NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Local structural transitions from the common B-DNA conformation into other DNA forms can be functionally important. This chapter describes the structures of DNA forms called alternative DNA conformations that are different from the canonical B-DNA helix. Also discussed are the requirements for the formation of alternative DNA structures, as well as their possible biological roles. The formation of non-B-DNA within certain sequence elements of DNA can be induced by changes in environmental conditions, protein binding and superhelical tension. Several lines of evidence indicate that alternative DNA structures exist in prokaryotic and eukaryotic cells. The data on their involvement in replication, gene expression, recombination and mutagenesis continues to accumulate.
Introduction
Genetic information is generally stored in long double-stranded DNA molecules. Hydrogen bonding between nucleobases keeps the complementary DNA strands organized into a right-handed helical structure called B-DNA. Structural transitions into other DNA forms can occur within certain sequence elements of DNA and these can be functionally important. Several non-B-DNA structures (oftentimes called unusual or alternative DNA structures) can be important for interactions with proteins involved in replication, gene expression and recombination. They may also play different roles in the formation of nucleosomes and other supramolecular structures involving DNA. DNA sequences characterized as “random” or “mixed sequence” typically only form A-DNA or B-DNA. Special sequence characteristics or defined symmetry elements are required to form alternative structures such as left-handed Z-DNA, cruciforms, intramolecular triplexes, quadruplex DNA, slipped-strand DNA, parallel-stranded DNA, and unpaired DNA structures.1 Together with variations in DNA supercoiling, local alternative structures provide enormous potential for autoregulation of DNA functions. This chapter will briefly review major alternative DNA structures and their potential involvement in biology.
B-DNA and A-DNA
Structure
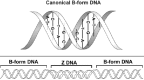
Table 1 lists structural parameters for three structural families of DNA helices. B-DNA is the term given for the canonical right-handed DNA helix that is the most common form of DNA. Canonical B-DNA is a double helix made of two antiparallel strands that are held together via hydrogen bonding in the A•T and G•C base pairs (fig. 1). One helical turn of B-DNA contains about 10.5 base pairs that are buried inside the helix and are almost perpendicular to the helical axis. DNA exists as a cylinder of 20 Å in diameter with two grooves, a major and a minor groove, spiraling around the cylinder. In B-DNA the distance between the bases (rise) is 3.4 Å. Studies of oligonucleotide duplexes in crystals showed significant sequence-dependent variability of the structural parameters listed in Table 1 that define the structure of the B-DNA helix. In bent DNA, for example, certain B-DNA parameters add up over a length of several base pairs to produce a permanently curved DNA helix. A- and Z-DNA are also double-helical but the spatial arrangement of base pairs differs significantly from that for B-DNA. Other DNA structures may have regions of unpaired strands or be composed of three and even four strands.
Table 1
Structural parameters of DNA helices.
A-DNA has 11 base pairs per helical turn, base pairs are tilted to about 20°, with respect to the helical axis, the grooves are not as deep as those in B-DNA, the sugar pucker is C3' endo compared to C2' endo for B-DNA, and the base pairs are shifted to the helix periphery which creates a 9 Å hole in the helix center. In A-DNA the average rise is 2.55 Å. A-DNA and B-DNA have different patterns of bound cations and water molecules2-4 that result in different stability conditions for these structures. B-DNA is stable under a broad variety of conditions, whereas A-DNA has been observed under conditions of reduced water content, such as in DNA fibers at 75% relative humidity or in solutions containing organic solvents or high salt concentrations. 5-7 The ease of conversion from B-DNA into the A-form is somewhat sequence-specific,3,8 being more difficult in sequences containing 5'-AA-3' steps, and easiest in sequences containing 5'-CC-3' and 5'-ACT-3' steps.8 Because of the variability in structural parameters for different di- and trinucleotides within the helix, B- and A-DNA actually consist of families of conformations. Experiments with oligonucleotide duplexes in crystals have shown that B- and A-DNA do not represent deep local energetic minima and that a number of intermediate structures may form upon a mild change in conditions.9-11
Biological Relevance of A-DNA
Biochemical, crystallographic and computer simulation analyses of the A-DNA structure and protein-DNA complexes indicate that an A-like DNA conformation may either form upon binding of certain proteins to DNA, or be an important intermediate step in forming the strongly distorted DNA conformation observed within at least some complexes with proteins.12-14 Several examples below illustrate these structural roles of A-DNA in biological processes.
TBP-Binding
Nanosecond scale molecular dynamics simulations in water using two different starting structures show that the DNA oligomer, GCGTATATAAAACGC, which contains a target site for the TATA-box binding protein (TBP), adopts an A-like conformation in the region of the TATA-box and undergoes bending related to that seen within the complex with the TBP.15 This is consistent with A-DNA being an important intermediate step in forming a strongly distorted DNA structure observed within its complex with TBP in crystals.9
CAP Binding
The Escherichia coli cyclic AMP receptor protein (CAP) has two symmetrically related inverted recognition elements separated by a spacer whose length may be either 6 or 8 bp (e.g., TGTGAxxxxxxTCACA).16 CAP binding induces DNA bending with DNA remaining in the B-form when the spacer is 6 bp. For the 8 bp spacer, an additional transition into the A-form is necessary to shorten the distance between TGTGA sites for CAP binding.17
Complexes with Polymerases
The B-to-A transition may occur in DNA complexes with enzymes that cut or seal at the (O3'-P) phosphodiester linkage. The transition is necessary to expose atoms of the sugar-phosphate backbone, such as the 3'-oxygen ordinarily buried within the chain backbone, for enzymatic attack.14 A polymerase-induced A-DNA conformation has been identified in crystallographic studies of HIV reverse transcriptase bound to DNA.18 The function of a conformational switch from the B-form to an underwound A-form DNA at the polymerase active site may provide discrimination between correct and incorrect base pairing19 because of a lower sequence-dependent structural variability in A-DNA compared with B-DNA. A-DNA in the vicinity of the DNA polymerase active site may improve the base pair fit in the nascent template-primer duplex and increase a reliability of proofreading thereby contributing to the fidelity of synthesis.13
Protection from DNA Damage
A-DNA stabilization by a group of proteins from sporulating bacteria Bacillus subtilis has been described.20 Nucleobases in A-DNA are an order of magnitude less susceptible to UV damage compared with B-DNA.21 Therefore, the conformational change on protein binding in the spores may be responsible for the well-known resistance of DNA in spores to UV damage.20
DNA Supercoiling
In most biological systems DNA is normally negatively supercoiled. Supercoiling is a property of topologically closed DNA molecules (those in which the free rotation of the DNA ends is restrained).1,22 Through changes in twisting and writhing, supercoiling makes the molecular shape and helix structure of DNA remarkably dynamic. The most important topological property of supercoiled DNA is its linking number, Lk, which is an integer number of times one strand crosses the other in a planar projection. Due to the continuity of DNA strands, the linking number can only change when at least one strand is cut by chemicals, ionizing radiation, or enzymes and then sealed. DNA topology is described by the equation
