NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
In our understanding of the mechanical performance of the heart as a pump we mostly rely on the famous studies of Otto Frank1 and Ernest Starling2 whose observations have been widely accepted for a century. Thus, the clinical therapeutic regimens contain volume substitution (preload), antihypertensive therapy (afterload), bradycardic agent (heart rate) and positive inotropic agent (contractility) drug administration. All these maneuvers involve the heart as a whole. In most of our patients, however, just parts of the ventricle are injured by insufficient energy supply. Therefore, if we treat the ventricle as a whole we may ask whether all the parts of the ventricle act in unison. When we look at heart preparations for morphological analyses (Fig. 1) it becomes obvious from the global as well as local anisotropy that there must be an inhomogeneity in myocardial contraction i) across the ventricular wall, ii) from site to site from apex to the base, and, moreover, iii) at any site along different angular orientations.
Regional blood supply measured by antegrade volume-flow through the coronary arteries may cause differential effects on the dependent myocardium. This will primarily be according to the specific amount of oxygen transported (limited oxygen supply by coronary artery stenosis or reduced oxygen transport due to hemodilution or perfusion with desaturated instead of arterial blood). Additionally the functional response of the myocardium to local impediment of the coronary blood flow might depend on the coronary artery architecture and on the absence or presence of collaterals—genetically preformed or acquired.
Thus, this chapter deals with regional differences in left ventricular performance, with species-related differences of myocardial functional response to coronary artery blood flow restriction, and with variations of oxygen delivery irrespective of blood volume-flow and their effects on myocardial function.
Local Differences in Left Ventricular Wall Motion
When we look directly at the beating heart during surgery or at its intracavitary ‘shadow’ of the contrast medium during cardiac catheterization, a heterogeneous motion becomes evident even in patients with regular ventricular wall function, i.e., in patients with intact coronary artery circulation. Two major reasons may be responsible for this: First, a twisted common band-like structure of the whole biventricular heart pump introduces a global anisotropy (Fig. 1).3 This has been demonstrated for more than a century by heart preparations for morphological studies which reached an artistic reading by Torrent-Guasp.4 Second, at any part of this muscle band forming the ventricular wall there is a local anisotropy across the wall which has been described by Streeter and Hanna5 as “a...nested set of fiber shells, with ... a fiber orientation like that of blades of an opened Japanese fan.” Although, the morphology of the heart has been extensively studied and the anisotropy has been taken into account in theoretical considerations, the functional consequences of this muscular structure could not be documented for a long time as there were no methods appropriate to assess local myocardial function in the beating heart. The pattern of local left ventricular wall motion could be first recorded continuously and even in chronic animal preparations by an ultrasound transit time technique called ‘sonomicrometry’.6 The method became even more reliable and easier to apply with the development of miniaturized implantable piezoceramic transducers with circular sound radiation which further allowed the study of geometrical changes in size and shape of a well-defined wall area.7 By using sonomicrometry, a mapping of the cyclic motion at several sites along the major axis of the canine left ventricular anterior free wall has been first performed by LeWinter et al.8 To do this, they implanted pairs of ultrasonic transducers in parallel to the myocardial fiber orientation into the middle layer of the wall and subepicardially, each near the base, at the midventricular level, and near the apex. In the epicardial segments which were oriented about 20° counter-clockwise to the long axis of the left ventricle there were no regional differences in shortening fraction across the ventricular free wall. When compared to these epicardial segments, an about four-fold shortening fraction of the hoop axis fibers had been observed in the midwall layers. Furthermore, in the middle layer of the ventricular wall, shortening fraction averaged 20% of the end-diastolic length near the apex, significantly more than shortening at the midventricular (13%) or basal (14%) levels. The gradient of shortening fraction became more even from the apex to the base (20%–13%–9%) during transient periods of aortic constriction, i.e., pressure loading of the left ventricle.
