NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Memories are believed to rely upon enduring morphologic and functional changes at synapses activated by learning events. Experiments carried out in the past two decades have indicated that several cellular mechanisms need to be activated in order for the synaptic changes to take place. Among these general cellular mechanisms, enzymatic cascades including the cAMP-dependent protein kinase (protein kinase A, PKA) signaling pathway in CA1 region of the hippocampus have been demonstrated to be crucial to memory processing.
The importance of the PKA pathway to memory formation is indicated by its unique profile of activation following learning experiences: PKA has two peaks of activity during long-term memory consolidation period, the first within the first few minutes after training, and the second in a protracted way, beginning 2-3 h after the experience, after most enzymatic cascades have ceased their contribution.
The coincident increase of nuclear phosphorylated form of the cAMP-responsive element binding protein (CREB) transcription factor at these specific periods, together with memory sensitiveness to inhibitors of gene transcription and protein synthesis during PKA active periods suggest this signaling pathway may contribute actively to the synthesis of new proteins, a crucial event for long-term memory (LTM) establishment. Simultaneously to its involvement in LTM formation, the PKA pathway in the hippocampus is critical in the first hour after training for the establishment of short-term memory (STM) and has contributed to the demonstration of STM and LTM independence.
Recently it has also been shown that PKA contributes crucially to memory retrieval and extinction, probably involving distinct mechanisms of activation among the variety of events that have been shown to influence PKA activity.
Introduction
Memories are considered since Ramón y Cajal60 to rely upon enduring morphologic and functional changes at synapses activated by learning processes (see also Geinisman et al, this book). Experiments carried out in the past two decades have indicated that several enzymatic cascades need to be activated in order for the synaptic changes to take place. It is clear that the enzymatic signaling pathways do not carry the mnemonic information themselves, but act instead as amplifying systems without which the protein synthesis dependent synaptic changes inherent to memory would not be correctly or sufficiently activated.
Some of these enzymatic cascades act directly at synapses in order to enhance transmitter release or receptor function in the CA1 region of the hippocampus: the Ca2+/calmodulin-dependent kinases (Medina and Cammarota, this volume), protein kinase C (see Noguès, this volume, and ref. 66) and perhaps tyrosine kinases (Gerlai, this volume; and also ref. 58). These mechanisms may be viewed as modulators of the input to the hippocampus; i.e., the connection between fibers afferent to CA1 and the postsynaptic membrane of CA1 pyramidal cells. Other enzymatic cascades boost mechanisms triggered by second messengers in order to activate general cellular cascades and, particularly, gene transcription and the resulting protein synthesis. These mechanisms are also activated in CA1 pyramidal cells, but in view of their nature may affect mainly the interactions of these cells with their output connections. Among these general cellular mechanisms, the cAMP-dependent protein kinase (protein kinase A, PKA) signaling pathway in CA1 stands out for several reasons.
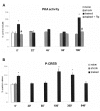
First and foremost, it is the only signaling pathway in the hippocampus that is activated twice after training experiences that produce memories: the first time briefly, within the first few minutes after training, and the second time in a protracted way, beginning 2-3 h after the experience (fig. 1A).6,71 The second peak of PKA activity occurs long after other major cascades involved in memory have ceased their function (see chapters by Noguès and Cammarota and Medina in this book, and also refs. 6,7 and 81).
Second, the two peaks of increased PKA activity are coincident in time both with an increase of nuclear phosphorylated form of the cAMP-responsive element binding protein (CREB) transcription factor (fig. 1B) (see refs. 6, 69 and Frankland and Josselyn, this book) and with periods in which memory formation is peculiarly sensitive to inhibitors of gene transcription42 and protein synthesis.47,48,59 PKA-mediated phosphorylation of CREB1, a constitutive transcription factor that is essential in CA1 for the maintenance both of LTM6,30 and LTP,33 is indeed a marker of LTM processing;75 its absence is a marker of amnesia.69 A large variety of plastic events in other nervous tissues has also been shown to depend on PKA and CREB phosphorylation.4,13 Thus, differently from the other kinases that are activated during or shortly after acquisition and remain active during a limited period following the learning experience, PKA seems more likely to be responsible for the mediation of the late and long-lasting cellular modifications thought to underlie long-term memory (LTM) storage.
