NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The Bacillus subtilis bacteriophage ø29 research team in Minneapolis has marveled at (and reveled in) the intricacies of ø29 assembly for more than 30 years. Here we highlight the current state of knowledge of ø29 DNA packaging. We describe the in vitro packaging system and focus on recent advances that address the mechanism of the packaging motor. Among advances, the head-tail connector has been visualized in proheads and the packaging motor resolved in partially packaged particles by electron microscopy, the structure of the connector has been solved by X-ray crystallography, and the force-velocity relationship of the motor has been established in single molecule studies. A challenge in the future is to determine the structure and interaction of motor components as well as the conformational changes in these components during energy transduction that define the mechanism of DNA translocation.
The compaction of ø29 DNA by more than 100-fold in length during packaging into the prohead is remarkable in that it overcomes the entropic, electrostatic and bending energies of DNA. The ø29 packaging motor is a multisubunit protein-RNA complex at the prohead portal vertex. The motor, driven by ATP hydrolysis, is force-generating and highly processive, and it opposes a strong internal force that builds up within the capsid as DNA is compressed. The motor can work against loads of 57 picoNewtons on average, making it one of the strongest molecular motors yet reported. We aim to identify and characterize the intermediates during the assembly and function of the motor and to determine the structure of each component of the motor at atomic resolution.
A brief overview of the ø29 DNA packaging system follows, and accordingly citation of published work is highly selective. For more detail, refer to a recent comprehensive review of ø29 DNA packaging.1 Also, a comparison of all the bacteriophage DNA packaging systems under study has been addressed (Jardine and Anderson, in press).
Components of the ø29 DNA Packaging Motor
The DNA packaging motor consists of the prohead, which contains the dodecameric head-tail connector at its portal vertex, a multimer of prohead RNA (pRNA) that is attached to the connector, and a multimer of the ATPase gp16 (gene product 16) that binds to pRNA (Table 1). The active motor also includes the packaging substrate DNA-gp3.
Table 1
Components of the ø29 DNA Packaging System.
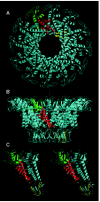
Proheads are produced in infection of the nonpermissive Bacillus subtilis host with the amber mutant sus16(300)-sus14(1241) that is defective for the packaging ATPase gp16 and that provides delayed lysis. The prohead is assembled from the head-tail connector (gp10), the scaffolding protein (gp7), the major capsid protein (gp8) and the head fibers (gp8.5) with the aid of an unidentified host protein.2 The prohead is prolate, an extended T=3 icosahedron with dimensions of 54 x 42 nm and a shell thickness of only 1.6 nm (ref. 3) (fig. 1). The shell contains 235 copies of the 49 kiloDalton (kDa) gp8, arranged as 11 pentamers at the vertices (the connector occupies the portal vertex) and 30 hexamers to expand the lattice. The 55 head fibers that project about 250Å from the quasi three-fold axes at the vertices are nonessential in both the prohead and virion. Occasionally most of the scaffolding protein is lost in cell lysis, yet the proheads retain full biological activity for in vitro DNA packaging (unpublished). Proheads can also be produced from products of the cloned genes 7, 8 and 10 in Escherichia coli, although the yield is about one-third that of infection in B. subtilis;4 these proheads must be reconstituted with pRNA prior to DNA packaging. Each prohead can be active in DNA packaging.
The head-tail connector, the crux of the DNA packaging motor, is a dodecamer of the 36 kDa gp10. The structure of the connector is solved to 3.2Å resolution5 and 2.1Å6,7 (fig. 2). It is 75Å in height, with three approximately cylindrical regions having external diameters of 138Å, 94Å and 66Å. The wide part is in the prohead, and the narrow part protrudes from the prohead and contains the RNA binding domain (fig.1A, B). The central part of the connector is comprised of three long alpha helices in each monomer that are arranged at an angle of about 40° relative to the central 12-fold axis. The outer surface of the connector is hydrophobic, which may facilitate connector rotation within the capsid.8 The inner surface of the connector channel is highly negatively charged, which may ease DNA passage. Investigation of the role of the lysine residues at positions 200 and 209 that may interact with DNA6 as well as the residues 229-246 in the wide region of the connector that are disordered in the crystal structure5 may be fruitful. Each monomer has positively and negatively charged sides that may stabilize the structure. The X-ray structure of the connector fits well into the cryo-EM 3D reconstruction of the prohead (fig. 3).
