NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Phagocytosis of immune-complexes is a dynamic process that is accompanied by the generation of inflammatory/tissue damaging products. Recent advances in the field indicate that this process is subject to regulation by inhibitory Fcγ receptors and intracellular phosphatases, including the inositol phosphatases SHIP-1, SHIP-2 and PTEN, and the protein tyrosine phosphatase SHP-1. This chapter will describe the role of the inhibitory Fc receptor, FcγRIIb, and the phosphatases in modulating the signaling events leading to phagocytosis and the accompanying inflammation.
Macrophages play an important role in the adaptive immune response by phagocytosing infectious particles via FcγR and complement receptors. This process is accompanied by the generation of reactive oxygen and nitrogen radicals and inflammatory cytokines in an effort to eliminate the infection. However, when produced in excess, these products can cause host tissue damage. The generation of these harmful byproducts mandates that the phagocytic process, specifically the production of inflammatory mediators, be subject to a tight regulation both in terms of the magnitude of the response, and in being contained to the locale in which the response takes place. Phagocytosis and in fact immune responses in general must be regulated, and must return to basal level after the infectious agent is eliminated. For many years the study of FcγR-mediated function of macrophages focused on molecular events leading to the activation process. Only recently have experiments revealed that FcγR-mediated activation processes are regulated; i.e., they are not simply turned on or off, but are subject to homeostatic control, resulting from the action of certain inhibitory receptors and enzymes that act alongside the activation cascade and serve to temper the biologic response.
Signaling Mechanisms Initiated by Macrophage ITAM-Associated (Activating) FcγR
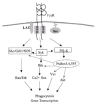
Macrophages express three classes of FcγR: FcγRI, FcγRII and FcγRIII.1 Although the expression of FcγRI and III is common to both mouse and human macrophages, the expression of FcγRII is different between the two species.2,3 Thus, human macrophages express two functionally different FcγRII, -IIa and IIb—the products of two separate genes. In contrast mouse macrophages express FcγRIIb but lack the gene for FcγRIIa. FcγRI, III and IIa are activating receptors, associated with tyrosine-based activation motifs (ITAM). Whereas the ITAM of FcγRIIa is present within its cytoplasmic tail,4 FcγRI and FcγRIIIa signal via the ITAMs present in the low molecular weight γ-subunit homodimers associated with these receptors.5 These ITAM-FcγR, when clustered, transduce signals in a manner similar to the B cell antigen receptor (BCR) and the T cell receptor (TCR).6-8 The earliest signaling events that occur upon FcγR clustering include activation of the receptor-associated Src kinases that phosphorylate the ITAM9-12 (fig. 1). The phosphorylated ITAM then serves as the docking site for Src Homology 2 (SH2) domain-containing cytoplasmic enzymes and adapterenzyme complexes. Thus, the tyrosine kinase Syk is recruited to the phosphorylated ITAM, following which Syk becomes activated and phosphorylates a number of cytoplasmic signaling proteins.13-15 The lipid raft-associated adapter protein LAT is also constitutively associated with FcγR and becomes phosphorylated upon receptor clustering.16 The phosphorylated receptor ITAMs, as well as the phosphorylated tyrosines of LAT provide multiple docking sites, recruiting and activating key SH2-domain-containing proteins. Such proteins include PLCγ,17 which hydrolyzes inositol phospholipids to generate intracellular mediators; the Ras activating GEF (guanine exchange factor) Sos, recruited through adapter proteins Shc and Grb2;18-20 and PtdIns3-Kinase, recruited through the p85 adapter protein.21-23 Recruitment of these effectors to the plasma membrane delivers them to the proximity of kinases that phosphorylate and activate these effectors, and also facilitates access to their protein and lipid substrates. The signaling pathways thus initiated result in a variety of functional outcomes. In the case of macrophages these outcomes include phagocytosis of IgG-opsonized particles, the generation of reactive oxygen species and the production of inflammatory cytokines.
The molecular details of FcγR-mediated phagocytosis and the production of inflammatory cytokines have been well described.24,25 The phagocytic process is initiated when an IgG-coated particle encounters a macrophage and engages FcγR. Macrophage FcγR thus clustered then initiate intracellular signaling pathways that direct pseudopod extension around the particle and its subsequent engulfment by the macrophage. Several obligatory molecular events have been identified, including the recruitment of PtdIns3-Kinase to the plasma membrane and the subsequent phosphorylation of its lipid substrates to generate 3-phosphoinositides,26,27 particularly PtdIns3,4,5P3, which is necessary for the activation of Vav. Vav is a guanine nucleotide exchange factor (GEF) for the low molecular weight GTPases of the Rho family that promote actin polymerization and cytoskeletal rearrangements necessary for the phagocytic process. Likewise, the molecular events leading to the generation of cytokines following FcγR clustering have been extensively investigated. Inflammatory cytokines such as IL-1, IL-8 and TNF-a are generated in response to FcγR clustering and are dependent upon the activation of transcription factors such as NFAT and NFκB.28
Paradoxically, the ITAM-FcγR not only recruit and activate positive signaling enzymes described above, but they also associate with inhibitory enzymes such as the inositol phosphatases SHIP-1, SHIP-2, and the protein tyrosine phosphatase SHP-1. Clustering of ITAMFcγR on human monocytes induces phosphorylation of SHIP-1 and SHIP-2 on tyrosine residues.29-31 The activation of SHIPs by the ITAM-FcγR results in a down regulation of both phagocytosis and cytokine gene expression.32,33 Likewise, SHP-1 phosphatase activity is turned on upon FcγRIIa clustering and results in the downregulation of phagocytosis and NFκB-dependent gene transcription.34 These observations suggest that the activation process initiated by ITAM-FcγR clustering is subject simultaneously to the inhibitory influence of phosphatases, such that the resultant biologic response is tempered. The role of each of these phosphatases will be dicussed in greater detail in the following sections.
