NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
As discussed in Chapter 5, embryonic spinal cord grafted into the injured spinal cord of neonatal and adult animals can serve as a relay tissue bridge for axonal growth and regeneration, and promote some degree of functional recovery in the host. In these experiments the pattern of host axonal projections to the grafts has been well characterized, but less emphasis has been placed on the efferent connections of the graft. Furthermore, much fewer attempts have been made to look more selectively at the behaviour of specific subpopulations of grafted neurones. At the time of transplantation embryonic spinal cord contains a mixed population of already committed neurones and neuronal precursors which might respond differently to transplantation. In this chapter we will discuss a different experimental approach which explores the possibility of replacing a defined population of the host neurones by homologous cells present in a graft. This approach, focusing on embryonic neurones of a particular type, also provides an interesting opportunity to follow up their fate after grafting.
Motoneurones are large cholinergic neurones localized to the motor nuclei of the brain stem and spinal cord, and they are the first neuronal population generated during development of the spinal cord.1 They are also the only central neurones which extend their axons outside the CNS to innervate a nonneuronal target - skeletal muscle. Their selective loss is a hallmark of certain human neurological disorders like spinal muscular atrophies, adult motoneurone disease and poliomyelitis. These disorders, where the loss of motoneurones is the most important cause of the disability underline the significance of studies focusing on grafting motoneurones into a spinal cord which has previously been depleted of its motoneurones. It remains a tantalizing possibility that some results of this research could be relevant not only to our understanding of pathology, but also to treatment of neurological disorders affecting motoneurones.
In the late 1980s different experimental models of motoneurone transplantation were introduced. A number of questions had to be addressed at the beginning of this research. First, what happens to the grafted motoneurones which, as the earliest-born neurones, either are already present in the grafts of embryonic spinal cord, or are due to be born shortly after grafting. It was noted in earlier studies that large motoneurone-like cells were relatively underrepresented in the grafts of embryonic spinal cord, especially if the grafted tissue was obtained from older embryos. This finding could be explained by their selective death or arrested maturation which rendered them indistinguishable from the other types of grafted neurones.
However, the experiments of Sotelo and Alvarado-Mallart on transplantation of Purkinje cells into the cerebellar cortex of mice2,3 suggested yet another possibility. In their studies fragments of cerebellar primordia or suspensions of embryonic cerebellar neurones were grafted into the cerebellar cortex of mutant mice with a hereditary ataxia due to the Purkinje cells degeneration (Purkinje Cell Degeneration or PCD mice). In this strain of mice the cerebellar cortex develops normally but about three weeks postnatally the Purkinje cells degenerate completely. Thus, this animal model provides an unique opportunity to study what happens when developing Purkinje cells from the graft are confronted with a mature cortex depleted of the homologous highly specialized neurones. Some Purkinje cells left the grafts and migrated tangentially into the host cortex settling in the upper third of the molecular layer. These cells subsequently developed a typical sagitally oriented dendritic tree and made synaptic contacts with appropriate host afferents. Few of them also extended their axons into their normal efferent projection field in the dentate nucleus. Thus, the graft-derived Purkinje cells were able to replace missing host neurones in a highly organized, point-to-point connected circuitry. These results suggested a possibility that perhaps some embryonic motoneurones could also migrate from the grafts of embryonic spinal cord into the host grey matter to replace the missing motoneurones of the host. If this was true then the next question was whether the grafted motoneurones would be able (a) to integrate into the host neuronal network, and (b) will they be able to innervate a skeletal muscle of the host.
Conditions for Survival of Transplanted Embryonic Motoneurones
Several factors concerning the graft and the host can influence the survival of the grafted embryonic motoneurones . Selection of the appropriate gestational age of the donor embryos is of obvious importance. Developmental studies using 3H thymidine revealed that motoneurone pools of the rat spinal cord are formed between embryonic day 11 and 13 (ED11 and ED13) (the day of insemination was counted as ED-1).1,4 In the rat the first motor axons extend outside the spinal cord towards their target muscles on ED 12.5 Therefore the best time to obtain embryonic spinal tissue for transplantation of motoneurons should be after the onset of motoneuron proliferation but before extension of motor axons to the periphery, i.e., between 11 and 13 days of gestation.
Some characteristic features of developing motoneurones should also be taken into consideration. During the earliest stages of development of the neuromuscular system motoneurones and mesenchymal cells giving rise to muscle primordia develop independently of each other. However, subsequently motoneurones and their target muscles become dependent on each other and together proceed through a sequence of events ultimately leading to formation of adult type motor units.
