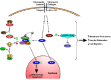
Integrins are cell surface receptors that mediate the interactions of cells with each other and the extracellular matrix. In this chapter, we review experiments indicating that the Abl family of nonreceptor tyrosine kinases, Abl and Arg in vertebrates, are important mediators of cellular responses to integrin engagement. During the early stages of cell spreading, integrins trigger the activation of Abl family kinases and their association with multiple focal adhesion proteins. These events lead to phosphorylation of several cytoskeletal regulatory proteins and changes in cell morphology and motility. Integrins may also utilize Abl family kinases to regulate nuclear processes such as gene expression, cell cycle progression and cell survival. Defects in the proper modulation of cell adhesive responses by Abl family kinases are thought to contribute to the progression of chronic myelogenous leukemia and could potentially underlie other human diseases and behavioral disorders.
Introduction
Cells live in a meshwork of proteins and polysaccharides known as the extracellular matrix (ECM). The ECM directs many aspects of localized cell behavior such as proliferation, differentiation, migration and polarity that are required for tissue organization and function. The interactions of cells with the ECM as well as with neighboring cells are mediated through several classes of cell surface adhesion molecules including integrins, cadherins, immunoglobulins, proteoglycans, and selectins.
Integrins are the principle receptors for binding most ECM proteins such as fibronectin, collagen, vitronectin, and laminin.1-4 Integrins are heterodimers consisting of one α- and one β-subunit. Eighteen different a-subunits and eight different β-subunits have been identified and are known to combine into 24 αβ heterodimers.4,5 Each integrin recognizes a specific set of ECM proteins and cell-surface ligands.4 Ligand binding to integrins results in changes in integrin conformation and clustering, and recruitment of numerous signaling and cytoskeletal proteins.3,5-7 The resulting aggregates of ECM proteins, integrins, cytoskeletal proteins and signaling molecules can form several different types of adhesions including focal complexes, focal adhesions, podosomes or fibrillar adhesions.8 These integrin-mediated adhesions comprise hubs where adhesion, signaling, cytoskeletal reorganization, and mechanical stresses all interact to drive diverse functions including cell shape, polarity, motility, survival, proliferation, and differentiation.1,3,7,9
Research conducted over the last decade has revealed that the Abl family nonreceptor tyrosine kinases, Abl and Arg in vertebrates, are important mediators of integrin signaling (fig. 1). Integrins regulate the subcellular localization, binding interactions and kinase activity of Abl family kinases to evoke both cytoskeletal and nuclear responses to cell adhesion. In this chapter, we review the currently known connections between Abl family kinases and cell adhesion responses.
The Bcr-Abl Fusion Protein Causes Defects in Integrin Function
The first link between Abl family kinases and cell adhesion came from studies of the Bcr-Abl fusion protein. Bcr-Abl is expressed as a result of a chromosomal translocation between chromosomes 9 and 22, and is the causative agent of nearly all cases of chronic myelogenous leukemia (CML).10-12 Expression of Bcr-Abl causes multiple abnormalities in cell adhesion and integrin signaling pathways.13,14 Hematopoietic progenitors from CML patients exhibit decreased adhesion to stromal layers and fibronectin but increased adhesion to laminin and collagen type IV.15-18 These changes have been hypothesized to mediate premature release of Bcr-Abl cells into the circulation. However, several groups have observed increased cell adhesion to fibronectin when Bcr-Abl is introduced into hematopoietic cell lines.13,19-21 These differences likely result from differences in cell type, length of adhesion, cytokines and culture conditions. Bcr-Abl transformed cells also exhibit enhanced and persistent motility on fibronectin-coated surfaces.22 While adhesion to fibronectin inhibits cell cycle progression in normal hematopoietic cells, this regulation is lost in Bcr-Abl transformed cells.23,24 Conversely, adhesion to ECM proteins is normally required for DNA proliferation in fibroblasts, but this requirement is relieved by expression of Bcr-Abl without diminishing the requirement for growth factors or serum.25 Overall, the disruption of integrin function by Bcr-Abl is suspected to contribute to the abnormal trafficking and expansion of CML progenitors.
