NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Ever since the discovery of the transforming retroviral v-sis oncogene, which encodes PDGF-B, PDGF signaling has been an interesting target for cancer treatment. In last few years, compelling evidence supports the essential role of PDGF signaling for cancer cell proliferation and tumor angiogenesis in several types of human cancers, including nonmelanoma skin cancers. BCCs, the commonest human cancer, contain activation of the hedgehog pathway, frequently through inactivated mutations of tumor suppressor gene PTCH1. How does activation of the hedgehog pathway promote cell proliferation in the tumor? Our data indicate that PDGFRα activation is important for hedgehog signaling-mediated cell proliferation in BCCs. These findings not only detail the molecular basis of hedgehog-mediated tumorigenesis, but also provide new designs for skin cancer therapeutics.
Signal Transduction by PDGFs and Their Receptors
Platelet-derived growth factor (PDGF) is one of the first growth factors to be characterized.1 PDGF functions as a potent mitogen in mesenchymal and glial cells. In addition, PDGF regulates cell morphology and cell movement, such as chemotaxis. PDGF signaling is involved in embryogenesis, carcinogenesis, atherosclerosis and wound healing.2-5 During cancinogenesis, PDGF receptor is frequently activated through over-expression of the ligand and its receptor.
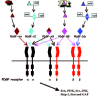
PDGF belongs to PDGF/VEGF family with several conserved cysteine residues. Four PDGF family members have been identified: the classical PDGF-A and PDGF-B, and the novel PDGF-C and PDGF-D.6-8 PDGF expression is observed in a variety of cell types, including fibroblasts, keratinocytes, neurons, endothelial and epithelial cells, which can be regulated by external factors such as cytokines and thrombin.4 PDGF subunits are synthesized as precursor molecules, which undergo proteolytic processing. A variety of PDGF dimers can be formed from these subunits (fig. 1), which then activate the PDGF receptor tyrosine kinases.
PDGF receptors contain an extracellular loop for association of the ligand, a transmembrance domain and an intracellular tail with a tyrosine kinase domain. PDGFs activate their receptors by promoting formation of receptor dimers: PDGFRαα, PDGFRαβ and PDGFRββ (fig. 1). PDGF-dependent receptor dimerization triggers receptor autophosphorylation and subsequent signal transduction. The specific cellular functions of each PDGF subunit will partly depend on the availability of the receptor dimers (fig.1). PDGF receptors function in the cell through activating downstream effectors, such as PLC-γ, Grb2/ SOS, PI3K, GAP and Stat (fig. 1).
In Vivo Functions of PDGFs and Their Receptors
Studies of knockout mice have shielded light on the in vivo roles of PDGFs and their receptors. All PDGF-A, PDGF-B, PDGFRα or PDGFRβ knockout are embryonic lethal whereas heterozygous mice are viable.4
PDGFRα null mice show the most severe phenotype. PDGFRα-/- mice display cleft face, spinal bifida, skeletal and cardial defects, resulting embryonic lethality between E8 and E16.9 In comparison, PDGF-A deletion causes defect in alveoli development of lung, and the mutant mice die perinatally.10 These studies indicate that PDGFRα might mediate functions of other PDGF isoforms in addition to PDGF-A. Analyses of PDGF-A and PDGFRα null mice reveal that PDGF-A is often expressed in epithelium, muscle or neural tissues whereas PDGFRα expresses in the mesenchymal compartment, suggesting a paracrine mechanism of signaling (Table 1). The expression pattern of PDGF-C is similar to that of PDGF-A, suggesting an overlapping function of the two. It is therefore reasonable to believe that PDGF-C may be accountable for the phenotypic differences between PDGF-A null mice and PDGFRα null mice. PDGF-A, PDGF-C and PDGFRα are reported to be involved in intestinal, lung, skin, hair, testis and oligodendrocyte development.5
Table 1
In vivo functions of classic PDGFs and their receptors.