Negative Regulation by the ITIM-Bearing FcγRIIb
In contrast to the ITAM-FcγR described above, FcγRIIb is a receptor that serves solely to inhibit the activation processes initiated by the ITAM-bearing immunoreceptors. Human hematopoetic cells express two forms of this receptor, FcγRIIb1 and FcγRIIb2, which result from the splicing of a 19 amino acid insertion into the cytoplasmic tail of FcγRIIb1. The two isoforms exhibit differential expression patterns, with FcγRIIb1 predominating in B cells and FcγRIIb2 in monocytes/macrophages. Until recently, the lack of specific antibodies for human FcγRIIb handicapped the identification and functional characterization of this receptor in human monocytes where multiple FcγR are expressed. However, although study of the function of FcγRIIb in macrophages has lagged, great advances have been made in the understanding of FcγRIIb1 function in B cells and mast cells. The function of FcγRIIb as an inhibitory receptor was first established in B cells where cellular activation by IgG anti-IgM crosslinking was abrogated when intact IgG was used, but not if (Fab')2 fragments of IgG antibody were used.35 It was concluded that the intact antibody co-clustered BCR with FcγRIIb, the only FcγR expressed in B cells. That the inhibitory potential of FcγRIIb resides in a 13 amino acid sequence that contains an ITIM was elegantly demonstrated by mutational analysis of the cytoplasmic tail of the receptor36-38 (fig. 2). Furthermore, the in vivo role of FcγRIIb in the regulation of B cell, mast cell, and macrophage function has been assessed in genetically altered mice that are deficient in FcγRIIb expression. Thus, FcγRIIb knockout mice display elevated levels of serum IgG in response to antigenic challenge suggesting that this receptor is required for negative feedback regulation of antibody production.39 Likewise, the mast cells of these mice also display augmented IgG-mediated degranulation, thereby supporting a regulatory role for FcγRIIb in mast cell reactivity to IgG-immune complexes. The inhibitory role of FcγRIIb phagocytosis of IgG-opsonized particles has been demonstrated by studies showing enhanced phagocytic capacity of FcγRIIb-deficient macrophages compared with wild-type macrophages.40
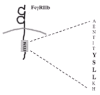
Figure 2
FcγRIIb. The cytoplasmic tail of FcγRIIb contains a tyrosine based inhibitory motif (ITIM), that becomes phosphorylated by membrane-associated Src kinases, leading to the recruitment of SH2 domaincontaining phosphatases.
The first studies to demonstrate that FcγRIIb inhibits phagocytosis of IgG-opsonized particles were done in a transfected COS-1 fibroblast model.41 In these studies it was shown that COS-1 fibroblasts transfected with ITAM containing FcγR were able to efficiently phagocytose IgG-coated particles. However, when the cells were also co-transfected to express FcγRIIb there was a dramatic decrease in phagocytic efficiency. These findings were later confirmed by studies showing that FcγRII-deficient murine macrophages display enhanced phagocytic ability. These observations suggested that when a monocyte/macrophage encounters an immunecomplex, both the activating FcγR and inhibitory FcγR are clustered so that the magnitude of the ensuing phagocytic response is dictated by the ratio of the activating to inhibitory FcγR. Accordingly, in human monocytic cells treated with the anti-inflammatory cytokine IL-4 expression of FcγRIIb was upregulated, accompanied by decrease in phagocytic efficiency.42,43
The mechanism by which FcγRIIb mediates its inhibitory effects was proposed to involve the recruitment SH2 domain-containing enzymes to the phosphorylated ITIM. Using as a bait a synthetic phosphopeptide corresponding to the phosphorylated ITIM of FcγRIIb, enzymes such as the protein tyrosine phosphatase SHP-1 and the inositol phosphatase SHIP-1 were initially identified as potential candidates.44-46 Further analyses revealed that SHIP-1 and SHP-1 do not serve redundant roles, but rather that SHIP-1 is the effector molecule of FcγRIIbmediated inhibition, whereas SHP-1 works in concert with other ITIM-bearing receptors such as the killer inhibitory receptor (KIR) expressed on NK cells.47-49
The SH2 Domain Containing Inositol 5' Phosphatase SHIP-1
γThe hematopoietic cell-specific inositol phosphatase SHIP-1 is a multi-domain cytosolic protein, containing an inositol 5' phosphatase domain and several protein interaction domains50-52 (fig. 3). The interaction domains of SHIP-1 include: (a) a SH2 domain that associates with high affinity to the phosphorylated ITIM of FcγRIIb,45,53,54 (b) a proline-rich domain that is constitutively associated with the SH3 domains of the Ras adapter protein Grb2,55 and 3) two sites of tyrosine phosphorylation set in a motif that preferentially binds proteins containing phosphotyrosine binding (PTB) domains, such as the Ras adapter Shc,51,56,57 and the RasGAP-binding protein Dok.58 SHIP-1 hydrolyzes PtdIns-3,4,5P3, a product of PtdIns3-Kinase, which is required for the activation of several key enzymes that contain a plextrin homology (PH) domain, such as the tyrosine kinase Btk,59 involved in intracellular calcium mobilization, Akt,60-62 an enzyme involved in protecting cells from apoptosis, and Vav.63 Although the enzyme activity of SHIP is constitutively turned on, SHIP must localize to the plasma membrane to access its lipid substrates. Thus, in resting cells SHIP is in the cytoplasm and translocates to the membrane upon activation of the cell. Membrane association of SHIP is mediated by the association of its SH2 domain with tyrosine phosphorylated receptors either directly, or through the adapter molecule Shc. The C-terminal region of SHIP appears to further enhance membrane-association, and the stability of SHIP at the membrane.64-66
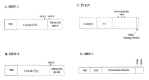
Figure 3
Phosphatases involved in FcγR signaling. A, B) SHIP-1 and SHIP-2 are inositol 5-phosphatases. C) PTEN is an inositol 3-phosphatase with some protein tyrosine phosphatase activity. D) SHP-1 is a protein tyrosine phosphatase.