Several properties of Bcr-Abl contribute to the disruption of normal integrin signaling. Fusion of Bcr to Abl hyperactivates Abl kinase activity and results in the phosphorylation of several focal adhesion proteins including paxillin, FAK, vinculin, talin, tensin, CRKL, p130Cas and Cbl.26-30 Constitutive phosphorylation of at least some of these proteins by Bcr-Abl can interfere with proper signaling in response to integrin engagement. For example, Bcr-Abl induces constitutive association of CRKL with paxillin and p130Cas, and disrupts the association of p130Cas and tensin.30,31 In addition to its elevated tyrosine kinase activity, direct cytoskeletal interactions mediated by Abl's C-terminal filamentous (F)-actin binding domain contribute to the effects of Bcr-Abl on cell adhesion.18,32 Bcr-Abl's effects on adhesion also require the Bcr coiled-coil domain,32 most likely because this domain mediates oligomerization of Bcr-Abl to enhance Abl kinase activity and the association of Bcr-Abl with actin filaments.33,34 Finally, deletion of the Abl proline-rich region in Bcr-Abl reduces the adhesion defects caused by Bcr-Abl, suggesting that interactions between Bcr-Abl and SH3-domain containing proteins are involved.18
Abl Family Kinases Mediate Cellular Responses to Integrin Engagement
Many of the interactions between Bcr-Abl and focal adhesion components likely result from changes in localization, binding partners, substrate affinities, and kinase activity caused by the fusion of Bcr and Abl. However, these observations raised the question whether normal Abl family kinases are involved in physiological integrin signaling. Indeed, genetic, pharmacological, and biochemical analyses have revealed that Abl family kinases are important mediators of both the cytoskeletal and nuclear responses to integrin engagement (fig. 1).
Cytoskeletal Responses
Abl stimulates the formation of actin microspikes and Arg promotes lamellipodial protrusions and retractions in fibroblasts spreading on fibronectin.35,36 Abl family kinases also modulate neuronal morphology in response to integrin engagement. When cultured on the integrin ligand laminin-1, cortical neurons from both abl-/- mice and arg-/- mice exhibit reduced neurite outgrowth and branching relative to wild type neurons (Eva M.Y. Moresco and Anthony J. Koleske, unpublished data).37 In contrast, arg-/- and wild type neurons elaborate neurites similarly when cultured on poly-ornithine, a substrate that does not engage integrin receptors (Eva M.Y. Moresco and Anthony J. Koleske, unpublished data). Further, laminin-1 mediated neurite outgrowth and branching in wild type neurons is prevented either by inhibiting β1 integrins with echistatin or Abl family kinases with STI571 (Eva M.Y. Moresco and Anthony J. Koleske, unpublished data). Together, these experiments indicate that Abl family kinases are required for integrin-mediated neurite outgrowth and branching, and that they contribute to the regulation of protrusive structures in fibroblasts adhering to ECM proteins.
Abl family kinases also regulate fibroblast motility on integrin ligands. Deletion of Abl or inhibition of Abl and Arg kinase activity with STI571 results in enhanced cell migration on fibronectin, while over-expression of Abl inhibits fibroblast migration on fibronectin.38,39 This inhibitory effect was linked to phosphorylation of a negative regulatory tyrosine on Crk.38 Phosphorylation of this site prevents Crk from binding to p130Cas and thereby decreases Rac activation and cell migration.38,40
Nuclear Responses
Although Arg is confined to the cytoplasm, Abl localizes to both the cytoplasm and the nucleus.41- 44 In the nucleus, Abl is involved in cellular responses to DNA damage, cell cycle progression and apoptosis.45,46 Abl is activated by DNA damage in adherent cells but not in suspended cells.47 Decreased Abl activity in suspended cells correlates with stabilization of the p53 homolog p73 and decreased apoptosis after DNA damage.47 These data reveal a surprising synergy between integrin pathways and the nuclear pathways that regulate the response to DNA damage.