In contrast, PDGF-B and PDGFRβ null mice appear to be identical, and the mice die perinatally from cardiovascular complications.5 Both PDGF-B and PDGFRβ have a similar pattern of expression. PDGFRβ positive mesencymal cells are frequently in close contact with PDGF-B expressing endothelial cells (Table 1), further indicating a paracrine signaling mechanism. In addition to blood vessel formation, PDGF-B and PDGFRβ are known to be involved in development of kidney and placenta.4,5
Development of skin and hair requires the PDGF-A/ PDGFRα signaling axis. PDGF-A expresses in skin epidermis while PDGFRα is in the mesenchyme. PDGFRα null embryos show severe dermal defects, with a general absence of dermal mesenchymal cells and formation of epidermal blisters.9 In contrast, the phenotype of PDGF-A null mutant embryos is quite mild,5 suggesting a substitutive role of PDGF-C in skin development. Development of hair follicle is a typical process of epithelium-mesenchymal interactions, with PDGF-A in hair follicle epithelium and PDGFRα in dermal papillae. Hair follicles of PDGF-A-/- mice are misshaped and smaller.5
PDGF pathways are altered in several physiological human conditions such as atherosclerosis, fibrosis of lung and kidney, oncogenesis and developmental defects of neural tubes. A number of reviews are available for PDGF pathways in human diseases.3-5 In this chapter, we will summarize the recent development in human cancer, particularly in nonmelanoma skin cancers.
PDGF Alterations in Cancer
The role of PDGFs in carcinogenesis is initially demonstrated by the fact that v-sis oncogene encodes a PDGF-B-like protein. Both v-sis and its cellular counterpart c-sis transform cultured cells through an autocrine mechanism. Studies in last two decades clearly indicate that PDGFs and their receptors are involved in human cancers through autocrine stimulation of tumor cell growth.2,3 There is compelling evidence for the role of PDGF signaling in Gliomas, Gastrointestinal stromal tumor (GIST) and myelomonocytic leukaemia. Only recently, the role of PDGF signaling for nonmelanoma skin cancer (particularly basal cell carcinomas and dermafibrosarcoma protuberans) has been demonstrated, which will be described in details later (see Table 2 for the summary).
Table 2
PDGF signaling in human cancers.
In human gliomas, PDGF-A and PDGFRα are over-expressed in the tumor with an increase in the high-grades.2 Interestingly, PDGFRα expression is particularly high in tumors with no amplification of EGF receptor. The protein over-expression is often accompanied by PDGFRα gene amplification. Compelling evidence is provided by Clarke and Dirks that an activated deletion mutant of PDGFRα is oncogenic and is amplified in subsets of primary gliomas.11 High levels of all PDGF subunits are detected in the tumor. As a result, the downstream effectors are activated. Additional evidence supports that autocrine stimulation of cell proliferation by PDGF is sufficient for glioma formation in mice. While injection of recombinant PDGF-B retrovirus into mouse brain leads to formation of gliomas,12 gene transfer of PDGF-B into mouse neural progenitor cells results in oligodendrogliomas.13 Furthermore, PDGFRα inhibitors14,15 specifically prevent cell growth in cultured tumor cells. Thus, it appears that activation of PDGFRα is critically important for glioma development.
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the human gastrointestinal tract. The gain-of-function mutations in the c-kit gene (90%) or platelet-derived growth factor receptor alpha (PDGFRα) gene (5%) are now considered to be causative for GIST.16 Mutations of PDGFRα and Kit are mutually exclusive, indicating that activation of one PDGF receptor is sufficient to activate the downstream signaling. Mice with knock-in of activated kit develop symptoms of GIST and later die of the disease,17 further supporting the role of Kit/PDGFRα in GIST. Furthermore, mutations of kit or PDGFRα are prognostic factors for GIST patients treated with PDGF receptor inhibitor Sleevec.18
In subsets of chronic myelomonocytic leukaemia (CML), the t(5;12) translocation results in fusion of the amino terminus of TEL to the transmembrane and cytoplasmic domains of the PDGFRβ.19 TEL-PDGFRβ fusion protein is constitutively active through autophosphorylation, which confers IL-3-independent cell growth.20 Similarly, a rare t(4;22) translocation in CML generates fusion of BCR-PDGFRα, resulting in constitutive activation of PDGFRα.21 Treatment of Gleevec, which inhibits both c-Abl and PDGFR activities, has shown a very good response in PDGF receptor activated-CML patients, providing additional evidence for the role of PDGF signaling in subsets of CML.