The inhibitory role of SHIP-1 on BCR-mediated activation of B cells, FcγRI-mediated activation of mast cells, and FcγR-mediated activation of macrophages is well established both in vitro using cell lines and in vivo using SHIP-1 knockout mice.67 Thus, the presence of SHIP-1 down regulates BCR-mediated calcium flux,45 MAPKinase activation,55 and Akt activation, 60,61 and FcγRI-mediated mast-cell degranulation.68 Likewise, SHIP has also been shown to dampen the activation events initiated by growth factor and cytokine receptors.67
The role of SHIP in phagocytosis is actively being investigated by several groups. The initial studies to examine the influence of SHIP on phagocytosis used macrophages derived from SHIP knockout mice and their wild type littermates, and found that deletion of SHIP resulted in enhanced phagocytic efficiency.69 Other studies soon followed, analyzing the molecular details of SHIP activation by macrophage FcγR. These studies established that SHIP is not only capable of associating with phosphorylated ITIMs, but is also able to associate with the phosphorylated ITAMs of the activating FcγR both directly as well as through the adapter molecule Shc.32,33 In addition to downregulating phagocytosis, SHIP is also capable of dampening FcγR-induced activation of NFκB in monocytic cells.33 Together these studies suggest that SHIP serves to temper FcγR-mediated phagocytosis and the accompanying inflammatory cytokine gene expression working through both activating and inhibiting FcγR.
Despite the close attention that SHIP has received in the last few years, there remain several unanswered questions with regard to the role of SHIP in macrophage FcγR function. For example, it is well known that SHIP can influence signaling pathways not only by its ability to hydrolyze PtdIns3,4,5P3, but also by its ability to interact with key signaling molecules via the SHIP interaction domains. In B cells, SHIP appears to downregulate the Ras signaling pathway by at least two mechanisms that do not involve SHIP catalytic function. In the first mechanism proposed SHIP competitively inhibits the association of Shc with the Grb2/Sos complex, preventing Grb2/Sos membrane translocation and the subsequent activation of Ras.70,71 Second, SHIP is reported to associate with p62dok, a RasGAP-binding protein, and promote the inactivation of Ras.58 At present, it is unclear whether SHIP influences macrophage FcγR function by solely its enzyme activity, hydrolyzing PtdIns3,4,5P3, or whether there are additional mechanisms involving the protein interaction domains of SHIP.
The Inositol Phosphatase SHIP-2
SHIP-2 is a SH2 domain-containing inositol 5' phosphatase that has a high level homology to SHIP-1 in its catalytic region (fig.3).72,73 The molecules are largely divergent in the C-terminal region consisting of a proline-rich domain that associates with unique SH3 domaincontaining proteins.74 Thus, while the proline-rich domain of SHIP-1 associates specifically with Grb2, the proline-rich domain of SHIP-2 associates with other SH3 domain containing proteins such as Abl. In addition, while SHIP-1 has two tyrosine residues in the C-terminus that conform to an NPXY motif shown to bind PTB (phosphotyrosine binding) domains upon phosphorylation, SHIP-2 has only one NPXY motif.75 Thus, these two enzymes while enzymatically similar, likely differ in functions that are related to their protein interactions via the C-terminal region. These two molecules also differ in their expression patterns: SHIP-1 is expressed predominantly in hematopoetic cells, while SHIP-2 is much more ubiquitously expressed. Recent studies have revealed a critical role for SHIP-2 in regulating insulin receptor signaling.76-78 Gene knockout mice that are deficient in SHIP-2 expression are hypoglycemic and do not survive past the first day. Other studies have demonstrated a role for SHIP-2 in compensating for the loss of SHIP-1 in SHIP-1-deficient B cell blasts, and mediating the inhibitory effect of FcγRIIb.79,80
SHIP-2 is abundantly expressed in macrophages but is present at virtually undetectable levels in human peripheral blood monocytes. However, SHIP-2 expression in PBM is dramatically upregulated upon treatment with bacterial lipopolysaccharide.30 Upon FcγR clustering, SHIP-2 is recruited to the phosphorylated ITAM of FcγRIIa, in a manner that is dependent on the SH2 domain of SHIP-2, and serves to down regulate FcγRIIa-induced activation of Akt and NFκB-dependent gene transcription. Although a role for SHIP-2 in phagocytosis has not yet been reported, based on the findings that SHIP-2, like SHIP-1, dampens FcγR-induced signaling events such as the activation of Akt and NFκB would suggest that SHIP-2 likely also downregulates phagocytosis.
