NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Fumonisins have considerable structural similarity to sphinganine, as illustrated in Figure 1, for one of the most prevalent species (fumonisin B1, FB1) and its backbone aminopentol (AP1). Fumonisins inhibit ceramide synthase, and perturbation of sphingolipid metabolism accounts for many of the pathological effects of consumption of these mycotoxins.1,2 In part because it is commercially available, many labs have utilized FB1 to explore the functions of complex sphingolipids (see ref. 3, for examples). Nonetheless, the cellular effects of fumonisins can be complex, constituting not only suppression of complex sphingolipid formation but also the accumulation of sphinganine and other bioactive metabolites.1,4,5 To understand how fumonisins affect sphingolipid metabolism as well as gain better insight into the behavior of other commonly encountered species, we have employed a variety of methods, including liquid chromatography electrospray tandem mass spectrometric (LC-MS/MS), to analyze essentially all of the pertinent bioactive sphingolipids (e.g., sphingoid bases, sphingoid base 1-phosphates, ceramides, sphingomyelins, glucosylceramides, inter alia)5 and to characterize fumonisin metabolites.6 Some of the findings will be summarized in this review.
Inhibition of Ceramide Synthase
In the current model for how FB1 inhibits ceramide synthase, it has been proposed1 that the aminopentol backbone competes for binding of the sphingoid base substrate whereas the tricarballylic acid side-chain interferes with utilization of the co-substrate fatty acyl-CoA, as can be envisioned by overlaying the structures in Figures 1A and B. Some of the evidence that supports this model is: 1) the potency of inhibition by FB1 depends on the concentrations of both sphingoid bases and fatty acyl-CoA6,7; 2) removal of the tricarballylic acids diminishes the potency of ceramide synthase inhibition6,8 and, 3) upon removing the tricarballylic acids, AP1 becomes a substrate for acylation (whereas FB1 is not acylated).6 As will be discussed later, this acylation may explain why aminopentols remain toxic to animals despite the lower potency of these compounds as ceramide synthase inhibitors.
When added to mammalian cells in culture, FB1 is usually effective in blocking the formation of ceramide, SM and glucosylceramide, and more complex sphingolipids, measured by incorporation of radiolabelled serine7 or of mass5,9 (see below). Blockage of complex sphingolipid formation must be confirmed for each case because some organisms, such as yeast,10 take up fumonisins poorly unless treated specially and there are cases where other enzymmatic reactions, such as reversal of ceramidase,11,12 can provide an alternative pathway for ceramide synthesis. It is also advisable to check the effectiveness of fumonisin stock solutions occasionally because potency is sometimes lost during storage, presumably due to hydrolysis of the ester-linked tricarballylic acid(s).
Analysis of Sphingolipids in Fumonisin-Treated Cells
Conventional methods for analysis of sphingolipids are sufficient to determine if FB1 is blocking de novo synthesis. Among these, the most convenient are measurement of the incorporation of radiolabelled serine or palmitic acid into sphingolipids13,14 or elevation of sphinganine (by HPLC with a fluorescence detector).15,16 In addition to the relative convenience of the latter, it serves to remind the investigator that the elevation in sphinganine can be dramatic, as high as several nmol/106 cells, amounts that are high enough to affect signaling pathways, such as the inhibition of protein kinase C.17 These conventional assays are labor intensive, provide little information about structural variants within each class (for example, the different molecular subspecies of ceramides), and are not very efficient at extracting and detecting polar compounds such as the sphingoid base 1-phosphates.
Analysis of Sphingolipids by Electrospray Tandem Mass Spectrometry
Heretofore, mass spectrometry has been used primarily for structural elucidation of sphingolipids (reviewed in ref. 18); however, the development of electrospray ionization (ESI) provides the ability to generate intact molecular ions of polar biomolecules directly from solution (i.e., the eluate from HPLC columns) and to perform quantitative analysis. Equally important has been the availability of a method to quantify different species (multiple reaction monitoring, illustrated in Fig. 3) and the commercial availability of internal standards for sphingoid bases (C20-sphingosine and -sphinganine), sphingoid base 1-phosphates (the C17 homologs), ceramides, sphingomyelins, glucosylceramide and lactosylceramide (all prepared as the C12-fatty acid homologs) (Avanti Polar Lipids, Alabaster, AL). With these capabilities, a step-by-step procedure has been developed for the extraction, analysis, and quantitation of all of these categories of sphingolipids (as well as lysosphingolipids and N-methylsphingoid bases) using HPLC electrospray tandem mass spectrometry.5 Its sensitivity allows analysis of all of these compounds in samples as small as a petri dish of cells in culture.