In order to obtain more clinically relevant data about regional ventricular wall motion we modified this study in that we implanted the transducers into subendocardial layers in parallel and perpendicular to the major axis of the left ventricle.9,10 Firstly, dimensional changes of the endocardium determine the shape and size of the instantaneous ventricular cavity and, thus, of the stroke volume, and, secondly, cineventriculographic studies are also based on analyses of the inner ventricular cross-sectional changes without respect to the myocardial fiber orientation. Though we used slightly different criteria for the calculation of shortening fraction (SF), the results from 46 canine experiments fitted well with the previous data from the literature:
with:
- SF: shortening fraction of subendocardial left ventricular wall segment;
- Lo: segment length at the time of aortic valve opening;
- Lc: segment length at the time of aortic valve closure;
- edL: segment length at the end of ventricular diastole.
This formula differs slightly from the algorithm used by some investigators who calculate SF from maximal segment length instead of Lo and minimal segment length instead of Lc at control conditions; their criteria, however, become inconsistent as soon as paradoxical systolic dilatation or bulging occurs due to hypoxic injury. This problem is overcome by the use of the above cited formula.
According to our data, SF of the hoop axis declines from 15.2% at the apex to 8.3% at the midventricular level and to 7.9% at the base during ‘control conditions’ with the dogs anesthetized, mechanically ventilated, and the thorax and pericardium open. The respective SF values from parallel to the major axis of the left ventricle were 6.7%–4.4%–6.9%. Afterload augmentation was performed by graded inflation of a saline-filled balloon catheter with its tip positioned just distal to the aortic arch. An elevation of the peak left ventricular pressure from 125 mm Hg to 151mm Hg caused an augmentation of the end-diastolic pressure from 7.3–11.4 mm Hg and a concomitant 6% increase in edL along the minor ventricular diameter but no evident changes in SF along any direction at the basal and midventricular site. At the apical site, however, SF decreased by 30% along the hoop axis whereas it increased by 50% along the major heart axis. This resulted in a piston pump-like motion of the pressure loaded ventricle during contraction. A similar contraction pattern was observed after positive inotropic stimulation (10 μg isoproterenol).
The effects of volume loading on regional ventricular wall motion were observed after rapid i.v. infusion of dextran 60 (30 ml/kg body weight). This augmented end-diastolic left ventricular pressure by 104% but increased left ventricular peak pressure moderately by only 14%; the lowered blood viscosity due to hemodilution yielded this primary increase in preload. Volume loading became evident in a 6–8% enlargement of the apical and midventricular end-diastolic dimensions. At the basal site, the segment dilation averaged 9% in parallel to the minor axis but only 3% along the major axis. Irrespective of this, the amount of systolic shortening increased in both directions by about 50% near the base and by about 30% at the equatorial level of the ventricle. Near the apex, changes in SF did not correlate with the extent of segment dilatation: SF changes averaged between −5% and +10%.
According to the data obtained by this functional mapping of the ventricular wall motion, we monitor routinely in our experiments the mechanical function of the ventricular wall region of interest—mostly areas of the anterior free wall supplied either by the circumflex or by the anterior descending branch of the left coronary artery—by measuring the cyclic motion of subendocardial wall segments which are oriented in parallel to the minor ventricular axis avoiding the apical third of the left ventricle. The reason for this is: Firstly, the sum of all the local dimensional changes of the endocardium yields the total pump function of the left ventricle. Secondly, the endocardium has been shown to be most sensitive to alterations in the energy demand/supply ratio. Thirdly, the ultrasonic transducers for detection of dimensional changes can be placed accurately in this deep wall layer and stay there in a stable position for hours, for days, or longer. Finally, segments oriented in parallel to the minor heart axis at basal or midventricular wall regions are found to be most representative in their dimensional changes to geometrical alterations of the total left ventricle. Superficially, in subepicardial layers the relative extent of systolic shortening SF amounts to only between 30% and 70% of that obtained in subendocardial layers.8,10,11 Thus, the response to functional alterations is less pronounced in subepicardial wall segments as compared to those of subendocardial layers.