High levels of PDGFs and their receptors are important diagnostic markers for medulloblastomas. PDGFRα is highly expressed in 30% of medulloblastomas. The activity of PDGFRα is likely driven by expression of PDGF-C.22 Gene expression profiling studies reveal a high level expression of PDGFRβ in metastatic medulloblastomas.23 PDGF-D also highly expresses in medulloblastomas.
In addition to the autocrinal regulation of tumor growth, PDGF signaling exerts paracrinal stimulation on stroma cells. Such an example is shown in tumor angiogenesis, which has been observed in breast cancer, colorectal cancer, melanoma and small cell lung cancer.3 The level of PDGFs is correlated with the density of blood vessel in the tumor. It is shown that xenotransplanted melanomas with expression of PDGF-B form highly vascularized and stromal-rich tumors whereas melanomas without PDGF form poorly vascularized and necrotizing tumors with undetectable stroma.2 In addition, PDGF signaling indirectly regulates angiogenesis. PDGF receptor beta can induce the transcription and secretion of VEGF, which plays an important role in tumor angiogenesis. Over-expression of PDGFs and their receptors is also observed in many other types of cancers, such as prostate cancer and nonsmall cell lung cancer. However, their roles for tumor proliferation remain to be demonstrated.
The Role of PDGF for Cell Proliferation in BCCs
The link between the hedgehog pathway and human cancer was first revealed in Gorlin syndrome, a disease with a high risk of basal cell carcinomas (BCCs).24-26 It is demonstrated that loss-of-function mutations of the patched gene (PTCH1) are cause of Gorlin syndrome. Basal cell carcinomas (BCCs), the commonest human cancer, consistently have abnormalities of the hedgehog pathway and often have lost the function of PTCH1 via point mutations and loss of the remaining allele.27-30 Most PTCH1 mutations lead to loss of the protein function. Mice heterozygous for a PTCH1 null mutation exhibit a high risk of cancers such as medulloblastomas, rhabdomyosarcomas and basal cell carcinomas, confirming that PTCH1 functions as a tumor suppressor.31-33 Currently, Ptch1+/- mice represent the most practical model of UV-mediated BCC formation.33 In addition to PTCH1, somatic mutations of smoothened (SMO), a putative seven-transmembrane protein of the hedgehog pathway, occur in sporadic BCCs.34-36 Activation of the hedgehog pathway is also found in medulloblastomas, small cell lung cancer and GI-tract cancers (Table 3).37-40 These findings provide additional insight into the role of the sonic hedgehog pathway in human cancer.
Table 3
Aberrant hedgehog signaling in human cancer.
Increasing evidence indicates the role of hedgehog signaling for cell proliferation. In the cerebellum, Shh, which is produced by Purkinje cells,41,42 induces proliferation of granule cell precursors in culture, and in vivo administration of Shh-neutralizing antibodies causes a dramatic reduction in the number of granule cells. Shh exerts its cell proliferation effects possibly through inducing expression of N-myc and cyclin D1.43 In epidermis, Shh promotes proliferation of hair lineage progenitors whereas Ihh stimulates proliferation of sebocyte precursors.44,45 Shh signaling is required for early development of hair follicles.46 Follicle development in the Shh mutant embryo arrests after the initial epidermal-dermal interactions. When grafted to nude mice (which lack T cells), large abnormal follicles with small dermal papillae can be seen but no hair is formed. In addition, Ptch1+/- mice are generally larger than the wild type mice, suggesting that the hedgehog pathway may be required for regulating the body size.47
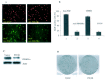
Gli1 has been frequently used as a biological probe to identify molecules that mediate BCC cell proliferation.48 In human BCCs, Gli1 is consistently up-regulated, suggesting that Gli1 may be a major downstream mediator for tumor formation. Using the hedgehog-responsive cell line C3H10T1/2, Gli1 activates PDGFRα expression and enhances its activation (fig. 2A). In consistent with this regulation, the level of phospho-Erk (fig. 2B), an effector of PDGF signaling, is up-regulated in Gli1 expressing cells. Similarly, a Ras/Erk pathway luciferase reporter gene can be activated by Gli1, indicating that Gli1 can activate the Ras/Erk pathway, possibly through functional activation of PDGFRα (fig. 2C).