Figure 3
Illustration of the advantage of using multiple reaction monitoring (MRM) in the quantitative analysis of sphingolipids. Data from ref. .
Effects of Fumonisin B1 on Sphingolipid Metabolism
Analyses of sphingolipid metabolism using diverse methods, most of which have now been confirmed by mass spectrometry, reveal interesting changes in the amounts of sphingolipids in cells. Pathways of sphingolipid metabolism that participate in the response to fumonisins are shown in Figure 2.
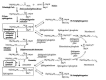
Figure 2
Pathway for sphingoid base biosynthesis with illustration of the side reactions (phosphorylation and acetylation) that can increase when sphinganine and sphingosine are elevated by (dihydro)ceramide synthase inhibition by fumonisins.
Elevation of Sphinganine
Elevations in cellular sphinganine remain the hallmark of fumonisin's effects on cells in culture and in vivo (see ref. 1 for review). An example of the magnitude of the elevations in sphinganine and sphingosine is shown in Figure 3. The amount of sphinganine in these cells (ca 2 nmol/106 cells) is at or above the levels that affect diverse cell signaling pathways when added to cells exogenously.17,19 Sphingosine is also sometimes elevated (presumably due to inhibition of the recycling of sphingosine produced during complex sphingolipid turnover), but the amounts are typically smaller than sphinganine (c.f. Fig. 2).
Depletion of More Complex Sphingolipids
Fumonisins can completely block synthesis of new sphingolipids and severely deplete the total mass of cellular sphingolipids.1,7,20,21 As an example of the findings from mass spectrometric analysis of the effects of FB1 on cellular amounts of SM, glucosylceramide and ceramide in NIH3T3 cells are shown in Figure 4. These changes can disrupt cell functions dependent on complex sphingolipids; for example, FB1 treatment of intestinal cells in culture blocks folate uptake because the folate transporter is a glycosylphosphatidylinositol-anchored protein, which typically require sphingolipids and cholesterol to function normally.22
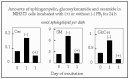
Figure 4
Amounts of ceramide (Cer), sphingomyelin (SM) and glucosylceramide (GlcCer) inIH 3T3 cells incubated for 24 h with (or without) 50 mM FB1 and analyzed by electrospray tanden mass spectrometry.
Production of Sphingoid Base 1-Phosphates and Down-Stream Metabolites (e.g., Ethanolamine Phosphate)
Sphingoid bases are catabolized by phosphorylation and lytic cleavage to a fatty aldehyde and ethanolamine phosphate (see Fig. 2). FB1 has been shown to increase the amounts of cellular sphingoid base 1-phosphates4,5 (see also Fig. 5) as well as to increase the amount of sphingolipid-derived ethanolamine phosphate that is incorporated into phosphatidylethanolamine.4
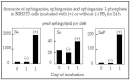
Figure 5
Amounts of sphinganine (Sa), sphingosine (So) and sphinganine 1-phosphate (SaP) in NIH 3T3 cells incubated for 24 h with (or without) 50 mM FB1 and analyzed by electrospray tandem mass spectrometry.