Similarly to other developing neuronal populations the final number of cells within the motor pools is a net result of the initial mitotic proliferation of neuroblasts in the germinal zone of the neural tube and naturally occurring cell death, recently attributed to apoptosis of “excess” cells.6 In mice this naturally occuring motoneurone death begins on ED13 and between ED13 and ED18 67% of cells initially present in the lateral motor columns die.7 In the rat the timing and magnitude of naturally occurring motoneurone death is similar and seems to be completed before birth.8
It has been recognized for a long time that the onset of naturally occurring cell death of motoneurones coincides with the arrival of the motor axons to the muscles.9-12 The magnitude of motoneurone death can be influenced by experimental manipulations leading to either reduction or expansion of the peripheral field of innervation. For example, removal of a limb bud of a chick embryo at a very early stage of development (ED2.5) does not affect proliferation of neuroblasts and migration of motoneurones to the mantle layer. However, later most motoneurones which would normally have innervated the missing limb died.13 By contrast, expansion of the peripheral field of innervation by implantation of a supernumerary limb bud can prevent death of some, but not all, motoneurones normally destined to die.14 This dependence on target for survival extends into the early postnatal period. In the neonatal rat, for example, ablation of the limb muscles causes massive death of motoneurones.15 Similarly, sciatic nerve crush at birth invariably produces death of a large proportion of motoneurones.16,17 Identical injury to the sciatic nerve inflicted on postnatal day 5 (PD 5) does not induce cell death indicating that, by that stage, motoneurones have acquired the ability to survive temporary isolation from the muscle. It is possible that the disastrous consequences of a peripheral nerve injury during the critical period of development are due to direct axotomy.18 However, a similar degree of motoneurone death can be induced by preventing neuromuscular transmission with a postsynaptic blocking agent.19 These experiments, in which direct surgical or pharmacological damage to motor axons was avoided, prove that motoneurone death after a neonatal nerve injury is, indeed, caused by the lack of interaction with the target during the critical period of development.
It can be expected that embryonic motoneurones grafted into the spinal cord could express a similar sensitivity to deprivation of target muscles. It has to be emphasized that so far no systematic studies addressing this issue have been reported. In some experimental models attempts have been made to give the grafted motoneurones a chance to innervate a surrogate target: one of the muscles of the host.20 Regardless of these purely neurobiological considerations, this approach could also satisfy the restorative purpose of motoneurone transplantation by showing that the grafted cells are capable of forming functional efferent connections with the host muscles.
If the grafted embryonic motoneurones are to innervate the muscles of the host their axons have to be able to exit the spinal cord of the host. Ideally, the best results could be expected if the axons of grafted motoneurones were able to use the existing anatomical routes like the ventral roots and peripheral nerves. However, studies of axonal regeneration from adult motoneurones axotomized close to their perikarya (within the spinal cord) have shown that regeneration into the ventral roots does not occur unless the roots are cut or avulsed and reimplanted directly in the grey matter.21-22 As discussed in Chapter 5, the environment of the adult CNS, and in particular that of the white matter, permits only abortive regeneration of axons of the central neurones. On the other hand, peripheral nerves implanted into the grey matter of the brain or spinal cord attract axons from the neighbouring neurones and provide an excellent environment for their long-distance (up to 30 mm in the rat) regeneration (reviewed by Aguayo,23 1985; Bray24 et al, 1987). Thus, even if the axons from the grafted neurones would have failed to exit the spinal cord using the existing routes, they still could be led out towards the periphery using a conduit of a nerve implanted in the vicinity of the graft.
The environment of the host spinal cord also influences the survival of grafted motoneurones. Many more grafted cells survive in a spinal cord where the host motoneurones have been depleted. However, since the main interest in grafting embryonic motoneurones into an adult spinal cord is to replace lost cells this factor may be an advantage. Thus the 3 factors that seem to determine the survival of grafted cells are: (i) depletion of host motoneurones, (ii) availability of a muscle that can be reinnervated via a peripheral nerve and (iii) the nature of the peripheral nerve implant. In the following sections the role of these factors is discussed.
Transplantation of Embryonic Motoneurones into the Adult Spinal Cord
Establishing Optimal Conditions for Survival of Grafted Embryonic Motoneurones in an Adult Host
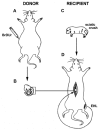
Attempts of grafting of embryonic motoneurones were carried out in the spinal cord of adult rats. The experimental model used in these studies was designed so as to provide favorable conditions for the survival of the grafted embryonic motoneurones20 i.e., the host cord was depleted of its motoneurones and a skeletal muscle with a nerve conduit provided (see fig. 1). A small fragment of the ventral part of the spinal cord from ED11-ED12 embryos was inserted into the lumbar segments of the host spinal cord (L4-L5). The host spinal cord's sciatic motor pool had been selectively depleted several weeks earlier by subjecting the future hosts to sciatic nerve crush at birth. This injury is known to produce death of sciatic motoneurones distributed at L4-L5 level.16 Another experimental manipulation used in this model was to supply the grafted motoneurones with a target muscle which they could innervate. One of the host's muscles (Extensor Hallucis Longus {EHL} or Soleus {SOL}) was dissected from the contralateral hindlimb of the host with a length of its nerve attached to it. This neuromuscular preparation was attached to the paravertebral muscles. The cut end of the nerve was then inserted into the spinal cord in the immediate proximity of the graft to create a conduit for axonal growth towards the muscle.