PTEN (Phosphatase and Tensin Homologue on Chromosome 10)
PTEN is a dual phosphatase that can dephosphorylate phospholipids at the 3' position, as well as tyrosine-phosphorylated proteins (fig. 3).81,82 The enzyme consists of a N-terminal phosphatase domain, a C2 domain and a C-terminal PDZ-binding sequence and multiple phosphorylation sites.67 The C2 domain and the phosphatase domain have been shown to be necessary for membrane localization of PTEN.83 In contrast, phosphorylation of PTEN is accompanied by dissociation of PTEN from the membrane and a reduction in phosphatase activity. PTEN has been reported to negatively regulate immune receptor and growth factor receptor-mediated events. Most of PTEN's biological effects are attributed to its inositol phosphatase activity. For example, PTEN is thought to serve as a tumor suppressor by hydrolyzing PtdIns3,4,5P3 and thereby downregulating the activation of the Akt. The protein tyrosine phosphatase activity of PTEN has been reported to regulate the Ras/Erk pathway by dephosphorylating the Ras adapter Shc,84 and influence adhesion and cell migration by dephosphorylating focal adhesion kinase (FAK).85,86
The role of PTEN in phagocytosis is largely unknown, although there is some evidence based on a transfected fibroblast model that PTEN may suppress phagocytosis through the downregulation of Rac activation.87 The molecular mechanisms involved in the activation of PTEN by FcγR, and the functional role of PTEN's lipid phosphatase and protein phosphatase activity in phagocytosis remain to be investigated in macrophages.
The Protein Tyrosine Phosphatase SHP-1
SHP-1 is 65kD protein tyrosine phosphatase expressed predominantly in hematopoetic cells. SHP-1 contains two N-terminal SH2 domains, a phosphatase domain and two tyrosine phosphorylation sites in the C-terminal region of the protein88-90 (fig. 3). The phosphatase activity of SHP-1 is regulated by intramolecular interactions, such that the N-terminal SH2 domain is folded over the phosphatase domain in the inactive state.91,92 The enzyme becomes activated when the N-terminal SH2 domain is engaged by a phosphopeptide, which allows the phosphatase domain to gain access to its substrate. Thus SHP-1 activity can be upregulated by either addition of a cognate phosphopeptide or by deletion of the N-terminal SH2 domain. In contrast, the C-terminal SH2 domain serves to recruit SHP-1 substrates but does not contribute to activation of the enzyme. Recent evidence suggests that of the two C-terminal tyrosines that become phosphorylated, modification of Y-536 results in a 4-fold increase in SHP-1 activity. In contrast, modification of Y564 did not alter SHP-1 phosphatase activity. Thus phosphorylation of SHP-1 on tyrosine 536 contributes to activation of the enzyme.93
The functional role of SHP-1 as a negative regulator emerged with the identification of mice with point mutations in the SHP-1 gene such that the result was the expression of a catalytically inactive splice variant in one mutant, or a complete loss of SHP-1 protein expression in the other. The animals bearing the former mutations are designated motheaten viable (Mev), and those bearing the latter mutation are designated as motheaten (Me).94-97 Both of these mutations result in a severe phenotype, with uncontrolled expansion of myeloid cells and a shortened life span of about 5-10 weeks. Specifically, T and B cells from SHP-1 deficient animals are hyperresponsive to immune receptor stimulation.98-102 In addition, SHP-1 has been demonstrated to negatively regulate a wide variety of growth factor receptors including the CSF-1 receptor and EGF receptor.89 Among the immunereceptors known to invoke SHP-1 are the Killer cell Inhibitory Receptors (KIR),49,103,104 CD22105 and gp49B.106,107 Although initial studies found association of SHP-1 to FcγRIIb derived peptides, more recent studies have conclusively demonstrated that FcγRIIb-mediated inhibition occurs via SHIP and not SHP-1.47-49
Recent studies have addressed the role of SHP-1 in FcγR-mediated activation of macrophages. Thus, Durden and colleagues found that human FcγRIIa-mediated phagocytosis was downregulated by SHP-1 in a transfected fibroblast model.108 In this system over-expression of SHP-1 was shown to lead to dephosphorylation of Cbl and downregulation of Rac activation. Other studies demonstrated that SHP-1 downregulates NFγB-dependent gene transcription in response to FcγRIIa clustering in monocytic cells.34 In these latter studies SHP-1 was reported to associate specifically with the phosphorylated N-terminal ITAM tyrosine of FcγRIIa. Taken together, these latter observations would imply that SHP-1 is activated in macrophages during FcγR-mediated phagocytosis, perhaps in a non-FcγRIIb-dependent manner. The molecular details of SHP-1 activation by FcγR and the precise nature of SHP-1 influence on FcγR-mediated signaling biology remains to be examined. Additional work is needed to identify the exact substrates of SHP-1 during the phagocytic process.
It is clear from the studies described above that immune-complex clearance is a highly regulated process, and that the absence of such regulation could lead to inflammation and tissue damage. However, it is not yet entirely clear why there exist so many different mechanisms of regulation. There remain many unanswered questions regarding the non-overlapping functions of the phosphatases involved. As well, these regulatory mechanisms may be separated by expression pattern of the phosphatases during differentiation stages of myeloid cells. As future work defines the exact molecular details of these regulatory mechanisms it will be interesting to see whether there might be molecules that will specifically downregulate the production of inflammatory mediators while maintaining the efficiency of engulfment and destruction of IgG-coated particles.
References
- 1.
- Anderson CL, Shen L, Eicher DM. et al. Phagocytosis mediated by three distinct Fcγ receptor classes on human leukocytes. J Exp Med. 1990;171:1333–1346. [PMC free article: PMC2187839] [PubMed: 2139103]
- 2.
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. [PubMed: 11244038]
- 3.
- Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. [PubMed: 9143687]
- 4.
- Van Den Herik-Oudijk IE, Capel PJ, Van der Bruggen T. et al. Identification of signaling motifs within human FcγRIIa and FcγRIIb isoforms. Blood. 1995;85:2202–2211. [PubMed: 7718892]
- 5.