Formation of N-Acetyl-Derivatives of Sphingoid Bases (C2-Ceramides)
The accumulation of free sphingoid bases allows them to “spill over” into other pathways to become substrates for N-acetyltransferases that participate in xenobiotic metabolism23 or that transfer the acetyl-group from platelet activating factor to sphingosine.24 In a recent study of this side pathway, the amounts of “C2-ceramides” were measured in livers from rats fed a fumonisin-free diet and rats fed 150 μg FB1/g diet. The amounts in the control rats were ca 0.6 nmol of N-acetylsphingosine/g and 0.3 nmol of N-acetyl-sphinganine; FB1 feeding had no effect on the amount of N-acetylsphingosine, but increased N-acetyl-sphinganine by 4-fold.1
Alteration of Other Lipid Metabolic Pathways
Changes in these sphingolipids can impact other important lipid metabolic/signaling pathways. For example, phosphatidic acid phosphatase is highly sensitive to cellular amounts of free sphingoid bases,10,25,26 and FB1 has been shown to alter this (and other) pathway(s) in yeast.27 Sphingoid bases28,29 and their 1-phosphates30 can also activate phospholipase; and, sphingoid bases inhibit monoacylglycerol acyltransferase.31
Additional Findings with Aminopentols and Other Sphingoid-Base Analogs
Animals (including humans) are naturally exposed to the fumonisin aminopentols because they are formed during the treatment of corn with lye, a common practice in the preparation of flour for tortillas; furthermore, the intestinal microflora of primates can hydrolyze FB1 to AP1. Although AP1 is a weak inhibitor of ceramide synthase, it is acylated to form derivatives such as the one shown in Figure 6 (N-palmitoyl-AP1 or PAP1).6 These acylated aminopentols are more potent inhibitors of ceramide synthase and are as, or more, cytotoxic than FB1.6
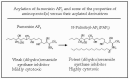
Figure 6
Acylation of AP1 and some of the properties of the original aminopentol and this metabolite.
Structural differences between the aminopentols and sphinganine include the absence of a hydroxyl group at the 1-position, the presence of an additional hydroxyl at position 5, and a threo- versus erythro-stereochemistry at positions 2 and 3. When the toxicities of naturally occurring fumonisin aminopentols (e.g., AP1) are compared to sphinganine with cells in culture, AP1 is less toxic. This may be due to the lower hydrophobicity of the aminopentol because when 1-deoxy-sphinganine analogs have been synthesized and compared (K. Desai et al, manuscript in preparation), they are approximately 10-fold more toxic than sphinganine. These types of compounds offer promise as probes for the mechanisms of action of both fumonosins and sphingoid bases.
Conclusions
It should be clear from this overview that perturbation of one aspect of sphingolipid metabolism has ramifications for other pathways. Of particular interest is the finding that fumonisins can increase not only sphinganine growth inhibitory and pro-apoptotic compound but also sphinganine 1-phosphate, a mitogenic and anti-apoptotic sphingolipid. This may account for the puzzling research literature for fumonisins in which they have been studied by toxicologists for their toxicity, but have been recently used by other researchers to protect cells against apoptosis (indeed, some companies market FB1 as an inhibitor of apoptosis). This may also have an in vivo correlate since fumonisins are toxic in vivo but also carcinogenic for liver, kidney and some other organs.
The possible mechanisms of action of fumonisins are probably quite complex, involving reductions in complex sphingolipids, elevations in sphingoid bases (sometimes counterbalanced with sphingoid base 1-phosphates), and formation of other interesting compounds such as the C2-(dihydro)ceramides and acylated aminopentols. One way to resolve some of these discrepancies is to use other inhibitors of sphingolipid biosynthesis, such as ISP1 or myriosin.32 Nonetheless, this complexity probably explains why these mycotoxins show such a wide spectrum of pathologies including neurotoxicity, pulmonary toxicity, liver and renal toxicity (and carcinogenicity) inter alia.1,2
Acknowledgments
Work from the lab of the corresponding author was supported by grants from the NIH (GM46368 and ES09204). We are also grateful to the many valuable collaborators and particularly Ron Riley and colleagues at the USDA laboratories in Athens, GA, who have contributed to findings discussed in this review.
References
- 1.
- Merrill A H Jr, Sullards MC, Wang E. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ Health Perspect. 2001;109(Suppl 2):283–289. [PMC free article: PMC1240677] [PubMed: 11359697]
- 2.
- Marasas W F O. Discovery and occurrence of fumonisins: A historical perspective. Environ Health Perspect. 2001;109(Suppl 2):239–243. [PMC free article: PMC1240671] [PubMed: 11359691]
- 3.
- Linn SC, Kim HS, Keane EM. Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption. Biochem Soc Trans. 2001;29:831–835. [PubMed: 11709083]
- 4.
- Smith ER, Merrill A H Jr. Regulation of the “burst” of free sphingosine and sphinganine, and their 1-phosphate and N-acyl-derivatives, that occurs upon changing the medium of cells in culture: Differential roles of de novo sphingolipid biosynthesis and turnover in J774A.1 macrophages. J Biol Chem. 1995;270:18749–18758. [PubMed: 7642524]
- 5.
