NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
OX40 (CD134) and its binding partner OX40-Ligand (OX40L) represent members of the TNFR and TNF superfamilies that appear to be crucial to many types of immune reaction mediated by T cells. Emerging data have now put these molecules at the forefront of the field of what has been termed T cell costimulation. Costimulation is defined as signals from membrane bound molecules that synergize with, or modify, signals provided when the T cell encounters its specific antigen. In large part, costimulation is essential for an efficient T cell response, whether it is protective or pathogenic, and without costimulatory interactions between membrane bound receptor-ligand pairs, a T cell is ineffective and may often succumb to death or become nonfunctional. OX40 is induced on the T cell surface a number of hours or days after recognition of antigen, and expression coincides with the appearance of OX40L on several cell types that can present antigen such as dendritic cells and B cells. Recent data show that OX40 can provide signals to a T cell to allow prolonged cell division after activation and to prevent excessive cell death. The OX40/OX40L interaction then controls the absolute number of pathogenic or protective effector T cells that are generated at the peak of the immune response and dictates the frequency of memory T cells that subsequently develop. This then has implications regarding strategies to suppress unwanted immune responses, and for vaccination to promote naturally weak immune responses. Reagents that interfere with the binding of OX40 to OX40L have been shown to inhibit T cell responses and pathogenic symptoms in a number of immune based diseases. Conversely reagents that augment OX40 signals have now shown therapeutic efficacy in models of cancer. This article will review the literature regarding these molecules and discuss their implications in T cell immunity.
Introduction to OX40 (CD134) and OX40-Ligand
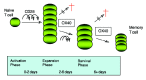
OX40 is a 50 Kd glycosylated type 1 transmembrane protein.1-4 The extracellular N-terminal portion of OX40 is 191 amino acids, and contains three cysteine-rich domains (CRDs) of approximately 40 amino acids, which are characteristic of the TNFR superfamily. The intracellular region consists of 36 amino acids. OX40L is a 34 Kd glycosylated type II transmembrane protein and as with other members of the TNF family is thought to be present on the surface of cells as a trimer.5-7 The extracellular C-terminal domain of OX40L has a 133 amino acid long TNF homology domain (THD) that organizes into a characteristic “jelly roll” beta-sandwich structure. The intracellular region consists of 50 amino acids. Based on sequence similarity with other members of the TNF/TNFR family, the quaternary organization of the signaling unit of OX40 interacting with OX40L is likely to be three OX40 molecules bound to one trimeric OX40L complex (fig. 1).8,9
The TNFR family of molecules can be divided into two groups based on the presence or absence of a cytoplasmic death domain that leads to apoptosis. OX40 lacks such a domain, and similar to other members of this family, extensive data from multiple systems now supports the idea that the main function of the OX40/OX40L interaction is to promote cell survival. This review will focus on recent studies of these molecules and discuss their role in immune function.
Expression Characteristics of OX40 and OX40L on T Cells and APC
OX40 was identified in 1987 with an antibody that reacted to activated rat CD4 T cells.10 Although OX40 has been primarily visualized on CD4 T cells,1,2 and the vast majority of studies to date have been directed towards this cell subset, CD8 T cells can also bear OX40 under certain conditions.5,7 Moreover, OX40 has now been visualized on other diverse cell types including B cells, dendritic cells, and eosinophils (M. Croft et al, unpublished observations), although as yet the physiological significance of this expression is unknown.