Third, the PKA pathway in the hippocampus is critical in the first hour after training for the establishment of short-term memory (STM).71,73 As will be seen in the next section, STM is a separate form of memory that lasts about 3-6 h and runs parallel to the consolidation phase of long-term memory (LTM).36,41
Finally, the PKA signaling pathway interacts strongly and at several points with the PKC and MAPK pathways,53 both of which are critical for memory processing in the hippocampus (see chapters by Noguès et al and Selcher et al in this book). The first peak of PKA activity may correlate with the need for MAPK or PKC activity in the hippocampus for the establishment of STM,44,71 and the second peak of PKA may correlate with the requirement for MAPK activity 3 h after training in order for long-term memory to become consolidated.77
Short- and Long-Term Memory
LTMs are not immediately established in their definitive form.50,51 This process takes hours and requires a sequence of molecular events that are believed to result in structural and functional long-lasting modifications at synapses of brain areas involved in memory storage.29,39,46 As was postulated years ago,50 a parallel STM system is in charge during the hours that it takes for LTM to become effectively consolidated.35-37,40,41,71-73
The findings that led to the discovery that STM pertains to separate system parallel to the first hours of LTM consolidation involved the demonstration that a variety of treatments can block STM while leaving LTM intact for the same task in a given individual. This was first shown for short- and long-term facilitation in Aplysia (see ref. 21) and then for one-trial inhibitory (passive) avoidance learning in rats.35-37,40,41
The method of using localized and timed infusions of drugs with specific molecular actions in order to extricate STM from LTM is the only procedure that can effectively separate the two memory types.36,37,40,41 The learning-associated metabolic changes that are seen in the CA1 region of the hippocampus and elsewhere in the first 3-6 hours that follow acquisition,12,39,58 could in principle underlie one or another form of memory. Only the specific inhibition of one or more of these changes at different times in the post-training period can determine whether they pertain to STM or to LTM formation.36,41,43
The hippocampal events that underlie the processing of STM and LTM begin with the activation of glutamate NMDA receptors in the CA1 region of the hippocampus. The changes include activation of the PKA and MAPK cascades and an increase of nuclear P-CREB1 and cfos protein levels.6,12,39 They are not seen in animals treated with posttraining intra-hippocampal infusions of the NMDA receptor blocker D-aminophosphonopentanoic acid (AP5), which also causes amnesia.12
One-Trial Avoidance
The task of choice for the study of variables that affect or are affected by memory formation has been for many years a form of contextual fear called one-trial inhibitory avoidance.39,74 This task is learned in a few seconds and therefore permits a clear-cut dichotomy between effects on acquisition and effects on the post-acquisition period in which STMs and LTMs are formed.27 In addition, this task offers a clear-cut definition between acquisition, consolidation, retrieval and extinction.74 This is not the case with multi-trial tasks, such as the diverse varieties of spatial learning; in these tasks, acquisition, consolidation and retrieval are distributed over several sessions and are impossible to extricate one from the other in each session. Further, many experiments carried out over the past decade have shown that one-trial avoidance depends mainly on the functional integrity of specific molecular mechanisms in the CA1 region of the hippocampus, and in several of its connections, namely, the entorhinal, posterior parietal and cingulate cortex,2 the basolateral amygdala and the medial septum.38,39
Many of the experiments to be commented here used one-trial step-down inhibitory (passive) avoidance. For this task, rats are placed on a platform (CS) and receive a footshock (US) when they step down from it onto a grid. Animals learn to remain longer on the platform than they do on the training session (CR). The name “avoidance” should not obscure the fact that this is not really an instrumental task: it is acquired through a single CS(context)-US (footshock) pairing, which implies an impossibility for the animal to actually use the CR (to stay in a safe platform or compartment) as an instrument to avoid the US. When the animals are tested, they are exposed to the CS alone, which is actually the method of choice for initiating extinction.74 In fact, one-trial avoidance is extinguished by repeated testing at 24 h intervals.74
The cAMP/PKA Signaling Pathway
The second-messenger cyclic 3'-5'adenosine monophosphate (cAMP) regulates several important pre- and post-synaptic events, mainly by the activation of the cAMP-dependent protein kinase (PKA) and subsequent targets. The intracellular levels of cAMP are directly regulated by cAMP-phosphodiesterase, a membrane bound enzyme that breaks down cAMP molecules, and by adenylyl cyclase (AC), the enzyme that produces cAMP from ATP molecules. Several types of adenylyl cyclase have been identified and their activation constitute an important regulatory step in the cascade.14,19 ACs are targets of dopaminergic D1, β-noradrenergic and serotonergic 1A receptor coupled G proteins that either can have stimulatory or inhibitory effect upon cAMP production. Also, AC can be directly sensitive to intracellular raises in calcium-calmodulin levels resulting from the activation of NMDA receptors, voltagedependent calcium channels, or intracellular Ca2+ release.12,55,78
PKA consists of a tetrameric holoenzyme composed by two regulatory subunits constitutively linked to two catalytic subunits. cAMP binds to the regulatory subunits, inducing a conformational change that results in the release of catalytic subunits. Once separated, catalytic subunits can phosphorylate Ser/Tre residues on its substrates either in the synapse and its periphery, or, when translocated to the soma, in the nucleus.24 Drugs acting specifically upon catalytic and regulatory PKA subunits have been extensively used to characterize the physiological role of PKA.1,6,71,73
Different PKA isoforms of both regulatory and catalytic subunits have been identified, resulting either from expression of different genes or from alternative mRNA splicing of the same precursor.24 This diversity permits several combinations between them. Several holoenzyme subtypes have been characterized, with specific catalytic dynamics, substrate affinity and cellular location. PKAs also vary in relation to the subcellular compartment in which they are more prevalent, and this seems to be related to specific anchoring proteins that bind PKA in resting conditions.20,22
Among PKA neural substrates, the constitutively expressed regulatory transcription factor CREB is a prominent candidate to mediate PKA mechanisms in LTM storage and has been proposed to act as molecular switch from short- to long-lasting synaptic modifications.6,13,23,33,69 When catalytic subunits of PKA translocate to the nucleus they phosphorylate CREB on133 Ser, activating the protein and directly linking cAMP transduction pathways to gene expression and protein synthesis.13,67,79 There are several molecular forms of CREB; the one most widely believed to participate in memory consolidation is CREB1 (see ref. 67 and Frankland and Josselyn, this book).
There is no direct evidence whatsoever as to what proteins are specifically synthetized through the activation of the cAMP/PKA/P-CREB pathway, with the possible exception of c-fos.12 However, very solid and abundant evidence suggests that: 1) many of these are new proteins; 2) some are cell adhesion molecules (Regan, this volume), and 3) the action of these mediates changes in synaptic ultrastructure (Geinisman et al, this volume) and synaptic organization. Thus, the cAMP/PKA/CREB signaling pathway is crucial for the regulation of the synaptic events that are at the core of memory formation. It does NOT carry the information, but enables it to be carried.
