation studies indicate that, at gastrulation, mesodermal cells have not yet committed to a particular mesodermal fate.5 These studies show that although these cells are incapable of forming structures other than mesoderm, the specific mesodermal fate chosen (i.e., somatic muscles, heart) depends on the final position that a cell occupies within the mesoderm and its location relative to other germ layers. Consistent with these studies, both intrinsic and extrinsic signals have been shown to affect the allocation of mesodermal cells into different fates (fig. 2).
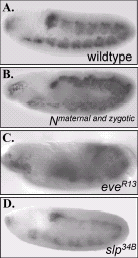
The coordinated activities of the segmentation genes even-skipped (eve) and sloppy-paired (slp) as well as the ectodermal signals Hedgehog (Hh) and Wingless (Wg) are required to divide each mesodermal segment into two domains across the anterior-posterior axis6,7 (fig. 2). Cells in the Eve/Hh domain differentiate into visceral muscle, fat body, heart and a subset of glia, while cells in the Slp/Wg domain differentiate into somatic muscles and heart. While Eve is detected transiently in the mesoderm at the onset of gastrulation, Slp is detected in the mesoderm both at gastrulation and during the subdivision period. Eve and Slp function as transcriptional regulators, which pattern the mesoderm both directly by regulating expression of mesodermal genes, and indirectly by activating the ectodermal signaling pathways Hh and Wg/Wnt. These signaling pathways reinforce and further refine the patterning initiated by Eve and Slp. As the mesoderm is subdivided along the anterior-posterior axis, the activity of the TGF-— family member Decapentaplegic (Dpp) stimulates the subdivision along the dorsal-ventral axis. Dpp, secreted by the dorsal ectoderm, acts on the underlying mesoderm to regulate dorsal fate genes, such as tinman, and to repress ventrally expressed genes such as pox meso8,9 (fig. 2).
From the view of somatic myogenesis, a critical output of this subdivision process is the modulation of Twist expression. The characteristic uniform high Twist expression required to specify mesoderm at gastrulation becomes modulated into a segmentally repeated pattern of low and high Twist expression along the anterior-posterior axis of the embryo.10-12 Cells expressing low Twist levels differentiate into visceral muscles and fat body, while cells expressing high Twist levels develop into somatic muscles.13,14 Increasing Twist expression in mesodermal domains where Twist levels are usually low blocks formation of visceral mesoderm and fat body and leads to formation of ectopic somatic muscles in their place. Conversely, uniform low Twist levels interfere with somatic myogenesis but permit the development of other tissues.15,16 These results indicate that the role of Twist during mesodermal subdivision is to promote formation of the somatic muscles.
During the subdivision period, the transcriptional regulators Slp and Eve as well as the Wg and Hh signaling pathways modulate Twist expression (fig. 3). Specifically, prior to and during subdivision, the expression of Slp in the mesoderm overlaps with the high Twist domain primordial.17 Genetic experiments revealed that Slp activates Twist expression; slp loss-of-function mutants express Twist uniformly at low levels throughout the mesoderm, while panmesodermal overexpression of Slp has the opposite effect: Twist is expressed at uniform high levels.7,17 Since Slp and Twist colocalize in the mesoderm and analysis of the twist promoter revealed several conserved Slp-binding sites, it is likely that Slp directly regulates twist.18 Slp activity is, in turn, regulated by Wg signaling and by Eve. Ectodermal Wg signaling directly induces mesodermal slp expression.17 However, contrary to what happens in slp mutant embryos, wg loss- and gain-of-function mutants properly modulate Twist into low and high domains. Thus, the effect that Slp has on Twist appears to be only partially mediated by Wg.7,18 Moreover, Eve and Hh signaling also affect Twist indirectly as Eve, which functions as both an ectodermal upstream regulator and a mesodermal target of Hh signaling, interferes with Slp function.6,7