In vivo most DNA is negatively supercoiled. This is easily understood for circular molecules such as plasmids and bacterial chromosomes in which the free rotation of DNA strands is restrained. Circular bacterial chromosomes are long enough to be additionally subdivided into smaller topological domains. In fact, the 2.9 Mb E. coli chromosome is organized into about 45 independent domains in vivo.23 For linear DNA to exist in a supercoiled state, it must be organized into one or more topological domains. Eukaryotic chromosomes may form independent loops stabilized by the interaction of specific DNA regions with proteins attached to the nuclear matrix. In addition, RNA polymerase can define topological domains in eukaryotic cells.24
Linking number in vivo is regulated by enzymes called topoisomerases that transiently break and reseal the DNA double helix. Type I topoisomerases break only one strand of the DNA, allowing one strand to rotate around the other. Type II topoisomerases break and reseal both DNA strands. Correspondingly, the linking number changes in increments of 1 and 2 for type I and type II topoisomerases, respectively. In bacterial cells the level of supercoiling is carefully maintained by topoisomerase I, that relaxes supercoils, and topoisomerase II (gyrase), that introduces negative supercoils. In bacterial cells about half of the free energy from DNA supercoiling (called unrestrained supercoiling) is available for biological reactions, while the other half is presumably restrained by virtue of stable left-handed toroidal coiling around proteins. On average in bulk eukaryotic DNA, supercoils are restrained by the organization into nucleosomes. However, DNA in individual genes can contain unrestrained negative supercoiling.24-27 Transient changes in the level of supercoiling can be caused by proteins tracking through the DNA. In particular, the movement of an RNA polymerase during transcription generates waves of negative supercoiling behind and positive supercoiling in front of the enzyme.28
The state of DNA supercoiling may be important for the regulation of cell functions in a number of ways. (i) The energy from DNA supercoiling can be used to facilitate the opening of the promoter or origin of replication regions by RNA polymerase or replication proteins.1 (ii) DNA supercoiling may facilitate functional enhancer-promoter communication over a large distance, probably by bringing the enhancer and promoter in the plectonemically wound DNA into close proximity.29 (iii) The supercoil-induced formation of alternative structures in the regulatory regions may also influence protein binding. One particular example, albeit an artificially created system, is a down-regulation of transcription from an inverted repeat-containing promoter where the cruciform formation possibly prevents an assembly of transcription machinery.30 An example of transcriptional up-regulation is a likely supercoil-driven Z-DNA formation in the Rous sarcoma virus promoter that prevents nucleosome formation and facilitates access of transcription proteins to the gene regulatory regions.31
Supercoil-Induced DNA Structures and Their Biological Roles
While DNA mostly has a seemingly random distribution of nucleobases in the sequence, defined order sequences may rather frequently occur. These include inverted repeats that can form cruciforms, mirror repeats that may adopt intramolecular triplex DNA conformations, and direct repeats, that can form slipped mispaired structures, and (GC)n and (GT)n tracts that can form Z-DNA.
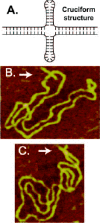
Cruciform Structure
An inverted repeat or a palindrome is a DNA sequence that reads the same from the 5' to 3' in either strand. For example, many type II restriction enzyme sites are palindromic. To form a cruciform the interstrand hydrogen bonds in the inverted repeat must be broken and intrastrand hydrogen bonds then established between complementary bases in each single strand, thus forming two hairpin-like arms with 3-4 unpaired bases at their tips (fig. 2A). As a whole, the cruciform consists of two rather long duplex DNA arms, and two comparatively short hairpin arms which form a four-way junction. The structure of the four-way junction is such that the nucleobases in and around the junction are fully involved in base pairing.32 Cruciforms can form in topologically closed molecules where they use energy from DNA supercoiling to melt the center of the inverted repeat, allowing the intrastrand hairpin nucleation.1,32 The thermodynamic stability of the cruciform comes from relaxation of one negative supercoil per 10.5 bp of DNA sequence that converts into the cruciform. The propensity for cruciform formation increases in longer inverted repeats that relax more supercoils than shorter ones. It also depends on temperature and the base composition of the inverted repeat, most importantly, in its center, in accordance with a requirement of partial DNA melting before the hairpin base pairing. Although schematically the cruciform is usually shown as having a cross shape as in the schematic representation in Figure 2, such an extended structure is favored only under the low-salt conditions, where electrostatic repulsion between phosphates pushes all four cruciform arms apart (fig. 2B). Under physiologically relevant salt conditions, where the phosphates are partially shielded and repulsion is reduced, the cruciform adopts an X-type structure with unequal inter-arm angles as seen in the AFM image in Figure 2C.33 The extended cruciform is rather stiff, as judged from little fluctuation of the inter-arm angles, whereas the X-type cruciform has a pronounced mobility of the hairpin arms observed by atomic force microscopy in liquid.33 The distribution of inverted repeats in eukaryotic DNA is nonrandom and they are clustered at or near genetic regulatory regions, which suggests that they are important biologically.34-36
Z-DNA Structure
Left-handed Z-DNA has been mostly found in alternating purine-pyrimidine sequences (CG)n and (TG)n.37 Z-DNA is thinner (18 Å) than B-DNA (20 Å), the bases are shifted to the periphery of the helix, and there is only one deep, narrow groove equivalent to the minor groove in B-DNA. In contrast to B-DNA where a repeating unit is 1 base pair, in Z-DNA the repeating unit is 2 bp. For Z-DNA in (CG)n sequences the twist angle for a CpG step is 9°, whereas it is 51° for the GpC step, totaling 60° in the 2 bp repeating unit. The helix repeat in Z-DNA is 12 bp/turn and an average rise is 3.7 Å/bp, compared with 10.5 bp/turn and 3.4 Å/bp in B-DNA. The backbone follows a zigzag path as opposed to a smooth path in B-DNA. The sugar and glycosidic bond conformations alternate: C2' endo in anti dC or dT and C3' endo in syn dG or dA. Electrostatic interactions play a crucial role in Z-DNA formation. Because of the zigzag backbone path, some phosphate groups are closer and electrostatic repulsion between them is greater than in B-DNA. Therefore, Z-DNA is stabilized by high salt concentrations or polyvalent cations that shield interphosphate repulsion better than monovalent cations. Other factors also contribute to Z-DNA stability. If an alternating purine-pyrimidine sequence occurs in a circular DNA molecule, DNA supercoiling is a major driving force for Z-DNA formation. Z-DNA formation unwinds DNA about two supercoils per 12 bp of DNA. The junctions between the B- and Z-DNA in supercoiled DNA span several base pairs in which nucleobases behave as if they were unpaired. In particular, they are partially reactive to single-strand specific chemicals. A computer analysis of over one million base pairs of human DNA, containing 137 complete genes identified 329 potential Z-DNA-forming sequences.38 Like inverted repeats, potential Z-DNA-forming sequences have a distinctly nonrandom distribution with a strong bias toward locations near the site of transcription initiation.