- Ernst LK, Duchemin A-M, Anderson CL. Association of the high affinity receptor for IgG (FcγRI) with the γ subunit of the IgE receptor. Proc Natl Acad Sci USA. 1993;90:6023–6027. [PMC free article: PMC46859] [PubMed: 8327478]
- 6.
- Weiss A. T cell antigen receptor signal transduction: A tale of tails and cytoplasmic proteintyrosine kinases. Cell. 1993:209–212. [PubMed: 8477442]
- 7.
- Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. [PubMed: 9203415]
- 8.
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed: 2927501]
- 9.
- Duchemin A-M, Anderson CL. Association of non-receptor protein tyrosine kinases with the FcγRI/ γ-chain complex in monocytic cells. J Immunol. 1997;158:865–871. [PubMed: 8993005]
- 10.
- Ghazizadeh S, Fleit HB. Tyrosine phosphorylation provides an early obligatory signal for FcγRIImediated endocytosis in the monocytic cell line THP-1. J Immunol. 1994;152:30–41. [PubMed: 8254198]
- 11.
- Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with FcγRII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–8884. [PubMed: 8132624]
- 12.
- Hamada F, Aoki M, Akiyama T. et al. Association of immunoglobulin FcγRII with Src-like protein- tyrosine kinase Fgr in neutrophils. Proc Natl Acad Sci USA. 1993;90:6305–6309. [PMC free article: PMC46917] [PubMed: 8327512]
- 13.
- Ghazizadeh S, Bolen JB, Fleit HB. Tyrosine phosphorylation and association of Syk with FcγRII in monocytic THP-1 cells. Biochem J. 1995;305:669–674. [PMC free article: PMC1136413] [PubMed: 7530449]
- 14.
- Chacko GW, Duchemin A-M, Coggeshall KM. et al. Clustering of the platelet FcγR induces noncovalent association with the tyrosine kinase p72syk. J Biol Chem. 1994;269:32435–32440. [PubMed: 7798242]
- 15.
- Kiener PA, Rankin BM, Burkhardt AL. et al. Cross-linking of Fcγ receptor I (FcγRI) and receptor II (FcγRII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993;268:24442– 24448. [PubMed: 8226994]
- 16.
- Tridandapani S, Lyden TW, Smith JL. et al. The adapter protein LAT enhances Fcγ receptormediated signal transduction in myeloid cells. J Biol Chem. 2000;275:20480–20487. [PubMed: 10781611]
- 17.
- Liao F, Shin HS, Rhee SG. Tyrosine phosphorylation of phospholipase C-γ1 induced by crosslinking of the high-affinity or low-affinity Fc receptor for IgG in U937 cells. Proc Natl Acad Sci USA. 1992;89:3659–3663. [PMC free article: PMC48928] [PubMed: 1373507]
- 18.
- Margolis B. The GRB family of SH2 domain proteins. Prog Biophys Mol Biol. 1994;62:223–244. [PubMed: 7892504]
- 19.
- Downward J. Control of ras activation. Cancer Surv. 1996;27:87–10087-100. [PubMed: 8909796]
- 20.
- Downward J. The GRB2/Sem-5 adaptor protein. FEBS Lett. 1994;338:113–117. [PubMed: 8307166]
- 21.
- Chacko GW, Brandt JT, Coggeshall KM. et al. Phosphatidylinositol 3-kinase and p72Syk noncovalently associate with the low affinity Fc gamma receptor on human platelets through an ITAM: reconstitution with synthetic phosphopeptides. J Bio Chem. 1996;271:10775–10781. [PubMed: 8631888]
- 22.
- Gibbins J, Briddon S, Shutes A. et al. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor. J Biological Chem. 1998;273:34437–34443. [PubMed: 9852111]
- 23.
- Cooney DS, Phee H, Jacob A. et al. Signal transduction by human-restricted FcγRIIa involves three distinct cytoplasmic kinase families leading to phagocytosis. J Immunol. 2001;167:844–854. [PubMed: 11441091]
- 24.
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. [PubMed: 10358769]
- 25.
- Sánchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. J Leukoc Biol. 1998;63:521–533. [PubMed: 9581795]
- 26.
- Lowry MB, Duchemin A-M, Coggeshall KM. et al. Chimeric receptors composed of PI3-kinase domains and Fcγ receptor ligand-binding domains mediate phagocytosis in COS fibroblasts. J Biol Chem. 1998;273:24513–24520. [PubMed: 9733745]
- 27.
- Cox D, Tseng CC, Bjekic G. et al. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. [PubMed: 9880492]
- 28.
- Sanchez-Mejorada G, Rosales C. Fcgamma receptor-mediated mitogen-activated protein kinase activation in monocytes is independent of Ras. J Biol Chem. 1998;273:27610–27619. [PubMed: 9765295]
- 29.
- Maresco DL, Osborne JM, Cooney D. et al. The SH2-containing 5'-inositol phosphatase (SHIP) is tyrosine phosphorylated after Fcγ receptor clustering in monocytes. J Immunol. 1999;162:6458–6465. [PubMed: 10352260]
- 30.
- Pengal RA, Ganesan LP, Fang H. et al. SHIP-2 inositol phosphatase is inducibly expressed in human monocytes and serves to regulate Fcγ receptor-mediated signaling. J Biol Chem. 2003;278:22657–63. [PubMed: 12690104]
- 31.
- Cameron AJ, Allen JM. The human high-affinity immunoglobulin G receptor activates SH2-containing inositol phosphatase (SHIP). Immunology. 1999;97:641–647. [PMC free article: PMC2326891] [PubMed: 10457218]
- 32.