Notch signaling also regulates Twist expression during the subdivision period.19 Embryos that completely lack Notch fail to modulate Twist into low and high domains, resulting in the uniform maintenance of high Twist levels (fig.3). Conversely, global mesodermal overexpression of a constitutively active form of the Notch receptor represses Twist expression: compared to wild-type embryos, most cells express low levels of Twist. Analysis of the mechanism utilized by Notch to regulate twist revealed that Notch and its transcriptional effector Suppressor of Hairless20 have multiple inputs into twist. Notch signaling regulates twist both directly, through a conserved Su(H) site on the twist promoter, and indirectly, by activating proteins that repress twist. Genetic data suggested that these “repressors of twist” are the bHLH transcriptional repressors encoded by the Enhancer of split [E(spl)] gene complex and the HLH protein Extramacrochaetae (Emc). E(spl) appears to repress Twist directly through sites in the twist promoter, whereas Emc inhibits Twist by sequestering the bHLH protein Daughterless, which is required to up-regulate Twist.19
Birth of a Muscle Founder Cell
Muscle founder cells are born as distinct sibling pairs by the division of progenitor cells. These progenitors, in turn, segregate from clusters of equivalent cells (“promuscle groups”) that express the bHLH protein Lethal of Scute (L'sc).21 L'sc is expressed in 19 clusters per hemisegment that appear sequentially in the embryonic mesoderm from late stage 10 (5h after egg laying) until stage 12 (7h 30 after egg laying). In each equivalence group, L'sc expression is progressively restricted to one cell, the progenitor. Expression of L'sc is lost when the progenitor divides, so that L'sc is no longer detected in founder cells. The number of L'sc-expressing clusters/progenitors can account for all the 30 founder cells required per hemisegment to seed each specific myofiber. The absence or excess of l'sc function leads to losses or duplications, respectively, of a subset of Slouch+-founder cells and somatic muscles. These phenotypes indicate a role of L'sc during early myogenesis. However, l'sc loss-of-function phenotype is weaker than that expected for its wide pattern of expression in all promuscle clusters/progenitors. This suggests that there are other factors that collaborate with L'sc to specify progenitor fate.
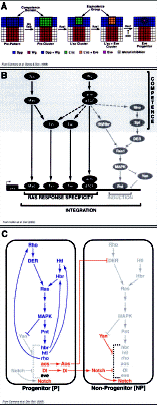
Different signaling pathways are required throughout the process of muscle progenitor specification. Particularly, for two-dorsal L'sc+ clusters/progenitors that also express the identity protein Eve (L'sc clusters 2 and 15), it has been shown that individual progenitors are progressively determined by unique combinations of intercellular signals.22 First, the intersecting domains of Wg and Dpp, secreted from the ectoderm, define a broad region in the dorsal mesoderm that is competent to respond to a subsequent Ras-Mitogen Activated Protein Kinase (MAPK) signaling. This prepatterned region is revealed by the expression of L'sc (i.e., “precluster”). Then, Ras-MAPK is locally activated, within the competent region, by two receptor tyrosine kinases (RTKs), the Drosophila EGF receptor (DER) and the FGF receptor Heartless (Htl).23,24 RTK local activation restricts L'sc expression to a subset of cells, within the precluster, which constitute a promuscle cluster or equivalence group. Subsequently, Ras activates Eve in all cells of the cluster. Finally, individual progenitors are singled out from each group of equivalent cells under the opposing influences of the positive Ras activity and the lateral inhibitory function of the Notch signaling pathway (fig. 4A).