Abl Family Kinases Are Recruited to Sites of Adhesive Contact
Abl is found in the nucleus and diffusely in the cytoplasm in stably adhered or suspended fibroblasts.41,42 However, during fibroblast attachment to the ECM proteins fibronectin, vitronectin, or collagen, Abl exits the nucleus and colocalizes with integrins at newly formed sites of adhesion (fig. 1).42 Levels of Abl in the nucleus are lowest 20 minutes after adhesion to ECM proteins and then slowly return to the levels observed in stably attached cells.42 These effects are specific to integrin-mediated adhesion as fibroblast adhesion to poly-L-lysine does not induce Abl export from the nucleus or localization to sites of adhesion.42
The mechanisms that trigger integrin-mediated export of Abl from the nucleus and the localization of Abl to focal adhesions have not been determined. As described below, Abl associates with several focal adhesion proteins upon integrin engagement and these interactions may recruit Abl to focal adhesions. It remains to be determined whether Arg, like Abl, is recruited to sites of focal contact.
Integrin Engagement Activates Abl Family Kinases
Abl kinase activity, measured in immunoprecipitates, declines about 3-fold upon detachment of stably adherent fibroblasts.42 Upon reattachment to fibronectin or to an antibody against integrin α5, Abl kinase activity transiently increases 4- to 5-fold and then returns to the plateau level observed in stably adherent cells.42 Fibroblast adhesion to fibronectin also stimulates Arg kinase activity with similar magnitude and kinetics as Abl (K.Q.T. and Anthony J. Koleske, unpublished). In contrast, no change in Abl42 or Arg (K.Q.T. and Anthony J. Koleske, unpublished) kinase activity is observed when fibroblasts adhere to poly-L-lysine. Further, integrin-mediated phosphorylation of the Arg substrate p190RhoGap is prevented by STI571 or by genetic deletion of Arg in fibroblasts or neurons (Samuel E. Hernández and Anthony J. Koleske, unpublished).48 Together, these experiments indicate that the kinase activities of both Abl and Arg are activated upon integrin engagement by ECM proteins (fig. 1).
As mentioned earlier, Abl returns to the nucleus at later times after adhesion.42 While enhanced Abl kinase activity is observed in the cytoplasmic pool within 5 minutes of cell adhesion to fibronectin, Abl kinase activity in the nucleus does not increase until after 20 minutes of cell adhesion, which parallels the transport of Abl back to the nucleus.42 Together, these data suggest that upon integrin engagement, nuclear Abl is exported to the cytoplasm where it is activated and then returned to the nucleus. This mechanism may allow Abl to relay signals from integrin receptors directly to the nucleus to mediate integrin regulation of gene expression, cell cycle progression, differentiation or survival.
It is poorly understood how integrins activate Abl and Arg. A recent study suggested that Abl and Arg activation by adhesion may be mediated in part by the release from inhibitory interactions with F-actin.49 F-actin was found to inhibit Abl kinase activity in vitro, and deletion of Abl's F-actin binding domain enhanced the in vivo kinase activity of Abl and reduced its adhesion-dependence.49 On the other hand, adhesion to fibronectin has been shown to stimulate Abl phosphorylation.50 Although the sites of phosphorylation have not been determined, Abl and Arg contain multiple phosphorylation sites that activate their kinase activities.51,52 Adhesion-dependent phosphorylation of Abl requires Abl kinase activity, implying either that it results from autophosphorylation or that Abl kinase activity is needed to recruit another kinase to phosphorylate Abl.50 However, it remains to be determined whether phosphorylation mediates adhesion-dependent activation of Abl.
Tyrosine kinases play a key role in relaying signals from integrin receptors. The focal adhesion kinase (FAK) is activated during integrin-mediated adhesion.53 Src kinases, which phosphorylate and activate Abl and Arg following stimulation of growth factor receptors, are recruited to activated FAK were they promote phosphorylation of numerous proteins including the Abl substrates, paxillin and Cas.52,54-56 PLC-γ1 also contributes to the activation of Abl and Arg by growth factors, and is recruited and activated by FAK upon integrin engagement.56-58 Further studies are needed to determine whether FAK, Src or PLC-γ1 participate in the activation of Abl and Arg by integrin receptors.