- Sullards MC, Merrill A H Jr. Analysis of sphingosine 1-phosphate, ceramides and other bioactive sphingolipids by liquid chromatography-tandem mass spectrometry, Science Signal Transduction Environment (STKE). 2000. [PubMed: 11752637]
- 6.
- Humpf HU, Schmelz EM, Meredith FI. et al. Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase: Formation of N-palmitoyl-aminopentol (PAP1) produces a toxic metabolite of hydrolyzed fumonisin (AP1), and a new category of ceramide synthase inhibitor. J Biol Chem. 1998;273: 19060–19064. [PubMed: 9668088]
- 7.
- Merrill A H Jr, van Echten G, Wang E. et al. Fumonisin B1 inhibits sphingosine (sphinganine) N-actyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J Biol Chem. 1993;268:27299–27306. [PubMed: 8262970]
- 8.
- Merrill A H Jr, Wang E, Gilchrist DG. et al. Fumonisins and other inhibitors of de novo sphingolipid biosynthesis. Adv Lipid Res. 1993;26:215–234. [PubMed: 8379451]
- 9.
- Yoo HS, Norred WP, Showker J. et al. Elevated sphingoid bases and complex sphingolipid depletion as contributing factors in fumonisin-induced cytotoxicity. Toxicol Appl Pharmacol. 1996;138:211–218. [PubMed: 8658522]
- 10.
- Wu W -I, Lin Y -P, Wang E. et al. Regulation of phosphatidate phospatase activity fom the yeast Saccharomyces cerevisiae by sphingoid bases. J Biol Chem. 1993;268:13830–13837. [PubMed: 8314751]
- 11.
- El Bawab S, Birbes H, Roddy P. et al. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. J Biol Chem. 2001;276:16758–16766. [PubMed: 11278489]
- 12.
- Mao C, Xu R, Bielawska A. et al. Cloning of an alkaline ceramidase from Saccharomyces cerevisiae. An enzyme with reverse (CoA-independent) ceramide synthase activity. J Biol Chem. 2000;275:6876–6884. [PubMed: 10702247]
- 13.
- Van Echten-Deckert G. Sphingolipid extraction and analysis by thin-layer chromatography. Meth Enzymol. 2000;312:64–79. [PubMed: 11070863]
- 14.
- Wang E, Norred WP, Bacon CW. et al. Inhibition of sphingolipid biosynthesis by fumonisins: Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed: 1860857]
- 15.
- Wang E, Ross PF, Wilson TM. et al. Alteration of serum sphingolipids upon exposure of ponies to feed containing fumonisins, mycotoxins produced by Fusarium moniliforme. J Nutr. 1992;122:1706–1716. [PubMed: 1640265]
- 16.
- Wang E, Riley RE, Meredith FI. et al. Fumonisin B1 consumption by rats causes reversible, dose-dependent increases in urinary sphinganine and sphingosine. J Nutr. 1999;129:214–220. [PubMed: 9915902]
- 17.
- Smith ER, Jones PL, Boss JM. et al. Changing J774A.1 cells to new medium perturbs multiple signaling pathways, including the modulation of protein kinase C by endogenous sphingoid bases. J Biol Chem. 1997;272:5640–5646. [PubMed: 9038174]
- 18.
- Adams J, Ann Q. Structure determination of sphingolipids by mass spectrometry. Mass Spectrom Rev. 1993;12:51–85.
- 19.
- Stevens VL, Nimkar S, Jamison WC. et al. Characteristics of the growth inhibition and cytotoxicity of long-chain (sphingoid) bases for Chinese hamster ovary (CHO) cells: Evidence for an involvement of protein kinase C. Biochim Biophys Acta. 1990;1051:37–45. [PubMed: 2297538]
- 20.
- Wang E, Norred WP, Bacon CW. et al. Inhibition of sphingolipid biosynthesis by fumonisins: Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed: 1860857]
- 21.
- Merrill A H Jr, Schmelz E -M, Dillehay DL. et al. Sphingolipids---the enigmatic lipid class: Biochemistry, physiology and pathophysiology. Toxicol & Appl Pharm. 1997;142:208–225. [PubMed: 9007051]
- 22.