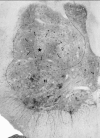
The motoneurones of donor embryos were prelabelled in utero with a synthetic analogue of thymidine-5-bromo-2'-deoxyuridine (BrdU) administered in pulsed injections to a pregnant female on ED10, i.e.,during proliferation of lumbar motoneurone pools in the embryos. The tissue was harvested for grafting one to two days later. Similarly to 3H-thymidine, BrdU labels DNA of dividing cells and can be used to mark birth dates of neurones. The label can be conveniently visualized in the nucleus by immunocytochemistry with the anti-BrdU antibody,25 allowing identification of embryonic motoneurones in the host spinal cord. Several weeks after transplantation viable and well integrated grafts could be found in the spinal cords of the majority of host animals. In light microscopy the grafts had typical cellular composition and organization, well described in earlier studies (see Chapter 5). Examining the grafts one is often confronted with a problem of deciding which cells fulfil the criteria for a motoneurone. Among numerous smaller neurones the grafts contain some larger perikarya, which on Nissl preparations resemble small motoneurones (they are usually smaller than typical alpha-motoneurones in the host ventral horn). However, a specific motoneurone marker is needed to ascertain the nature of these cells. There are a few possible markers, for example (i) the labelling cells according to their birth date with BrdU or a similar substance, or (ii) the expression of the enzymes involved in cholinergic transmission, like acetylcholinesterase (AChE) or choline acetyltransferase (ChAT). Although these markers are not entirely specific, because a number of other spinal neurones also have cholinergic phenotype,26,27 the motoneurones are the most numerous subpopulation of the cholinergic cells in the spinal cord. More specific markers for motoneurones have recently been described, but these were not available when the first grafting experiments were carried out. Typically, AChE histochemistry reveals variable numbers of AChE-positive neurones, which sometimes form well defined clusters strikingly resembling a ventral horn of the developing spinal cord28 (fig. 2). It has been proposed that at least a proportion of these cells could be motoneurones which survived transplantation but became arrested in their maturation.28 The ventral horn-like areas can be seen in some grafts but are by no means a regular feature. These appearances may depend on a number of technical variables, such as dissection of embryonic tissue including different proportions of the ventral spinal cord, or on the orientation of the tissue during transplantation.
Interestingly, it was observed that the large AChE positive neurones appeared to be more frequent in occasional “bad” experiments, where the graft was unintentionally contaminated with mesenchymal cells, including muscle (Sieradzan and Vrbová, personal unpublished observations). In these cases mature muscle fibres, fat and connective tissue developed intraspinally in the vicinity, or even within, the graft. These muscle fibres often had well differentiated end-plates which stained for AChE. This finding is consistent with recent findings where motoneurones grafted into a denervated skeletal muscle or peripheral nerve are able to survive and establish connections with the host muscle fibres.29,30
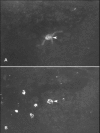
One way of distinguishing embryonic motoneurones from both other cell types present in the grafts, and from neurones of the host, is to prelabel them with BrdU. Immunocytochemistry against BrdU revealed a typical pattern of distribution of the labelled nuclei of embryonic cells in the host spinal cord (see fig. 3). Within the grafts variable numbers of labelled cells were dispersed in a random fashion. The BrdU positive nuclei were of different sizes and varied in intensity of staining for BrdU. In contrast, the cells with nuclei heavily labelled with BrdU were often distributed on the graft-host interface, particularly in the areas of apposition to the host ventral horn. Moreover, some of these intensely labelled nuclei were found outside the grafts in the adjacent grey matter of the host, most often in the ventral horn. The intense labelling of these cells with BrdU showed that their birth-date coincided with administration of the marker, i.e.,they were motoneurones from the embryonic grafts. Indeed, some of these heavily labelled nuclei were found to belong to the cell bodies with motoneurone-like morphological features.