The specific integrin receptors that regulate Abl and Arg also remain to be carefully determined. An antibody that crosslinks α5 integrins activates Abl kinase activity, suggesting that the α5β1 fibronectin receptor can activate Abl.42 Other data implicate Abl and Arg in neuronal responses to laminin-1 which binds multiple integrins including α1β1 and α6β1 in neurons (Eva M.Y. Moresco and Anthony J. Koleske, unpublished).37,59 Abl localization to adhesion sites is observed in fibroblasts plated on collagen and vitronectin, which implicates integrins α2β1 and αvβ3.42 Thus, Abl family kinases are most likely regulated by multiple integrin receptors, but detailed analysis of kinase regulation by these receptors remains to be carried out. Determining the spectrum of integrin receptors and their ligands that regulate Abl and Arg will give important insights into the physiological processes that utilize the cell adhesion responses mediated by Abl family kinases.
Abl Family Kinases Interact with Multiple Proteins upon Integrin Engagement
Upon fibroblast adhesion to ECM proteins, Abl family kinases are known to associate with multiple proteins and to phosphorylate several substrates (fig. 1). These interactions are described below and likely contribute to the ability of Abl family kinases to regulate the cytoskeletal and nuclear responses to integrin engagement.
Paxillin
Paxillin is an adapter protein that localizes to focal adhesions and modulates cell motility and gene expression.60,61 Plating suspended fibroblasts on fibronectin stimulates transient co-immunoprecipitation of paxillin and Abl.50 This association is lost at later times of adhesion.
Immunoprecipitated Abl can phosphorylate paxillin in vitro, and its ability to do so is enhanced following cell adhesion to fibronectin.50 It is not known which paxillin tyrosines are phosphorylated by Abl, whether Abl or Arg phosphorylate paxillin in vivo, or what physiological consequences these phosphorylation events may have. However, as tyrosine phosphorylation of paxillin recruits multiple proteins to focal adhesions and regulates cell motility, Abl phosphorylation of paxillin has the potential to regulate these events.60,61
VASP
The vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and localizes to focal adhesions and to the leading edge of membrane protrusions.62 Abl co-immunoprecipitates with VASP in adherent cells, and this association is enhanced during the early stages of cell spreading when Abl kinase activity is elevated.63 Although the consequences of this interaction remain to be determined, VASP may localize Abl to adhesive structures and/or to membrane protrusions, and may mediate the membrane protrusive effects of Abl and Arg.
Grb2
Another protein that co-immunoprecipitates with Abl specifically during cell spreading is Grb2, an adaptor protein that couples cell-surface receptors to MAP kinase signaling.64 Integrin-mediated activation of MAP kinase signaling regulates cell-cycle progression and migration.65-67 Over-expression of kinase-inactive Abl decreases the activation of Erk2 upon integrin engagement, suggesting that Abl may contribute to integrin activation of MAP kinase signaling.64 Abl induces phosphorylation of the Grb2-associated guanine nucleotide-exchange factor Sos-1 during growth factor stimulation, resulting in Rac activation and membrane ruffles.68 This suggests that Abl may also contribute to the activation of Sos-1 and Rac during integrin engagement, although this hypothesis remains to be tested.
Dok1
Dok1 (down stream of tyrosine kinases) is hyper-phosphorylated on tyrosine in Bcr-Abl transformed cells and was recently found to be an in vivo substrate of Abl in wild type fibroblasts.36,69-71 Abl mediated phosphorylation of Dok1 is enhanced by adhesion to fibronectin, and is required for Abl-induced filopodial extensions during cell spreading.36 Abl/Dok1-mediated filopodial extensions require Nck, an adaptor protein that under some circumstances can induce localized actin polymerization.36,72,73 Phosphorylation of Dok1 by Abl stimulates the association of Dok1 and Nck.36 Together, these experiments suggest that upon activation by integrin receptors, Abl phosphorylates Dok1 to promote its association with Nck and the formation of filopodial extensions.