OX40 is not expressed on resting T cells, but can be induced by peptide/MHC interaction with the T cell receptor (TCR) or reagents that cross-link the TCR/CD3 complex, and initially appears 12-24 hr after stimulation of naïve T cells. Peak expression is seen after 2-3 days and then OX40 is downregulated several days later, implying a delayed mode of action in primary immune responses. OX40 has been visualized in vivo in T cell zones of spleen or lymph nodes several days after immunization with protein antigen, directly coinciding with the peak of the primary T cell response.11,12 In contrast, antigen-experienced memory/effector T cells can rapidly reexpress OX40 within 4 hr of reactivation, suggesting an earlier role in secondary immune responses of memory T cells when antigens are reencountered.13
OX40L was first identified on the surface of HTLV-infected leukemic T cells.5 However, expression on nontransformed T cells appears to be rare and may depend on as yet undefined inflammatory conditions, or be restricted to specialized sites such as the gut.14,15 The majority of OX40L is found on professional antigen-presenting cells (APC) such as dendritic cells, B cells and macrophages and as with OX40 on T cells, OX40L is induced many hours to days after APC receive an activating stimulus.6,16-18 In the case of dendritic cells and B cells, Toll-like receptor signals induced by LPS can promote OX40L expression in addition to contributions from Ig signals and CD40 signals.16-18 Additionally, OX40L has been visualized on activated endothelial cells in vitro, and in tissues from patients with lupus nephritis and inflammatory bowel disease, implying a role in promoting migration of OX40-expressing T cells into inflamed tissues, or providing signals to T cells to augment their activity in these peripheral sites.19-21
Function of OX40 on T Cells
Since its discovery, a number of in vitro studies using either receptor specific agonist antibodies, or cells transfected with OX40L, have shown that signals through OX40 can augment T cell responses, either in isolation, or in combination with signals from the Ig superfamily member, CD28, when it interacts with its ligands B7-1 or B7-2.5,6,10,13,22-25 Although a number of activities have been described in vitro such as enhancing T cell proliferation, cytokine secretion, and cell survival, recent studies of knockout animals, with antagonist and agonist reagents in vivo, or receptor-deficient T cells, have aided tremendously in defining the physiological role of OX40 signals when they are received in the context of multiple other signals provided when an APC presents antigen to a T cell.
The initial reports of OX40- and OX40L-deficient mice demonstrated that CD4 T cell responses to the viruses LCMV and influenza, to several common protein antigens, and in contact hypersensitivity reactions, were markedly reduced.12,18,26-28 Further in vivo data in OX40-deficient mice provided an indication of the role of OX40 in governing T cell immunity when a frequency analysis of antigen-specific CD4 T cells generated after immunization showed dramatically reduced numbers late in the primary response, and additionally after 5 weeks when T cell memory was formed.12 Corresponding data was produced when agonist anti-OX40 reagents, injected in vivo shortly after immunization, augmented the number of antigen-reactive CD4 cells that accumulated over time.12,29 Along the same lines, transgenic expression of OX40L on dendritic cells in vivo led to greater numbers of primed CD4 cells30 and blocking OX40L in another model reduced the accumulation of CD4 cells.31
Collectively, these studies have implied that a major role of OX40/OX40L interactions is to regulate the number of effector (protective or pathogenic) T cells that accumulate in primary immune responses, and consequently to promote a large number of memory T cells to subsequently develop (fig. 2). More recent data obtained with antigen-specific TCR transgenic CD4 cells lacking OX40 have now provided greater insight into the mechanism of action.32 These studies have directly demonstrated that OX40 signals contribute little to the initial response of a CD4 cell that occurs within 2-3 days of an encounter with antigen. OX40-deficient T cells become activated, secrete cytokines fairly normally, and go through a number of rounds of cell division. This directly contrasts with CD28-deficient T cells in that this molecule is required for much of the early T cell response. The major phenotype seen in the absence of OX40 signals was reduced proliferation 4-5 days into the response, and a great defect in the ability to survive over the long-term. The lack of survival was shown to be due to apoptotic cell death and could be rescued by inhibitors of the caspase cascade.32 OX40 expression is not dependent on CD28 signals, but several systems have shown that CD28 engagement can augment the level of OX40 expressed on a T cell.31,32 As CD28 is constitutively expressed on a T cell, and then will provide signals prior to OX40 expression, this reinforces the concept that the two molecules most likely cooperate together in a sequential manner.
Therefore, in summary, the data at this point in time suggest a model whereby OX40 signals act in a temporal manner after CD28 signals, and enable effector T cells to survive and continue proliferating over an extended period of time, predominantly by transmitting anti-apoptotic signals that prevent excessive T cell death (fig. 2). This ultimately results in greater numbers of T cells surviving the primary immune response and developing into memory T cells that can then respond in secondary immune reactions when antigen is reencountered at a later time.
Signals Transduced by OX40
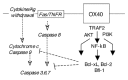
How do OX40 signals regulate T cells and suppress cell death. As is the case with other members of the TNFR family, the intracellular tail of OX40 can bind several members of the TNFR-associated factor (TRAF) family of signaling molecules, in this case TRAF-2, -3 and -5.33,34 The C-terminal region of TRAF2, which is conserved among the TRAF members, enables self-association into trimers, suggesting that OX40L binding brings three OX40 molecules into close proximity on the surface of a T cell and provides an opportunity for trimeric TRAF molecules to engage in multivalent interactions.35 The interaction of the OX40 cytoplasmic tail and the C-terminal domain of TRAF molecules requires only a short stretch of conserved acidic amino acids, which contains the QEE motif.