Thus, at least a proportion of embryonic motoneurones present in the grafts at the time of transplantation survived in the host spinal cord and migrated from the graft into the host grey matter showing a preference to the motoneurone-depleted ventral horn.20,31,33 Thus, after a long interval after depletion of the host motoneurone pool, when the spinal circuitry had probably been extensively remodelled, selective depletion of the host ventral horn promoted survival of the homologous embryonic neurones. These results were obtained from animals that had their a considerable proportion of their own motoneurones depleted at birth. In animals that had their normal number of motoneurones the survival of grafted motoneurones was very poor.31
Attempts have also been made to graft cell suspensions enriched in motoneurones. Cultured motoneurones were grafted into the intact spinal cord and the intact brain.32,33 Surprisingly, only 5% of the grafted motoneurones survived, and some of them attached to the ependyma of cerebral ventricles. These embryonic motoneurones migrated for up to 2 mm from the site of injection both in the host grey and white matter and appeared to be relatively small and immature.
Later studies of motoneurone-enriched cell suspensions,34 or purified motoneurone fractions cultured and labelled in vitro prior to grafting,35 showed that some of these cells express AChE and CGRP, but expression of ChAT was generally poor, similarly to the solid grafts of embryonic spinal cord.
Innervation of Host Muscles by the Grafted Motoneurones
As described above, grafted embryonic neurones were able to innervate a host muscle by extending their axons along the peripheral nerve conduit coimplanted in the host spinal cord. Using retrograde labelling from the muscle implant, many labelled cells were found in the host tissue, and majority of these cells were motoneurones. The majority of cells were at the graft-host interface. Retrograde labelling of peripheral nerve implants that had no access to muscle, when inserted into the cervical spinal cord of adult rats showed extensive growth of axons from all types of neurones. However the numbers of axons from grafted motoneurone-like cells were small.36 Since the origin of the cells was not examined in these experiments some of the retrogradely labelled neurones found in the host grey matter could be motoneurone-like cells of embryonic origin which migrated from the graft.
To provide a more detailed description of the origin of retrogradely labelled cells a double-labelling method was used. Neurones retrogradely labelled with fluorescent tracers from the target muscle were predominantly distributed in the grey matter of the host and about 15% of the total number of these cells contained BrdU in their nuclei confirming their embryonic origin.37 These findings indicate that a small number of the BrdU-positive embryonic motoneurone-like cells not only migrated from the graft into the host ventral horn, but also extended their axons outside the host spinal cord to innervate the target muscle.
Morphological studies of the target host muscles showed features consistent with denervation and reinnervation. There was a variability of muscle fiber sizes with some proliferation of connective tissue, and fibre type grouping was detected on histochemical staining. Most importantly the muscles were functionally reinnervated; since strong contractions similar to those produced by the original muscle in its usual position could be elicited by electrical stimulation applied to the nerve bridge.20 Numerous motor axons contacting well developed end-plates were visualized by acetylcholinesterase-silver stain. Thus, despite a profound developmental mismatch between grafted mebryonic motoneurones and the target muscle, the embryonic motoneurones were able to interact with the adult denervated muscle and could be of potential functional value.
In a recent study some of these results obtained on the lumbar cord of adult rats were confirmed in the more rostral cord. Motoneurone enriched grafts from embryos were transplanted into the cervical cord of adult rats and connected to the musculocutaneuos nerve. A large proportion of these motoneurones extended their axons to the biceps muscle and like in the lumbar cord established connections with the denervated biceps muscle.38
Reinnervation of an Avulsed and Reimplanted Lumbar Ventral Root by Axons of Grafted Motoneurones
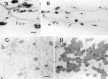
Although axons of grafted neurones were able to enter the nerve conduit to a paravertebrally placed hindlimb muscle (EHL or SOL) they were unable to enter the lumbar ventral roots.39-41 However, it has been shown that axons of injured adult motoneurones can enter a ventral root that had been avulsed at the exit from the spinal cord and then reimplanted into the ventral horn.21,22,42 Such an avulsed ventral root reimplanted close to the graft is also an excellent conduit for axons of grafted motoneurones for many motoneurones of embryonic origin extended their axons into the reimplanted ventral root.43 Interestingly, many more motoneurones entered the reimplanted ventral root than the EHL nerve-muscle implant. Approximately 20% of these reinnervating neurones was confirmed to be of embryonic origin with BrdU labelling43 and 83% of them proved to be motoneurone (Nógrádi and Vrbová, unpublished results). At least 75% of the neurones innervating the reimplanted ventral root reached and reinnervated the hindlimb muscles. The reinnervation of the denervated hindlimb muscles was proven both histologically and functionally (fig. 4). Animals that had their L4 ventral root avulsed and reimplanted without an embryonic graft were severely handicapped, but those that had embryonic grafts walked without any major visible deficit.