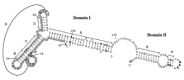
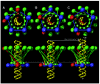
Prohead RNA is a 174-base transcript of the far left end of the genome and is essential for DNA packaging9 (fig. 4). A 120-base form of pRNA (domain I of fig.4), generated by the action of adventitious ribonucleases during prohead isolation, is fully active in DNA packaging and phage assembly.10 The pRNA can be released from the prohead and reattached, with concomitant loss and restoration of biological activity, facilitating mutational analysis of RNA structure and function.11-16 The number of copies of pRNA needed for DNA packaging is six by genetic methods,17,18 and hexamers and dimers are the predominant forms of pRNA in solution by analytical ultracentrifugation.17 The hexamer is formed by a novel intermolecular base pairing between identical monomers (helix G of fig.4), and dimers are intermediates in formation of the hexamer.17-19 However, cryo-EM 3D reconstruction demonstrates five-fold symmetry of pRNA attached to the prohead,5,20 or six-fold symmetry using different methods,21 thus the symmetry of pRNA in the packaging motor is controversial. The electron density due to pRNA is visualized on the connector by cryo-EM as a ring around its protruding end, with a skirt of five dense spokes5 (fig. 1C). If the five-fold symmetric pRNA ring contacts the icosahedral capsid as proposed, then the capsid-pRNA-gp16 complex may act as a stator around the dodecameric connector (see model of the mechanism below).
The ATPase gp16 is produced by sucrose induction of B. subtilis (pSac16) that contains the cloned gene 16 (unpublished). The 39 kDa protein is the larger of the two ø29 DNA packaging proteins and contains ATP binding consensus sequences and the predicted secondary structure of ATPases.22 The ATPase activity of gp16 is pRNA-dependent.23 gp16 has an RNA recognition motif and binds to pRNA on the prohead to complete assembly of the packaging motor. gp16 is needed in multiple copies for DNA packaging in vitro,24 and at least six copies of purified gp16 per prohead are added to achieve full packaging activity in the defined system. However, the number of copies of gp16 in the motor is unknown. gp16 is purportedly visualized by cryo-EM attached to the pRNA spokes on the partially packaged particle5 (fig. 1D). pRNA and gp16 exit the filled head after packaging and are not components of the virion.9,25
The ø29 genome is 19.3 kilobasepairs (kbp) and has a single subunit of gp3 covalently attached at each 5' terminus (DNA-gp3). The 31 kDa gp3 is essential for both DNA replication and DNA packaging. gp3 primes DNA replication, which proceeds by a strand-displacement mechanism, producing unit-length genomes for packaging (for a review see ref. 26). gp3 is the smaller of the two packaging proteins commonly found in phage systems. It also confers structure on the DNA, as the gp3 ends of DNA-gp3 interact to yield circles, and gp3 loops back on the DNA to form lariats (see packaging events below).27 DNA-gp3 is produced in restrictive infection with the mutant sus4(369)-sus8(22).
The DNA Packaging Assay and Provisional Packaging Events
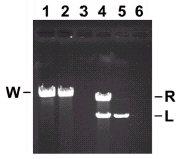
The packaging assay is based on protection of DNA in filled heads from DNase treatment.28 Proheads, DNA-gp3 and gp16 are generally mixed in a ratio of 2:1:12 to provide an estimated two-fold excess of proheads and gp16, and the ternary complex forms during a 5 minute incubation at room temperature. ATP is added to initiate packaging, and incubation is continued for 10 minutes. DNase I is added to digest unpackaged DNA, and the protected packaged DNA is extracted and quantified by agarose gel electrophoresis (fig. 5). Packaging efficiency approaches 100% (lanes 1 and 2). Packaging is oriented, as left-end restriction fragments are packaged preferentially29 (lanes 4 and 5).