NF-κB is most likely one of the central mediators of OX40 signals. After the recruitment of TRAF molecules to the cytoplasmic tail of activated OX40, TRAF-2 and -5 appear to play an important role in modulating an early step in activation of NF-κB by using their N-terminal RING and zinc finger domains. Studies using dominant negative forms of TRAF-2 and -5, which lack the N-terminal domains, demonstrated the critical contribution in OX40-induced NF-κB activation.33,34 The introduction of TRAF3 together with the dominant negative mutants of TRAF2 or TRAF5 further reduced NF-κB activation,36 suggesting that OX40 signals may be negatively modulated by TRAF3.
How OX40 provides survival signals remains incompletely understood (fig. 3). Anti-apoptotic Bcl-2 family members (Bcl-2, Bcl-xL and Bfl-1) and pro-apoptotic Bcl-2-related proteins (Bim, Bad or Bid) have been identified that play key roles in regulating cell death in T cells. Recent data have shown that OX40-deficient CD4 cells cannot maintain high levels of Bcl-xL, Bcl-2, and Bfl-1 over the long-term following antigen stimulation.32 Moreover, forced expression of Bcl-xL or Bcl-2 completely reversed the survival defect of these cells and conferred resistance to spontaneous apoptosis.32 This data directly suggests that signals from OX40 positively affect molecules that inhibit the T cell from dying. Additionally, OX40 may also negatively affect the expression or activity of the pro-apoptotic molecules (J. Song and M. Croft, unpublished data) implying there may be two alternate coordinated modes of enhancing T cell survival. Whether TRAF2 or TRAF5, or activation of NF-κB activation, are required for these effects is presently unknown, but is obviously a distinct possibility.
Other recent studies have shown that a phosphatidylinostol 3-kinase (PI3K)-mediated signaling cascade mediates survival signals in multiple cell types. The serine/threonine protein kinase Bα (AKT/PKB) is a downstream target of PI3K, and is also known to play an essential role in some forms of apopotic cell death. Recent data have also implicated PI3K and AKT in OX40 signaling. Ligation of OX40 induces PI3K recruitment and activation of AKT, and forced expression of active AKT in OX40-deficient T cells reverses their survival defect (J. Song and M. Croft, submitted for publication). How AKT inhibits T cell apoptosis is not clear. It has been shown to be capable of phosphorylating and inactivating Bad, directly up-regulating the expression of Bcl-xL and Bcl-2, and altering the function of transcription factors that can also lead to cellular apoptosis. It remains to be determined how PI3K and AKT modulate survival following OX40 ligation, but these molecules and NF-κB may be the principal intermediaries that regulate this activity of OX40 (fig. 3).
Function and Signaling of OX40L on Accessory Cells
Although a lot of data exists on the role of OX40 on CD4 T cells, and obviously the interaction with OX40L is required for promoting OX40 signaling, it is not clear whether OX40L itself is essential for the response of the cell that bears it. Data gathered a number of years ago with reagents that cross-linked OX40L on B cells in vitro suggested that signals were transduced to allow a B cell to differentiate into a plasma cell secreting high levels of immunoglobulin.16 This idea was initially supported when antibody production in vivo was suppressed by a polyclonal serum to OX40.11 However, more recent data with OX40- and OX40L-deficient animals have now shown that these animals can mount relatively normal antibody responses,26-28 questioning whether OX40L is required for a B cell response. A similar idea regarding APC activation was also put forward from other in vitro studies of dendritic cells that showed that these cells produced elevated levels of inflammatory cytokines such as IL-1 and IL-12 after OX40L was cross-linked on their surface.17 As with the B cell studies, these data suggest there is the potential for OX40L to transduce signals to the APC at the time of encounter with an OX40-expressing T cell. However, more physiological data is needed from in vivo studies before it can be concluded that this is a major consequence of OX40/OX40L interaction.
As previously detailed, OX40L has also been seen on some activated endothelial cells19 and the only study on signaling through OX40L has concentrated on this cell type. This data demonstrated that binding OX40L resulted in an increase in c-jun and c-fos mRNA, which is likely to be mediated by a cytoplasmic RPRF motif.37 The endothelial cells were shown to upregulate production of a CC chemokine RANTES/CCL5 after OX40L engagement.38 As this chemokine has been implicated in promoting migration of T cells into peripheral sites, these data suggest a possible link between OX40/OX40L and extravasation of T cells into inflamed tissues. However, again, it remains to be determined under more physiological conditions in vivo whether OX40L expression on endothelial cells is essential for a T cell response to develop at a site of inflammation.