Local ventricular wall function also could be assessed by measuring the cyclic variations in wall thickening.12 This method requires very accurate transducer alignment to avoid misreadings which might result from transmural shear motion.13 And, moreover, systematic mapping of the wall thickening patterns across the left ventricle has not yet been performed. As long as no manipulations are made which alter left ventricular wall mass or volume, measurements of either wall thickness or segment length should reveal equivalent statements. In most animal experiments, myocardial mass and volume of the ventricular wall can be assumed to be constant for the investigational period. It might slowly increase by developing edema and is rapidly enlarged by excessive antegrade as well as retrograde coronary artery hyperperfusion. Antegrade coronary artery perfusion with coronary perfusion pressure more than 20% above the aortic pressure results in an expansion of left ventricular wall thickness and in a diminution of the subendocardial wall dimensions.14 Obviously, with increasing wall thickness an intramural ballooning causes an enlargement of the external ventricular silhouette and a compression of the intraventricular cavity. Similar observations were made during intermittent coronary sinus occlusion in dogs at intact or only moderately impeded antegrade coronary artery perfusion but disappeared in the presence of total occlusion of the left anterior descending coronary artery.15 An increase in end-diastolic wall thickness which might indicate a ballooning of the wall became evident during coronary venous retroperfusion in pigs when the retrograde flow markedly exceeded the amount of antegrade control flow.16 As an increase in end-diastolic ventricular wall thickness may indicate a return from ischemic dilation as well as a progressive swelling or ballooning, correct interpretation of the mechanisms calls for additional simultaneous measurements of changes in corresponding wall segments when alterations of the wall volume are to be suspected.
In the past, most studies dealing with local or regional differences in left ventricular wall motion were performed in anesthetized dogs. In the meantime, most experiments concerning the cardiovascular system and its autoregulatory mechanisms are performed in pigs. From my experience, SF of the swine left ventricular free wall in the acute experiment at rest exceeds that of the canine one by up to 70%. Systematical mapping of the contraction pattern of the porcine left ventricle, however, has not yet been performed.
Mapping of the mechanical action of the heart by sonomicrometry is a highly invasive and time-consuming procedure and, unfortunately, the results originate mainly from acute experiments with anesthetized animals. Forthcoming noninvasive techniques like nuclear magnetic resonance imaging with its sophisticated technical and analytical features3,17 might enable us to institute reference maps of local myocardial function in the healthy human with respect to his physical activities or life style as well as in the ill patient on account of his disease.
Variability in Ventricular Wall Motion Pattern
If one questions now whether a SF of 10% indicates regular or hypokinetic wall motion, you should ask for the circumstances like site and orientation of measurement, species, at rest or during exercise, etc. To classify a ventricular wall segment as a regularly or less efficient contracting part of the ventricle is possible only after the response to variations in pressure load or wall stress, in work and power is analyzed. As soon as ventricular wall regions dilate during systole (dyskinesia) or are almost akinetic for the whole cardiac cycle, physical load variations are in general inappropriate to test the viability of the myocardium; such tests are reserved for methods which are based on the detection of local metabolism, e.g., positron emission tomography.18 The main problem is to differentiate between regular contraction and little to moderate hypokinesia. In the acute animal experiment, we commonly start from normal myocardial function at control conditions and, thus, we are able to install graded regional impairment of left ventricular function and to model coronary artery disease of well-defined severity. By modeling coronary artery disease we discriminate between ‘critical’ and ‘functionally effective’ coronary artery stenosis. A coronary artery stenosis might be defined to be ‘critical’ or ‘sub-critical’ when the coronary artery is narrowed to a degree just not affecting the extent of contraction of the dependent myocardial region19 or when the stenosis reaches a degree where reactive hyperemia following a 15-second coronary artery occlusion is just abolished.20 ‘Functionally effective’ coronary artery stenosis always results in an impairment of the mechanical function and in an at least minimal hypokinesia of the afflicted wall region.