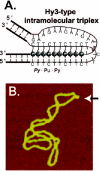
Triplex DNA Structure
When the hydrogen bonds in the A•T and G•C base pairs in canonical B-form DNA are formed, several hydrogen bond donor and acceptor groups in nucleobases remain unused. Each purine base has two such groups on the edges that are exposed in the major groove. These groups can be used to form base triads that are unit blocks of triple-stranded (triplex) DNA that consists of the B-form double helix and the third strand bound in the major groove.39-42 The third strand bases form the so-called Hoogsteen-type hydrogen bonds with purines in the B-form duplex. Energetically favorable triplexes have duplex pyrimidines (Py) and purines (Pu) segregated in complementary strands (Py•Pu duplex). For a snug fit in the duplex major groove, the third strands are made of either only pyrimidines (Py•Pu•Py triplex), or mostly purines with a fraction of pyrimidines (Py•Pu•Pu triplex). In the Py•Pu•Py triplex, the usual base triads are T•A•T and C•G•C+ (cytosine is protonated and this requires pH < 5). In the Py•Pu•Pu triplex the usual triads are T•A•A and C•G•G, and less frequently T•A•T. Triplex DNA may form intermolecularly, between a duplex target and a third oligonucleotide strand. It may also form intramolecularly in supercoiled DNA within a Py•Pu sequence of mirror repeat symmetry. For this, half of the mirror repeat Py•Pu sequence unpairs and one of the unpaired strands folds back and binds as a third strand to purines in the repeat's double-stranded half. The resulting local structure contains three notable features: a triple-stranded region; a fourth, unpaired strand; and a short (3-4 nt) stretch of unpaired bases in the fold-back strand (fig. 3A). The Py•Pu•Py triplex/single strand combination is termed H-DNA to reflect the necessity of cytosine protonation in the C•G•C+ triads.43 By analogy, the Py•Pu•Pu triplex/single strand combination is termed H'-DNA.44 Similar to the cruciform, H (H')-DNA may only form under torsional stress in a topologically closed DNA (fig. 3B).41,42 Among other factors that promote H (H')-DNA are longer lengths of Py•Pu mirror repeats and the presence of multivalent cations.42,45 The presence of single-stranded regions provides the DNA molecule with local increased flexibility akin to a hinge, which is incidentally another reason for calling the structure H-DNA. However, the angle between the outgoing duplex arms in the H-DNA structure fluctuates over a smaller range than in the X-type cruciform.46 Analysis of the genomic databases showed that in eukaryotes mirror repeated sequences occur more frequently than statistically expected.34,36 In the human genome, H-DNA-forming sequences may occur as frequently as 1 in 50,000 bp, whereas in the E. coli genome they are not abundant.34
Search for Unusual DNA Structures in Vivo
Numerous attempts have been undertaken to show the formation of supercoil-induced alternative DNA structures in living cells. The differential chemical susceptibility of double and single-stranded DNA regions in cruciforms and H-DNA as well as of structural junctions in Z-DNA has been exploited to probe for the formation of alternative structures in vivo. Using OsO4 reactivity with unpaired thymines, the cruciforms,47 Z-DNA48 and H-DNA49 were detected in supercoiled plasmids propagated in E. coli cells. The differences in photochemical reactivity of TA dinucleotides with psoralen (reactive in double-stranded DNA but not in single-stranded DNA or in the junctions between the B- and Z-DNA regions) were used to show the formation of cruciforms, Z-DNA, and H-DNA in E. coli.50,51 H'-DNA formation in E. coli was also detected by chloroacetaldehyde reactivity with unpaired adenines and cytosines.52 Elevated plasmid supercoiling in E. coli was interpreted as a combination of (i) supercoil relaxation by the formation of Z-DNA or cruciforms and (ii) a compensatory supercoiling increase by DNA gyrase.53,54 The analysis of sites differentially susceptible to DNA methylase in B- and Z-DNA showed that (GC)n sequences in plasmids or integrated in the E. coli chromosome form Z-DNA in vivo.55,56 Monoclonal antibodies were raised that recognize structural features of either cruciforms, Z-DNA, or triple-stranded DNA. These were then used to probe eukaryotic chromosomal DNA for the structures in question. Local structural transitions into cruciforms,57 Z-DNA58 and triplex DNA59 were detected by immunofluorescence. Thus, several lines of evidence indicate the presence of alternative DNA structures in prokaryotic and eukaryotic cells. The existence of proteins that specifically bind to alternative DNA structures also supports the notion of H-, Z- and cruciform DNA formation in vivo.
Cruciform, Z-DNA and H-DNA-Binding Proteins
An integral part of our understanding of the biological roles of alternative DNA structures comes from the identification of proteins that specifically interact with these structures. A number of proteins bind to different structural elements in cruciforms.35 They include HMG proteins, a replication initiation protein RepC, the cruciform binding protein CBP,35 and four-way junction resolvases.60,61 Among the Z-DNA-binding proteins are the highly specific binders, such as Zα domain-contatining proteins ADAR1 and ESL3,37,62 and relatively low specific proteins, such as HMG proteins, zeta crystalline and type III intermediate filament proteins.63 Proteins that bind to triple-stranded DNA have been identified in the HeLa cell extracts and keratinocyte cDNA expression library.64-66 In addition, several proteins that bind single-stranded Py or Pu sequences have been partially characterized.42,67-69
Possible Biological Roles of Supercoil-Driven Alternative Structures
Many similar biological roles for alternative DNA structures including cruciforms, Z-DNA, and H-DNA have been proposed.1 This is perhaps not surprising because these sequences often occur in the regulatory regions of genes that may use different structures for the same purposes. The dependence of all three structures on DNA supercoiling as well as the preference of structures to form in certain locations of topological domains also add to the apparent similarity in their functions.