Once progenitors are specified, they move into close contact with the ectoderm and divide asymmetrically, giving rise to two founder cells.25,26 Different proteins, known to have a crucial function during neural asymmetric cell divisions, have also been implicated in the asymmetric division of muscle progenitors.25-29 Inscuteable (Insc), an adaptor cytoskeletal protein, and Numb, a membrane associated protein that contains a phosphotyrosine-binding domain, localize as cortical crescents on opposite sides of dividing progenitor cells. Insc provides positional information required for Numb asymmetric segregation into one of the sibling founder cells. This is critical for the specification of distinct sibling cell fates, as Numb binds and suppresses Notch signaling in the sibling cell into which it segregates.30 Two different models, which are not mutually exclusive, have been proposed to explain the mechanism by which Numb inhibits Notch during neural asymmetric cell divisions. One model shows that Numb inhibits Notch through α-Adaptin, a protein involved in receptor-mediated endocytosis.31 The authors show that Numb contributes to the endocytosis of Notch and the consequent repression of Notch signal by polarizing the distribution of α-Adaptin. The second model points to Sanpodo (Spdo), a four-pass transmembrane protein that interacts physically with Notch and is required for Notch signaling.32,33 According to this model, Numb inhibits Notch by regulating Spdo membrane localization: Numb binds Spdo and inhibits Spdo localization at the membrane; consequently, Notch is inactive in this cell. This last mechanism of Notch inhibition by Numb may be also operating in the mesoderm, as Spdo has been shown to have an important role in this tissue during asymmetric cell divisions.27,29 Another protein necessary during both neural and muscle asymmetric cell divisions is Partner of Numb (Pon).28 Pon colocalizes and interacts physically with Numb. Loss- and gain-of-function mutant analysis of pon shows that Pon forms part of the molecular machinery crucial for Numb asymmetric localization. Finally, a new protein implicated in Insc regulation has been identified very recently.34 This protein, called Abstrakt (Abs), belongs to a family of RNA-dependent ATPases or DEAD-box proteins. The authors show that Abs binds the insc mRNA and is essential in the posttranscriptional regulation of insc. In abs mutant embryos, the levels of Insc protein drop significantly with the consequent failure in the proper localization of cell fate determinants (i.e., Insc, Pon). Abs is also required for both neural and muscle progenitors' asymmetric divisions.
Thus, both intrinsic and extrinsic cues are required for the assignation of specific cell fates to muscle founders. Shortly after they are born, both sibling founder cells express a specific set of identity proteins, which usually are already expressed in the progenitor and occasionally at the cluster stage. However, as the siblings mature, they begin to display distinct characteristics. For example, one of the two siblings loses the expression of identity proteins that were expressed in the progenitor, whereas the other sibling keeps the expression of such proteins.21,25,35 In other cases, only one sibling starts to express specific markers that were not previously expressed in the progenitor.36 These observations reveal the asymmetric nature of the progenitor division and the unique fate of each founder cell. Founder cells will start to fuse with neighboring fusion-competent myoblasts, seeding the formation of individual and distinctive muscle fibers.
Integration of Signal Transduction Pathways during Muscle Progenitor Specification
Multiple signaling pathways and transcriptional regulators are involved in the determination of muscle progenitors. However, how mesodermal cells integrate this information is not completely understood. Transcriptional convergence has been shown to be an important mechanism for integrating signals and for achieving tissue-specific responses.37,38 In these studies, the authors identify an enhancer from the muscle identity gene eve that recapitulates Eve expression during muscle and heart progenitor specification (MHE, Muscle and Heart Enhancer). This enhancer contains binding sites for two classes of transcriptional regulators: signal-activated and tissue-restricted factors. Among the first class are the nuclear effectors of Wg (dTCF), Dpp (Mad) and Ras (Pointed and Yan) signaling pathways; among the mesoderm-specific factors are Tinman and Twist. All these factors are integrated at the MHE where they function synergistically to promote eve transcription. This enhancer model explains how Wg and Dpp provide competence to mesodermal cells to respond to Ras-MAPK, by regulating proximal components of the Ras-MAPK pathway, and how the specificity of Ras response is achieved, since Wg and Dpp also regulate Twist and Tinman expression (fig. 4B).
Additional networks of signal cross-regulation have been described during muscle progenitor specification. For example, extensive reciprocal interactions occur between opposing Ras/MAPK and Notch signaling pathways during the formation of Eve+ progenitors.36 Notch competes with Ras by down-regulating different components of the Ras-MAPK cascade, including Htl, Heartbroken (Hbr, also known as Stumps and Dof)39-41 and Rhomboid (Rho).23,42 Cooperation between Ras and Notch signals was also observed: Ras upregulates the Notch ligand Delta and the epidermal growth factor receptor antagonist Argos.43 Then, Delta and Argos synergize to inhibit nonautonomously the Ras-MAPK signaling cascade. In addition, multiple feedback loops, positive and negative, are established. For instance, Ras upregulates proximal components of its own pathway, including Htl, Hbr and Rho, in the prospective progenitor. In addition, Notch upregulates its own expression and represses Delta and Argos in nonprogenitor cells (fig. 4C). All these reciprocal interactions combine to generate the signal thresholds that are essential for proper specification of progenitors and nonprogenitors from groups of initially equivalent cells.