- Stevens VL, Tang J. Fumonisin B1-induced sphingolipid depletion inhibits vitamin uptake via the glycosylphosphatidylinositol-anchored folate receptor. J Biol Chem. 1997;272:18020–18025. [PubMed: 9218430]
- 23.
- King CM, Land SJ, Jones RF. et al. Role of acetyltransferases in the metabolism and carcinogenicity of aromatic amines. Mutat Res. 1997;376:123–128. [PubMed: 9202747]
- 24.
- Lee TC, Ou MC, Shinozaki K. et al. Biosynthesis of N-acetylsphingosine by platelet-activating factor: Sphingosine CoA-independent transacetylase in HL-60 cells. J Biol Chem. 1995;271:209–217. [PubMed: 8550561]
- 25.
- Aridor-Piterman O, Lavie Y, Liscovitch M. Bimodal distribution of phosphatidic acid phosphohydrolase in NG108-15 cells. Modulation by the amphiphilic lipids oleic acid and sphingosine. Eur J Biochem. 1992;204:561–568. [PubMed: 1541271]
- 26.
- Perry DK, Hand WL, Edmondson DE. et al. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase. Inhibition by phosphatidic acid phosphohydrolase inhibitors. J Immunol. 1992;149:2749–2758. [PubMed: 1328385]
- 27.
- Wu W -I, McDonough M, Nickels JT. et al. Regulation of lipid biosynthesis in Saccharomyces cerevisiae by fumonisin B1. J Biol Chem. 1995;270:13171–13178. [PubMed: 7768913]
- 28.
- Lavie Y, Liscovitch M. Activation of phospholipase D by sphingoid bases in NG108-15 neural-derived cells. J Biol Chem. 1990;265:3868–3872. [PubMed: 2303483]
- 29.
- Kiss Z, Crilly KS, Rossi MA. et al. Selective inhibition by 4-hydroxynonenal of sphingosine-stimulated phospholipase D in NIH 3T3 cells. Biochim Biophys Acta. 1992;1124:300–302. [PubMed: 1576170]
- 30.
- Desai NN, Zhang H, Olivera A. et al. Sphingosine-1-phosphate, a metabolite of sphingosine, increases phosphatidic acid levels by phospholipase D activation. J Biol Chem. 1992;267:23122–23128. [PubMed: 1429659]
- 31.
- Coleman RA, Wang P, Bhat BG. Fatty acids and anionic phospholipids alter the palmitoyl coenzyme A kinetics of hepatic monoacylglycerol acyltransferase in Triton X-100 mixed micelles. Biochemistry. 1996;35:9576–9583. [PubMed: 8755739]
- 32.
- Schmelz EM, Dombrink-Kurtzman MA, Roberts PC. et al. Induction of apoptosis by Fumonisin B1 in HT-29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol Appl Pharmacol. 1998;148:252–260. [PubMed: 9473533]
- Inhibition of Ceramide Synthase
- Analysis of Sphingolipids in Fumonisin-Treated Cells
- Analysis of Sphingolipids by Electrospray Tandem Mass Spectrometry
- Effects of Fumonisin B1 on Sphingolipid Metabolism
- Additional Findings with Aminopentols and Other Sphingoid-Base Analogs
- Conclusions
- Acknowledgments
- References
- Insights into the Modulation of Ceramide Metabolism by Naturally Occurring and S...Insights into the Modulation of Ceramide Metabolism by Naturally Occurring and Synthetic Sphingolipid Analogs as Monitored by Electrospray Tandem Mass Spectrometry - Madame Curie Bioscience Database
- Modeling Structure-Activity Relationships - Madame Curie Bioscience DatabaseModeling Structure-Activity Relationships - Madame Curie Bioscience Database
- Regional Differences and Variability in Left Ventricular Wall Motion - Madame Cu...Regional Differences and Variability in Left Ventricular Wall Motion - Madame Curie Bioscience Database
- UI-H-BW1-anf-g-03-0-UI.s1 NCI_CGAP_Sub7 Homo sapiens cDNA clone IMAGE:3082180 3'...UI-H-BW1-anf-g-03-0-UI.s1 NCI_CGAP_Sub7 Homo sapiens cDNA clone IMAGE:3082180 3', mRNA sequencegi|11599688|gnl|dbEST|7041874|gb|BF 9.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...