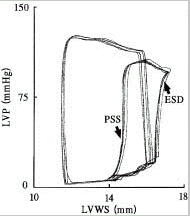
There are only weak discriminations between normal and hypokinetic ventricular wall motion unless verified by functional stress tests. If one is familiar with it, the left ventricular pressure-dimension loop may best and easiest inform about the actual functional state of the ventricular wall segment under study.21,22 It is mentioned that there are some peculiarities in the shape of the pressure-length loop which might indicate an acute ischemic insult (Fig. 2). Depending on the site and layer where the ultrasonic transducers are implanted, the recorded tracing may show conformational changes of the ventricle which appear as length changes during the isovolumic contraction and relaxation. Pronounced regional wall shortening during isovolumic ventricular relaxation (postsystolic shortening (PSS)) also has been observed in the acutely ischemic myocardium and has been identified to be a predictor of the early and late recovery of function after coronary artery reperfusion.23 In addition to PSS, the oxygen-deprived myocardial muscle often dilates during the very early systole which is followed by a seemingly regular shortening for the remaining ejection phase. Such early-systolic dilatation (ESD) might result from inhomogeneous force distribution inside the heart chamber generated by maximal blood acceleration at the onset of ejection.24 As shown in Figure 6.2, PSS and ESD are immediately recognized in left ventricular pressure-length loops; otherwise cumbersome data analysis would be necessary to detect these signs of hypofunction.
Essentially, pressure-length loops represent local increments of the global pressure-volume diagram and, consequently, changes in size of the pressure-length loop indicate changes in work of the corresponding ventricular wall region. As with to the pressure-volume area, a reduction of the area covered by the pressure-length loop does not necessarily indicate an impairment of the wall region. Mainly heart rate variations cause marked alterations in the pressure-length loop by variations in the extent of shortening of the unimpeded myocardial contraction. Thus, pressure-length loops are the most prominent aid to recognize variations in the functional state and work of the myocardium. However, to validate the energetic situation of myocardial regions heart rate and/or ejection time has to enter into the evaluation. This enables an analysis of myocardial function in terms of physical power and of the amount of local contribution to the total power processed by the heart. The following index of regional myocardial power (RMP) has been proven to be highly sensitive to an unbalance of energy demand/supply ratio.25
with:
- SF: shortening fraction of left ventricular wall segment;
- AoPej : aortic mean pressure during ventricular ejection;
- HR: heart rate in beats per minute;
- tej : duration of ventricular ejection from aortic valve opening to closure.
Functional State of the Myocardium and Oxygen Delivery: Rules and Exceptions
The most common way to model myocardial ischemia in acute animal experiments is to narrow a main branch of the coronary artery by a mechanical obstructer. To what degree the blood flow through the coronary artery will be restricted depends on the aim of the study. Although this procedure directly affects the coronary blood flow, other parameters may serve as a measure of impairment. In our experimental studies on ischemic myocardium a branch of the left coronary artery is narrowed by a micrometer-driven snare until the extent of systolic shortening of the dependent ventricular wall area diminishes to the limit set in advance. A retrospective evaluation of the relation between reduction in coronary artery blood flow and myocardial systolic shortening revealed that a 50% decrease in blood flow through the left anterior descending coronary artery (LAD) curtails systolic wall segment shortening by about 50% in dogs but by more than 80% in pigs (Table 1). It is assumed that this discrepancy arises from differences in the coronary vascular bed: acute support for energy supply to the oxygen-deprived region can be activated by preformed collaterals which exist in dogs but do not in pigs. In humans, the presence of collaterals may follow genetic orders, collaterals may be acquired by physical activities, or they might have ‘grown’ to compensate for gradual occlusions of adjoining coronary vessels. Thus, without cardiac catheterization it is uncertain in human individuals whether there are collaterals and to what degree they might be able to support the oxygen supply. Comparable functional consequences to ischemic injury can be expected only from individuals with similar coronary architecture. Presence or absence of collaterals is one factor which determines regional myocardial blood supply. Although the antegrade volume-flow of blood through the coronary artery is commonly measured as representative for myocardial energy supply, the energy delivery is in fact determined by the oxygen dissolved and transported by the fluid.