Apart from the findings on the reinnervation of hindlimb muscles, immunocytochemical analysis of the grafted cells showed that considerably higher number of grafted neurones expressed ChAT44 and CGRP than in experiments where a small nerve-muscle implant was connected to the graft. This made it possible to perform double labelling on the retrogradely labelled neurones with one of these markers. Almost all the CGRP positive cells in the graft were retrogradely labelled, although a considerable number of reinnervating cells did not contain the marker, suggesting that not all the reinnervating neurones reached the same level of maturity and differentiation.
Transplantation in Models of Neurodegeneration
Indiscriminate degeneration of neurones can be induced by injection of high doses of kainic acid. Grafts of cell suspensions from embryonic spinal cord into such neurone-depleted regions formed a well defined “neonucleus” repopulating the lesioned area.45,46 The transplants contained mixed neuronal populations with small, medium and few large-size somata which resembled motoneurones. Presumed motoneurones did contain CGRP in their cytoplasm, although expression of the peptide was less intense than in the host motoneurones in the adjacent intact ventral horn. Similarly to the findings in other experimental models, this feature could reflect abnormal maturation and/or connectivity of the grafted motoneurones.
A selective depletion of adult motoneurones with neurotoxic lectins provides a relatively good experimental model of human motoneurone disease of adult onset.47,48 Following injection of a neurotoxic lectin, volkensin or ricin into the muscle or peripheral nerve, the lectin is retrogradely transported to motoneurone somata causing their death, but there is no evidence of transsynaptic spread of the toxin.49 Embryonic grafts placed into these motoneurone-depleted areas behaved in a similar manner to that seen in the spinal cord depleted of motoneurones by neonatal nerve injury. The grafted motoneurone-like cells labelled with BrdU were predominantly localized to the graft-host interface. Some of them also migrated into the host grey matter and were typically found near the junction with the host ventral horn. In this experimental situation the BrdU labelled neurones remained confined to the graft and little migration into the host tissue occurred.41
Transplantation of Embryonic Motoneurones into the Spinal Cord of Immature Animals
As discussed in Chapter 5, integration of the embryonic grafts into the spinal cord of young animals appears to be more complete than in the adult. This finding is mirrored by the functional improvement in these young animals. It could therefore be expected that grafted embryonic motoneurones would also survive better in the immature spinal cord. Surprisingly, the opposite seems to be the case.39,50
In 5-12 days-old rat pups local depletion of the host motoneurones was induced by neonatal sciatic nerve crush so that at the time of grafting 70-80% died.16,17 The axons of the grafted motoneurones were given a chance to innervate an autologous muscle implant through a bridge of the peripheral nerve coimplanted in the vicinity of the graft.
Survival and general development of the grafts was satisfactory, but retrograde labelling with fluorescent tracers to the nerve-muscle implant revealed very few reinnervating neurones in the host spinal cords. The muscle implants were severely atrophic and did not show any signs of reinnervation. The negative outcome of these experiments, much worse than in the adult animals, was surprising. There was an almost complete absence of embryonic motoneurone-like cells in the host neuropil and it appeared that neither host motoneurones nor embryonic grafted cells were able to extend their axons into this immature nerve-muscle implant. Whether this failure of grafted motoneurones to survive and extend their axons into the immature graft can be attributed to the unfavourable environment of the less mature spinal cord, or to the nerve muscle conduit, was established next.
Therefore the immature autologous target muscle and nerve conduit was replaced by a similar nerve-muscle implant obtained from adult immunocompatible rats.50 These adult implants were much more efficient in attracting axonal outgrowth from the spinal neurones. However, double-labelling for the presence of BrdU showed that only about 3% of the neurones that sent their axons to the target muscle were of embryonic origin. Thus, even when the target muscle was accessible, the numbers of surviving motoneurone-like cells derived from the graft were much lower in the developing than in the adult animals.
All these data strongly suggested that, apart from the nonpermissive nature of an immature peripheral nerve bridge, the environment of the developing spinal cord was also responsible for poor survival of the grafted embryonic motoneurones. In the rat spinal cord most of axonal growth and synaptogenesis occur during the first postnatal weeks but the latter is not complete until about 30 days after birth.51 In such dynamically developing environment the chances for migrating embryonic motoneurones to be exposed to the growing host afferents, many of which utilize glutamate as a neurotransmitter, could be higher than in a relatively quiescent adult spinal cord, and at a critical stage of development excessive exposure to glutamate is toxic to spinal neurones.52-54 (for review see Lowrie and Vrbová,17 1992). Thus, in the developing spinal cord excessive activation by the growing glutaminergic inputs from the host could aggravate the stress already imposed upon the grafted embryonic motoneurones by deprivation of target in critical stage of development.