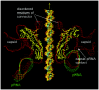
Provisional events of ø29 DNA packaging are illustrated in Figure 6. We begin with a description of the packaging substrate DNA-gp3, which has a maturation pathway for packaging that is independent of prohead assembly.27 Supercoiling of the packaging substrate occurs by gp3-dependent (but sequence independent) formation of a lariat, binding of multiple copies of gp16 to the lariat loop junction, and supercoiling of the lariat loop by concerted gp3-gp16 action (fig. 6A). The prohead then binds to the lariat loop of the supercoiled DNA-gp3-gp16 (not to the tail of the lariat), as observed by electron microscopy (fig. 6B; unpublished). The supercoiled DNA-gp3-gp16 is shown wrapped around the connector, a projection of our finding that the prohead connector wraps supercoiled plasmid DNA, reducing the DNA contour length (unpublished). Thus the connector of the prohead behaves as free connectors, which wrap supercoiled plasmid DNA around the outside to reduce DNA contour length and restrain negative supercoils.30 Linear plasmid DNA is not wrapped by free connectors or connectors in proheads. Efficient prohead connector-DNA binding may be due to prohead recognition of the helical pitch of the supercoiled DNA or affinity for DNA crossovers. Supercoiled DNA-gp3 is preferentially packaged over linear DNA-gp3.27 Although both the left and right termini of DNA-gp3 are supercoiled in the presence of gp16, the prohead recognizes and packages the left end preferentially; gp3 may be posttranslationally modified, or the pRNA may hybridize transiently to its coding sequence near the left end.
Once DNA is wrapped around the connector, the prohead may slide on the DNA to the lariat loop junction, to position the DNA left end at the connector channel (fig. 6C). Proheads are observed at the lariat loop junction by electron microscopy (unpublished). Then gp16 is hypothesized to bind to pRNA to complete the assembly of the packaging motor, and concurrently the left end of the DNA is freed and positioned for packaging (fig. 6C and D; the pRNA and gp16 are not shown). It is not known if the copies of gp16 bound to DNA are sufficient for DNA packaging. However, it is clear that gp16 is needed in multiple copies,24 and at least six copies of purified gp16 per prohead are added to achieve full packaging in vitro (unpublished). Hydrolysis of ATP by gp16-pRNA energizes sequential connector conformational change that results in DNA translocation (fig. 6E), as described below in the model of the motor mechanism.
Several aspects of the ø29 packaging system and model are currently unique to ø29: RNA is an essential component of the packaging motor, the packaging substrate is supercoiled by the packaging proteins, and the supercoiled DNA is wrapped around free connectors and connectors in the prohead. We hope to determine if other phage DNA packaging systems share these features.
A Model of the Packaging Mechanism
The most striking structural characteristic of the connector is the 36 long alpha helices in the central region, inclined roughly obliquely to the DNA helix, that give the connector the appearance of a compressible spring. The idea that the connector can expand and contract is the basis of a ratchet mechanism model5 (fig. 7). There are three concentric symmetries of the active motor: the 10-fold symmetry of DNA in the connector channel, the 12-fold symmetry of the connector, and the five-fold symmetry of the capsid-pRNA-gp16. To start, the active ATPase I contacts connector monomer A, which in turn interacts with the DNA (fig. 7A). The connector extends down the DNA by 2 bp, and concurrently the connector narrow end rotates counterclockwise by 12°; this aligns ATPase II with the connector monomer C and in turn monomer C with the DNA backbone (fig. 7B). Then the connector contracts by 6.8Å to advance 2 bp of DNA into the prohead, and the wide end of the connector concurrently and passively rotates counterclockwise by 12° to return to the position for activation of ATPase II in the next cycle (fig.7C). Thus the rotation of the connector does not actively drive DNA translation, as a rotating nut advances a bolt,31 but rather the rotation acts to index ordered firing of ATPases and thus coordinate sequential conformational changes in the connector to translocate DNA. Atomic force microscopy shows that the connector can be compressed reversibly by about one-third of its height,32 lending support to our model.
The ø29 packaging motor, in translocating DNA in one direction through the connector channel, is different in kind from the other molecular motors under study. We have proposed that the ø29 motor is a ratchet mechanism that depends on passive rotation of the connector for sequential ATP-energized protein conformational change.5 The F1-ATPase33 and the bacterial flagellar34 rotary motors use sequential rotary conformational change to turn a spindle and are normally driven by a proton gradient. Although the F1 motor uses ATP hydrolysis to rotate in one direction, the F0 motor attached to the common shaft uses an electrochemical gradient to turn in the opposite direction. The linear motors of the myosin group that drive muscle contraction and move cargo along actin filaments, and motors of the kinesin group that move along microtubules are track motors that use conformational fluctuations to effect translation in steps (for a review see ref. 35). In the putative ø29 ratchet mechanism, the spindle or bar (DNA) is translocated, and the connector serves as the pawl.