Taken together, these findings show that signal transduction pathways do not act as isolated linear cascades during muscle progenitor specification. Rather, their effects are intertwined at multiple levels to form an integrated network of cross-talk nodes and feedback loops.
Muscle Identity Is Regulated by Muscle Founder Identity Genes
While recent work has given us insight to the specification of founder cells, we know little about the next step in muscle morphogenesis, when the information and processes within a founder cell coordinates and elaborates specific muscle traits. Some clues to this next step have come from the observation that the specific combinations of signaling inputs that a given founder cell receives results in the production of a unique set of molecular determinants, founder cell identity proteins, that gives each muscle fiber its shape, size and connection pattern. Eleven identity genes, including the homeobox genes S59 (also named slouch),2,21,44 apterous (ap),45 muscle segment homeobox (msh),46 eve,22,47,48 ladybird,49 the myc-related HLH encoding gene collier,50 the bHLH encoding gene, nautilus (nau),51,52 vestigial53 and the zinc finger encoding gene Krüppel (Kr)54 have been found to date to be expressed in different, sometimes overlapping, subsets of founder cells. These genes have been called “muscle or founder cell identity genes”, initially by virtue of the fact that they identify, by their expression pattern, specific founder cells and muscles. Loss and gain-of-function experiments have been performed on 7 of the 11 known founder cell identity genes.3,55 These data indicated a role for these regulators in determining final muscle characteristics; for example, loss of Kr function leads not only to complete muscle transformations (i.e., specific muscle losses and duplications) but also incomplete transformations of specific muscles (the specific muscle forms in the correct position but has aberrant features such as changes in shape and attachments). Hence, a gene is called a muscle/founder cell identity gene if it fulfils the following: (1) it encodes a transcriptional regulator; (2) it is expressed in subsets of founder cells; and (3) it has a known or presumed role in specifying the developmental fates of individual muscles, leading to complete and/or partial transformations in muscle identity.
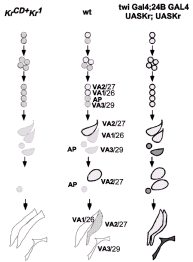
Both the overlapping pattern of founder cell identity gene expression in different sets of muscle founder cells and genetic analyses have led to the hypothesis that individual muscle identities are specified by combinatorial codes of identity genes. For example, the muscle VA2/ 27 (muscle letter/number hereon) founder cell expresses and maintains Kr, Slouch, Ap, and Nau and are thought to all contribute to VA2/27 identity.44,54 However, loss- and gain-of-function experiments revealed added complexity to the combinatorial hypothesis, suggesting that these transcriptional regulators may target different aspects of the morphogenic process in different founder cells. It appears that some founder cell identity genes may control other founder cell transcriptional regulators whereas others appear to control directly specific attributes of the muscle morphogenesis. For example, Kr and Slouch are expressed in the muscle progenitor that divides to give rise to the founder cells for muscles VA2/27 and VA1/26 (fig. 5). Both proteins are maintained in the VA2/27 muscle founder cell and fade in the VA1/26 muscle founder cell. In the absence of mesodermal Kr, however, Slouch expression is initiated in the muscle progenitor, but is lost in the VA2/27 founder. This VA2/27 muscle founder cell fails to give rise to a VA2/27 muscle; instead the VA2/27 founder cell has been transformed into its sibling VA1/26 founder, which subsequently develops into a second VA1/26 muscle. Thus the embryo has two VA1/26 muscles and no VA2/27 muscles. Ectopic Kr expression in both VA1/26 and VA2/27 muscle founder cells leads to expression of Slouch in both VA1/26 and VA2/27 muscle founders and the subsequent loss of the VA1/26 muscle and duplication of the VA2/27 muscle54 (fig. 5). Loss of Slouch function itself in VA2/27 founder cell does not lead to a complete VA2/27 → VA1/26 transformation as would have been predicted from a simple combinational model in which all transcriptional regulators act together on the same target promoters. In this case, muscle VA2/27 is partially transformed: the muscle forms but with aberrant features (altered shape and attachment) and altered gene expression {maintained Kr and Nau (VA2/27 markers) but failed to express Ap}. Loss of Nau or Ap, which are also expressed in the VA2/27 founder cell, has no effect on VA2/27 development, possibly due to functional redundancy with Kr, Slouch or yet unknown proteins.44 Taken together, these experiments indicated that there may be a hierarchy among identity genes in different founder cells: some identity genes regulate the expression of others (e.g., Kr can regulate Slouch and presumably other regulators for VA2/27) to dictate a complete muscle identity, others (Slouch for VA2/27) regulate only a subset of the specific program and still others (Ap, Nau for VA2/ 27) have no effect possibly due to redundancy.