Modulation of Supercoiling
The extent of supercoiling is known to affect transcription, recombination, and replication such that an optimum DNA topology may be required for these processes.1,70 The formation of cruciforms, Z-DNA and H-DNA may cause partial relaxation of excessive superhelicity in a topological domain. Specific cases of DNA replication and gene expression have been described that depend on superhelicity changes induced by the formation of cruciforms, Z-DNA and H-DNA.30,35,71
Nucleosome Exclusion
DNA wrapping around histones in nucleosomes interferes with the protein binding to promoters and origins of replication.72 Nucleosome formation, on the one hand, and the formation of cruciforms, Z-DNA and triplex DNA, on the other hand, are mutually exclusive. 31,73-75 Thus, the alternative structure-forming DNA sequences may expose nucleosome-free DNA, making them accessible to transcription, replication and recombination proteins.
Positioning of Sequence Elements, Molecular Switch
Supercoiled DNA at physiological ionic strength forms a plectonemic superhelix in which distant parts of the double helix are intertwined. The slithering motion of one duplex region on the other results in a wide distribution of distances between any two pre-selected remote sites. Similar to strongly bent DNA,76 the X-type cruciforms and H-DNA tend to occupy the apical positions in plectonemic DNA structures33,46 and therefore, may specifically position distant DNA sites. This was first realized for H-DNA whose fold-back structure seemed suitable for bringing remote sequence elements into close proximity. In agreement with this idea, increased recombination rates were observed when homologous sequences were separated by H-DNA-forming elements.77,78 It is likely that the X-type cruciforms may also position DNA elements for recombination or for promoter-enhancer interactions. Moreover, cruciform transitions between the X-type and extended conformations may serve to switch between the favorable and unfavorable arrangements of interacting DNA sites.79
Roles in Transcription
Analyses of genomic databases show that sequences capable of forming cruciforms, Z- and H-DNA are frequently found around transcription initiation sites.34,36,38 The formation of alternative DNA structures in these sequences may influence transcription by changing the supercoiling levels within a domain thereby changing the energy cost for protein-DNA binding. The formation of an alternative structure may also alter interactions between transcription factors bound to different sites due to a change in their spatial positioning. At least two of the structures, cruciforms and H-DNA, may spatially organize DNA around their formation sites so that certain DNA segments are brought into close proximity.33,46 Gene expression may also depend on protein binding to unusual DNA structures. For example, poly(ADP-ribose) polymerase (PARP) may bind to the junction-containing DNA structures such as cruciforms.80 Repressive PARP binding to potential cruciforms in a promoter of its own gene and dissociation upon DNA strand break-induced autoribosylation are parts of the mechanism of autoregulation of PARP expression.80,81 In another example, in the human proenkephalin gene switching of a region of DNA between the linear and cruciform form provides a mechanism of gene regulation.82 More correlations of transcrption with the formation of non-B-DNA structures are discussed in detail in other chapters of this book.
Roles in Replication
One of the well-studied effects of alternative structures on replication is a block to polymerases due to template folding, which was shown for cruciforms/hairpins83,84 and H-DNA.85-87 Unless unwound by the replication accessory proteins, including helicases,88 polymerization blocks may result in genetic mutations that lead to the development of human diseases, such as polycystic kidney disease and Friedrich ataxia. Single-stranded parts of the cruciform and H-DNA may serve as recognition elements for the replication initiation proteins.35,89 Protein binding may also be directed to the four-way junction of the cruciform to initiate replication as shown for CBP in HeLa cells.35
Roles in Recombination
There are several relationships between the formation of alternative structures and DNA recombination. Consistent with an idea of sequence positioning by a fold-back structure of H-DNA, facilitated recombination was observed between distant elements separated by the Py•Pu tract.77,78 Several models of Z-DNA assisted recombination have been proposed.90 DNA strand exchange during recombination requires initial duplex-duplex interaction. For this, exposed N7 and C8 of guanosines in one Z-DNA duplex are available for interaction with another Z-DNA duplex so as to initiate recombination. During the synapsis step in homologous recombination a paranemic joint, a nascent heteroduplex where strands from different DNA molecules base pair without breaking them, can be formed from the alternating left-handed and right-handed turns.
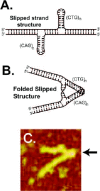
Slipped-Strand DNA
If a region of DNA contains a block of several nucleotides that repeats many times, there are multiple opportunities for the formation of base pairs in an out-of-register or “slipped” fashion. A slipped-strand DNA (S-DNA) structure forms when a section of the repeating duplex unwinds so that one region of the direct repeat forms the Watson-Crick base pairs with another region of the repetitive sequence forming two loop-out regions in opposite strands (fig. 4A).1,91 The likelihood of DNA slippage increases with increasing length of the repeats and increasing potential for partial base pairing in the looped-out single strand. The out-of-register base pairing is more probable in the GC-rich repeats because they have a better propensity for nucleation of the double-stranded structure than the average 50% GC flanking sequences if the DNA strands are temporarily separated and then allowed to re-form the duplex. Interruptions in the direct repeat tracts significantly reduce the number of possible out-of-register configurations and, therefore, the probability of S-DNA formation.
Biological Significance
S-DNA has been of considerable interest in the last decade. Fourteen genetic neurodegenerative diseases and three fragile sites have been associated with the expansion of (CTG)n•(CAG)n, (CGG)n•(CCG)n, or (GAA)n•(TTC)n repeat tracts. Different models have been proposed for the expansion of triplet repeats, most of which presume the formation of alternative DNA structures in repeat tracts. One of the most likely structures, S-DNA, can stably and reproducibly form within the GC-rich triplet repeat sequences, (CTG)n•(CAG)n, (CGG)n•(CCG)n. In fact, given that the loops of the slipped out arms are complementary, good evidence exists that there is a further conformational transition to a folded slipped strand structure (fig.4B), as formed by the model slipped strand structure shown in Figure 4C. S-DNA may be involved in triplet repeat mutagenesis in several ways, such as a simple primer/template misalignment or reiterative synthesis, involving repetitive slippage events. More details on the S-DNA structure and its role in the triplet repeat mutagenesis may be found in recent reviews.91,92
Slipped misalignment during DNA replication is very important in spontaneous frameshift mutagenesis. In 1966, Streisinger et al proposed a model that explained frameshift mutations within runs of a single base by a slippage of the nascent DNA strand on the replication template strand.93 Since the genetic code is read as triplets, adding or deleting a single base shifts the reading frame of all bases downstream of the mutation. As a result, part of the mRNA encodes amino acids that are different from those in the wild-type protein. Further work has shown that direct repeats and more complex DNA repeats often contribute to frameshift mutagenesis.94-96 The hairpin-forming sequences intervening the repeats have been shown to stabilize S-DNA and promote mutations.