Table 1
Species-related effects of coronary artery blood flow obstruction on left ventricular wall segment shortening in anesthetized dogs vs. pigs.
As shown in Table 8.1, a 50% reduction of systolic shortening in the canine myocardium was achieved by diminishing the coronary blood volume flow and, consequently, by diminishing the oxygen delivery to 50% (Table 2A). The oxygen flow to the myocardium, however, can be varied independently from blood volume flow. This has been shown in a study about the effects of hemodilution on myocardial function when the coronary reserve capacity was exhausted.19 For this reason, a ‘subcritical’ stenosis of the LAD has been established by narrowing the vessel to a degree which did just not affect the dependent wall motion. This ‘subcritical’ LAD stenosis restricted the coronary blood flow by less than 15%, but the shortening fraction of anterior wall segments was not influenced. In the presence of this stenosis the hematocrit was lowered from 45% to 15% in steps of 5% by isovolumic exchange of blood for dextran 60. At a hematocrit of 15% the blood flow through the narrowed coronary artery had increased by 47% due to the lowered viscosity. At the same time, however, the oxygen transport capacity of the diluted blood was reduced by 51% which resulted in a concomitant 46% decrease in the amount of systolic shortening (Table 2B). When the coronary artery stenosis had been removed, the blood flow increased due to both the lowered viscosity and reactive hyperemia to three-fold the control flow. With respect to oxygen transport this flow augmentation compensated for the lowered hematocrit; the oxygen supply reached again 94% of control and myocardial shortening fraction also returned to 94 + 6% of the control value before hemodilution.
Table 2
Alterations in coronary artery blood volume flow, oxygen delivery, and segmental ventricular wall shortening by different types of intervention in anesthetized dogs.
The fact that the myocardial shortening fraction almost linearly follows changes of oxygen delivery is further documented in an experimental model of anomalous origin of left coronary artery from the pulmonary artery—a rare congenital cardiac malformation.26 To study the effects of antegrade coronary artery perfusion with venous blood in dogs, the LAD has been cannulated and perfused via a flow-controlled pump with either arterial blood from the aorta or venous blood from the pulmonary artery. Oxygen content of the arterial blood was 193 + 7 ml O2 per liter of blood and it was 145 + 7 ml O2 per liter of venous blood. From actual coronary blood flow times oxygen content of the perfusate (arterial or venous blood) divided by heart rate, oxygen delivery to the myocardium has been calculated in microliter of oxygen per heart beat. Data corresponding to an oxygen delivery between 45–55% of control perfusion with arterial blood are averaged in Table 2C. At half the oxygen delivery, coronary perfusion with venous blood was reduced by only 29% in blood flow; at the same time systolic wall shortening decreased by 42% which was in the order of the averaged 49% reduction in oxygen delivery.
These results demonstrate that the systolic function of the myocardium directly depends on the actual oxygen delivery. On the other hand we know, of course, from many studies that acute improvements in oxygen supply do not always myocardial contraction. This leads to the definitions of hibernating, stunned, or infarcted myocardium.27 The experimental data presented in Tables 1 and 2 describe acute responses in the mechanical function of ventricular wall regions to acute reductions in oxygen supply. This close interplay gets lost after a prolonged period of severe oxygen deficiency when myocardial stunning develops. In 44 experiments with domestic pigs, myocardial stunning has been provoked according to the protocol described in detail by Schad et al.23 After 90 minutes of unimpeded reperfusion with arterial blood the spontaneous coronary blood flow has been recovered to 106 ± 4% of control value whereas systolic shortening of the dependent wall region remained markedly depressed at 36 ± 2%.