Loss- and gain-of-function experiments of these muscle gene regulators also suggested that the specific founder cell context determines the role or place of muscle regulators within the hierarchy. For example, Slouch is required for the normal development of all abdominal muscles that are derived from Slouch founder cell. However loss of Slouch activity has different effects in individual muscle types. As stated above, slouch mutant embryos form muscle VA2/27 with aberrant features. However, muscles VT1/25 and VA3/29, which also normally express Slouch, are completely transformed into muscles with different identities.44 Hence the combination of transcriptional regulators, the place within the hierarchy and thus, the specific role of a transcriptional regulator within a specific founder cell appears to control the unique traits elaborated during muscle morphogenesis.
A Molecular Definition of Muscle Founder Cell and Fusion-Competent Myoblasts
Founder cell identity genes have given us a glimpse into the regulation of muscle identity. However, additional studies are needed to determine how these regulators translate the specific aspects of muscle morphogenesis. For example, we do not know the genes or the mechanisms that control how the final number of fusion events is regulated, how a particular tendon cell is selected or how final shape of the muscle is achieved. In addition, while many founder cells have had identity genes assigned to them, there are many muscle founder cells in which no such genes have been localized. Recently, a step forward has been taken to better understand the translation of founder cell identity into muscle morphogenesis. A large-scale gene expression analysis was performed to identify genes differentially expressed in founder cells versus fusion-competent myoblasts. To enrich for each cell type, embryos derived from Toll10b mutants to obtain primarily muscle-forming mesoderm, and expression of activated forms of Ras or Notch to induce founder cell or fusion-competent myoblast fate, respectively, were collected. The transcripts present in embryos of each genotype were compared using hybridization to cDNA microarrays encompassing 40% of the Drosophila genome. Among the 83 genes differentially expressed, genes known to be enriched in founder cells or fusion-competent myoblasts such as htl or hibris, previously characterized genes with unknown roles in muscle development, and predicted genes of unknown function were found. Additional studies of newly identified genes discovered new patterns of gene expression restricted to one of the two types of myoblasts and also remarkable muscle phenotypes. Some genes such as phyllopod play a critical role during specification of particular muscles, while others including tartan and cadmus are necessary for normal muscle morphogenesis.56
The simplest view of the “founder cell” hypothesis is that each founder cell contains all the information for the development of a particular muscle. Fusion-competent myoblasts, in contrast, have been seen as a naïve group of myoblasts, entrained to a particular muscle program upon fusion to the founder cell. The array data indicate that these two groups of myoblasts have distinct transcriptional profiles and raise the possibility of a greater role for fusion-competent myoblasts in determining the final muscle morphology. The mechanisms underlying the complex morphological changes that occur during migration and cell fusion, as well as changes in cell shape and cell physiology, require a rich and dynamic program of transcriptional activity. A major challenge for the future of in muscle biology lies in exploring the function of the new genes identified in this and similar screens during the period of muscle morphogenesis. The key to understanding this process will be to find the molecular interactions among these newly identified genes and the known molecular networks that build and coordinate the thirty unique muscle elements in each Drosophila embryonic hemisegment.
Acknowledgements
A.C and M.B were supported by a Human Frontiers Postdoctoral Fellowship and by National Institutes of Health Grant GM56989.
References
- 1.
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. [PubMed: 2100994]
- 2.
- Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4:2098–2111. [PubMed: 1980118]
- 3.
- Bate M. The mesoderm and its derivatives in the development of Drosophila melanogasterIn: Bate M. Martinez-Arias A, eds. New York: Cold Spring Harbor: CSH Laboratory Press,19931013–1090.