PDGFRα signaling requires association with its ligands. PDGF-A is highly transcribed in C3H10T1/2 cells regardless of Gli1 expression, and the protein can be detected by ELISA. PDGF-A neutralizing antibodies, which deplete active PDGF-A in the culture medium, significantly reduce Gli1-mediated Ras/Erk activation, indicating the role of PDGF/PDGFR signaling axis for Gli1 functions. Similarly, PDGFRα neutralizing antibodies, which inactivate PDGFRα, prevents Gli1-mediated Ras/Erk activation (fig. 2C). These data demonstrate that PDGFRα mediates Gli1-induced Ras/Erk activation.
The in vitro data in C3H10T1/2 cells suggest that functional up-regulation of PDGFRα could be an important consequence following Gli1 upregulation in BCCs in vivo. PDGFRα expression is high in BCCs derived from Ptch1+/- mice (fig. 3A). The ligand, PDGF-A, is also expressed in the tumor (fig. 3B). This immunohistostaining is further confirmed by Western blot analysis in a mouse BCC cell line ASZ001 (fig. 3C). Thus, in this well-defined genetic system where the hedgehog pathway is constitutively activated, PDGFRα expression is very high, suggesting in vivo regulation of PDGFRα by the hedgehog pathway.48 In most human BCCs, PDGFRα are expressed in the tumor (fig. 3). This protein is distributed mostly in the tumor nest (fig. 3, 11 of 14). Sometimes, strong staining of PDGFRα is also seen in the stroma (fig. 3EE). The ligand PDGF-A is highly expressed in the tumor (fig. 3F). These immunohistochemstry data are confirmed by western blotting analysis (fig. 3). PDGFRα expression in the tumor is always accompanied by a high level of phosphorylated Erk, indicating an activation of the Ras/Erk pathway. The high levels of PDGFRα and of its ligand PDGF-A in the tumor imply their importance for BCC development.
The role of PDGFRα for cell proliferation of BCCs is demonstrated using PDGFRα neutralizing antibodies in ASZ001 cells. Both copies of the Ptch1 gene are lost in the ASZ001 cells, thus resembling the PTCH1 status in human BCCs. This cell line contains a high level of PDGFRα (fig. 3). After treatment with anti-PDGFRα neutralizing antibodies, DNA synthesis index is reduced by 70% (fig. 4B). In contrast, FGF neutralizing antibody or purified goat IgG do not affect DNA synthesis. Furthermore, a downstream MEK inhibitor U0126 inhibits DNA synthesis in this cell line (fig. 4). Therefore, increased expression of PDGFRα and activation of the ERK pathway appears to be important for cell proliferation in the ASZ001 cells.
To test for a direct link between the hedgehog pathway and PDGFRα, PTCH1 is reexpressed in Ptch1 null ASZ001 cells, which leads to down-regulation of PDGFRα protein (fig. 4C). This reduced expression of PDGFRα is correlated with reduced cell proliferation (fig. 4). Thus, down-regulation of the hedgehog pathway can reduce the expression of PDGFRα and can inhibit cell proliferation.
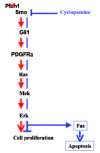
All these data indicate that PDGFRα can be regulated by Gli1 and that PDGFRα mediates Gli1-induced Ras/Erk activation. The expression of PDGFRα is up-regulated in BCCs of mice and humans. In the mouse BCC cell line ASZ001, perturbation of PDGFRα function, whether directly by neutralizing antibodies or indirectly by PTCH1, leads to decreased cell proliferation (fig. 5). Therefore, up-regulation of PDGFRα appears to be an important mechanism by which hedgehog signaling induces basal cell carcinomas.