PKA Involvement in Long-Term Memory Formation
The first inklings of involvement of the cAMP/PKA/P-CREB pathway in the maintenance of CA1 LTP49 and in LTM storage appeared several years ago.17 Subsequently, the participation of this pathway in memory formation was characterized in different plastic processes in several species, from facilitation in Aplysia17 to odor conditioning in Drosophila,70,79 spatial learning in the mouse,10,11,30 and aversive learning in the chick65,81 and the rat.6,69,71,72 The various experiments were performed using both genetically modified animals that were unable to express CREB or PKA correctly,10,11,79 and PKA inhibitors,1,6,71,73 cAMP analogues,1 or anti-sense CREB30 infused at different times after the original training The studies on transgenic or knockout animals were useful to establish the need of PKA or CREB for memory formation, and the pharmacologic experiments revealed both this and the precise timing of the intervention of cAMP, PKA or CREB in the process.39
As mentioned above, the cAMP/PKA/P-CREB pathway is activated twice after inhibitory avoidance training: briefly within the first few minutes after acquisition, and again 2-6 h later (fig.1A).6,12,69,71-73 The two posttraining peaks of PKA activity in the CA1 region of the rat hippocampus are accompanied by increased levels of P-CREB (fig. 1B)6,69 and are necessary for LTM formation.71 Also, they are coincident in time with the two phases in which memory of the one-trial task is sensitive to the infusion into CA1 of inhibitors of transcription42 or of protein synthesis.59 Inhibition of AC or PKA at the time of either peak blocks LTM formation. 1,6,8
The second peak of post-training PKA activity depends on the first: if this is abolished by Rp-cAMPs given into CA1, the second peak of PKA is not seen.71 Further, the second peak of post-training PKA activity depends on the prior activation of glutamate NMDA receptors at the time of training: if these are blocked by AP5 given post-training, the increase of intranuclear PKA activity that takes place 2 h later is not seen.12
The reliance of memory formation on a double wave of metabolic activity in the hippocampus was first described by Matthies and his collaborators in the ‘80s,28,47,48 and confirmed by many others using various forms of aversive conditioning in the rat6,10,39and the chick.65,81
A peak of increased PKA activity is seen in the entorhinal cortex but not in the parietal cortex 3 h after training in the one-trial task.58 The hippocampus is interconnected through the entorhinal cortex to several other regions of the cortex.34
The first posttraining peak of PKA activity occurs without any detectable concomitant change in cellular cAMP levels.6 It must, then, result from a quick activation of the enzyme somehow triggered by glutamate or by noradrenergic receptor activation (see below). The early PKA peak could also result from cross-talk with the concomitant activation of other enzymatic systems, such as CaMKII (Cammarota and Medina, this volume), PKC or Src.53,80 In the hippocampus, cAMP levels increase slowly 60 min after inhibitory avoidance training, and attain a peak at 180-360 min. The maximum rise in cAMP levels is supposed to trigger PKA activation: it correlates with the second PKA peak of activity.6 The cAMP increase cannot be attributed to changes in cAMP-specific phosphodiesterase, and might be consequence of enhanced adenylylcyclase activity.6
Various neurotransmitter systems associated with alertness, anxiety, emotion or mood affect PKA activity indirectly, through actions on G-protein coupled receptors that regulate AC. Dopaminergic D1 and β-noradrenergic receptors enhance, and serotonin 1A (5HT1A) receptors inhibit adenylyl cyclase activity (see Buhot et al, de Bruin, and also Gibbs and Summers in this book), and thereby alter cellular cAMP levels. Forskolin stimulates adenylyl cyclase; 8-BrcAMP mimicks the effects of cAMP, including that upon the regulatory subunits of PKA. It was found that the infusion of the D1 agonist, SKF38393, of norepinephrine, of the 5HT1A receptor antagonist, NAN-190, of forskolin or of 8-Br-cAMP enhances LTM when given posttraining into CA1, the entorhinal cortex or the posterior parietal cortex; in contrast, infusions of the PKA inhibitor, KT5720, of the D1 antagonist. SCH23390, of the β-blocker timolol, or of the 5HT1A agonist, 8-HO-DPAT hinders LTM formation.1,6 The effect of these substances on memory is probably related to the well-known fluctuations of memory processes that occur in relation to mood, anxiety levels or emotion. The effect of these substances on memory varies with the time after training at which they are given, and with the brain structure into which they are infused. The time-windows of the effectiveness of each drug may or may not correlate with the occurrence of PKA activity peaks. Thus, at the immediate posttraining period, norepinephrine enhances and KT5720 inhibits memory consolidation when given into CA1; but only 3 or 6 h later all the drugs become effective as mentioned when given into this structure.1,8 In contrast, SKF38393, SCH23390, norepinephrine, timolol, 8-HO-DPAT, NAN-190, KT5720, forskolin and 8-Br-cAMP were effective when given into the entorhinal cortex 0, 3 or 6 h after training, or into the parietal cortex 3 or 6 h after training.1 Obviously, these studies point to the need of AC/PKA activity at precise moments of the post-training period, regardless of whether this activity is at a peak or not.
It is interesting to note here that despite the similarity in nature and time-course between the second peak of PKA activation in memory formation6 and in the involvement of PKA in CA1 LTP,33 there are some significant differences. First, the existence of an early peak of PKA activity in LTP has not been clearly demonstrated and whether this may be at all necessary for the occurrence of the second peak, as is the case in memory formation.71 Second, the second, late peak of PKA activity and P-CREB levels that follows training and is necessary for LTM is modulated by dopaminergic D1, β-noradrenergic and 5HT1A receptors in CA11,6 and by the muscarinic cholinergic input coming from the medial septum.69 The late CREB-dependent phase of LTP in CA1 is apparently only modulated by D1 receptors in CA1;31 it is instead modulated by β-noradrenergic receptors only in CA3.32 The differences may of course be due to the fact that the LTP work was carried out in tissue slices, in which modulatory input is absent.