DNA Unwinding Elements
DNA unwinding elements (DUE) have been identified in both prokaryotic and eukaryotic DNA sequences (see ref. 1, for review). DUEs are AT-rich sequences about 30-100 bp long. They have little sequence similarity except for being AT-rich. Under torsional stress, unwinding of the double helix occurs first in AT-rich sequences, therefore, DUEs can be maintained as unpaired DNA regions in the presence of negative supercoiling (fig. 5A). In the presence of Mg2+, DUEs tend to remain double-stranded and other regions (such as inverted repeats) unwind to partially relieve superhelical tension. Thus, the ability of DUEs to form denaturation bubbles may be dependent on the level of unrestrained supercoiling and the local ionic environment in cells.
Biological Significance
DUEs are commonly associated with replication origins and chromosomal matrix attachment regions. DUEs are a common feature of DNA replication origins in E. coli and yeast.97 Replication from the yeast origins shows a correlation between the extent of DNA unwinding and the proficiency of the DUEs as replication origins. Similarly, DNA unwinding is also required at the E. coli origin of replication. The AT-rich DUEs are also found in at least some mammalian origins.98 Thus, DUEs seem to fulfill a primary requirement for the initiation of DNA replication in all systems, which is the formation of an unpaired region in DNA where the replication complex assembles. Recent studies showed that an AT-rich repetitive sequence (ATTCT)n•(AGAAT)n, whose spontaneous length expansion has been associated with the development of the disease, spinocerebellar ataxia type 10, has the properties of DUE (fig. 5B).99 Under superhelical stress, the repeating sequence preferentially unpairs and may potentially bind the proteins of the replication complex. Unscheduled initiation of replication from the false origin in combination with a possible primer/template slippage in the repeated sequence may produce longer than expected products of DNA replication. These may be incorporated into the repeat tract leading to the expansion of repeat length and eventually to the development of the disease.99
Conclusion
Local structural transitions from the common B-DNA conformation into other DNA forms can be functionally important. Such transitions within certain sequence elements of DNA can be induced by changes in environmental conditions, protein binding and superhelical tension. Several lines of evidence indicate that alternative DNA structures may exist in prokaryotic and eukaryotic cells. The data on their involvement in replication, gene expression, recombination and mutagenesis continues to accumulate.
References
- 1.
- Sinden RR. DNA Structure and FunctionSan Diego: Academic Press,1994 .
- 2.
- Cheatham TEIII, Kollman PA. Insight into the stabilization of A-DNA by specific ion association: spontaneous B-DNA to A-DNA transitions observed in molecular dynamics simulations of d[ACCCGCGGGT]2 in the presence of hexaamminecobalt(III) Structure 1997. | 51297–1311. [PubMed: 9351805]
- 3.
- Feig M, Pettitt BM. A molecular simulation picture of DNA hydration around A- and B-DNA. Biopolymers. 1998;48:199–209. [PubMed: 10699840]
- 4.
- Egli M. DNA-cation interactions: quo vadis? Chem Biol. 2002;9:277–286. [PubMed: 11927253]
- 5.
- Fuller W, Wilkins MHF, Wilson HR. et al. The molecular configuration of deoxyribonucleic acid. IV. X-ray diffraction of the A form. J Mol Biol. 1965;12:60–80. [PubMed: 14343297]
- 6.
- Ivanov VI, Minchenkova LE, Minyat EE. et al. The B to A transition of DNA in solution. J Mol Biol. 1974;87:817–833. [PubMed: 4427376]
- 7.
- Nishimura Y, Torigoe C, Tsuboi M. Salt induced B-A transition of poly(dG)•poly(dC) and the stabilization of A form by its methylation. Nucleic Acids Res. 1986;14:2737–2748. [PMC free article: PMC339695] [PubMed: 3960732]
- 8.
- Ivanov VI, Minchenkova LE. The A-form of DNA: in search of the biological role. Mol Biol (Mosk). 1994;28:1258–1271 Engl Transl 1995 28780-788. [PubMed: 7885327]
- 9.
- Guzikevich-Guerstein G, Shakked Z. A novel form of the DNA double helix imposed on the TATA-box by the TATA-binding protein. Nat Struct Biol. 1996;3:32–37. [PubMed: 8548452]
- 10.
- Vargason JM, Henderson K, Ho PS. A crystallographic map of the transition from B-DNA to A-DNA. Proc Natl Acad Sci USA. 2001;98:7265–7270. [PMC free article: PMC34657] [PubMed: 11390969]
- 11.
- Dickerson RE, Ng HL. DNA structure from A to B. Proc Natl Acad Sci USA. 2001;98:6986–6988. [PMC free article: PMC34608] [PubMed: 11416174]
- 12.
- Nekludova L, Pabo CO. Distinctive DNA conformation with enlarged major groove is found in Zn-finger-DNA and other protein-DNA complexes. Proc Natl Acad Sci USA. 1994;91:6948–6952. [PMC free article: PMC44315] [PubMed: 8041727]
- 13.
- Timsit Y. DNA structure and polymerase fidelity. J Mol Biol. 1999;293:835–853. [PubMed: 10543971]
- 14.
- Lu XJ, Shakked Z, Olson WK. A-form conformational motifs in ligand-bound DNA structures. J Mol Biol. 2000;300:819–840. [PubMed: 10891271]
- 15.
- Flatters D, Young M, Beveridge DL. et al. Conformational properties of the TATA-box binding sequence of DNA. J Biomol Struct Dyn. 1997;14:757–765. [PubMed: 9195344]
- 16.
- Barber AM, Zhurkin VB, Adhya S. CRP-binding sites: evidence for two structural classes with 6-bp and 8-bp spacers. Gene. 1993;130:1–8. [PubMed: 8393822]
- 17.