>Regulation of T Cell Tolerance and Cancer Immunity by OX40
It has been known for some time that recognition of antigen in a noninflammatory environment can lead to T cell tolerance,39,40 and this process is characterized by defective survival of a large number of cells and hypo-responsiveness of those T cells that do survive. Because OX40 can be expressed on a T cell at low levels in the absence of inflammatory signals and CD28 signals are not essential, this suggests that OX40 may be a realistic target for providing signals to affect the tolerance process. This was directly shown in a recent study using agonist antibodies to OX40, where it was demonstrated that OX40 signals given within two days of encountering soluble antigen in vivo could prevent CD4 T cell deletion and stop T cells from entering a hypo-responsive state.41 Similar data were also obtained assessing T cell deletion in response to a superantigen where agonist anti-OX40 also significantly enhanced the survival of CD4 T cells.29 Moreover, additional data demonstrated that anti-OX40, given after T cell deletion and hyporesponsiveness had occurred, could target the small number of antigen-specific tolerant T cells and cause them to expand in numbers and to regain responsiveness.41 The ability to reverse tolerance by providing costimulatory signals through OX40 indicates the possibility of a similar mechanism operating in vivo, if inadvertent OX40L expression were to occur, which would lead to autoimmunity. This was recently partly confirmed when transgenic mice were produced that constitutively expressed OX40L and were shown to spontaneously develop interstitial pneumonia, inflammatory bowel disease, and antibodies to double-stranded DNA.42
Therefore, if certain conditions are encountered that promote prolonged expression of both OX40 and OX40L, tolerant self-reactive T cells may gain normal function if they encounter their specific antigen and lead to development of late-onset autoimmune diseases. Blocking the interaction of OX40 and its ligand then represents a potential therapeutic target for limiting autoimmunity. On the other hand, treatment with agonist reagents to OX40 might be beneficial in situations where autoimmunity is desired.
Tumors can evade an immune response through an active tolerance mechanism by which T cells reactive with tumor self-peptides are deleted and/or made hyporesponsive, raising the possibility that OX40 targeted immunotherapy may be beneficial in augmenting anti-tumor immunity. OX40-expressing T cells have now been found at the sites of inflammation in patients with solid tumors,43 directly on T lymphomas,44-46 and within infiltrates of various types of tumors, including melanoma, head and neck cancer, breast cancer and colon cancer.43,47,48 Treatment with reagents that bind to and signal through OX40 have recently been shown to delay tumor growth and enhance memory CD4 T cells reactive against tumor antigens.49,50 For optimal systemic anti-tumor immunity, it is pertinent to develop strategies that would activate both CD4 and CD8 T cell responses against tumors. CD8 T cells upregulate OX40 upon activation, similar to CD4 T cells. Although the majority of data on OX40 have been directed towards CD4 T cells, agonistic anti-OX40 has also now been shown to enhance CD8 T cell responses to antigen challenge in vivo (P. Bansal-Pakala and M. Croft, submitted for publication). OX40 signals can also reverse CD8 T cell tolerance (P. Bansal-Pakala and M. Croft, submitted), similar to CD4 cells.41 This then holds great promise for anti-OX40 in tumor therapy, since by the time of therapeutic intervention, it is likely that T cells are already tolerized to existing tumors.
Expression and Role of OX40 in T Cell-mediated Disease
The expression of OX40 has now been detected on T cells at the site of inflammation in patients and rodents during clinical signs of a wide range of immunologically mediated diseases (Table 1) including: experimental allergic encephalomyelitis (EAE), the mouse model of MS,51,52 graft-vs-host disease,53-55 rheumatoid arthritis,56,57 myasthenia gravis,58 inflammatory bowel disease,20 celiac disease, Crohn's disease, ulcerative colitis,59 and inflammatory muscle disease.60
Table 1
Patterns of OX40 and OX40L expression in human and animal models of disease.