As mentioned, both PKA peaks correlate with an increase in nuclear CREB1 phosphorylation at133 Ser in CA1, and with a sensitivity of memory to inhibitors of transcription or of protein synthesis. Antisense CREB infused into CA1 blocks the persistence of spatial LTM beyond 4h (Frankland and Josselyn, this book and also ref. 30). Inhibitors of transcription42 or of protein synthesis10,59 given at the time of training or 3 h later but not in the period in between also block LTM. There is evidence that the key proteins syntetized 3-5 or more h after training for the construction of long-lasting memories involve glycoproteins related to cell adhesion (Regan, this volume) promoting morphological changes at the synapses involved in each particular learning experience (see Geinisman et al, this book).
The intracellular mediators of glutamatergic, monoaminergic or cholinergic transmission to protein synthesis stimulation remain unclear. The hypothesis that there must be a relation between the receptors and PKA involvement is substantiated by the following findings: a) intact NMDA receptors are needed for the second peak of PKA activity;12 b) D1, β, 5HT1A and cholinergic receptors in the hippocampus modulate PKA and produce the changes in memory formation that would be predicted from their biochemical effects; c) PKA is the only kinase described to follow the double wave activation pattern that coincides with protein synthesis requirement during LTM consolidation, and this depends on the early participation of glutamate AMPA, NMDA and metabotropic receptors (mGluRs);39 d) PKA activates CREB at the times in which changes in gene activation and protein synthesis are essential for memory formation: around the time of training and again 2-6 h later.59,74
The role of mGluRs in the activation of PKA should be investigated. Such a role is to be predicted from their physiological action.62 Intact mGluRs in rat CA1 are necessary for memory formation in the first few minutes posttraining,8a as they have shown to be for the establishment of LTP.9,64 The participation of class I metabotropic receptor and specifically mGluR5 in memory formation has been recently ascertained.15,63 Moreover, their different contribution to short- and long-term memories suggest a distinct contribution to short- and long-term memory (see Riedel et al, this book).
The metabolic intracellular scenarios of each period of PKA activation, ranging from neurotransmitter actions and their consequences on second messengers to the cross-talk between PKA and other signaling pathways53 are different, and this will have to be taken into account when a fully descriptive formal hypothesis on the cellular processes necessary for memory formation is established. The formulation of such a hypothesis is still several experiments away from current knowledge.
PKA Involvement in Short-Term Memory Formation
PKA is separately involved in STM and in LTM. In fact, this separation contributed to demonstrate the dichotomy between STM and LTM.71,73
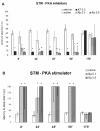
When infused into CA1 immediately after training, canceling the first peak of hippocampal PKA activity,71 competitive inhibitors of the catalytic (KT5720) and the regulatory (Rp-cAMP) subunits of PKA cause amnesia for STM and LTM. (fig. 2A).41,71,73 In contrast, the stimulant of the regulatory subunit, Sp-cAMP, enhanced retention of both memory types (fig. 2B).71 Therefore in the immediate posttraining period, PKA is obviously necessary for the formation of both STM and LTM.
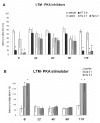
However, the infusion into CA1 of KT5720 or Rp-cAMPs from 22 to 90 min after training blocks STM completely but has no effect on LTM (fig.3A); Sp-cAMPs given 22-90 min posttraining selectively enhances STM.71,73 When given 170-180 min post-training, these drugs affect LTM again (fig.3A), while having no influence on the retrieval of STM (see refs. 41,71, 73). Stimulators of PKA subserve the same time profile than inhibitors influencing LTM (fig. 3B). Therefore, PKA is necessary for STM formation during the first hour or so after training, and it is necessary for LTM formation only at the time of its two peaks.71 The PKA substrates(s) involved in its role in STM are not known; clearly, they do not include P-CREB, which remains at basal levels in the period between 5 and 180 min post-training.8,69 In principle, the PKA substrates needed for its influence on STM may include receptors, enzymes or cytoskeletal synaptic components whose function can be temporary modulated by phosphorylation.23
STM is also modulated by dopamine D1, β-noradrenergic and 5HT1A receptors in CA1 and in the entorhinal cortex. As mentioned, these receptors regulate PKA indirectly through influences on AC. However, this modulation is quite different in both brain structures, and it is also different from that observed for LTM.37 Thus, overall, there is a very strong monoaminergic modulation of memory processes by pathways and receptors involved in the perception of and reaction to changes in alertness, mood, emotion or anxiety levels, but the final outcome of this modulation in terms of cognitive events is difficult to predict. Monoaminergic receptors in the CA1 region and in the entorhinal cortex acting simultaneously may have similar, different or even opposite effects on STM and LTM depending on the degree to which each of these receptors is activated.41,42 In animals, including humans, this will probably depend on the particular mood, degree of alertness or emotional state of the subjects, and these, as is known, vary subtly from minute to minute in daily life.
KA Involvement in Memory Retrieval
For reasons that escape us, most research on the molecular mechanisms in memory has centered on memory formation.38,39,46 Until 2 years ago there were very few experiments on the mechanisms of memory retrieval. This is so in spite of the obvious fact that memories can only be measured indirectly, through retrieval.