- Nakamura K, Malykhin A, Coggeshall KM. The Src homology 2 domain-containing inositol 5- phosphatase negatively regulates Fcγ receptor-mediated phagocytosis through immunoreceptor tyrosine- based activation motif-bearing phagocytic receptors. Blood. 2002;100:3374–3382. [PubMed: 12384440]
- 33.
- Tridandapani S, Wang Y, Marsh CB. et al. Src homology 2 domain-containing inositol polyphosphate phosphatase regulates NF-γB-mediated gene transcription by phagocytic FcγRs in human myeloid cells. J Immunol. 2000;169:4370–8. [PubMed: 12370370]
- 34.
- Ganesan LP, Fang H, Marsh CB. et al. The protein-tyrosine phosphatase SHP-1 associates with the phosphorylated immunoreceptor tyrosine-based activation motif of FcγRIIa to modulate signaling events in myeloid cells. J Biol Chem. 2003;278:35710–35717. [PubMed: 12832410]
- 35.
- Phillips NE, Parker DC. Cross-linking of B lymphocyte Fcγ receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J Immunol. 1984;132:627–632. [PubMed: 6228594]
- 36.
- Muta T, Kurosaki T, Misulovin Z. et al. A 13-amino-acid motif in the cytoplasmic domain of Fcγ RIIB modulates B-cell receptor signalling [published erratum appears in Nature 1994; 369(6478):340] Nature. 1994;368:70–73. [PubMed: 8107887]
- 37.
- Amigorena S, Bonnerot C, Drake JR. et al. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. [PubMed: 1535455]
- 38.
- Choquet D, Ku G, Cassard S. et al. Different patterns of calcium signaling triggered through two components of the B lymphocyte antigen receptor. J Biol Chem. 1994;269:6491–6497. [PubMed: 7509805]
- 39.
- Takai T, Ono M, Hikida M. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379:346–349. [PubMed: 8552190]
- 40.
- Clynes R, Maizes JS, Guinamard R. et al. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–185. [PMC free article: PMC1887693] [PubMed: 9874574]
- 41.
- Hunter S, Indik ZK, Kim MK. et al. Inhibition of Fcγ receptor-mediated phagocytosis by a nonphagocytic Fcγ receptor. Blood. 1998;91:1762–1768. [PubMed: 9473244]
- 42.
- Pricop L, Redecha P, Teillaud J-L. et al. Differential modulation of stimulatory and inhibitory Fcγ receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001;166:531–537. [PubMed: 11123333]
- 43.
- Tridandapani S, Siefker K, Teillaud J-L. et al. Regulated expression and inhibitory function of FcγRIIb in human monocytic cells. J Biol Chem. 2001;277:5082–9. [PubMed: 11741917]
- 44.
- D'Ambrosio D, Hippen KL, Minskoff SA. et al. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcγRIIBI. Science. 1995;268:293–296. [PubMed: 7716523]
- 45.
- Ono M, Bolland S, Tempst P. et al. Role of the inositol phosphatase SHIP in negative regulation the of immune system by the receptor FcγRIIB. Nature. 1996;383:263–266. [PubMed: 8805703]
- 46.
- Chacko GW, Tridandapani S, Damen JE. et al. Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5-phosphatase, SHIP. J Immunol. 1996;157:2234–2238. [PubMed: 8805618]
- 47.
- Ono M, Okada H, Bolland S. et al. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. [PubMed: 9244303]
- 48.
- Nadler MJS, Chen B, Anderson JS. et al. Protein-tyrosine phosphatase SHP-1 is dispensable for FcγRIIB-mediated inhibition of B cell antigen receptor activation. J Bio Chem. 1997;272:20038–20043. [PubMed: 9242674]
- 49.
- Gupta N, Scharenberg AM, Burshtyn DN. et al. Negative signaling pathways of the killer cell inhibitory receptor and FcγRIIb1 require distinct phosphatases. J Exp Med. 1997;186:473–478. [PMC free article: PMC2199004] [PubMed: 9236201]
- 50.
- Damen JE, Liu L, Rosten P. et al. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. [PMC free article: PMC40003] [PubMed: 8643691]
- 51.
- Lioubin MN, Algate PA, Tsai S. et al. p150 SHIP, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. [PubMed: 8654924]
- 52.
- Kavanaugh WM, Pot DA, Chin SM. et al. Multiple forms of an inositol polyphosphate 5-phosphatase form signaling complexes with Shc and Grb2. Current Biology. 1996;6:438–445. [PubMed: 8723348]
- 53.
- Tridandapani S, Kelley T, Pradhan M. et al. Recruitment and phosphorylation of SHIP and Shc to the B cell Fcγ ITIM peptide motif. Mol Cell Biol. 1997;17:4305–4311. [PMC free article: PMC232283] [PubMed: 9234687]
- 54.
- Tridandapani S, Pradhan M, LaDine JR. et al. Protein interactions of SHIP: association with Shc displaces SHIP from FcγRIIb in B cells. J Immunol. 1998;162:1408–1414. [PubMed: 9973396]
- 55.
- Tridandapani S, Phee H, Shivakumar L. et al. Role of Ship in FcγRIIb-mediated inhibition of Ras activation in B cells. Mol Immunol. 1998;35:1135–1146. [PubMed: 10395202]
- 56.
- Pradhan M, Coggeshall KM. Activation-induced bi-dentate interaction of SHIP and Shc in B lymphocytes. J Cell Biochem. 1997;67:32–42. [PubMed: 9328837]
- 57.
- Lamkin TD, Walk SF, Liu L. et al. Shc interaction with Src homology 2 domain containing inositol phosphatase (SHIP) in vivo requires the Shc-phosphotyrosine binding domain and two specific phosphotyrosines on SHIP. J Bio Chem. 1997;272:10396–10401. [PubMed: 9099679]
- 58.