Most Important Points
The available data shows that a number of grafted motoneurones survive transplantation for prolonged periods of time. The grafted motoneurones have a better chance of survival when the host cord is depleted of it's own motoneurones. The motoneurones of graft origin are able to survive and establish functional connections with adult denervated muscle. It is interesting that reducing this developmental mismatch by grafting embryonic motoneurones into a developing spinal cord reduces the chances of survival of grafted cells. In the adult spinal cord the grafted motoneurone-like cells display preferential migration into the ventral horn of the host.
From the practical point of view it is important to define the conditions which could improve survival of grafted embryonic motoneurones. The importance of making contact with the target muscle for the survival of developing motoneurones has already been discussed. Even if the experimental design creates a possibility of innervating a surrogate target, such as a muscle of the host, this obviously would not have a chance to occur during the most vulnerable stage after grafting. Thus, the presence of the muscle is more likely to be of benefit for maturation of the grafted embryonic motoneurones, and possibly for their long-term maintenance, but not for their immediate survival.
However, the major challenge is how to guide the axons of the grafted motoneurones towards the appropriate muscles of the host. At present the ventral root avulsion and reimplantation procedure makes it possible for the axons of grafted motoneurones to reach the denervated hindlimb muscles and induce functional reinnervation.
References
- 1.
- Altman J, Bayer SA. The development of the rat spinal cord. Adv Anat Embryol Cell Biol. 1984;85:1–166. [PubMed: 6741688]
- 2.
- Sotelo C, Alvarado-Mallart RM. Reconstruction of the defective cerebellar circuitry in the adult Purkinje cell degeneration mutant mice by Purkinje cell replacement through transplantation of solid embryonic implants. Neuroscience. 1987;20:1–22. [PubMed: 3561760]
- 3.
- Sotelo C, Alvarado-Mallart RM, Gardette R. et al. The fate of grafted embryonic Purkinje cells in the cerebellum of the adult “Purkinje cell degeneration” mutant mouse. I. Development of reciprocal graft-host interactions. J Comp Neurol. 1990;295:165–187. [PubMed: 2358510]
- 4.
- Nornes HO, Das GD. Temporal pattern of neurogenesis in spinal cord of rat. I. An autoradiographic study - time and sites of origin and migration and settling patterns of neuroblasts. Brain Res. 1974;73:121–138. [PubMed: 4407392]
- 5.
- Reynolds ML, Fitzgerald M, Benowitz LJ. GAP-43 expression in developing cutaneous and muscle nerves in the rat hindlimb. Neuroscience. 1991;41:201–211. [PubMed: 1829143]
- 6.
- Oppenheim RW. Cell death during development of the central nervous system. Annu Rev Neurosci. 1991;14:453–501. [PubMed: 2031577]
- 7.
- Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Dev Brain Res. 4:473–479. [PubMed: 7127154]
- 8.
- Oppenheim RW. The absence of significant postnatal motoneuron death in the brachial and lumbar spinal cord of the rat. J Comp Neurol. 1986;246:281–286. [PubMed: 3958254]
- 9.
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160:535–546. [PubMed: 1123466]
- 10.
- Hamburger V. The Developmental History of the Motor Unit: The F.O. Schmidt Lecture in Neuroscience In: Neuroscience Research Programme BulletinCambridge, MA: MIT Press,19761–37.
- 11.
- Hamburger V, Oppenheim RW. Naturally occurring cell death in vertebrates. Neurosci Comment. 1982;1(2):39–55.
- 12.
- Oppenheim RW, Chu-Wang I-W. Aspects of naturally-occurring motoneuron death in the chick spinal cord during embryonic developmentIn: Burnstock G, O'Brien RAD, Vrbová G, eds.Somatic and Autonomic Nerve InteractionsAmsterdam: Elsevier Science Publishers BV,198356–107.
- 13.
- Hamburger V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958;102:365–410. [PubMed: 13617221]
- 14.
- Hollyday M, Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170:311–320. [PubMed: 993371]
- 15.
- Romanes GJ. Motor localization and the effects of nerve injury on the ventral horn cells of the spinal cord. J Anat. 1946;80:117–131. [PubMed: 20996683]
- 16.
- Lowrie MB, Subramaniam K, Vrbová G. Permanent changes in muscles and motoneurones induced by nerve injury during a critical period of development of the rat. Dev Brain Res. 1987;31:91–101. [PubMed: 3815121]
- 17.
- Lowrie MB, Vrbová G. Dependence of postnatal motoneurones on their targets: Review and hypothesis. Trends Neurosci. 1992;15:80–84. [PubMed: 1373920]
- 18.