- 4.
- Rushton E, Drysdale R, Abmayr SM. et al. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. [PubMed: 7635046]
- 5.
- Beer J, Technau G, Campos-Ortega J. Lineage analysis of transplanted individual cells in embryos of Drosophila melanogaster: IV commitment and proliferative capabilities of individual mesodermal cells. Roux's Arch Dev Biol. 1987;196:222–230. [PubMed: 28305697]
- 6.
- Azpiazu N, Lawrence PA, Vincent J-P. et al. Segmentation and specification of the Drosophila mesoderm. Genes Dev. 1996;10:3183–3194. [PubMed: 8985186]
- 7.
- Riechmann V, Irion U, Wilson R. et al. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124:2915–2922. [PubMed: 9247334]
- 8.
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. [PubMed: 7700357]
- 9.
- Staehling-Hampton K, Hoffman FM, Baylies MK. et al. dpp induces mesodermal gene expression in Drosophila. Nature. 1994;372:783–786. [PubMed: 7997266]
- 10.
- Simpson P. Maternal-zygotic gene interactions during the formation of the dorso-ventral pattern in Drososphila embryos. Genetics. 1983;105:615–632. [PMC free article: PMC1202177] [PubMed: 17246169]
- 11.
- Thisse B, Stoetzel C, Gorosotiza-Thisse C. et al. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988;7:2175–2183. [PMC free article: PMC454534] [PubMed: 3416836]
- 12.
- Leptin M. Twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes & Development. 1991;5:1568–1576. [PubMed: 1884999]
- 13.
- Baylies MK, Bate M. twist: A myogenic switch in Drosophila. Science. 1996;272:1481–1484. [PubMed: 8633240]
- 14.
- Dunin-Borkowski O, Bate M, Brown N. Anterior-posterior subdivision and the diversification of the mesoderm in Drosophila. Development. 1995;121:4183–4193. [PubMed: 8575318]
- 15.
- Bate M, Baylies MK. Intrinsic and extrinsic determinants of mesodermal differentiation in Drosophila. Semin Cell Dev Biol. 1996;7:103–111.
- 16.
- Castanon I, Von StetinaS, Kass J. et al. Dimerization partners determine the activity of the Twist bHLH protein during Drosophila mesoderm development. Development. 2001;128(16):3145–3159. [PubMed: 11688563]
- 17.
- Lee HH, Frasch M. Wingless effects mesoderm patterning and ectoderm segmentation events via induction of its downstream target sloppy paired. Development. 2000;127(24):5497–5508. [PubMed: 11076769]
- 18.
- Cox V. Signal transport, levels and integration: New lessons from wingless regulation of Drosophila mesoderm development [Ph.D] New York: Development Biology, Weill School of Medicine at Cornell University. 2004
- 19.
- Tapanes-Castillo A, Baylies M. Notch signaling patterns Drosophila mesodermal segments by regulating the bHLH transcription factor twist. Development. 2004;131:2359–2372. [PubMed: 15128668]
- 20.
- Bray S, Furriols M. Notch pathway: Making sense of Suppressor of Hairless. Curr Biol. 2001;11(6):R217–221. [PubMed: 11301266]
- 21.
- Carmena A, Bate M, Jiménez F. lethal of scute, a proneural gene, participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev. 1995;9:2373–2383. [PubMed: 7557389]
- 22.
- Carmena A, Gisselbrecht S, Harrison J. et al. Combinatorial signaling codes for the progressive determination of cell fates in the Drosophila embryonic mesoderm. Genes & Development. 1998a;15:3910–3922. [PMC free article: PMC317272] [PubMed: 9869644]
- 23.
- Buff E, Carmena A, Gisselbrecht S. et al. Signalling by the Drosophila epidermal growth factor receptor is required for the specification and diversification of embryonic muscle progenitors. Development. 1998;125:2075–2086. [PubMed: 9570772]
- 24.
- Michelson AM, Gisselbrecht S, Zhou Y. et al. Dual functions of the heartless fibroblast growth factor receptor in development of the Drosophila embryonic mesoderm. Dev Genet. 1998b;22(3):212–229. [PubMed: 9621429]
- 25.