Again, most research on this topic centered on the one-trial step-down avoidance task. Retrieval of this task measured 3 h after training (STM retrieval) is blocked by the infusion into CA1 of the glutamate AMPA receptor antagonists, CNQX or DNQX, or by the generic mGluR receptor blocker, MCPG; it is unaffected by NMDA antagonists or by inhibitors of PKA or MAPK.44 Therefore, STM retrieval appears to depend only on the integrity of the regular glutamatergic transmission through AMPA and metabotropic receptors in CA1. The relation between glutamate receptor activation, particularly mGluRs, and PKA in retrieval deserves to be studied as well, as has been pointed out above in connection with consolidation. Actually, more is known about the role of mGluRs in retrieval than about that in consolidation and its down-stream effectors. In the hippocampus, together with AMPA glutamatergic receptors, mGluRs are crucial for retrieval and its involvement is suggested to be responsible for PKA and MAPK signaling pathways activation.68
In contrast, retrieval of the one-trial avoidance task measured 24 h or 31 days after training depends on mGluRs and on the PKA and MAPK cascade: it is blocked by the infusion into CA1 of MCPG (or, at the 24 h interval, CNQX or DNQX), or of the inhibitor of PKA, RpcAMPs, or of the MAPK inhibitor PD098059.2,43,68 Retrieval measured 1 or 31 days after training is enhanced by the pre-test infusion into CA1 of the PKA activator, Sp-cAMPs, which underscores a key role of PKA in retention test performance.2 AP5 given into CA1 has no effect on retrieval, and DNQX given into this structure at the time of testing 31 days after training also has no effect.2,43
Very importantly, all these substances have similar effects to those observed in CA1, when given into the entorhinal, posterior parietal or anterior cingulate cortex prior to the test session, with two exceptions: AP5 also blocked retrieval when given into the parietal or cingulate cortex, and DNQX was ineffective when given into the cingulate cortex.2 This shows that the retrieval of a task as deceivingly simple as one-trial avoidance requires similar and simultaneous metabolic activity in many regions of the brain.42
The basolateral amygdala, which plays a major role in the consolidation of various forms of fear conditioning, including one-trial avoidance38 also participates in retrieval. Among the molecular systems studied (see above), only DNQX was able to block retrieval when infused into the basolateral amygdala prior to testing.2 This does not detract from the role of that structure on retrieval. It merely shows that this role is metabolically simpler than that of the cortex. The basolateral amygdala is a site of action of glucocorticoids in the modulation of retrieval18 and there is evidence for a role of it in the modulation of the emotional content of memories both at the time of consolidation and at the time of retrieval.38
The involvement of PKA in retrieval occurs without any detectable change of the activity of the enzyme in CA1. This stands in contrast to the MAPK pathway enzymes, p42 and p44, which are increased following the test session (ref. 68 and see also Selcher et al in this book).
The need for regular ongoing PKA activity in CA1, entorhinal, parietal and cingulate cortex in order for retrieval to take place is underlined by the fact that the infusion of D1 or β receptor agonists or of a 5HT1A antagonist in all these structures prior to testing enhances retrieval, whereas that of D1 or β receptor antagonists or of a 5HT1A antagonist depresses retrieval of the one-trial task.3 Again, this modulation by the pathways and receptors involved in emotion, mood, alertness or anxiety occurs simultaneously in all the brain structures mentioned.
PKA Involvement in Extinction
Retrieval of the one-trial avoidance task, or of most fear conditioning procedures for that matter, is usually carried out without the unconditioned stimulus, i.e., the footshock(s).42,74 This is precisely the necessary condition for extinction to take place.57 Recent evidence indicates that extinction of fear-motivated tasks requires the integrity of the hippocampus.16 Extinction involves a new learning of opposite sign to the original learning: animals learn a CS-no shock contingency instead of the previously acquired CS-US contingency.
We have observed that extinction of the one-trial avoidance task really begins in the first test session. If further test sessions are repeated at 24 h intervals, retrieval becomes gradually diminished. 74 A variety of treatments given into CA1 either before or after the first test sessions hinders extinction. Among these, the most relevant are the transcription blocker, DRB,42 the protein synthesis inhibitor, anisomycin,74 the NMDA receptor antagonist, AP5, the CaMKII inhibitor, KN-62, the MAPK inhibitor, PD098059, and the PKA inhibitor, Rp-cAMPs.42,74
These pharmacological findings indicate that extinction is indeed a new learning, requiring transcription and protein synthesis (see also ref. 5) as much as the original learning does, as well as a key role of NMDA receptors, CaMKII, PKA and MAPK. The main difference between memory formation of aversive learning and its extinction is that in the former all these molecular processes act in a sequential way, whereas in extinction they appear to act simultaneously at the time of the first retrieval test.42
Summary
PKA plays a pivotal role in the consolidation, retrieval and extinction of memories. The cAMP/PKA/P-CREB pathway is involved twice in the consolidation of LTM: first at the time of training, and then again 2-6 h later. PKA is involved in STM formation during at least the first hour after training. The role of PKA in memory formation is not restricted to the hippocampus: PKA is also necessary in the entorhinal and posterior parietal cortex, where it can be up- or down-regulated by receptors involved in anxiety or mood. It is believed that the protein synthesis that mediates the effective laying down of memory traces through changes at the synaptic level is specifically activated by the PKA signaling pathway.
Further, PKA activity is necessary for retrieval in CA1, entorhinal cortex, posterior parietal and anterior cingulate cortex. Extinction is normally initiated by the first retrieval test after a training experience. Extinction also requires intact on-going PKA activity in the CA1 region.
Cross-talk between the PKA pathway and others may occur and it may be crucial for memory processes.53 It remains to be studied and analysed in detail.
References
- 1.