Consistent with those findings, we have found that inhibition of the hedgehog signaling by SMO antagonist cyclopamine decreases the levels of PDGFRα and phospho-Erk, resulting apoptosis in ASZ001 cell (fig. 6A). (Athar et al, 2004, submitted). Because cyclopamine inhibits PDGFRα expression, PDGF-A is unable to prevent cyclopamine-mediated cell death. By contrast, EGF, which can activate the Ras-Erk pathway independent of PDGFRα, inhibits cyclopamine-mediated cell death (fig. 6B). Thus, it appears that SMO antagonist cyclopamine inhibits PDGFRα expression and down-regulates Ras-Erk signaling in BCC cells.
The Role of PDGF/VEGF Signaling in Angiogenesis of SCCs
The role of PDGF for tumor development of squamous cell carcinomas is not well studied. However, there is a good correlation of angiogenic cytokine secretion with the microvessel density in the primary tumors of SCC.49 In both BCCs and SCCs, PDGF-A is elevated, suggesting the PDGF/PDGFR signaling axis may be a common mediator of epidermal hyperproliferation. In head and neck SCCs, VEGF and PDGF-AB are secreted in high amounts.50 Keratinocytes are a major source of cutaneous PDGF whereas human dermal fibroblasts do not produce any detectable PDGF.51
The role of PDGF for angiogenesis is demonstrated by Dr. Fusenig's group using HaCaT cells.52 After transfection with PDGF-B cDNA, HaCaT cells overexpress PDGF-B but are negative for the PDGF receptors alpha and beta (mRNA). Thus, they do not exhibit autocrine growth stimulation in vitro, but proliferation of cocultured fibroblasts is enhanced and this effect can be inhibited by neutralizing antibodies to PDGF-BB. After subcutaneous injection into nude mice, the transfected cells maintain high PDGF expression and form rapidly proliferating cysts, classified as benign tumors. During early tumor development (up to 2 months), PDGF-B transfectants induce marked mesenchymal cell proliferation and angiogenesis, yet this effect vanished at later stages (2-6 months) concomitantly with increased epithelial cell proliferation and enhanced tumor growth. These results demonstrate that an activated stromal environment can promote tumorigenic conversion of nontumorigenic keratinocytes by inducing sustained epithelial hyperproliferation. Thus, PDGF-BB appears to promote tumor growth by inducing angiogenesis and stromal formation, and PDGF-activated stromal cells maintain elevated keratinocyte proliferation via a paracrine mechanism.
PDGF Signaling in Dermatofibrosarcoma Protuberans and Giant Cell Fibroblastoma
Dermatofibrosarcoma protuberans (DFSP) and giant cell fibroblastoma (GCF) are recurrent, infiltrative skin tumors of intermediate malignancy, presents specific features such as reciprocal translocations t(17;22)(q22;q13) and supernumerary ring chromosomes derived from the t(17;22). This chromosomal rearrangement generates a fusion gene of the collagen type I to the PDGF-B.53 This gene fusion deletes exon 1 of PDGF and released the growth factor from its normal regulation and is capable of transforming cultured cells.54 PDGF receptors are activated in primary cultures derived from DFSP and GCF tumors. Autocrine PDGF receptor stimulation is therefore predicted to contribute to DFSP and GCF development.
Evidence suggests that the primary cultured cells of DFSP and GCF are sensitive to treatment of PDGF receptor inhibitor Sleevec,55 resulting in reduced cell proliferation. Treatment of DFSP tumor-bearing mice with this inhibitor also decreases tumor growth. These findings suggest targeting of PDGF receptors as a novel treatment strategy for DFSP patients. In fact, clinical trials of STI571 have some good responses in DFSP and GCF patients,56 further supporting the role of PDGF signaling in the development of DFSP and GCF.