- Tamir I, Stolpa JC, Helgason CD. et al. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immun. 2000;12:347–358. [PubMed: 10755621]
- 59.
- Scharenberg AM, El-Hillal O, Fruman DA. et al. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns- 3,4,5-P3) Tec kinase-dependent calcium signaling pathway: A target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. [PMC free article: PMC1170542] [PubMed: 9524119]
- 60.
- Jacob A, Cooney D, Tridandapani S. et al. FcγRIIB modulation of surface immunoglobulin-induced Akt activation in murine B cells. J Biol Chem. 1999;274:13704–13710. [PubMed: 10224144]
- 61.
- Aman MJ, Lamkin TD, Okada H. et al. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem. 1998;273:33922–33928. [PubMed: 9852043]
- 62.
- Carver DJ, Aman MJ, Ravichandran KS. SHIP inhibits Akt activation in B cells through regulation of Akt membrane localization. Blood. 2000;96:1449–1456. [PubMed: 10942391]
- 63.
- Ma AD, Metjian A, Bagrodia S. et al. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase gamma, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. [PMC free article: PMC109060] [PubMed: 9671484]
- 64.
- Aman MJ, Walk SF, March ME. et al. Essential role for the C-terminal noncatalytic region of SHIP in FcγRIIB1-mediated inhibitory signaling. Mol Cell Biol. 2000;20:3576–3589. [PMC free article: PMC85650] [PubMed: 10779347]
- 65.
- Damen JE, Ware MD, Kalesnikoff J. et al. SHIP's C-terminus is essential for its hydrolysis of PIP3 and inhibition of mast cell degranulation. Blood. 2001;97:1343–1351. [PubMed: 11222379]
- 66.
- Baran CP, Tridandapani S, Helgason CD. et al. The inositol 5'-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem. 2003;278:38628–38636. [PubMed: 12882960]
- 67.
- Krystal G. Lipid phosphatases in the immune system. Immunology. 2000;12:397–403. [PubMed: 10995586]
- 68.
- Huber M, Helgason CD, Damen JE. et al. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci. 1998;95:11330–11335. [PMC free article: PMC21642] [PubMed: 9736736]
- 69.
- Cox D, Dale BM, Kishiwada M. et al. A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fcγ receptors and complement receptor 3 (α(M)β(2); CD11b/CD18. J Exp Med. 2001;193:61–71. [PMC free article: PMC2195884] [PubMed: 11136821]
- 70.
- Tridandapani S, Chacko GW, Van Bruggen MCJ. et al. Negative signaling in B cells Causes reduced Ras activity by reducing Shc-Grb2 interactions. J Immunol. 1997;158:1125–1132. [PubMed: 9013951]
- 71.
- Tridandapani S, Kelly T, Cooney D. et al. Negative signaling in B cells: SHIP Grbs Shc. Immunol Today. 1997;18:424–427. [PubMed: 9293157]
- 72.
- Pesesse X, Moreau C, Drayer AL. et al. The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett. 1998;437:301–303. [PubMed: 9824312]
- 73.
- Pesesse X, Dewaste V, De Smedt F. et al. The Src homology 2 domain containing inositol 5- phosphatase SHIP2 is recruited to the epidermal growth factor (EGF) receptor and dephosphorylates phosphatidylinositol 3,4,5-trisphosphate in EGF-stimulated COS-7 cells. J Biol Chem. 2001;276:28348–28355. [PubMed: 11349134]
- 74.
- Wisniewski D, Strife A, Swendeman S. et al. A novel SH2-containing phosphatidylinositol 3,4,5- trisphosphate 5-phosphatase (SHIP2) is constitutively tyrosine phosphorylated and associated with src homologous and collagen gene (SHC) in chronic myelogenous leukemia progenitor cells. Blood. 1999;93:2707–2720. [PubMed: 10194451]
- 75.
- Erneux C, Govaerts C, Communi D. et al. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim Biophys Acta. 1998;1436:185–199. [PubMed: 9838104]
- 76.
- Blero D, De SmedtF, Pesesse X. et al. The SH2 domain containing inositol 5-phosphatase SHIP2 controls phosphatidylinositol 3,4,5-trisphosphate levels in CHO-IR cells stimulated by insulin. Biochem Biophys Res Commun. 2001;282:839–843. [PubMed: 11401540]
- 77.
- Wada T, Sasaoka T, Funaki M. et al. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5'- phosphatase catalytic activity. Mol Cell Biol. 2001;21:1633–1646. [PMC free article: PMC86709] [PubMed: 11238900]
- 78.
- Clement S, Krause U, Desmedt F. et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature. 2001;409:92–97. [PubMed: 11343120]
- 79.
- Brauweiler A, Tamir I, Marschner S. et al. Partially distinct molecular mechanisms mediate inhibitory FcgammaRIIB signaling in resting and activated B cells. J Immunol. 2001;167:204–211. [PubMed: 11418650]
- 80.
- Muraille E, Pesesse X, Kuntz C. et al. Distribution of the src-homology-2-domain-containing inositol 5-phosphatase SHIP-2 in both non-haemopoietic and haemopoietic cells and possible involvement of SHIP-2 in negative signalling of B-cells. Biochem J. 1999;342:697–705. [PMC free article: PMC1220512] [PubMed: 10477282]
- 81.
- Sulis ML, Parsons R. PTEN: From pathology to biology. Trends Cell Biol. 2003;13:478–483. [PubMed: 12946627]
- 82.
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. [PMC free article: PMC33561] [PubMed: 10200246]
- 83.
- Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA. 2003;100:7491–7496. [PMC free article: PMC164614] [PubMed: 12808147]
- 84.
- Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factormediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375–1383. [PMC free article: PMC2133067] [PubMed: 9832564]
- 85.
- Gu J, Tamura M, Pankov R. et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146:389–403. [PMC free article: PMC2156182] [PubMed: 10427092]
- 86.
- Tamura M, Gu J, Takino T. et al. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed: 9927060]
- 87.
- Kim JS, Peng X, De PK. et al. PTEN controls immunoreceptor (immunoreceptor tyrosine-based activation motif) signaling and the activation of Rac. Blood. 2002;99:694–697. [PubMed: 11781256]
- 88.
- Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Immunology. 2000;12:361–378. [PubMed: 10995583]
- 89.
- Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. [PubMed: 9069265]
- 90.
- Neel BG. Role of phosphatases in lymphocyte activation. Curr Opin Immunol. 1997;9:405–420. [PubMed: 9203419]
- 91.
- Pei D, Lorenz U, Klingmuller U. et al. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: A new function for Src homology 2 domains. Biochemistry. 1994;33:15483–15493. [PubMed: 7528537]
- 92.
- Pei D, Wang J, Walsh CT. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA. 1996;93:1141–1145. [PMC free article: PMC40045] [PubMed: 8577729]
- 93.
- Zhang Z, Shen K, Lu W. et al. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biological Chem. 2003;278:4668–74. [PubMed: 12468540]
- 94.
- Shultz LD, Schweitzer PA, Rajan TV. et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. [PubMed: 8324828]
- 95.
- Shultz LD, Green MC. Motheaten, an immunodeficient mutant of the mouse. II. Depressed immune competence and elevated serum immunoglobulins. J Immunol. 1976;116:936–943. [PubMed: 56406]
- 96.
- Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975;66:250–258. [PubMed: 1184950]
- 97.
- Tsui HW, Siminovitch KA, de Souza L. et al. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. [PubMed: 8348149]
- 98.
- Cornall RJ, Goodnow CC, Cyster JG. Regulation of B cell antigen receptor signaling by the Lyn/ CD22/SHP1 pathway. Curr Top Microbiol Immunol. 1999;244:57–6857-68. [PubMed: 10453649]
- 99.
- Doody GM, Justement LB, Delibrias CC. et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. [PubMed: 7618087]
- 100.
- Plas DR, Johnson R, Pingel JT. et al. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. [PubMed: 8638162]
- 101.
- Pani G, Fischer KD, Mlinaric-Rascan I. et al. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J Exp Med. 1996;184:839–852. [PMC free article: PMC2192780] [PubMed: 9064344]
- 102.
- Pani G, Kozlowski M, Cambier JC. et al. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med. 1995;181:2077–2084. [PMC free article: PMC2192043] [PubMed: 7539038]
- 103.
- Berg KL, Carlberg K, Rohrschneider LR. et al. The major SHP-1-binding, tyrosine-phosphorylated protein in macrophages is a member of the KIR/LIR family and an SHP-1 substrate. Oncogene. 1998;17:2535–2541. [PubMed: 9824165]
- 104.
- Burshtyn DN, Lam AS, Weston M. et al. Conserved residues amino-terminal of cytoplasmic tyrosines contribute to the SHP-1-mediated inhibitory function of killer cell Ig-like receptors. J Immunol. 1999;162:897–902. [PubMed: 9916713]
- 105.
- Blasioli J, Goodnow CC. Lyn/CD22/SHP-1 and their importance in autoimmunity. Curr Dir Autoimmun. 2002;5:151–160. [PubMed: 11826756]
- 106.
- Kuroiwa A, Yamashita Y, Inui M. et al. Association of tyrosine phosphatases SHP-1 and SHP-2, inositol 5-phosphatase SHIP with gp49B1, and chromosomal assignment of the gene. J Biol Chem. 1998;273:1070–1074. [PubMed: 9422771]
- 107.
- Lu-Kuo JM, Joyal DM, Austen KF. et al. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domaincontaining phosphatase-1, and suppression of early and late calcium mobilization. J Biol Chem. 1999;274:5791–5796. [PubMed: 10026201]
- 108.
- Kant AM, De P, Peng X. et al. SHP-1 regulates Fcγ receptor-mediated phagocytosis and the activation of RAC. Blood. 2002;100:1852–1859. [PubMed: 12176909]
- Signaling Mechanisms Initiated by Macrophage ITAM-Associated (Activating) FcγR
- Negative Regulation by the ITIM-Bearing FcγRIIb
- The SH2 Domain Containing Inositol 5' Phosphatase SHIP-1
- The Inositol Phosphatase SHIP-2
- PTEN (Phosphatase and Tensin Homologue on Chromosome 10)
- The Protein Tyrosine Phosphatase SHP-1
- References
- Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience...Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience Database
- Inflammatory Myopathies: Dermatomyositis, Polymyositis and Inclusion Body Myosit...Inflammatory Myopathies: Dermatomyositis, Polymyositis and Inclusion Body Myositis - Madame Curie Bioscience Database
- Caspases as Targets for Drug Development - Madame Curie Bioscience DatabaseCaspases as Targets for Drug Development - Madame Curie Bioscience Database
- Insights into the Modulation of Ceramide Metabolism by Naturally Occurring and S...Insights into the Modulation of Ceramide Metabolism by Naturally Occurring and Synthetic Sphingolipid Analogs as Monitored by Electrospray Tandem Mass Spectrometry - Madame Curie Bioscience Database
- Modeling Structure-Activity Relationships - Madame Curie Bioscience DatabaseModeling Structure-Activity Relationships - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...