- Carmena A, Murugasu-Oei B, Menon D. et al. Inscuteable and numb mediate asymmetric muscle progenitor cell divisions during Drosophila myogenesis. Genes Dev. 1998b;12:304–315. [PMC free article: PMC316482] [PubMed: 9450926]
- 26.
- Baylies MK, Bate M, Ruiz-Gomez M. The specification of muscle in Drosophila. Cold Spring Harb Symp Quant Biol. 1997;LXII:385–393. [PubMed: 9598373]
- 27.
- Park M, Yaich LE, Bodmer R. Mesodermal cell fate decisions in Drosophila are under the control of the lineage genes numb, Notch, and sanpodo. Mech Dev. 1998;75(1-2):117–126. [PubMed: 9739121]
- 28.
- Lu B, Rothenberg M, Jan LY. et al. Partner of numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell. 1998;95(2):225–235. [PubMed: 9790529]
- 29.
- Ward EJ, Skeath JB. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development. 2000;127(22):4959–4969. [PubMed: 11044409]
- 30.
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: Interaction of Numb and Notch. Neuron. 1996;17(1):27–41. [PubMed: 8755476]
- 31.
- Berdnik D, Torok T, Gonzalez-Gaitan M. et al. The endocytic protein alpha-Adaptin is required for Numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002a;3(2):221–231. [PubMed: 12194853]
- 32.
- Dye CA, Lee JK, Atkinson RC. et al. The Drosophila sanpodo gene controls sibling cell fate and encodes a tropomodulin homolog, an actin/tropomyosin-associated protein. Development. 1998;125(10):1845–1856. [PubMed: 9550717]
- 33.
- O'Connor-Giles KM, Skeath JB. Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev Cell. 2003;5(2):231–243. [PubMed: 12919675]
- 34.
- Irion U, Leptin M. Developmental and cell biological functions of the Drosophila DEAD-box protein Abstrakt. Curr Biol. 1999;9(23):1373–1381. [PubMed: 10607561]
- 35.
- Ruiz-Gomez M, Bate M. Segregation of myogenic lineages in Drosophila requires numb. Development. 1997a;124:4857–4866. [PubMed: 9428422]
- 36.
- Carmena A, Buff E, Halfon M. et al. Reciprocal regulatory interactions between the Notch and Ras signaling pathways in the Drosophila embryonic mesoderm. Dev Biol. 2002;244:226–242. [PubMed: 11944933]
- 37.
- Halfon MS, Carmena A, Gisselbrecht S. et al. Ras pathway specificity is determined by the integration of multiple signal-activated and tissue-restricted transcription factors. Cell. 2000;103:63–74. [PubMed: 11051548]
- 38.
- Knirr S, Frasch M. Molecular integration of inductive and mesoderm-intrinsic inputs governs even-skipped enhancer activity in a subset of pericardial and dorsal muscle progenitors. Dev Biol. 2001;238(1):13–26. [PubMed: 11783990]
- 39.
- Imam F, Sutherland D, Huang W. et al. stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics. 1999;152:307–318. [PMC free article: PMC1460608] [PubMed: 10224263]
- 40.
- Michelson AM, Gisselbrecht S, Buff E. et al. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development. 1998a;125:4379–4389. [PubMed: 9778498]
- 41.
- Nolan K, Barrett K, Lu Y. et al. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes & Development. 1998;12:3337–3342. [PMC free article: PMC317223] [PubMed: 9808621]
- 42.
- Freeman M. Feedback control of intercellular signalling in development. Nature. 2000;408(6810):313–319. [PubMed: 11099031]
- 43.
- Freeman M, Klambt C, Goodman CS. et al. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell. 1992;69(6):963–975. [PubMed: 1606617]
- 44.
- Knirr S, Azpiazu N, Frasch M. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development. 1999;126(20):4525–4535. [PubMed: 10498687]
- 45.
- Bourgouin C, Lundgren SE, Thomas JB. apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron. 1992;9:549–561. [PubMed: 1524829]
- 46.