The expression patterns firstly suggest that OX40 may be a useful marker for identifying antigen-specific pathogenic T cells in a wide range of immune related diseases. Direct experimental evidence for this was first provided in EAE where the disease course was abrogated by administration of an anti-OX40 immunotoxin that directly killed CNS-infiltrating T cells.61 More recently, it was shown that intravenous injection of immunoglobulin (IVIg) prevented the development of acute GVHD by decreasing the number of CD4+OX40+ donor alloreactive T-cells.62
Secondly, expression of OX40 in sites of immune-mediated inflammation suggests that as well as OX40 providing a target for augmenting T cell function and enhancing immunity, the interaction of OX40 with OX40L is an attractive target for suppressing immune responses that may be detrimental to the host (Table 2). Studies of OX40-deficient mice showed that they exhibited reduced primary CD4 responses to the viruses LCMV and influenza, characterized by lower numbers of IFN-γ-secreting cells and fewer T-cells infiltrating the lungs of infected animals.26 OX40-deficient mice were also shown to have an impaired ability to generate Th2 immune responses and develop pulmonary lung inflammation and airway hyperreactivity in a murine model of asthma,63 and this observation was confirmed in OX40L-deficient mice.64 In other studies, OX40L-deficient mice were also found to be defective in primary contact hypersensitivity responses to oxazalone and DNBS.27
Table 2
Major features of OX40 and OX40L deficient or transgenic mice.
Several studies in experimental animal models have now not only stressed the importance of OX40 and OX40L for their manifestations, but shown that inhibiting this interaction can be useful therapeutically (Table 3). For example, anti-OX40L antibodies, or OX40-Ig fusion proteins that bind specifically to OX40L, can abrogate Th2 or Th1-induced pathologies in experimental leishmaniasis,65 EAE,66,67 acute graft-versus-host disease,54,55 inflammatory bowel disease,68,69 and collagen-induced arthritis.56 These studies have highlighted the broad reaching control of T cell responses by OX40 and OX40L, particularly those mediated by CD4 cells, and promoted this interaction to the forefront of potential therapies aimed at dampening T cell driven immune diseases.
Table 3
Consequence of OX40 inhibtion or OX40 engagement in animal models of disease.
Summary
In conclusion, there is now a considerable body of literature that shows the importance of OX40 and OX40L in the generation of T cell immunity. Strong evidence has been presented that OX40 signaling to a T cell regulates expansion and cell division and is critical to the long-term survival of T cells. This not only impacts the magnitude of the primary immune response and its efficiency, but also directly affects the number of T cells that can go on to form the memory pool. Augmenting signals through OX40 have shown great promise in experimental models of tolerance and tumor immunity, and demonstrated that agonist reagents targeting OX40 may represent useful tools in the future as adjuvants for vaccination, and for treating ongoing immunological diseases that require a greater T cell response for protection. In addition, reagents that can inhibit endogenous OX40/OX40L interactions also show great promise as novel immunotherapeutic approaches for the treatment of autoimmune and allergic diseases that are characterized by exaggerated T cell responses.
Future Considerations
Although much is known regarding the function of OX40 expressed on a T cell, there are many questions that remain unanswered not only regarding T cells but other cell types that have now been visualized to express OX40. For example, does OX40 contribute to the function of eosinophils or dendritic cells and if so, is it an analogous action to that on T cells. Additionally, even though a great amount of functional data has been obtained regarding the control of CD4 T cell responses, there is little data to date on the importance of OX40 signals to a CD8 T cell. Moreover, a number of CD4 T cell mediated responses such as those to helminth parasites, as well as several virally induced CD8 T cell responses, appear to be OX40 independent and the question is raised as to why OX40 is critical to certain T cell reactions but not others and whether there is a logical rationale for where and when OX40 plays a dominant role. Lastly, there is little information regarding the importance of OX40L signals to the cell type that expresses this molecule and whether it largely serves as an aggregation partner to induce OX40 signals, or whether there is a direct dialogue with the OX40L-expressing cell that is critical for the ultimate immune response. Answers to these questions will no doubt be forthcoming in the next few years and should provide a great framework for determining the therapeutic applications of targeting OX40 or OX40L in either positive or negative ways.
References
- 1.
- Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–8. [PMC free article: PMC551780] [PubMed: 2157591]
- 2.
- Calderhead DM, Buhlmann JE, van et al. Cloning of mouse Ox40: A T cell activation marker that may mediate T-B cell interactions. J Immunol. 1993;151:5261–71. [PubMed: 8228223]
- 3.
- Latza U, Durkop H, Schnittger S. et al. The human OX40 homolog: cDNA structure, expression and chromosomal assignment of the ACT35 antigen. Eur J Immunol. 1994;24:677–83. [PubMed: 7510240]
- 4.