- Lieberman AR. Some factors affecting retrograde neuronal responses to axonal lesionsIn: Bellairs R, Gray EG, eds.Essays on the Nervous System and Festschrift for Professor JZ YoungOxford: Oxford University Press,197477–105.
- 19.
- Greensmith L, Vrbová G. Alterations of nerve-muscle interactions during postnatal development influence motoneurone survival in rat. Dev Brain Res. 1992;69:125–131. [PubMed: 1424084]
- 20.
- Sieradzan K, Vrbová G. Replacement of missing motoneurons by embryonic grafts in the rat spinal cord. Neuroscience. 1989;31:115–130. [PubMed: 2771053]
- 21.
- Cullheim S, Carlstedt T, Linda H. et al. Motoneurons reinnervate skeletal muscle after ventral root implantation into the spinal cord of the cat. Neuroscience. 1989;29:725–733. [PubMed: 2739906]
- 22.
- Carlstedt T, Risling M, Linda H. et al. Regeneration after spinal nerve root injury. Restor Neurol Neurosci. 1990:289–295. [PubMed: 21551569]
- 23.
- Aguayo AJ. Axonal regeneration from injured neurons in the adult mammalian central nervous systemIn: Cotman CW, ed.Synaptic PlasticityNew York: The Guilford Press,1985457–484.
- 24.
- Bray GM, Villegas-Peres MP, Vidal-Sanz M. et al. The use of peripheral nerve grafts to enhance neuronal survival, promote growth and permit terminal reconnections in the central nervous system of adult rats. J Exp Biol. 1987;132:5–13. [PubMed: 3323406]
- 25.
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. [PubMed: 3167568]
- 26.
- Barber RP, Phelps PE, Houser CR. et al. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: An immunocytochemical study. J Comp Neurol. 1984;229:329–346. [PubMed: 6389613]
- 27.
- Phelps PE, Barber RP, Brennan LA. et al. Embryonic development of four different subsets of cholinergic neurons in rat cervical spinal cord. J Comp Neurol. 1990;291:9–26. [PubMed: 2298930]
- 28.
- Clowry GJ, Sieradzan K, Vrbová G. Expression of cholinergic phenotype by embryonic ventral horn neurones transplanted into the spinal cord in the rat. Restor Neurol Neurosci. 1994;6:209–219. [PubMed: 21551751]
- 29.
- Thomas CK, Erb DE, Grumbles RM. et al. Embryonic cord transplants in peripheral nerve restore skeletal muscle function. J Neurophysiol. 2000;84:591–595. [PubMed: 10899232]
- 30.
- Guangliang J, Yudong G. The observation of transplanted embryonic motoneurons in the denervated muscles of adult rats. Chinese Med J. 1998;111:63–66. [PubMed: 10322657]
- 31.
- Sieradzan K, Vrbová G. Factors influencing survival of transplanted embryonic motoneurones in the spinal cord of adult rats. Exp Neurol. 1991;114:286–299. [PubMed: 1748203]
- 32.
- Demierre B, Martinou J-C, Kato A. Embryonic motoneurones grafted into the adult CNS differentiate and migrate. Brain Res. 1990;510:355–359. [PubMed: 2331607]
- 33.
- Demierre B, Ruiz-Flandes P, Martinou J-C. et al. Grafting of embryonic motoneurones into adult spinal cord and brain. Prog Brain Res. 1990;82:233–237. [PubMed: 2290938]
- 34.
- Sieradzan K, Clowry G, Haynes L. et al. The ability of isolated embryonic motoneurones grafted into rat spinal cord to express cholinergic markers and innervate skeletal muscle. Restor Neurol Neurosci. 1992;4:220.
- 35.
- Peschanski M, Nothias F, Cadusseau J. Is there a therapeutic potential for intraspinal transplantation of fetal spinal neurons in motoneuronal diseases? Restor Neurol Neurosci. 1992;4:227.
- 36.
- Horvat JC, Pecot-Dechavassine M, Mira JC. et al. Formation of functional endplates by spinal axons regenerating through a peripheral nerve graft: A study in the adult rat. Brain Res Bull. 1989;22:103–14. [PubMed: 2713708]
- 37.
- Clowry GJ, Vrbová G. Observations on the development of transplanted embryonic ventral horn neurones grafted into adult spinal cord and connected to skeletal muscle implants via peripheral nerve. Exp Brain Res. 1992;91:249–258. [PubMed: 1459227]
- 38.