p190RhoGap
The 190-KDa GTPase activating protein for Rho (p190RhoGAP) was recently identified as an Arg substrate.48 RhoA promotes the formation of focal adhesions and actin stress fibers.74 Phosphorylation of p190RhoGAP upon fibroblast adhesion to ECM proteins increases its GAP activity towards RhoA.75 This results in transient RhoA inactivation to promote efficient cell spreading.75 Integrin-induced phosphorylation of p190RhoGAP and inactivation of Rho was previously found to be mediated by Src and FAK.76,77 However, Arg is also required for integrin-mediated p190RhoGAP phosphorylation in both fibroblasts and neurons.48 The relationship between Arg and FAK/Src in this pathway remains to be investigated. Arg mediated inactivation of Rho contributes to the formation of membrane protrusions, and may explain the increased actin stress fibers observed in arg-/- fibroblasts.35,44,48
Crk
Although it has not been shown explicitly, Abl and Arg are likely to also phosphorylate the Crk adapter protein following activation by integrins. Both Crk and Abl associate with paxillin following integrin engagement, potentially positioning Crk to be phosphorylated by Abl.50,78 As mentioned previously, Abl phosphorylates Crk at an inhibitory tyrosine that disrupts the Crk-p130Cas complex and decreases cell migration.38,40
Lasp-1
Cell adhesion or exposure to growth factors induces the translocation of the actin binding protein Lasp-1 from the cell periphery to focal adhesions.79 Abl was recently reported to phosphorylate Lasp-1 upon activation by DNA damage or oxidative stress, and phosphorylation of Lasp-1 by Abl was found to prevent its translocation to focal adhesions.79 This effect is specific to stress pathways, since activation of Abl by cell adhesion or growth factors did not result in detectable phosphorylation of Lasp-1 or prevent Lasp-1 relocalization.79 In this study, loss of Lasp-1 from focal adhesions was linked to increased apoptosis in response to stresses, suggesting that Abl promotes cell death partly by inhibiting an integrin-dependent survival pathway mediated by Lasp-1.
Together, these studies indicate that integrins modulate the interactions of Abl family kinases with multiple proteins that regulate focal adhesion signaling and cytoskeletal dynamics. It should be noted that these identified interactions represent only a few pieces to a complex puzzle. Additional proteins and signaling events likely contribute to the adhesive responses mediated by Abl family kinases.
Concluding Remarks and Future Challenges
The findings discussed in this chapter clearly indicate that Abl family kinases are functionally relevant mediators of cellular responses to integrin engagement. During the early stages of cell spreading, integrins trigger the association of Abl family kinases with multiple focal adhesion proteins and elevate the kinase activities of Abl and Arg to promote the phosphorylation of several cytoskeletal regulatory proteins. By modulating cytoskeletal dynamics in response to integrin engagement, Abl and Arg produce adhesion dependent changes in cell morphology and motility. Integrins may also utilize Abl family kinases to regulate nuclear processes such as gene expression, cell cycle progression and cell survival. However, much remains to be learned about the biochemical mechanisms whereby Abl family kinases link integrin receptors to downstream cellular responses and about the physiological consequences of these signaling events.
A comprehensive understanding of the cell adhesion responses mediated by Abl family kinases and their biological significance requires the completion of several future challenges. The first challenge is to determine how Abl family kinase signaling is regulated by adhesive cues. This includes determining the specific integrin receptors that regulate Abl and Arg and the biochemical mechanisms by which they transiently induce Abl and Arg relocalization, kinase activation, and signaling protein interactions. It should also be determined how integrins coordinate with other stimuli to regulate Abl and Arg. It will be particularly interesting to determine how cell adhesion regulates the activation of Abl by DNA damage and whether growth factor and integrin receptors cooperate to produce additive enhancements of Abl and Arg signaling cascades. The second challenge is identify the complete list of Abl and Arg signaling interactions regulated by adhesion and the biochemical mechanisms whereby these interactions produce downstream cellular responses. The third challenge is to understand the physiological consequences of these cellular responses and how they impact the function of tissues.
The last and most important challenge is to determine how defects in the cell adhesion responses of Abl family kinases contribute to human disease. As discussed above, improper regulation of integrin signaling cascades by Bcr-Abl likely contributes to the abnormal expansion and trafficking of CML progenitors. The inability to properly respond to adhesive cues may also explain some of the defects observed in mice lacking Abl or Arg.80-82 For example, mice lacking Arg exhibit multiple behavior abnormalities such as reduced mating and aggression that may result from the reduced dendrite arborization observed in these mice (Eva M.Y. Moresco and Anthony J. Koleske, unpublished data).82 Reduced dendrite arbors are also observed in several human disorders such as mood disorders, and mental retardation syndromes.83-85 More work is required to determine whether defects in Abl family kinase signaling contribute to these or other human disorders.