The ø29 Packaging Motor Is Processive and Powerful
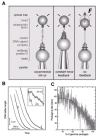
In single molecule studies, optical tweezers pull on ø29 DNA as it is packaged and demonstrate that the packaging motor is force-generating.36 Tethering a single packaging complex between two beads allows measurement of the packaging rates in real time and the forces during packaging. DNA packaging is initiated and then stalled after about 15% packaging by the addition of the ATP analogue, γS-ATP. The partially packaged complexes are attached to a streptavidin-coated polystyrene bead via the biotinylated unpackaged end of the DNA and captured in the optical trap. This bead is brought into contact with a second bead, coated with anti-head antibodies, that is held by a pipette to form a stable tether (fig. 8A, left). In the “constant force feedback” mode, the bead distance is adjusted to maintain a constant DNA tension of 5 picoNewtons (pN) (fig. 8A, middle). Only when ATP is restored do the beads move closer together, as DNA is packaged (fig. 8B). Although packaging is highly processive, in that packaging of 5 μm lengths of DNA are observed, pauses and slips occur. On average there are about three pauses per μm of DNA packaged, and pausing frequency increases with higher capsid fillings. During a pause the motor remains engaged with the DNA, despite the 5 pN of applied external force (fig. 8B, inset). During slips, an abrupt increase in tether length occurs, suggesting that the motor loses grip on the DNA, but then the packaging motor recovers and resumes packaging.
Figure 8C shows a plot of the packaging rate of a ø29-lambda hybrid DNA versus the percent of the genome packaged. Interestingly, the packaging rate is not constant. The packaging rate drops dramatically when about 50% of the DNA is packaged, from an initial rate of about 100 bp/second to zero when about 105% of the ø29 genome length is packaged. This rate decrease that is specific to the last half of packaging likely results from the build-up of internal force due to DNA confinement that opposes and slows the motor.
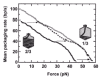
To investigate the build-up of internal force, the “no feedback” mode, where the positions of the trap and pipette are fixed (fig. 8A, right), is used with a DNA substrate about 0.8x the length of ø29 DNA to obtain Force-Velocity (F-V) traces. As the DNA is reeled into the prohead during packaging, the bead is displaced in the laser trap and tension in the DNA molecule increases. Eventually the force reaches a high enough level to stall the motor. Shown are mean F-V curves at two points, when one-third and when two-thirds of the genome is packaged (fig. 9). At one-third filling no internal force opposes the motor, as there is no velocity decrease, while at two-thirds filling there is an internal force since it takes about 14 pN less external force to stall the motor. Shifting of the two-thirds curve over 14 pN (in the direction of the arrows) shows that there is good overlap with the one-third curve. Thus the internal and external forces add together to act on the motor, and the inherent F-V relationship of the motor in the absence of any internal forces is obtained. The packaging rate decreases even for small forces, and it is estimated that a rate-limiting step produces a mechanical displacement of only 1Å, much less than the estimated movement of 2 bp per ATP hydrolyzed in bulk experiments.22 When the packaging velocity decreases sharply at about 45 pN, a second step in the cycle of the motor associated with a larger movement becomes rate-limiting.36
The motor is one of the strongest molecular motors yet reported, working against loads of up to about 70 pN. The average stall force of 57 pN times the reported 0.68 nm (2 bp) moved per ATP gives a work done of 39 pN•nm, representing an energy conversion efficiency of about 30% of the estimated 120 pN•nm associated with ATP hydrolysis. The step size of the motor is less than 5 bp if the maximum force is 70 pN (120 pN•nm/70 pN = 1.7 nm).
By combining rate dependence on external force (fig.9) and on the fraction of DNA packaged (fig. 8C), the build-up of internal force is calculated to reach about 50 pN when the whole genome is packaged. Dividing this force by the hexagonal cell surface area37 gives an estimate of a pressure of about 6 megaPascals inside the phage head, assuming there is no significant dissipation of energy. This force builds only after about one-half of the DNA is packaged and may be due in part to rearrangements of DNA as it is progressively compressed. Total work done in packaging is estimated at 2 X 104 kT by integrating the curve of internal force (pN) versus percentage of genome packaged (not shown). If dissipation is not great, internal pressure may supply a part of the driving force for DNA injection into the host cell.