- Ivanov VI, Minchenkova LE, Chernov BK. et al. CRP-DNA complexes: inducing the A-like form in the binding sites with an extended central spacer. J Mol Biol. 1995;245:228–240. [PubMed: 7844815]
- 18.
- Jacobo-Molina A, Ding J, Nanni RG. et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc Natl Acad Sci USA. 1993;90:6320–6324. [PMC free article: PMC46920] [PubMed: 7687065]
- 19.
- Kiefer JR, Mao C, Braman JC. et al. Visualizing DNA replication in a catalytically active Bacillus DNA polymerase crystal. Nature. 1998;391:304–307. [PubMed: 9440698]
- 20.
- Mohr SC, Sokolov NV, He CM. et al. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci USA. 1991;88:77–81. [PMC free article: PMC50751] [PubMed: 1898779]
- 21.
- Becker MM, Wang Z. B-A transitions within a 5 S ribosomal RNA gene are highly sequence-specific. J Biol Chem. 1989;264:4163–4167. [PubMed: 2917994]
- 22.
- Vologodskii AV. Topology and Physics of Circular DNABoca Raton: CRC Press,1992 .
- 23.
- Sinden RR, Pettijohn DE. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci USA. 1981;78:224–228. [PMC free article: PMC319024] [PubMed: 6165987]
- 24.
- Kramer PR, Fragoso G, Pennie W. et al. Transcriptional state of the mouse mammary tumor virus promoter can affect topological domain size in vivo. J Biol Chem. 1999;274:28590–28597. [PubMed: 10497225]
- 25.
- Ljungman M, Hanawalt PC. Presence of negative torsional tension in the promoter region of the transcriptionally poised dihydrofolate reductase gene in vivo. Nucleic Acids Res. 1995;23: 1782–1789. [PMC free article: PMC306936] [PubMed: 7784183]
- 26.
- Jupe ER, Sinden RR, Cartwright IL. Specialized chromatin structure domain boundary elements flanking a Drosophila heat shock gene locus are under torsional strain in vivo. Biochemistry. 1995;34:2628–2633. [PubMed: 7873544]
- 27.
- Kramer PR, Sinden RR. Measurement of unrestrained negative supercoiling and topological domain size in living human cells. Biochemistry. 1997;36:3151–3158. [PubMed: 9115991]
- 28.
- Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci USA. 1987;84:7024–7027. [PMC free article: PMC299221] [PubMed: 2823250]
- 29.
- Liu Y, Bondarenko V, Ninfa A. et al. DNA supercoiling allows enhancer action over a large distance. Proc Natl Acad Sci USA. 2001;98:14883–14888. [PMC free article: PMC64953] [PubMed: 11742093]
- 30.
- Horwitz MS, Loeb LA. An E. coli promoter that regulates transcription by DNA superhelix-induced cruciform extrusion. Science. 1988;241:703–705. [PubMed: 2456617]
- 31.
- Krajewski WA. Enhancement of transcription by short alternating C•G tracts incorporated within a Rous sarcoma virus-based chimeric promoter: in vivo studies. Mol Gen Genet. 1996;252: 249–254. [PubMed: 8842144]
- 32.
- Murchie AI, Lilley DM. Supercoiled DNA and cruciform structures. Methods Enzymol. 1992;211:158–180. [PubMed: 1406306]
- 33.
- Shlyakhtenko LS, Potaman VN, Sinden RR. et al. Structure and dynamics of supercoil-stabilized DNA cruciforms. J Mol Biol. 1998;280:61–72. [PubMed: 9653031]
- 34.
- Schroth GP, Ho PS. Occurrence of potential cruciform and H-DNA forming sequences in genomic DNA. Nucleic Acids Res. 1995;23:1977–1983. [PMC free article: PMC306972] [PubMed: 7596826]
- 35.
- Pearson CE, Zorbas H, Price GB. et al. Inverted repeats, stem-loops, and cruciforms: significance for initiation of DNA replication. J Cell Biochem. 1996;63:1–22. [PubMed: 8891900]
- 36.
- Cox R, Mirkin SM. Characteristic enrichment of DNA repeats in different genomes. Proc Natl Acad Sci USA. 1997;94:5237–5242. [PMC free article: PMC24662] [PubMed: 9144221]
- 37.
- Herbert A, Rich A. Left-handed Z-DNA: structure and function. Genetica. 1999;106:37–47. [PubMed: 10710708]
- 38.
- Schroth GP, Chou PJ, Ho PS. Mapping Z-DNA in the human genome. Computer-aided mapping reveals a nonrandom distribution of potential Z-DNA-forming sequences in human genes. J Biol Chem. 1992;267:11846–11855. [PubMed: 1601856]
- 39.
- Wells RD, Collier DA, Hanvey JC. et al. The chemistry and biology of unusual DNA structures adopted by oligopurine•oligopyrimidine sequences. FASEB J. 1988;2:2939–2949. [PubMed: 3053307]
- 40.
- Htun H, Dahlberg JE. Topology and formation of triple-stranded H-DNA. Science. 1989;243:1571–1576. [PubMed: 2648571]
- 41.
- Frank-Kamenetskii MD, Mirkin SM. Triplex DNA structures. Annu Rev Biochem. 1995;64:65–95. [PubMed: 7574496]
- 42.
- Soyfer VN, Potaman VN. 1996 . Triple-Helical Nucleic Acids. New York: Springer.
- 43.
- Lyamichev VI, Mirkin SM, Frank-Kamenetskii MD. Structures of homopurine-homopyrimidine tract in superhelical DNA. J Biomol Struct Dyn. 1986;3:667–669. [PubMed: 3271043]
- 44.
- Kohwi Y, Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci USA. 1988;85:3781–3785. [PMC free article: PMC280302] [PubMed: 3375241]
- 45.
- Potaman VN, Sinden RR. Stabilization of intramolecular triple/single-strand structure by cationic peptides. Biochemistry. 1998;37:12952–12961. [PubMed: 9737875]
- 46.
- Tiner WJSr, Potaman VN, Sinden RR. et al. The structure of intramolecular triplex DNA: atomic force microscopy study. J Mol Biol. 2001;314:353–357. [PubMed: 11846549]
- 47.
- McClellan JA, Boublikova P, Palecek E. et al. Superhelical torsion in cellular DNA responds directly to environmental and genetic factors. Proc Natl Acad Sci USA. 1990;87:8373–8377. [PMC free article: PMC54958] [PubMed: 2172986]
- 48.