- Duchossoy Y, Kassar-Duchossoy L, Orsal D. et al. Reinnervation of the biceps brachii muscle following cotransplantation of fetal spinal cord and analogous periphral nerve into the injured spinal cordof the adult rat. Exp Neurol. 2001;167:329–340. [PubMed: 11161621]
- 39.
- Sieradzan K, Vrbová G. The ability of developing spinal neurons to reinnervate a muscle through a peripheral nerve conduit is enhanced by cografted embryonic spinal cord. Exp Neurol. 1993a;122:232–243. [PubMed: 8405261]
- 40.
- Nógrádi A, Vrbová G. The use of embryonic spinal cord grafts to replace identified motoneurone pools depleted by a neurotoxic lectin, volkensin. Restor Neurol Neurosci. 1992;4:219. [PubMed: 7925835]
- 41.
- Nógrádi A, Vrbová G. The use of embryonic spinal cord grafts to replace identified motoneurone pools depleted by a neurotoxic lectin, volkensin. Exp Neurol. 1994;129:130–141. [PubMed: 7925835]
- 42.
- Carlstedt T, Grane P, Hallin RG. et al. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323–1325. [PubMed: 7475770]
- 43.
- Nógrádi A, Vrbová G. Improved motor function of denervated rat hindlimb muscles induced by embryoni spinal cord grafts. Eur J Neurosci. 1996;8:2198–2203. [PubMed: 8921311]
- 44.
- Nógrádi A, Vrbová G. The effect of riluzole treatment in rats on the survival of injured adult and grafted embryonic motoneurons. Eur J Neurosci. 2001;13:113–118. [PubMed: 11135009]
- 45.
- Nothias F, Peschanski M. Homotypic fetal transplants into an experimental model of spinal cord neurodegeneration. J Comp Neurol. 1990;301:520–534. [PubMed: 2273098]
- 46.
- Nothias F, Cadusseau J, Dusart I. et al. Fetal neural transplants into the area of neurodegeneration in the spinal cord of adult rat. Restor Neurol Neurosci. 1991;2:283–238. [PubMed: 21551614]
- 47.
- de la Cruz RR, Baker R, Delgado-Garcia JM. Behaviour of cat abducens motoneurons following the injection of toxic ricin into the lateral rectus muscle. Brain Res. 1991;544:260–268. [PubMed: 2039942]
- 48.
- Nógrádi A, Vrbová G. The use of a neurotoxic lectin, volkensin, to induce loss of identified motoneurone pools. Neuroscience. 1992;50:975–986. [PubMed: 1448208]
- 49.
- de la Cruz RR, Pastor AM, Delgado-Garcia JM. Long-term effects of selective target removal on brainstem premotor neurons in the adult cat. Eur J Neurosci. 1993;5:232–239. [PubMed: 8261104]
- 50.
- Sieradzan K, Vrbová G. Observations on the survival of grafted embryonic motoneurons in the spinal cord of developing rats. Exp Neurol. 1993b;122:223–231. [PubMed: 8405260]
- 51.
- Weber ED, Stelzner DD. Synaptogenesis in the intermediate grey region of the lumbar spinal cord in the postnatal rat. Brain Res. 1980;185:17–37. [PubMed: 7353175]
- 52.
- Brenneman DE, Forsythe ID, Nicol T. et al. N-Methyl-D-Aspartate receptors influence neuronal survival in developing spinal cord cultures. Dev Brain Res. 1990;51:63–68. [PubMed: 1967564]
- 53.
- Meldrum B, Garthwaite J. Excitatory amino acids and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. [PubMed: 2238094]
- 54.
- Regan RF, Choi DW. Glutamate neurotoxicity in spinal cord cell culture. Neuroscience. 1991;43:585–591. [PubMed: 1681469]
Footnotes
- *
Based on chapter in previous edition written by Katarzyna Sieradzan and Gerta Vrbová.
- PubMedLinks to PubMed
- Replacement of Specific Neuronal Populations in the Spinal Cord - Madame Curie B...Replacement of Specific Neuronal Populations in the Spinal Cord - Madame Curie Bioscience Database
- The End in Sight: Poly(A), Translation and mRNA Stability in Eukaryotes - Madame...The End in Sight: Poly(A), Translation and mRNA Stability in Eukaryotes - Madame Curie Bioscience Database
- Origin and Evolution of DNA and DNA Replication Machineries - Madame Curie Biosc...Origin and Evolution of DNA and DNA Replication Machineries - Madame Curie Bioscience Database
- Polyomavirus Allograft Nephropathy: Clinico-Pathological Correlations - Madame C...Polyomavirus Allograft Nephropathy: Clinico-Pathological Correlations - Madame Curie Bioscience Database
- STATs and Infection - Madame Curie Bioscience DatabaseSTATs and Infection - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...