Ongoing Analysis of ø29 DNA Packaging: The Path to Enlightenment
The paradigm of analysis of any cell biological process at the molecular level, attributed to Tom Pollard and published in a review of the meeting Proteins as Machines in reference 38, is reproduced in Figure 10. The approach is to identify all components, describe all reaction intermediates, determine the rates of all transitions, and determine component structures at atomic resolution. Although arduous, all parts of the approach are vital to a complete understanding of the biological process. Multisubunit protein or protein-nucleic acid complexes dominate cell biochemical processes, for example DNA replication, targeting and transport of proteins, pre-mRNA splicing, or signal transduction. In machines of diverse cellular processes, the energy of nucleotide hydrolysis is coupled to conformational changes in protein subunits. The ø29 DNA packaging motor is such a multisubunit complex, with its connector, pRNA and gp16 components attached to the prohead and DNA-gp3 in its channel. We hope to determine the mechanism of the ø29 packaging motor by use of the approach outlined in Figure 10.
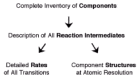
Figure 10
The path to enlightenment in dissection of complex biological processes. Reprinted with permssion from: Alberts B, Miake-Lye R. Cell 1992; 68:415-420. © 1999-2001 Elsevier Science.
The inventory of components of the ø29 packaging motor is complete, but the order of interactions in motor assembly and the identity of packaging intermediates are not known with certainty. Considering motor assembly, it is likely that gp16 of the supercoiled DNA-gp3-gp16 complex binds to pRNA on the prohead to complete the assembly of the motor. The number of copies of gp16 and its structure on DNA-gp3, as well as the mechanism and extent of supercoiling of the DNA substrate, are to be determined. The structure of the packaging initiation complex, i.e., the prohead with DNA-gp3 wrapped around the connector in the absence of ATP, is to be determined by high resolution cryo-EM 3D reconstruction. The steps by which the supercoiled DNA is resolved to a linear form for packaging are unknown. Above all, the precise location and structure of pRNA and gp16 on the partially packaged particle (packaging intermediate) are to be determined by high resolution cryo-EM 3D reconstruction. As a complement to electron microscopy, the approach of obtaining atomic resolution structures of ø29 packaging motor components and substructures by X-ray crystallography is ongoing, and when possible these high resolution structures will be fitted into cryo-EM 3D reconstructions (fig. 3).
Dynamic motor function is being investigated with a battery of methods to explore macromolecular interactions, reaction intermediates, and protein movement. Connector rotation is being investigated by polarization of single fluorophores. Packaging intermediates in ATP binding and hydrolysis are being isolated in an attempt to characterize the steps in chemo-mechanical energy transduction and to correlate these steps with protein conformational change. Electron paramagnetic resonance (EPR) and fluorescence resonant energy transfer (FRET) are being employed to detect and characterize conformational change in the connector, pRNA and gp16. Single molecule packaging in the laser tweezers is expected to reveal properties of the packaging motor that are inaccessible using packaging in bulk, such as precise measurement of the rate (and variations in rate) of packaging, the forces exerted on the DNA, effects of perturbation of local DNA structure, details of the catalytic cycle, and the step size of the motor.
Acknowledgements
We thank Charlene Peterson for preparation of the manuscript and Paul Jardine for comments. The work was supported by NIH grants to DA.
References
- 1.
- Grimes S, Jardine PJ, Anderson D. Bacteriophage ø29 DNA packaging. Adv Virus Res. 2002;58:255–294. [PubMed: 12205781]
- 2.
- Rajagopal BS, Reilly BE, Anderson DL. Bacillus subtilis mutants defective in bacteriophage ø29 head assembly. J Bacteriol. 1993;175:2357–2362. [PMC free article: PMC204524] [PubMed: 8096839]
- 3.
- Tao Y, Olson NH, Xu W. et al. Assembly of a tailed bacterial virus and its genome release studied in three dimensions. Cell. 1998;95:431–437. [PMC free article: PMC4167676] [PubMed: 9814712]
- 4.
- Guo P, Erickson S, Xu W. et al. Regulation of the phage ø29 prohead shape and size by the portal vertex. Virology. 1991;183:366–373. [PMC free article: PMC4167689] [PubMed: 1905079]
- 5.