Acknowledgments
We would like to thank Dr. Anthony Koleske, Bill Bradley, Scott Boyle, and Stefanie Lapetina for helpful comments on this manuscript. This work was supported by National Institutes of Health grants RO1 47214 (M.A.S.) and MH67388 (K.Q.T.).
References
- 1.
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. [PubMed: 1555235]
- 2.
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. [PubMed: 8689569]
- 3.
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. [PubMed: 10446041]
- 4.
- Plow EF, Haas TA, Zhang L. et al. Ligand binding to integrins. J Biol Chem. 2000;275(29):21785–21788. [PubMed: 10801897]
- 5.
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305(3):285–298. [PubMed: 11572082]
- 6.
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. [PubMed: 12297042]
- 7.
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4(4):E83–90. [PubMed: 11944041]
- 8.
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114(Pt 20):3583–3590. [PubMed: 11707510]
- 9.
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4(4):E65–68. [PubMed: 11944032]
- 10.
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed: 14427847]
- 11.
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. [PubMed: 4126434]
- 12.
- Bartram CR, de KleinA, Hagemeijer A. et al. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983;306(5940):277–280. [PubMed: 6580527]
- 13.
- Salesse S, Verfaillie CM. Mechanisms underlying abnormal trafficking and expansion of malignant progenitors in CML: BCR/ABL-induced defects in integrin function in CML. Oncogene. 2002;21(56):8605–8611. [PubMed: 12476307]
- 14.
- Wertheim JA, Miller JP, Xu L. et al. The biology of chronic myelogenous leukemia:mouse models and cell adhesion. Oncogene. 2002;21(56):8612–8628. [PubMed: 12476308]
- 15.
- Gordon MY, Dowding CR, Riley GP. et al. Altered adhesive interactions with marrow stroma of haematopoietic progenitor cells in chronic myeloid leukaemia. Nature. 1987;328(6128):342–344. [PubMed: 3474529]
- 16.
- Verfaillie CM, McCarthy JB, McGlave PB. Mechanisms underlying abnormal trafficking of malignant progenitors in chronic myelogenous leukemia. Decreased adhesion to stroma and fibronectin but increased adhesion to the basement membrane components laminin and collagen type IV. J Clin Invest. 1992;90(4):1232–1241. [PMC free article: PMC443164] [PubMed: 1383271]
- 17.
- Bhatia R, Munthe HA, Verfaillie CM. Role of abnormal integrin-cytoskeletal interactions in impaired beta1 integrin function in chronic myelogenous leukemia hematopoietic progenitors. Exp Hematol. 1999;27(9):1384–1396. [PubMed: 10480429]
- 18.
- Ramaraj P, Singh H, Niu N. et al. Effect of mutational inactivation of tyrosine kinase activity on BCR/ABL-induced abnormalities in cell growth and adhesion in human hematopoietic progenitors. Cancer Res. 2004;64(15):5322–5331. [PubMed: 15289338]
- 19.
- Bazzoni G, Carlesso N, Griffin JD. et al. Bcr/Abl expression stimulates integrin function in hematopoietic cell lines. J Clin Invest. 1996;98(2):521–528. [PMC free article: PMC507458] [PubMed: 8755665]
- 20.
- Kramer A, Horner S, Willer A. et al. Adhesion to fibronectin stimulates proliferation of wild-type and bcr/abl-transfected murine hematopoietic cells. Proc Natl Acad Sci USA. 1999;96(5):2087–2092. [PMC free article: PMC26741] [PubMed: 10051599]
- 21.
- Wertheim JA, Forsythe K, Druker BJ. et al. BCR-ABL-induced adhesion defects are tyrosine kinase- independent. Blood. 2002;99(11):4122–4130. [PubMed: 12010816]
- 22.
- Salgia R, Li JL, Ewaniuk DS. et al. BCR/ABL induces multiple abnormalities of cytoskeletal function. J Clin Invest. 1997;100(1):46–57. [PMC free article: PMC508164] [PubMed: 9202056]
- 23.