In 1978, we presented a clinical study about the acute effects of aortocoronary bypass surgery on left ventricular function and regional myocardial mechanics.28 At that time, neither the term ‘stunning’ nor ‘hibernation’ in myocardial ischemia had been coined. The study was performed in 22 men divided into two groups of 11 (Table 3). Patients were grouped according to the clinical status: Patients with a short history (< 6 months) of coronary artery disease but rapid progression of symptoms were appointed to the group of ‘unstable angina’, the others with documented history of previous myocardial infarction but free of symptoms at rest were classified as ‘stable angina’. All patients underwent aorto-coronary venous bypass grafting (ACVBG). Intraoperatively, during stable conditions 30 minutes after completion of cardiopulmonary bypass left ventricular pressure, ACVBG flow, and—by sonomicrometry—regional myocardial wall motion were assessed before, during, and after graft cross-clamping. Exclusively in patients (9 of 11 patients) considered to suffer previously from unstable angina, an acute positive functional response to revascularization could be verified. No acute functional improvements were observed in the other patients despite almost regular blood flow through the grafts and at least moderate reactive hyperemic response to graft cross-clamping in six patients. In two patients with intraoperatively continuing contraction disorders, postoperative examination revealed regular ventricular contraction. From these findings it was suggested 20 years ago “that there is some potential for long-term recovery in these hearts”.28 Nowadays, the lack of acute functional improvement might be interpreted as a property of ‘stunned myocardium’. In contrast, acute improvement of mechanical function after restoration of blood flow and, thus, energy supply as seen in 9 of 11 patients with unstable angina suggests to ‘hibernating myocardium’.
Table 3
Functional response of the myocardium to coronary revascularization in patients with aorto-coronary venous bypass graft (ACVBG) operation.
Unfortunately, hypokinesia per se gives no indication whether it is a transient expression to an acute energy deficiency or a lasting sign of previous severe ischemic injury. There are only a few less invasive examination procedures which might be suited in patients to unmask viable hibernating myocardium. Synchronized diastolic coronary venous retroperfusion seems to be one of these techniques.29,30 Whether this technique is of value to elucidate the anatomic location of viable myocardium depends on whether all the ventricular regions of interest are accessible to the retrograde perfusion in like manner.31,32
Conclusion
Performance of the heart results from a complex interplay between rate of fiber contraction, the fiber stress-strain relationship, the active state of the muscle, the force-velocity relationship, etc. Global pump performance is a function of locally different amount of mechanical wall function. This might be seen as a heterogeneity in myocardial function or as a consequence of “heart structure conditions function and vice versa”.33 In any case, left ventricular wall motion differs from region to region and is modulated by load alterations and by the response to changing metabolic requirements of the peripheral tissue. Thus, there are no definite set values which have to be fulfilled at any time by any part of the ventricle. Temporal and regional differences in left ventricular wall motion have to be validated with respect to their functional reserve. Functional reserve is assessed most reliably by load tests which are quite similar to those performed on an engine test bench. If a sufficient functional reserve can be mobilized we might suppose that the myocardial region under study works highly efficient at rest. If there is no positive response of locally hypokinetic regions to physical stress tests their possible viability might be discovered by studying local metabolism e.g., by PET. Recent developments in magnetic resonance imaging techniques and in three-dimensional echocardiographicy leave us confident that regional differences and variability in left ventricular wall motion will be ascertained in the near future, rapidly, and noninvasively. This should enable us to prepare maps and isograms of the heart with respect to mechanical function and efficiency as a base against which we can discriminate between regular myocardial contraction, apparently normal contraction but marginal energy supply, and ischemic hypokinesia.
Acknowledgment
The reported investigations were performed at the Department of Cardiovascular Surgery (Director: Prof. Fritz Sebening, MD; Prof. Hans Meisner, MD) and in the Cardiovascular Research Laboratory (Head: Prof. Nikolaus Mendler, MD) of the German Heart Center Munich, Germany.
References
- 1.
- Frank O. Zur Dynamik des Herzmuskels. Z Biol. 1895;32:370–437.
- 2.
- Starling EH. The Arris and Gale lectures on some points in the pathology of the heart disease. Lecture I: On the compensatory mechanisms of the heart. Lancet. 1897;1:569–572.
- 3.