- Simpson AA, Tao Y, Leiman PG. et al. Structure of the bacteriophage ø29 DNA packaging motor. Nature. 2000;408:745–750. [PMC free article: PMC4151180] [PubMed: 11130079]
- 6.
- Guasch A, Pous J, Ibarra B. et al. Detailed architecture of a DNA translocating machine: The high-resolution structure of the bacteriophage ø29 connector particle. J Mol Biol. 2002;315:663–676. [PubMed: 11812138]
- 7.
- Guasch A, Pous J, Ibarra B. et al. Detailed architecture of a DNA translocating machine: The high-resolution structure of the bacteriophage ø29 connector particle. (Note to ref. 6). J Mol Biol. 2002;321:379–380. [PubMed: 11812138]
- 8.
- Simpson AA, Leiman PG, Tao Y. et al. Structure determination of the head-tail connector of bacteriophage ø29. Acta Cryst. 2001;D57:1260–1269. [PubMed: 11526317]
- 9.
- Guo P, Erickson S, Anderson DL. A small viral RNA is required for in vitro packaging of bacteriophage ø29 DNA. Science. 1987;236:690–694. [PubMed: 3107124]
- 10.
- Wichitwechkarn J, Bailey S, Bodley JW. et al. Prohead RNA of bacteriophage ø29: Size, stoichiometry, and biological activity. Nucl Acids Res. 1989;17:3459–3468. [PMC free article: PMC317788] [PubMed: 2498842]
- 11.
- Guo P, Bailey S, Bodley JW. et al. Characterization of the small RNA of the bacteriophage ø29 DNA packaging machine. Nucl Acids Res. 1987;15:7081–7090. [PMC free article: PMC306194] [PubMed: 3116499]
- 12.
- Wichitwechkarn J, Johnson D, Anderson D. Mutant prohead RNAs in the in vitro packaging of bacteriophage ø29 DNA-gp3. J Mol Biol. 1992;223:991–998. [PubMed: 1538407]
- 13.
- Reid RJD, Bodley JW, Anderson D. Identification of bacteriophage ø29 prohead RNA domains necessary for in vitro DNA-gp3 packaging. J Biol Chem. 1994;269:9084–9089. [PubMed: 8132646]
- 14.
- Reid RJD, Zhang F, Benson S. et al. Probing the structure of bacteriophage ø29 prohead RNA with specific mutations. J Biol Chem. 1994;269:18656–18661. [PubMed: 8034614]
- 15.
- Zhang C, Lee CS, Guo P. The proximate 5' and 3' ends of the 120-base viral RNA (pRNA) are crucial for the packaging of bacteriophage ø29. Virol. 1994;201:77–85. [PubMed: 8178491]
- 16.
- Zhang C, Tellinghuisen T, Guo P. Use of circular permutation to assess six bulges and four loops of DNA-packaging pRNA of bacteriophage ø29. RNA. 1997;3:315–323. [PMC free article: PMC1369483] [PubMed: 9056768]
- 17.
- Zhang F, Lemieux S, Wu X. et al. Function of hexameric RNA in packaging of bacteriophage ø29 DNA in vitro. Mol Cell. 1998;2:141–147. [PubMed: 9702201]
- 18.
- Guo P, Zhang C, Chen C. et al. Inter-RNA interaction of phage ø29 pRNA to form a hexameric complex for viral DNA transportation. Mol Cell. 1998;2:149–155. [PubMed: 9702202]
- 19.
- Chen C, Sheng S, Shao Z. et al. A dimer as a building block in assembling RNA. J Biol Chem. 2000;275:17510–17516. [PubMed: 10748150]
- 20.
- Morais MC, Tao Y, Olson NH. et al. Cryo-EM image reconstruction of symmetry mismatches in bacteriophage ø29. J Struct Biol. 2001;135:38–46. [PMC free article: PMC5595366] [PubMed: 11562164]
- 21.
- Ibarra B, Caston JR, Llorca O. et al. Topology of the components of the DNA packaging machinery in the phage ø29 prohead. J Mol Biol. 2000;298:807–815. [PubMed: 10801350]
- 22.
- Guo P, Peterson C, Anderson D. Prohead and DNA-gp3-dependent ATPase activity of the DNA packaging protein gp16 of bacteriophage ø29. J Mol Biol. 1987;197:229–236. [PubMed: 2960820]
- 23.