- Rahmouni AR, Wells RD. Stabilization of Z DNA in vivo by localized supercoiling. Science. 1989;246:358–363. [PubMed: 2678475]
- 49.
- Karlovsky P, Pecinka P, Vojtiskova M. et al. Protonated triplex DNA in E. coli cells as detected by chemical probing. FEBS Lett. 1990;274:39–42. [PubMed: 2253780]
- 50.
- Zheng GX, Kochel T, Hoepfner RW. et al. Torsionally tuned cruciform and Z-DNA probes for measuring unrestrained supercoiling at specific sites in DNA of living cells. J Mol Biol. 1991;221:107–122. [PubMed: 1920399]
- 51.
- Ussery DW, Sinden RR. Environmental influences on the in vivo level of intramolecular triplex DNA in Escherichia coli. Biochemistry. 1993;32:6206–6213. [PubMed: 8512930]
- 52.
- Kohwi Y, Malkhosyan SR, Kohwi-Shigematsu T. Intramolecular dG•dG•dC triplex detected in Escherichia coli cells. J Mol Biol. 1992;223:817–822. [PubMed: 1538396]
- 53.
- Haniford DB, Pulleyblank DE. The in vivo occurrence of Z DNA. J Biomol Struct Dyn. 1983;1:593–609. [PubMed: 6401120]
- 54.
- Haniford DB, Pulleyblank DE. Transition of a cloned d(AT)n-d(AT)n tract to a cruciform in vivo. Nucleic Acids Res. 1985;13:4343–4363. [PMC free article: PMC321792] [PubMed: 4011446]
- 55.
- Jaworski A, Hsieh WT, Blaho JA. et al. Left-handed DNA in vivo. Science. 1987;238:773–777. [PubMed: 3313728]
- 56.
- Lukomski S, Wells RD. Left-handed Z-DNA and in vivo supercoil density in the Escherichia coli chromosome. Proc Natl Acad Sci USA. 1994;91:9980–9984. [PMC free article: PMC44941] [PubMed: 7937930]
- 57.
- Ward GK, Shihab-el-Deen A, Zannis-Hadjopoulos M. et al. DNA cruciforms and the nuclear supporting structure. Exp Cell Res. 1991;195:92–98. [PubMed: 1905239]
- 58.
- Nordheim A, Lafer EM, Peck LJ. et al. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982;31:309–318. [PubMed: 7159926]
- 59.
- Agazie YM, Burkholder GD, Lee JS. Triplex DNA in the nucleus: direct binding of triplex-specific antibodies and their effect on transcription, replication and cell growth. Biochem J. 1996;316(Pt 2):461–466. [PMC free article: PMC1217372] [PubMed: 8687388]
- 60.
- Sharples GJ. The X philes: structure-specific endonucleases that resolve Holliday junctions. Mol Microbiol. 2001;39:823–834. [PubMed: 11251805]
- 61.
- Constantinou A, Chen XB, McGowan CH. et al. Holliday junction resolution in human cells: two junction endonucleases with distinct substrate specificities. EMBO J. 2002;21:5577–5585. [PMC free article: PMC129086] [PubMed: 12374758]
- 62.
- Kim YG, Muralinath M, Brandt T. et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci USA. 2003;100:6974–6979. [PMC free article: PMC165815] [PubMed: 12777633]
- 63.
- Li G, Tolstonog GV, Traub P. Interaction in vitro of type III intermediate filament proteins with Z-DNA and B-Z-DNA junctions. DNA Cell Biol. 2003;22:141–169. [PubMed: 12804114]
- 64.
- Kiyama R, Camerini-Otero RD. A triplex DNA-binding protein from human cells: purification and characterization. Proc Natl Acad Sci USA. 1991;88:10450–10454. [PMC free article: PMC52946] [PubMed: 1961709]
- 65.
- Guieysse AL, Praseuth D, Helene C. Identification of a triplex DNA-binding protein from human cells. J Mol Biol. 1997;267:289–298. [PubMed: 9096226]
- 66.
- Ciotti P, Van DykeMW, Bianchi-Scarra G. et al. Characterization of a triplex DNA-binding protein encoded by an alternative reading frame of loricrin. Eur J Biochem. 2001;268:225–234 and references therein. [PubMed: 11168355]
- 67.
- Hildebrandt M, Lacombe ML, Mesnildrey S. et al. A human NDP-kinase B specifically binds single-stranded poly-pyrimidine sequences. Nucleic Acids Res. 1995;23:3858–3864. [PMC free article: PMC307302] [PubMed: 7479028]
- 68.
- Brunel F, Zakin MM, Buc H. et al. The polypyrimidine tract binding (PTB) protein interacts with single-stranded DNA in a sequence-specific manner. Nucleic Acids Res. 1996;24:1608–1615. [PMC free article: PMC145841] [PubMed: 8649976]
- 69.
- Farokhzad OC, Teodoridis JM, Park H. et al. CD43 gene expression is mediated by a nuclear factor which binds pyrimidine-rich single-stranded DNA. Nucleic Acids Res. 2000;28:2256–2267. [PMC free article: PMC102628] [PubMed: 10871347]
- 70.
- Wang JC, Lynch AS. Transcription and DNA supercoiling. Curr Opin Genet Dev. 1993;3:764–768. [PubMed: 8274860]
- 71.
- Kato M, Shimizu N. Effect of the potential triplex DNA region on the in vitro expression of bacterial β-lactamase gene in superhelical recombinant plasmids. J Biochem. 1992;112:492–494. [PubMed: 1491004]
- 72.
- Simpson RT. Nucleosome positioning: occurrence, mechanisms, and functional consequences. Prog Nucleic Acid Res Mol Biol. 1991;40:143–184. [PubMed: 2031082]
- 73.
- Nickol J, Martin RG. DNA stem-loop structures bind poorly to histone octamer cores. Proc Natl Acad Sci USA. 1983;80:4669–4673. [PMC free article: PMC384105] [PubMed: 6308638]
- 74.
- Casasnovas JM, Azorin F. Supercoiled induced transition to the Z-DNA conformation affects the ability of a d(CG/GC)12 sequence to be organized into nucleosome-cores. Nucleic Acids Res. 1987;15:8899–8918. [PMC free article: PMC306412] [PubMed: 3684575]
- 75.
- Westin L, Blomquist P, Milligan JF. et al. Triple helix DNA alters nucleosomal histone-DNA interactions and acts as a nucleosome barrier. Nucleic Acids Res. 1995;23:2184–2191. [PMC free article: PMC307006] [PubMed: 7610046]
- 76.