- Ardenghi P, Barros D, Izquierdo LA. et al. Late and prolonged post-training memory modulation in entorhinal and parietal cortex by drugs acting on the cAMP/protein kinase A signalling pathway. Behav Pharmacol. 1997;8:745–751. [PubMed: 9832961]
- 2.
- Barros DM, Izquierdo LA, Mello e Souza T. et al. Molecular signaling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav Brain Res. 2000;114:183–192. [PubMed: 10996059]
- 3.
- Barros DM, Mello e Souza T, De David T. et al. Simultaneous modulation of retrieval by dopaminergic D1, b-noradrenergic, serotoninergic 1A and cholinergic muscarinic receptors in cortical structures of the rat. Behav Brain Res. 2001;124:1–7. [PubMed: 11423160]
- 4.
- Bartsch D, Ghirardi M, Skehel PA. et al. Aplysia CREB2 represses long-term facilitation: Relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. [PubMed: 8521521]
- 5.
- Berman DE, Dudai Y. Memory extinction, learning anew and learning the new: Dissociations in the molecular machinery of learning in the cortex. Science. 2001;291:2417–2419. [PubMed: 11264539]
- 6.
- Bernabeu R, Bevilaqua L, Ardenghi P. et al. Involvement of hippocampal D1/D5 receptor-cAMP signaling pathways in a late memory consolidation phase of an aversively-motivated task in rats. Proc Natl Acad Sci USA. 1997;94:7041–7046. [PMC free article: PMC21281] [PubMed: 9192688]
- 7.
- Bernabeu R, Schmitz P, Faillace MP. et al. Hippocampal cGMP and cAMP are differentially involved in memory processing of an inhibitory avoidance learning. Neuro Report. 1996;7:585–588. [PubMed: 8730835]
- 8.
- Bevilaqua L, Ardenghi P, Schröder N. et al. Drugs acting upon the protein kinase A/CREB pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav Pharmacol. 1997;8:331–338. [PubMed: 9832992]
- 9.
- Bortolotto ZA, Fitzjohn SM, Collingridge GL. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol. 1999;9:299–304. [PubMed: 10395580]
- 10.
- Bourchuladze R, Abel T, Berman N. et al. Differential training procedures recruit either one or two critical periods for contextual memory consolidation. Learn Mem. 1998;5:465–474. [PMC free article: PMC311273] [PubMed: 10454361]
- 11.
- Bourtchouladze R, Freguelli B, Blendy J. et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element binding protein. Cell. 1994;79:59–68. [PubMed: 7923378]
- 12.
- Cammarota M, Bevilaqua LR, Ardenghi P. et al. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: Abolition by NMDA receptor blockade. Mol Brain Res. 2000;76:36–46. [PubMed: 10719213]
- 13.
- Carew TJ. Molecular enhancement of memory formation. Neuron. 1996;16:5–8. [PubMed: 8562090]
- 14.
- Choi EJ, Xia Z, Villacres EC. et al. The regulatory diversity of the mammalian adenylyl cyclases. Curr Opin Cell Biol. 1993;5:269–73. [PubMed: 8507499]
- 15.
- Christoffersen GR, Christensen LH, Harrington NR. et al. Task-specific enhancement of shortterm, but not long-term, memory by class I metabotropic glutamate receptor antagonist 1- aminoindan-1,5-dicarboxylic acid in rats. Behav Brain Res. 1999;101:215–226. [PubMed: 10372576]
- 16.
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. [PMC free article: PMC6762930] [PubMed: 11222661]
- 17.
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–21. [PubMed: 2141668]
- 18.
- de QuervainDJK, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of longterm memory. Nature. 1998;394:787–790. [PubMed: 9723618]
- 19.
- Edelhoff S, Villacres EC, Storm D. et al. Mapping adenylyl cyclase genes type I, II, III, IV, V and VI in mouse. Mamm Genome. 1995;6:111–113. [PubMed: 7766992]
- 20.
- Edwards AS, Scott JD. A-kinase anchoring proteins: Protein kinase A and beyond. Curr Opin Cell Biol. 2000;12:217–221. [PubMed: 10712918]
- 21.
- Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. [PubMed: 8211146]
- 22.
- Felciello A, Gottesman MX, Avedimento EV. The biological functions of A-kinase anchor proteins. J Mol Biol. 2001;308:99–114. [PubMed: 11327755]
- 23.
- Ferrer I, Blanco R, Rivera R. et al. CREB-1 and CREB-2 immunoreactivity in the rat brain. Brain Res. 1996;712:159–164. [PubMed: 8705300]
- 24.
- Francis SH, Corbin JD. Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol. 1994;56:237–272. [PubMed: 8010741]
- 25.
- Geinisman Y. Synaptogenesis and structural remodellingIn: Riedel G, Platt B, eds.Memories are made of theseAustin: RG Landes Co.,2002. in press .
- 26.
- Gerlai RT. EphrinsIn: Riedel G, Platt B, eds.Memories are made of theseAustin: RG Landes Co.,2002. in press .
- 27.
- Gold PE. The use of avoidance training in studies of modulation of memory storage. Behav Neural Biol. 1986;46:87–98. [PubMed: 3015121]
- 28.
- Grecksch G, Matthies H. Two sensitiv periods for the amnestic effect of anisomycin. Pharmacol Biochem Behav. 1979;12:663–665. [PubMed: 7393961]
- 29.
- Greenough WT. Morphological and molecular studies of synaptic memory mechanisms: Links to the fragile X mental retardation syndromeIn: Gold PE, Greenough WT, eds.Memory Consolidation: Essays in honor of James L McGaugh — A time to rememberWashington: American Psychological Association2001 .
- 30.