Perspectives
The platelet-derived growth factors (PDGF) are a pleotrophic family of peptide growth factors that signal through cell surface, tyrosine kinase receptors (PDGFR) and stimulate various cellular functions including growth, proliferation, and differentiation. Ever since the discovery of the transforming retroviral v-sis oncogene, PDGF signaling has been an interesting target for cancer treatment. To date, PDGF expression has been demonstrated in a number of different solid tumors, from glioblastomas to prostate carcinomas. Only in recent years, the role of PDGF for proliferation of nonmelanoma skin cancers has been established. The autocrine growth stimulation of tumor cells by PDGF is well demonstrated in many types of tumors including DFSP and BCCs of the skin. In addition, PDGF can regulate stromal cells through a paracrine mechanism, which is observed in skin SCCs and melanomas. Improved methods for detection of activated PDGF receptors would be most useful for screens of PDGF signaling activated tumors. Just as the discovery of ErbB2/Nu in breast cancer leads to a revolution of breast cancer treatment, clinically useful PDGF receptor antagonists, like Gleevec (STI571/Glivec), now allows for an evaluation of the importance of PDGF receptor signaling in a variety of cancers, including BCCs and SCCs of the skin. Thus, discovering the importance of PDGF signaling for the development of BCCs not only provides a molecular basis of hedgehog signaling-mediated tumorigenesis, but also has significant therapeutic implications. Thus far, there are several emerging possibilities for BCC treatments in addition to cryosurgery: suppression of the sonic hedgehog pathway (Athar et al, 2004, submitted), interferon alpha treatment57 and inhibition of PDGF signaling. Additional research of PDGF signaling in other cutaneous malignancies will certainly help us design better ways to treat these cancers.
Acknowledgement
This work was supported in part by National Institute of Health (NIH) grant R01- CA94160 and Department of Defense grant PC030429. Due to space limitation, only selected references are listed in this review.
References
- 1.
- Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378(1):F79–113. [PubMed: 9739761]
- 2.
- Ostman A, Heldin CH. Involvement of platelet-derived growth factor in disease: Development of specific antagonists. Adv Cancer Res. 2001;80:1–38. [PubMed: 11034538]
- 3.
- Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol. 2003;36(1):49–59. [PubMed: 12542975]
- 4.
- Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. [PubMed: 12952899]
- 5.
- Betsholtz C. Biology of platelet-derived growth factors in development. Birth Defects Res Part C Embryo Today. 2003;69(4):272–285. [PubMed: 14745969]
- 6.
- Li X, Ponten A, Aase K. et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302–309. [PubMed: 10806482]
- 7.
- LaRochelle WJ, Jeffers M, McDonald WF. et al. PDGF-D, a new protease-activated growth factor. Nat Cell Biol. 2001;3(5):517–521. [PubMed: 11331882]
- 8.
- Bergsten E, Uutela M, Li X. et al. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3(5):512–516. [PubMed: 11331881]
- 9.
- riano P. e PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. velopment. 97;4(4):91–2700. [PubMed: 9226440]
- 10.
- Bostrom H, Willetts K, Pekny M. et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85(6):863–873. [PubMed: 8681381]
- 11.
- Clarke ID, Dirks PB. A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene. 2003;22(5):722–733. [PubMed: 12569364]
- 12.
- Uhrbom L, Hesselager G, Nister M. et al. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58(23):5275–5279. [PubMed: 9850047]
- 13.
- Dai C, Celestino JC, Okada Y. et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. [PMC free article: PMC312748] [PubMed: 11485986]
- 14.
- Kilic T, Alberta JA, Zdunek PR. et al. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 2000;60(18):5143–5150. [PubMed: 11016641]
- 15.
- Lokker NA, Sullivan CM, Hollenbach SJ. et al. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62(13):3729–3735. [PubMed: 12097282]
- 16.
- Heinrich MC, Corless CL, Duensing A. et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. [PubMed: 12522257]
- 17.
- Sommer G, Agosti V, Ehlers I. et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the kit receptor tyrosine kinase. Proc Natl Acad Sci USA. 2003;100(11):6706–6711. [PMC free article: PMC164511] [PubMed: 12754375]
- 18.
- Heinrich MC, Corless CL, Demetri GD. et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349. [PubMed: 14645423]
- 19.
- Golub TR, Barker GF, Lovett M. et al. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77(2):307–316. [PubMed: 8168137]
- 20.