- Schmid P, Niederer P, Lunkenheimer PP. et al. The anisotropic structure of the human left and right ventricles. Technol Health Care. 1997;5:29–43. [PubMed: 9134617]
- 4.
- Torrent-Guasp F, Whimster WF, Redmann K. A silicone rubber mould of the heart. Technol Health Care. 1997;5:13–20. [PubMed: 9134615]
- 5.
- Streeter D D Jr, Hanna WT. Engineering mechanics for successive states in canine left ventricular myocardium. Circ Res. 1973;33:639–664. [PubMed: 4762006]
- 6.
- Franklin DL, Kemper WS, Patrick T. et al. Techniques for continuous measurement of regional myocardial segment dimensions in chronic animal preparations. Fed Proc. 1973;32:343.
- 7.
- Heimisch W, Hagl S, Gebhardt K. et al. Direct measurement of cyclic changes in regional wall geometry in the left ventricle of the dog. Innov Tech Biol Med. 1981;2:487–501.
- 8.
- LeWinter M, Kent RS, Kroener JM. et al. Regional differences in myocardial performance in the left ventricle of the dog. Circ Res. 1975;37:191–199. [PubMed: 1149193]
- 9.
- Heimisch W, Hagl S, Janeczka I. et al. Regional differences in left ventricular wall motion. Eur Surg Res. 1981;13:85.
- 10.
- Heimisch W. Die Sonomikrometrie in der Herz- und Kreislaufforschung (Sonomicrometry in cardiovascular research) [Dr. rer. biol. hum. (PhD)]. Munich - Germany: Ludwig-Maximilians-Universität München, 1989.
- 11.
- Heimisch W, Hagl S, Mendler N. et al. Ventricular wall thickening determines the endo/epicardial shortening ratio: agreement of experimental results with geometric model. Eur Heart J. 1983;4(suppl E):35.
- 12.
- Ross J Jr, Franklin D. Analysis of regional myocardial function, dimensions, and wall thickness in the characterization of myocardial ischemia and infarction. Circulation. 1976;53(Suppl 1):I88–I92. [PubMed: 1253375]
- 13.
- Osakada G, Sasayama S, Kawai C. et al. The analysis of left ventricular wall thickness and shear by an ultrasonic triangulation technique in the dog. Circ Res. 1980;47:173–181. [PubMed: 7397950]
- 14.
- Heimisch W, Schad H, Mendler N. Coronary hyperperfusion in vivo: Effects on left ventricular wall geometry, myocardial performance, and coronary hemodynamics. Phys Med Biol. 1994;39a:200.
- 15.
- Heimisch W, Mohl W, Mendler N. et al. Intermittent coronary sinus occlusion: Effects on regional function of the normal and ischemic myocardium In: Mohl W, Wolner E, GlogarD, eds. The Coronary Sinus Darmstadt NewYork: Steinkopff, Springer-Verlag, 1984465–472.
- 16.
- Oh BH, Volpini M, Kambayashi M. et al. Myocardial function and transmural blood flow during coronary venous retroperfusion in pigs. Circulation. 1992;86:1265–1279. [PubMed: 1394933]
- 17.
- Maier SE, Fischer SE, McKinnon GC. et al. Evaluation of left ventricular segmental wall motion in hypertrophic cardiomyopathy with myocardial tagging. Circulation. 1992;86:1919–1928. [PubMed: 1451263]
- 18.
- Haas F, Haehnel CJ, Picker W. et al. Preoperative positron emission tomographic viability assessment and perioperative and postoperative risk in patients with advanced ischemic heart disease. J Am Coll Cardiol. 1997;30:1693–1700. [PubMed: 9385895]
- 19.
- Hagl S, Heimisch W, Meisner H. et al. The effect of hemodilution on regional myocardial function in the presence of coronary stenosis. Basic Res Cardiol. 1977;72:344–364. [PubMed: 901378]
- 20.
- Schad H, Heimisch W, Mendler N. Models of coronary artery disease: “critical” versus “functional” coronary artery stenosis. Thorac Cardiovasc Surg. 1991;39:13–18. [PubMed: 2011842]
- 21.