- Nose A, Isshiki T, Takeichi M. Regional specification of muscle progenitors in Drosophila: The role of the msh homeobox gene. Development. 1998;125:215–223. [PubMed: 9486795]
- 47.
- Frasch M, Hoey T, Rushlow C. et al. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987;6(3):749–759. [PMC free article: PMC553460] [PubMed: 2884106]
- 48.
- Su M, Fujioka M, Goto T. et al. The Drosophila homeobox genes zfh-1 and even-skipped are required for cardiac-specific differentiation of a numb-dependent lineage decision. Development. 1999;126:3241–3251. [PubMed: 10375513]
- 49.
- Jagla T, Bellard F, Lutz Y. et al. ladybird determines cell fate decisions during diversification of Drosophila somatic muscles. Development. 1998;125:3699–3708. [PubMed: 9716535]
- 50.
- Crozatier M, Vincent A. Requirement for the Drosophila COE transcription factor collier in formation of an embryonic muscle: Transcriptional response to Notch signalling. Development. 1999;126(7):1495–1504. [PubMed: 10068642]
- 51.
- Michelson A, Abmayr S, Bate M. et al. Expression of a MyoD family member prefigures muscle pattern in Drosophila embryos. Genes Dev. 1990;4:2086–2097. [PubMed: 2176634]
- 52.
- Paterson BM, Walldorf U, Eldridge J. et al. The Drosophila homologue of vertebrate myogenic-determination genes encodes a transiently expressed nuclear protein marking primary myogenic cells. Proc Natl Acad Sci USA. 1991;88(9):3782–3786. [PMC free article: PMC51537] [PubMed: 1902570]
- 53.
- Bate M, Rushton E. Myogenesis and muscle patterning in Drosophila. C R Acad Sci III. 1993;316(9):1047–1061. [PubMed: 8076205]
- 54.
- Ruiz-Gómez M, Romani S, Hartmann C. et al. Specific muscle identities are regulated by Krüppel during Drosophila embryogenesis. Development. 1997;124(17): 3407–14. [PubMed: 9310335]
- 55.
- Frasch M. Controls in patterning and diversification of somatic muscles during Drosophila embryogenesis. Current opinion in Genetics & Development. 1999;9:522–529. [PubMed: 10508697]
- 56.
- Artero R, Furlong E, Becket E. et al. Notch and Ras signaling pathway effector genes expressed in fusioncompetent and founder vells during Drosophila myogenesis. Development. 2003;130(25):6257–72. [PubMed: 14602676]
- 57.
- Landgraf M, Baylies M, Bate M. Muscle founder cells regulate defasciculation and targeting of motor axons in the Drosophila embryo. Curr Biol. 1999;9(11):589–592. [PubMed: 10359699]
- 58.
- Crossley AC. The morphology and development of the Drosophila muscular systemIn: Ashburner M, Wright T, eds.The genetics and biology of DrosophilaNew York: Academic Press,19782b499–560.
- Introduction
- Allocation of Somatic Mesoderm-Mesodermal Subdivision
- Integration of Signal Transduction Pathways during Muscle Progenitor Specification
- Muscle Identity Is Regulated by Muscle Founder Identity Genes
- A Molecular Definition of Muscle Founder Cell and Fusion-Competent Myoblasts
- Acknowledgements
- References
- Development of the Larval Somatic Musculature - Madame Curie Bioscience DatabaseDevelopment of the Larval Somatic Musculature - Madame Curie Bioscience Database
- Tissue Engineering Constructs and Commercialization - Madame Curie Bioscience Da...Tissue Engineering Constructs and Commercialization - Madame Curie Bioscience Database
- Electrophysiological Simulation of Developmental Changes in Action Potentials of...Electrophysiological Simulation of Developmental Changes in Action Potentials of Cardiomyocytes - Madame Curie Bioscience Database
- A Guide to Modeling Reaction-Diffusion of Molecules with the E-Cell System - Mad...A Guide to Modeling Reaction-Diffusion of Molecules with the E-Cell System - Madame Curie Bioscience Database
- Therapeutic Utilities of SOD Mimetics: Cancer, Radiotherapy and SOD Mimetics - M...Therapeutic Utilities of SOD Mimetics: Cancer, Radiotherapy and SOD Mimetics - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...