- Carroll M, Tomasson MH, Barker GF. et al. The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways. Proc Natl Acad Sci USA. 1996;93(25):14845–14850. [PMC free article: PMC26224] [PubMed: 8962143]
- 21.
- Baxter EJ, Hochhaus A, Bolufer P. et al. The t(4;22)(q12;q11) in atypical chronic myeloid leukaemia fuses BCR to PDGFRA. Hum Mol Genet. 2002;11(12):1391–1397. [PubMed: 12023981]
- 22.
- Andrae J, Molander C, Smits A. et al. Platelet-derived growth factor-B and -C and active alpha-receptors in medulloblastoma cells. Biochem Biophys Res Commun. 2002;296(3):604–611. [PubMed: 12176024]
- 23.
- Gilbertson RJ, Clifford SC. PDGFRB is overexpressed in metastatic medulloblastoma. Nat Genet. 2003;35(3):197–198. [PubMed: 14593398]
- 24.
- Johnson RL, Rothman AL, Xie J. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272(5268):1668–1671. [PubMed: 8658145]
- 25.
- Hahn H, Wicking C, Zaphiropoulous PG. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. [PubMed: 8681379]
- 26.
- Xie J, Quinn A, Zhang X. et al. Physical mapping of the 5 Mb D9S196-D9S180 interval harboring the basal cell nevus syndrome gene and localization of six genes in this region. Genes Chromosomes Cancer. 1997;18(4):305–309. [PubMed: 9087571]
- 27.
- Quinn AG, Epstein JrE. Patched, hedgehog, and skin cancer. Methods Mol Biol. 2003;222:85–95. [PubMed: 12710681]
- 28.
- Bale AE, Yu KP. The hedgehog pathway and basal cell carcinomas. Hum Mol Genet. 2001;10(7):757–762. [PubMed: 11257109]
- 29.
- Toftgard R. Hedgehog signaling in cancer. Cell Mol Life Sci. 2000;57(12):1720–1731. [PubMed: 11130178]
- 30.
- Wicking C, Bale AE. Molecular basis of the nevoid basal cell carcinoma syndrome. Curr Opin Pediatr. 1997;9(6):630–635. [PubMed: 9425597]
- 31.
- Goodrich LV, Milenkovic L, Higgins KM. et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–1113. [PubMed: 9262482]
- 32.
- Hahn H, Wojnowski L, Zimmer AM. et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4(5):619–622. [PubMed: 9585239]
- 33.
- Aszterbaum M, Epstein J, Oro A. et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5(11):1285–1291. [PubMed: 10545995]
- 34.
- Xie J, Murone M, Luoh SM. et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. [PubMed: 9422511]
- 35.
- Lam CW, Xie J, To KF. et al. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18(3):833–836. [PubMed: 9989836]
- 36.
- Reifenberger J, Wolter M, Weber RG. et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58(9):1798–1803. [PubMed: 9581815]
- 37.
- Xie J, Johnson RL, Zhang X. et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57(12):2369–2372. [PubMed: 9192811]
- 38.
- Berman DM, Karhadkar SS, Maitra A. et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumors. Nature. 2003;425:846–51. [PubMed: 14520411]
- 39.
- Thayer S. et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. [PMC free article: PMC3688051] [PubMed: 14520413]
- 40.
- Watkins DN, Berman DM, Burkholder SG. et al. Hedgehog signaling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–317. [PubMed: 12629553]
- 41.
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–114. [PubMed: 10027293]
- 42.
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9(8):445–448. [PubMed: 10226030]
- 43.
- Oliver TG, Grasfeder LL, Carroll AL. et al. Transcriptional profiling of the Sonic hedgehog response: A critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci USA. 2003;100(12):7331–7336. [PMC free article: PMC165875] [PubMed: 12777630]
- 44.
- Allen M, Grachtchouk M, Sheng H. et al. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163(6):2173–2178. [PMC free article: PMC1892397] [PubMed: 14633591]
- 45.
- Niemann C, Unden AB, Lyle S. et al. Indian hedgehog and beta-catenin signaling: Role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11873–11880. [PMC free article: PMC304101] [PubMed: 12917489]
- 46.