- Birkeland ML, Copeland NG, Gilbert DJ. et al. Gene structure and chromosomal localization of the mouse homologue of rat OX40 protein. Eur J Immunol. 1995;25:926–30. [PubMed: 7737295]
- 5.
- Baum PR, Gayle RB, Ramsdell F. et al. Molecular characterization of murine and human OX40/ OX40 ligand systems: Identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. [PMC free article: PMC395319] [PubMed: 8076595]
- 6.
- Godfrey WR, Fagnoni FF, Harara MA. et al. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–62. [PMC free article: PMC2191595] [PubMed: 7913952]
- 7.
- Al-Shamkhani A, Birkeland ML, Puklavec M. et al. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur J Immunol. 1996;26:1695–9. [PubMed: 8765008]
- 8.
- Banner DW, D'Arcy A, Janes W. et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: Implications for TNF receptor activation. Cell. 1993;73:431–45. [PubMed: 8387891]
- 9.
- Al-Shamkhani A, Mallett S, Brown MH. et al. Affinity and kinetics of the interaction between soluble trimeric OX40 ligand, a member of the tumor necrosis factor superfamily, and its receptor OX40 on activated T cells. J Biol Chem. 1997;272:5275–82. [PubMed: 9030600]
- 10.
- Paterson DJ, Jefferies WA, Green JR. et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. [PubMed: 2828930]
- 11.
- Stuber E, Strober W. The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–89. [PMC free article: PMC2192367] [PubMed: 8642301]
- 12.
- Gramaglia I, Jember A, Pippig SD. et al. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. [PubMed: 10975814]
- 13.
- Gramaglia I, Weinberg AD, Lemon M. et al. Ox-40 ligand: A potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed: 9862675]
- 14.
- Takasawa N, Ishii N, Higashimura N. et al. Expression of gp34 (OX40 ligand) and OX40 on human T cell clones. Jpn J Cancer Res. 2001;92:377–82. [PMC free article: PMC5926725] [PubMed: 11346458]
- 15.
- Wang HC, Klein JR. Multiple levels of activation of murine CD8(+) intraepithelial lymphocytes defined by OX40 (CD134) expression: Effects on cell-mediated cytotoxicity, IFN-gamma, and IL-10 regulation. J Immunol. 2001;167:6717–23. [PubMed: 11739485]
- 16.
- Stuber E, Neurath M, Calderhead D. et al. Cross-linking of OX40 ligand, a member of the TNF/ NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–21. [PubMed: 7749983]
- 17.
- Ohshima Y, Tanaka Y, Tozawa H. et al. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–48. [PubMed: 9378971]
- 18.
- Murata K, Ishii N, Takano H. et al. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. [PMC free article: PMC2195745] [PubMed: 10637280]
- 19.
- Imura A, Hori T, Imada K. et al. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–95. [PMC free article: PMC2192546] [PubMed: 8642328]
- 20.
- Souza HS, Elia CC, Spencer J. et al. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. [PMC free article: PMC1727744] [PubMed: 10562584]
- 21.
- Aten J, Roos A, Claessen N. et al. Strong and selective glomerular localization of CD134 ligand and TNF receptor-1 in proliferative lupus nephritis. J Am Soc Nephrol. 2000;11:1426–38. [PubMed: 10906156]
- 22.
- Flynn S, Toellner KM, Raykundalia C. et al. CD4 T cell cytokine differentiation: The B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, blr-1. J Exp Med. 1998;188:297–304. [PMC free article: PMC2212448] [PubMed: 9670042]
- 23.
- Kaleeba JA, Offner H, Vandenbark AA. et al. The OX-40 receptor provides a potent costimulatory signal capable of inducing encephalitogenicity in myelin-specific CD4+ T cells. Int Immunol. 1998;10:453–61. [PubMed: 9620601]
- 24.
- Ohshima Y, Yang LP, Uchiyama T. et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed: 9787171]
- 25.
- Akiba H, Oshima H, Takeda K. et al. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–66. [PubMed: 10358148]
- 26.
- Kopf M, Ruedl C, Schmitz N. et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. [PubMed: 10626892]
- 27.
- Chen AI, McAdam AJ, Buhlmann JE. et al. Ox40-ligand has a critical costimulatory role in dendritic cell: T cell interactions. Immunity. 1999;11:689–98. [PubMed: 10626891]
- 28.
- Pippig SD, Pena-Rossi C, Long J. et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (Ox40). J Immunol. 1999;163:6520–6529. [PubMed: 10586044]
- 29.