- Heimisch W, Mendler N, Schad H. et al. The pressure-dimension loop: At-a-glance indicator of the actual functional state of the myocardium In: Amlaner CJ, Jr., ed. Tenth International Symposium on Biotelemetry Fayetteville, AR, USA: University of Arkansas Press, Fayetteville London, 1989633–639.
- 22.
- Settergren G. The loop displayed before your eyes could one day tell you the secrets of the heart. J Cardiothorac Vasc Anesth. 1991;5:537–538. [PubMed: 1768814]
- 23.
- Takayama M, Norris RM, Brown MA. et al. Postsystolic shortening of acutely ischemic canine myocardium predicts early and late recovery of function after coronary artery reperfusion. Circulation. 1988;78:994–1007. [PubMed: 3168201]
- 24.
- Heimisch W, Schad H, Mendler N. et al. Cyclic motion of ischemic ventricular wall area and hydrodynamics of the blood during ejection. Thorac Cardiovasc Surg. 1991;39 Suppl 3:205–210. [PubMed: 1803631]
- 25.
- Schad H, Heimisch W, Eising GP. et al. Effect of milrinone and atrial pacing on stunned myocardium. Eur J Cardiothorac Surg. 1997;11:1125–1132. [PubMed: 9237598]
- 26.
- Heimisch W, Schad H, Mendler N. Coronary artery perfusion: significance of blood volume-flow, perfusion pressure, and oxygen delivery. Phys Med Biol. 1994;39a:202.
- 27.
- Opie LH. ed. Stunning, hibernation, and calcium in myocardial ischemia and reperfusion Boston Dordrecht London: Kluwer Academic Publ. 1992.
- 28.
- Hagl S, Meisner H, Heimisch W. et al. Acute effects of aortocoronary bypass surgery on left ventricular function and regional myocardial mechanics: a clinical study. Ann Thorac Surg. 1978;26:548–558. [PubMed: 313767]
- 29.
- Hajduczki I, Kar S, Areeda J. et al. Reversal of chronic regional myocardial dysfunction (hibernating myocardium) by synchronized diastolic coronary venous retroperfusion during coronary angioplasty. J Am Coll Cardiol. 1990;15:238–242. [PubMed: 2295736]
- 30.
- Nienaber CA, Abend M, Rehders TC. et al. Synchronized coronary venous retroperfusion: Can retrograde delivery of flow identify “hibernating myocardium”? Z Kardiol. 1993;82:415–424. [PubMed: 8379241]
- 31.
- Feld F, Ekas RD, Felli P. et al. Differential effects of synchronized coronary sinus retroperfusion on regional myocardial function during brief occlusion of the left anterior descending and circumflex coronary arteries. Cathet Cardiovasc Diagn. 1994;32:70–78. [PubMed: 8039224]
- 32.
- Wakida Y, Haendchen RV, Kobayashi S. et al. Percutaneous cooling of ischemic myocardium by hypothermic retroperfusion of autologous arterial blood: effects on regional myocardial temperature distribution and infarct size. J Am Coll Cardiol. 1991;18:293–300. [PubMed: 2050933]
- 33.
- Lunkenheimer PP. Structure conditions function and vice versa [editorial] Technol Health Care. 1997;5:1–12. [PubMed: 9134614]
- PubMedLinks to PubMed
- Regional Differences and Variability in Left Ventricular Wall Motion - Madame Cu...Regional Differences and Variability in Left Ventricular Wall Motion - Madame Curie Bioscience Database
- UI-H-BW1-anf-g-03-0-UI.s1 NCI_CGAP_Sub7 Homo sapiens cDNA clone IMAGE:3082180 3'...UI-H-BW1-anf-g-03-0-UI.s1 NCI_CGAP_Sub7 Homo sapiens cDNA clone IMAGE:3082180 3', mRNA sequencegi|11599688|gnl|dbEST|7041874|gb|BF 9.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...