- Mill P, Mo R, Fu H. et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17(2):282–294. [PMC free article: PMC195973] [PubMed: 12533516]
- 47.
- Milenkovic L, Goodrich LV, Higgins KM. et al. Mouse patched1 controls body size determination and limb patterning. Development. 1999;126(20):4431–4440. [PubMed: 10498679]
- 48.
- Xie J, Aszterbaum M, Zhang X. et al. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci USA. 2001;98(16):9255–9259. [PMC free article: PMC55407] [PubMed: 11481486]
- 49.
- Ninck S, Reisser C, Dyckhoff G. et al. Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck. Int J Cancer. 2003;106(1):34–44. [PubMed: 12794754]
- 50.
- Gleich LL, Srivastava L, Gluckman JL. Plasma platelet-derived growth factor: Preliminary study of a potential marker in head and neck cancer. Ann Otol Rhinol Laryngol. 1996;105(9):710–712. [PubMed: 8800057]
- 51.
- Zhang JZ, Maruyama K, Ono I. et al. Production and secretion of platelet-derived growth factor AB by cultured human keratinocytes: Regulatory effects of phorbol 12-myristate 13-acetate, etretinate, 1,25-dihydroxyvitamin D3, and several cytokines. J Dermatol. 1995;22(5):305–309. [PubMed: 7673548]
- 52.
- Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70(9-10):486–497. [PubMed: 12492491]
- 53.
- Simon MP, Pedeutour F, Sirvent N. et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15(1):95–98. [PubMed: 8988177]
- 54.
- Greco A, Fusetti L, Villa R. et al. Transforming activity of the chimeric sequence formed by the fusion of collagen gene COL1A1 and the platelet derived growth factor b-chain gene in dermatofibrosarcoma protuberans. Oncogene. 1998;17(10):1313–1319. [PubMed: 9771975]
- 55.
- Sjoblom T, Shimizu A, O'Brien KP. et al. Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res. 2001;61(15):5778–5783. [PubMed: 11479215]
- 56.
- Rubin BP, Schuetze SM, Eary JF. et al. Molecular targeting of platelet-derived growth factor B by imatinib mesylate in a patient with metastatic dermatofibrosarcoma protuberans. J Clin Oncol. 2002;20(17):3586–3591. [PubMed: 12202658]
- 57.
- Li CX, Chi S, He N. et al. INF alpha induces Fas expression and apoptosis in hedgehog pathway activated BCC cells through inhibiting Ras-Erk signaling. Oncogene. 2004;23(8):1608–17. [PubMed: 14647422]
- Signal Transduction by PDGFs and Their Receptors
- In Vivo Functions of PDGFs and Their Receptors
- PDGF Alterations in Cancer
- The Role of PDGF for Cell Proliferation in BCCs
- The Role of PDGF/VEGF Signaling in Angiogenesis of SCCs
- PDGF Signaling in Dermatofibrosarcoma Protuberans and Giant Cell Fibroblastoma
- Perspectives
- References
- PDGF Pathways and Growth of Basal Cell and Squamous Cell Carcinomas - Madame Cur...PDGF Pathways and Growth of Basal Cell and Squamous Cell Carcinomas - Madame Curie Bioscience Database
- Phylogenetic, Structural and Functional Relationships between WD-and Kelch-Repea...Phylogenetic, Structural and Functional Relationships between WD-and Kelch-Repeat Proteins - Madame Curie Bioscience Database
- PROMOTER-ASSOCIATED LONG NONCODING RNAs REPRESS TRANSCRIPTION THROUGH AN RNA BIN...PROMOTER-ASSOCIATED LONG NONCODING RNAs REPRESS TRANSCRIPTION THROUGH AN RNA BINDING PROTEIN TLS - Madame Curie Bioscience Database
- The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exoc...The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exocytosis - Madame Curie Bioscience Database
- Circadian Organization in the Algal Flagellate Euglena - Madame Curie Bioscience...Circadian Organization in the Algal Flagellate Euglena - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...