- Grimes S, Anderson D. RNA dependence of the bacteriophage ø29 DNA packaging ATPase. J Mol Biol. 1990;215:559–566. [PubMed: 1700132]
- 24.
- Bjornsti MA, Reilly BE, Anderson DL. Bacteriophage ø29 proteins required for in vitro DNA-gp3 packaging. J Virol. 1984;50:766–772. [PMC free article: PMC255735] [PubMed: 6427474]
- 25.
- Chen C, Guo P. Sequential action of six virus-encoded DNA-packaging RNAs during phage ø29 genomic DNA translocation. J Virol. 1997;71:3864–3871. [PMC free article: PMC191537] [PubMed: 9094662]
- 26.
- Meijer WJJ, Horcajadas JA, Salas M. ø29 Family of Phages. Microbiol and Mol Biol Rev. 2001;65:261–287. [PMC free article: PMC99027] [PubMed: 11381102]
- 27.
- Grimes S, Anderson D. The bacteriophage ø29 packaging proteins supercoil the DNA ends. J Mol Biol. 1997;266:901–914. [PubMed: 9086269]
- 28.
- Grimes S, Anderson D. In vitro packaging of bacteriophage ø29 DNA restriction fragments and the role of the terminal protein gp3. J Mol Biol. 1989;209:91–100. [PubMed: 2530357]
- 29.
- Bjornsti MA, Reilly BE, Anderson DL. Morphogenesis of bacteriophage ø29 of Bacillus subtilis: Oriented and quantized in vitro packaging of DNA-gp3. J Virol. 1983;45:383–396. [PMC free article: PMC256420] [PubMed: 6185695]
- 30.
- Turnquist S, Simon M, Egelman E. et al. Supercoiled DNA wraps around the bacteriophage ø29 head-tail connector. Proc Natl Acad Sci USA. 1992;89:10479–10483. [PMC free article: PMC50362] [PubMed: 1438237]
- 31.
- Hendrix RW. Symmetry mismatch and DNA packaging in large bacteriophages. Proc Natl Acad Sci USA. 1978;75:4779–4783. [PMC free article: PMC336203] [PubMed: 283391]
- 32.
- Muller DJ, Engel A, Carrascosa JL. et al. The bacteriophage ø29 head-tail connector imaged at high resolution with the atomic force microscope in buffer solution. EMBO J. 1997;16:2547–2553. [PMC free article: PMC1169866] [PubMed: 9184202]
- 33.
- Kinosita JrK, Yasuda R, Noji H. et al. F1-ATPase: A rotary motor made of a single molecule. Cell. 1998;93:21–24. [PubMed: 9546388]
- 34.
- Silverman MR, Simon MI. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974;249:73–74. [PubMed: 4598030]
- 35.
- Vale RD, Milligan RA. The way things move: Looking under the hood of molecular motor proteins. Science. 2000;288:88–95. [PubMed: 10753125]
- 36.
- Smith DE, Tans SJ, Smith SJ. et al. The bacteriophage ø29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. [PubMed: 11607035]
- 37.
- Earnshaw WC, Casjens SR. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980;21:319–331. [PubMed: 6447542]
- 38.
- Alberts B, Miaki-Lye R. Unscrambling the puzzle of biological machines: The importance of the details. Cell. 1992;68:415–420. [PubMed: 1531448]
- The ø29 DNA Packaging Motor: Seeking the Mechanism - Madame Curie Bioscience Dat...The ø29 DNA Packaging Motor: Seeking the Mechanism - Madame Curie Bioscience Database
- Molecular Phylogeny and Evolution of the Coronin Gene Family - Madame Curie Bios...Molecular Phylogeny and Evolution of the Coronin Gene Family - Madame Curie Bioscience Database
- Cell-Cell Fusion: Transient Channels Leading to Plasma Membrane Merger - Madame ...Cell-Cell Fusion: Transient Channels Leading to Plasma Membrane Merger - Madame Curie Bioscience Database
- Replacement of Specific Neuronal Populations in the Spinal Cord - Madame Curie B...Replacement of Specific Neuronal Populations in the Spinal Cord - Madame Curie Bioscience Database
- The End in Sight: Poly(A), Translation and mRNA Stability in Eukaryotes - Madame...The End in Sight: Poly(A), Translation and mRNA Stability in Eukaryotes - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...