- Laundon CH, Griffith JD. Curved helix segments can uniquely orient the topology of supertwisted DNA. Cell. 1988;52:545–549. [PubMed: 2830027]
- 77.
- Kohwi Y, Panchenko Y. Transcription-dependent recombination induced by triple-helix formation. Genes Dev. 1993;7:1766–1778. [PubMed: 8370525]
- 78.
- Rooney SM, Moore PD. Antiparallel, intramolecular triplex DNA stimulates homologous recombination in human cells. Proc Natl Acad Sci USA. 1995;92:2141–2144. [PMC free article: PMC42439] [PubMed: 7892237]
- 79.
- Shlyakhtenko LS, Hsieh P, Grigoriev M. et al. A cruciform structural transition provides a molecular switch for chromosome structure and dynamics. J Mol Biol. 2000;296:1169–1173. [PubMed: 10698623]
- 80.
- Soldatenkov VA, Chasovskikh S, Potaman VN. et al. Transcriptional repression by binding of poly(ADP-ribose) polymerase to promoter sequences. J Biol Chem. 2002;277:665–670. [PubMed: 11684688]
- 81.
- Oei SL, Herzog H, Hirsch-Kauffmann M. et al. Transcriptional regulation and autoregulation of the human gene for ADP-ribosyltransferase. Mol Cell Biochem. 1994;138:99–104. [PubMed: 7898482]
- 82.
- Spiro C, McMurray CT. Switching of DNA secondary structure in proenkephalin transcriptional regulation. J Biol Chem. 1997;272:33145–33152. [PubMed: 9407101]
- 83.
- Bedinger P, Munn M, Alberts BM. Sequence-specific pausing during in vitro DNA replication on double-stranded DNA templates. J Biol Chem. 1989;264:16880–16886. [PubMed: 2674143]
- 84.
- Hacker JK, Alberts BM. The rapid dissociation of the T4 DNA polymerase holoenzyme when stopped by a DNA hairpin helix. A model for polymerase release following the termination of each Okazaki fragment. J Biol Chem. 1994;269:24221–24228. [PubMed: 7929078]
- 85.
- Baran N, Lapidot A, Manor H. Formation of DNA triplexes accounts for arrests of DNA synthesis at d(TC)n and d(GA)n tracts. Proc Natl Acad Sci USA. 1991;88:507–511. [PMC free article: PMC50840] [PubMed: 1988950]
- 86.
- Dayn A, Samadashwily GM, Mirkin SM. Intramolecular DNA triplexes: unusual sequence requirements and influence on DNA polymerization. Proc Natl Acad Sci USA. 1992;89:11406–11410. [PMC free article: PMC50559] [PubMed: 1454828]
- 87.
- Potaman VN, Bissler JJ. Overcoming a barrier for DNA polymerization in triplex-forming sequences. Nucleic Acids Res. 1999;27:e5. [PMC free article: PMC148516] [PubMed: 10454624]
- 88.
- Kopel V, Pozner A, Baran N. et al. Unwinding of the third strand of a DNA triple helix, a novel activity of the SV40 large T-antigen helicase. Nucleic Acids Res. 1996;24:330–335. [PMC free article: PMC145642] [PubMed: 8628658]
- 89.
- Liu G, Malott M, Leffak M. Multiple functional elements comprise a mammalian chromosomal replicator. Mol Cell Biol. 2003;23:1832–1842. [PMC free article: PMC151694] [PubMed: 12589000]
- 90.
- Blaho JA, Wells RD. Left-handed Z-DNA and genetic recombination. Prog Nucleic Acid Res Mol Biol. 1989;37:107–126. [PubMed: 2672108]
- 91.
- Pearson CE, Sinden RR. Trinucleotide repeat DNA structures: dynamic mutations from dynamic DNA. Curr Opin Struct Biol. 1998;8:321–330. [PubMed: 9666328]
- 92.
- Sinden RR, Potaman VN, Oussatcheva EA. et al. Triplet repeat DNA structures and human genetic disease: dynamic mutations from dynamic DNA. J Biosci. 2002;27(Suppl 1):53–65. [PubMed: 11927777]
- 93.
- Streisinger G, Okada Y, Emrich J. et al. Frameshift mutations and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. [PubMed: 5237214]
- 94.
- Ripley LS. Frameshift mutation: determinants of specificity. Annu Rev Genet. 1990;24:189–213. [PubMed: 2088167]
- 95.
- Sinden RR, Wells RD. DNA structure, mutations, and human genetic disease. Curr Opin Biotechnol. 1992;3:612–622. [PubMed: 1369117]
- 96.
- Strauss BS. Frameshift mutation, microsatellites and mismatch repair. Mutat Res. 1999;437: 195–203. [PubMed: 10592327]
- 97.
- Miller CA, Umek RM, Kowalski D. The inefficient replication origin from yeast ribosomal DNA is naturally impaired in the ARS consensus sequence and in DNA unwinding. Nucleic Acids Res. 1999;27:3921–3930 and references therein. [PMC free article: PMC148656] [PubMed: 10481032]
- 98.
- Berberich S, Trivedi A, Daniel DC. et al. In vitro replication of plasmids containing human c-myc DNA. J Mol Biol. 1995;245:92–109. [PubMed: 7799437]
- 99.
- Potaman VN, Bissler JJ, Hashem VI. et al. Unpaired structures in SCA10 (ATTCT)n•(AGAAT)n repeats. J Mol Biol. 2003;326:1095–1111. [PubMed: 12589756]
- DNA: Alternative Conformations and Biology - Madame Curie Bioscience DatabaseDNA: Alternative Conformations and Biology - Madame Curie Bioscience Database
- NMD in Drosophila: A Snapshot into the Evolution of a Conserved mRNA Surveillanc...NMD in Drosophila: A Snapshot into the Evolution of a Conserved mRNA Surveillance Pathway - Madame Curie Bioscience Database
- A Prelude to Translational (Re)Initiation - Madame Curie Bioscience DatabaseA Prelude to Translational (Re)Initiation - Madame Curie Bioscience Database
- Adding Complexity to Phagocytic Signaling: Phagocytosis-Associated Cell Response...Adding Complexity to Phagocytic Signaling: Phagocytosis-Associated Cell Responses and Phagocytic Efficiency - Madame Curie Bioscience Database
- Asparaginyl-tRNA Synthetases - Madame Curie Bioscience DatabaseAsparaginyl-tRNA Synthetases - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...