- Maxwell J, Weinberg AD, Prell RA. et al. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. [PubMed: 10605000]
- 30.
- Brocker T, Gulbranson-Judge A, Flynn S. et al. CD4 T cell traffic control: In vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–6. [PubMed: 10359115]
- 31.
- Walker LS, Gulbranson-Judge A, Flynn S. et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–22. [PMC free article: PMC2195670] [PubMed: 10523609]
- 32.
- Rogers PR, Song J, Gramaglia I. et al. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. [PubMed: 11567634]
- 33.
- Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18:558–65. [PMC free article: PMC121523] [PubMed: 9418902]
- 34.
- Kawamata S, Hori T, Imura A. et al. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF- kappaB activation. J Biol Chem. 1998;273:5808–14. [PubMed: 9488716]
- 35.
- Ye H, Park YC, Kreishman M. et al. The structural basis for the recognition of diverse receptor sequences by TRAF2. Mol Cell. 1999;4:321–30. [PubMed: 10518213]
- 36.
- Takaori-Kondo A, Hori T, Fukunaga K. et al. Both amino- and carboxyl-terminal domains of TRAF3 negatively regulate NF-kappaB activation induced by OX40 signaling. Biochem Biophys Res Commun. 2000;272:856–63. [PubMed: 10860842]
- 37.
- Matsumura Y, Hori T, Kawamata S. et al. Intracellular signaling of gp34, the OX40 ligand: Induction of c-jun and c-fos mRNA expression through gp34 upon binding of its receptor, OX40. J Immunol. 1999;163:3007–11. [PubMed: 10477563]
- 38.
- Kotani A, Hori T, Matsumura Y. et al. Signaling of gp34 (OX40 ligand) induces vascular endothelial cells to produce a CC chemokine RANTES/CCL5. Immunol Lett. 2002;84:1. [PubMed: 12161277]
- 39.
- Schwartz RH. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–81. [PubMed: 2525422]
- 40.
- Schwartz RH. T cell clonal anergy. Curr Opin Immunol. 1997;9:351–7. [PubMed: 9203408]
- 41.
- Bansal-Pakala P, Gebre-Hiwot JemberA, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–12. [PubMed: 11479622]
- 42.
- Murata K, Nose M, Ndhlovu LC. et al. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–36. [PubMed: 12370402]
- 43.
- Vetto JT, Lum S, Morris A. et al. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg. 1997;174:258–65. [PubMed: 9324133]
- 44.
- Durkop H, Latza U, Himmelreich P. et al. Expression of the human OX40 (hOX40) antigen in normal and neoplastic tissues. Br J Haematol. 1995;91:927–31. [PubMed: 8547142]
- 45.
- Imura A, Hori T, Imada K. et al. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: Implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–8. [PubMed: 9108415]
- 46.
- Jones D, Fletcher CD, Pulford K. et al. The T-cell activation markers CD30 and OX40/CD134 are expressed in nonoverlapping subsets of peripheral T-cell lymphoma. Blood. 1999;93:3487–93. [PubMed: 10233901]
- 47.
- Ramstad T, Lawnicki L, Vetto J. et al. Immunohistochemical analysis of primary breast tumors and tumor- draining lymph nodes by means of the T-cell costimulatory molecule OX- 40. Am J Surg. 2000;179:400–6. [PubMed: 10930490]
- 48.
- Petty JK, He K, Corless CL. et al. Survival in human colorectal cancer correlates with expression of the T- cell costimulatory molecule OX-40 (CD134). Am J Surg. 2002;183:512–8. [PubMed: 12034383]
- 49.
- Weinberg AD, Rivera MM, Prell R. et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–9. [PubMed: 10657670]
- 50.
- Kjaergaard J, Tanaka J, Kim JA. et al. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–21. [PubMed: 11034096]
- 51.
- Weinberg AD, Wallin JJ, Jones RE. et al. Target organ-specific up-regulation of the MRC OX-40 marker and selective production of Th1 lymphokine mRNA by encephalitogenic T helper cells isolated from the spinal cord of rats with experimental autoimmune encephalomyelitis. J Immunol. 1994;152:4712–21. [PubMed: 7512604]
- 52.
- Weinberg AD, Lemon M, Jones AJ. et al. OX-40 antibody enhances for autoantigen specific V beta 8.2+ T cells within the spinal cord of Lewis rats with autoimmune encephalomyelitis. J Neurosci Res. 1996;43:42–9. [PubMed: 8838572]
- 53.