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA. 1997;94:2693–2698. [PMC free article: PMC20151] [PubMed: 9122258]
- 31.
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92:2446–2450. [PMC free article: PMC42234] [PubMed: 7708662]
- 32.
- Huang Y-Y, Kandel ER. Modulation of both the early and the late phase of mossy fiber LTP by the activation of beta-adrenergic receptors. Neuron. 1996;16:611–617. [PubMed: 8785058]
- 33.
- Huang Y-Y, Li X-C, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and a macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. [PubMed: 7923379]
- 34.
- Hyman BT, Van HoesenGW, Damasio AR. Memory-related neural systems in Alzheimer's disease: An anatomic study. Neurology. 1990;40:1721–1730. [PubMed: 2234428]
- 35.
- Izquierdo I, Ardenghi PG, Barros DM. et al. Consolidation of short- and long-term memoryIn: Gold PE, Greenough WT, eds.Memory ConsolidationWashington American Psychological Association,200179–112.
- 36.
- Izquierdo I, Barros DM, Mello e Souza T. et al. Mechanisms for memory types differ. Nature. 1998;293:635–636. [PubMed: 9641675]
- 37.
- Izquierdo I, Izquierdo LA, Barros DM. et al. Differential involvement of cortical receptor mechanisms in working, short- and long-term memory. Behav Pharmacol. 1998;9:421–427. [PubMed: 9832927]
- 38.
- Izquierdo I, McGaugh JL. Behavioural pharmacology and its contribution to the molecular basis of memory consolidation. Behav Pharmacol. 2000;11:517–534. [PubMed: 11198125]
- 39.
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. [PubMed: 9398590]
- 40.
- Izquierdo I, Medina JH, Izquierdo LA. et al. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem. 1998;69:219–224. [PubMed: 9707486]
- 41.
- Izquierdo I, Medina JH, Vianna MRM. et al. Separate mechanisms for short- and long-term memory. Behav Brain Res. 1999;103:1–11. [PubMed: 10475159]
- 42.
- Izquierdo I, Vianna MRM, Izquierdo LA. et al. Memory retrieval and its lasting consequencesIn: Palomo T, Archer T, Beninger RJ, Borrell J, Murray R eds.Neurodevelopmental liabilities in brain disease statesMadrid: Fundacion Cerebro y Mente.2002 .
- 43.
- Izquierdo LA, Barros DM, Ardenghi PG. et al. Different hippocampal molecular requirements for short- and long-term retrieval of one-trial avoidance learning. Behav Brain Res. 2000;111:93–98. [PubMed: 10840135]
- 44.
- Izquierdo LA, Barros DM, Medina JH. et al. Novelty enhances retrieval of one-trial avoidance learning in rats 1 or 31 days after training unless the hippocampus is inactivated by different receptor antagonists and enzyme inhibitors. Behav Brain Res. 2000;117:215–220. [PubMed: 11099775]
- 45.
- Izquierdo LA, Vianna M, Barros DM. et al. Short- and long-term memory are differentially affected by metabolic inhibitors given into hippocampus and entorhinal cortex. Neurobiol Learn Mem. 2000;73:141–149. [PubMed: 10704324]
- 46.
- Kandel ER, Squire LR. Neuroscience: Breaking down scientific barriers to the study of brain and mind. Science. 2000;290:1113–20. [PubMed: 11185010]
- 47.
- Matthies H. Neurobiological aspects of learning and memory. Ann Rev Psychol. 1989;40:381–409. [PubMed: 2648980]
- 48.
- Matthies H. In search of the cellular mechanism of learning. Prog Neurobiol. 1989;32:277–349. [PubMed: 2566189]
- 49.
- Matthies H, Reymann KG. Protein kinase A inhibitors prevent the maintenance of hippocam pal long-term potentiation. Neuro Report. 1993;4:712–714. [PubMed: 8347813]
- 50.
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1359. [PubMed: 5917768]
- 51.
- McGaugh JL. Memory: A century of consolidation. Science. 2000;287:248–251. [PubMed: 10634773]
- 52.
- Medina JH, Cammarota M. Ca+2/calmodulin-dependent kinase IIIn: Riedel G, Platt B, eds.Memories are made of theseAustin: RG Landes Co.,2002. in press .
- 53.
- Micheau J, Riedel G. Protein kinases: Which one is the memory molecule? Cell Molec Life Sci. 1999;55:534–548. [PubMed: 10357224]
- 54.
- Mileusnic R. Protein synthesis: II new proteinsIn: Riedel G, Platt B, eds.Memories are made of theseAustin: RG Landes Co.,2002. in press .
- 55.
- Mons N, Guillou JL, Jaffard R. The role of Ca+2/calmodulin-stimulable adenylyl ciclase as molecular coincidence detectors in memory formation. Cell Mol Life Sci. 1999;55:525–533. [PubMed: 10357223]
- 56.
- Nógues X, Pascale A, Micheau J. et al. Protein kinase CIn: Riedel G, Platt B, eds.Memories are made of theseAustin: RG Landes Co.,2002. in press .
- 57.
- Pavlov IP. Conditioned Reflexes. New York: Dover. 1956 .
- 58.
- Pereira P, Ardenghi P, de Souza MM. et al. Effects of infusions of the tyrosine kinase inhibitor radicicol into the hippocampus on short- and long-term memory of the inhibitory avoidance task. Behav Pharmacol. 2001;12:299–302. [PubMed: 11548116]
- 59.
- Quevedo J, Vianna MRM, Roesler R. et al. Two time-windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: Protection from amnesia by pretraining but not pre-exposure to the apparatus. Learn Mem. 1999;6:600–607. [PMC free article: PMC311311] [PubMed: 10641764]
- 60.
- Ramón y Cajal S. Neue Darstellung vom histologischen Bau des Zentralnervösen System. Archiv Anat Physiol (Anat). 1893:319–428.
- 61.
- Regan C. Cell adhesion moleculesIn: Riedel G, Platt B, eds.Memories are made of theseAustin: RG Landes Co.,2002. in press .
- 62.
- Riedel G. Function of metabotropic glutamate receptors in learning and memory. Trends Neurosci. 1996;19:219–224. [PubMed: 8761955]
- 63.
- Riedel G, Casabona G, Platt B. et al. Fear conditioning-induced time- and subregion-specific increase in expression of mGlu5 receptor protein in rat hippocampus. Neuropharmacol. 2000;39:1943–1951. [PubMed: 10963738]
- 64.
- Riedel G, Reymann KG. Metabotropic glutamate receptors in hippocampal long-term potentiation and learning and memory. Acta Physiol Scand. 1996;15:1–19. [PubMed: 8735650]
- 65.
- Rose SPR. Time-dependent processes in memory formation revisitedIn: Gold PE, Greenough WT, eds.Memory ConsolidationWashington: American Psychological Association,2001113–128.
- 66.
- Routtenberg A. It's about timeIn: Gold PE, Greenough WT, eds.Memory ConsolidationWashington: American Psychological Association,2001113–128.
- 67.
- Silva AJ, Kogan JH, Frankland PW. et al. CREB and memory. Ann Rev Neurosci. 1998;21:127–148. [PubMed: 9530494]
- 68.
- Szapiro G, Izquierdo LA, Alonso M. et al. Participation of hippocampal metabotropic receptors, protein kinase A and mitogen-activated protein kinases in memory retrieval. Neurosci. 2000;99:1–5. [PubMed: 10924946]
- 69.
- Taubenfeld SM, Wiig KA, Bear MF. et al. A molecular correlate of memory and amnesia in the hippocampus. Nature Neurosci. 1999;2:309–310. [PubMed: 10204535]
- 70.
- Tully T. Discovery of genes involved with learning and memory: An experimental synthesis of Hirschian and Benzerian perspectives. Proc Natl Acad Sci USA. 1996;93:13460–13467. [PMC free article: PMC33631] [PubMed: 8942957]
- 71.
- Vianna MR, Izquierdo LA, Barros DM. et al. Differential role of hippocampal cAMP-dependent protein kinase in short- and long-term memory. Neurochem Res. 2000;25:621–626. [PubMed: 10905623]
- 72.
- Vianna MRM, Barros DM, Silva T. et al. Pharmacological demonstration of the differential involvement of protein kinase C isoforms in short- and long-term memory formation and retrieval in rats. Psychopharmacol. 2000;150:77–84. [PubMed: 10867979]
- 73.
- Vianna MRM, Izquierdo LA, Barros DM. et al. Intrahippocampal infusion of an inhibitor of protein kinase A separates short- from long-term memory. Behav Pharmacol. 1999;10:223–227. [PubMed: 10780835]
- 74.
- Vianna MRM, Szapiro G, McGaugh JL. et al. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci USA. 2001;98:12251–12254. [PMC free article: PMC59800] [PubMed: 11572949]
- 75.
- Viola H, Furman M, Izquierdo LA. et al. Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing: Effect of novelty. J Neurosci. 2000;20: 1–5. [PMC free article: PMC6773084] [PubMed: 11090612]
- 76.
- Walz R, Roesler R, Barros DM. et al. Effects of post-training infusions of a mitogen-activated protein kinase kinase inhibitor into the hippocampus or entorhinal cortex on short- and long-term retention of inhibitory avoidance. Behav Pharmacol. 1999;10:723–730. [PubMed: 10780287]
- 77.
- Walz R, Roesler R, Quevedo J. et al. Time-dependent impairment of inhibitory avoidance retention in rats by posttraining infusion of a mitogen-activated protein kinase kinase inhibitor into cortical and limbic structures. Neurobiol Learn Mem. 2000;73:11–20. [PubMed: 10686120]
- 78.
- Wong ST, Athos J, Figueroa XA. et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampal dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. [PubMed: 10482244]
- 79.
- Yin JCP, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. [PubMed: 8725970]
- 80.
- Zhao W, Cavallaro S, Gusev P. et al. Nonreceptor tyrosine protein kinase pp60c-src in spatial learning: Synapse-specific changes in its gene expression, tyrosine phosphorylation, and proteinprotein interactions. Proc Natl Acad Sci USA. 2000;97:8098–8103. [PMC free article: PMC16676] [PubMed: 10884433]
- 81.
- Zhao WQ, Polya GM, Wang BH. et al. Inhibitors of cAMP-dependent protein kinase impair longterm memory formation in day-old chicks. Neurobiol Learn Mem. 1995;64:106–18. [PubMed: 7582818]
- Protein Kinase A - Madame Curie Bioscience DatabaseProtein Kinase A - Madame Curie Bioscience Database
- Opioid Receptors on Peripheral Sensory Neurons - Madame Curie Bioscience Databas...Opioid Receptors on Peripheral Sensory Neurons - Madame Curie Bioscience Database
- Nucleotide Exchange Factors for Hsp70 Molecular Chaperones - Madame Curie Biosci...Nucleotide Exchange Factors for Hsp70 Molecular Chaperones - Madame Curie Bioscience Database
- Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of...Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms - Madame Curie Bioscience Database
- Structural Components and Architectures of RNA Exosomes - Madame Curie Bioscienc...Structural Components and Architectures of RNA Exosomes - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...