- Tittle TV, Weinberg AD, Steinkeler CN. et al. Expression of the T-cell activation antigen, OX-40, identifies alloreactive T cells in acute graft-versus-host disease. Blood. 1997;89:4652–8. [PubMed: 9192792]
- 54.
- Stuber E, Von FreierA, Marinescu D. et al. Involvement of OX40-OX40L interactions in the intestinal manifestations of the murine acute graft-versus-host disease. Gastroenterology. 1998;115:1205–15. [PubMed: 9797376]
- 55.
- Tsukada N, Akiba H, Kobata T. et al. Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–9. [PubMed: 10733518]
- 56.
- Yoshioka T, Nakajima A, Akiba H. et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–23. [PubMed: 11069062]
- 57.
- Saijo S, Asano M, Horai R. et al. Suppression of autoimmune arthritis in interleukin-1-deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum. 2002;46:533–44. [PubMed: 11840457]
- 58.
- Onodera J, Nagata T, Fujihara K. et al. Expression of OX40 and OX40 ligand (gp34) in the normal and myasthenic thymus [In Process Citation] Acta Neurol Scand. 2000;102:236–43. [PubMed: 11071109]
- 59.
- Stuber E, Buschenfeld A, Luttges J. et al. The expression of OX40 in immunologically mediated diseases of the gastrointestinal tract (celiac disease, Crohn's disease, ulcerative colitis). Eur J Clin Invest. 2000;30:594–9. [PubMed: 10886299]
- 60.
- Tateyama M, Fujihara K, Ishii N. et al. Expression of OX40 in muscles of polymyositis and granulomatous myopathy. J Neurol Sci. 2002;194:29–34. [PubMed: 11809163]
- 61.
- Weinberg AD, Bourdette DN, Sullivan TJ. et al. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–9. [PubMed: 8574963]
- 62.
- Caccavelli L, Field AC, Betin V. et al. Normal IgG protects against acute graft-versus-host disease by targeting CD4(+)CD134(+) donor alloreactive T cells. Eur J Immunol. 2001;31:2781–90. [PubMed: 11536177]
- 63.
- Jember AG, Zuberi R, Liu FT. et al. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193:387–392. [PMC free article: PMC2195923] [PubMed: 11157058]
- 64.
- Arestides RS, He H, Westlake RM. et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–80. [PubMed: 12355440]
- 65.
- Akiba H, Miyahira Y, Atsuta M. et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–80. [PMC free article: PMC2195752] [PubMed: 10637281]
- 66.
- Weinberg AD, Wegmann KW, Funatake C. et al. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–26. [PubMed: 9973447]
- 67.
- Nohara C, Akiba H, Nakajima A. et al. Amelioration of experimental autoimmune encephalomyelitis with anti- OX40 ligand monoclonal antibody: A critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166:2108–15. [PubMed: 11160262]
- 68.
- Higgins LM, McDonald SA, Whittle N. et al. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: Amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162:486–93. [PubMed: 9886424]
- 69.
- Malmstrom V, Shipton D, Singh B. et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:6972–81. [PubMed: 11359859]
- Introduction to OX40 (CD134) and OX40-Ligand
- Expression Characteristics of OX40 and OX40L on T Cells and APC
- Function of OX40 on T Cells
- Signals Transduced by OX40
- Function and Signaling of OX40L on Accessory Cells
- >Regulation of T Cell Tolerance and Cancer Immunity by OX40
- Expression and Role of OX40 in T Cell-mediated Disease
- Summary
- Future Considerations
- References
- Regulation of T Cell Immunity by OX40 and OX40L - Madame Curie Bioscience Databa...Regulation of T Cell Immunity by OX40 and OX40L - Madame Curie Bioscience Database
- Overview Αβ Metabolism: From Alzheimer Research to Brain Aging Control - Madame ...Overview Αβ Metabolism: From Alzheimer Research to Brain Aging Control - Madame Curie Bioscience Database
- Metastatic Genitourinary Malignancies - Madame Curie Bioscience DatabaseMetastatic Genitourinary Malignancies - Madame Curie Bioscience Database
- Lipocalins in Arthropoda: Diversification and Functional Explorations - Madame C...Lipocalins in Arthropoda: Diversification and Functional Explorations - Madame Curie Bioscience Database
- Role of Superoxide in Post-Ischemic Liver Injury - Madame Curie Bioscience Datab...Role of Superoxide in Post-Ischemic Liver Injury - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...