- Hurley RW, McCarthy JB, Verfaillie CM. Direct adhesion to bone marrow stroma via fibronectin receptors inhibits hematopoietic progenitor proliferation. J Clin Invest. 1995;96(1):511–519. [PMC free article: PMC185225] [PubMed: 7542285]
- 24.
- Lundell BI, McCarthy JB, Kovach NL. et al. Activation-dependent alpha5beta1 integrin-mediated adhesion to fibronectin decreases proliferation of chronic myelogenous leukemia progenitors and K562 cells. Blood. 1996;87(6):2450–2458. [PubMed: 8630410]
- 25.
- Renshaw MW, McWhirter JR, Wang JY. The human leukemia oncogene bcr-abl abrogates the anchorage requirement but not the growth factor requirement for proliferation. Mol Cell Biol. 1995;15(3):1286–1293. [PMC free article: PMC230351] [PubMed: 7862122]
- 26.
- Oda T, Heaney C, Hagopian JR. et al. Crkl is the major tyrosine-phosphorylated protein in neutrophils from patients with chronic myelogenous leukemia. J Biol Chem. 1994;269(37):22925–22928. [PubMed: 8083188]
- 27.
- Gotoh A, Miyazawa K, Ohyashiki K. et al. Tyrosine phosphorylation and activation of focal adhesion kinase (p125FAK) by BCR-ABL oncoprotein. Exp Hematol. 1995;23(11):1153–1159. [PubMed: 7556524]
- 28.
- Salgia R, Brunkhorst B, Pisick E. et al. Increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell lines expressing p210BCR/ABL. Oncogene. 1995;11(6):1149–1155. [PubMed: 7566975]
- 29.
- Salgia R, Sattler M, Pisick E. et al. p210BCR/ABL induces formation of complexes containing focal adhesion proteins and the protooncogene product p120c-Cbl. Exp Hematol. 1996;24(2):310–313. [PubMed: 8641358]
- 30.
- Salgia R, Pisick E, Sattler M. et al. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J Biol Chem. 1996;271(41):25198–25203. [PubMed: 8810278]
- 31.
- Salgia R, Uemura N, Okuda K. et al. CRKL links p210BCR/ABL with paxillin in chronic myelogenous leukemia cells. J Biol Chem. 1995;270(49):29145–29150. [PubMed: 7493940]
- 32.
- Wertheim JA, Perera SA, Hammer DA. et al. Localization of BCR-ABL to F-actin regulates cell adhesion but does not attenuate CML development. Blood. 2003;102(6):2220–2228. [PubMed: 12791659]
- 33.
- McWhirter JR, Wang JY. Activation of tyrosinase kinase and microfilament-binding functions of c-abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991;11(3):1553–1565. [PMC free article: PMC369443] [PubMed: 1705008]
- 34.
- McWhirter JR, Galasso DL, Wang JY. A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins. Mol Cell Biol. 1993;13(12):7587–7595. [PMC free article: PMC364830] [PubMed: 8246975]
- 35.
- Miller AL, Wang Y, Mooseker MS. et al. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J Cell Biol. 2004;165(3):407–419. [PMC free article: PMC2172189] [PubMed: 15138293]
- 36.
- Woodring PJ, Meisenhelder J, Johnson SA. et al. c-Abl phosphorylates Dok1 to promote filopodia during cell spreading. J Cell Biol. 2004;165(4):493–503. [PMC free article: PMC2172353] [PubMed: 15148308]
- 37.
- Woodring PJ, Litwack ED, O'Leary DD. et al. Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J Cell Biol. 2002;156(5):879–892. [PMC free article: PMC2173320] [PubMed: 11864995]
- 38.
- Kain KH, Klemke RL. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J Biol Chem. 2001;276(19):16185–16192. [PubMed: 11279004]
- 39.
- Frasca F, Vigneri P, Vella V. et al. Tyrosine kinase inhibitor STI571 enhances thyroid cancer cell motile response to Hepatocyte Growth Factor. Oncogene. 2001;20(29):3845–3856. [PubMed: 11439348]
- 40.
- Feller SM, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 1994;13(10):2341–2351. [PMC free article: PMC395099] [PubMed: 8194526]
- 41.
- Van EttenRA, Jackson P, Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989;58(4):669–678. [PubMed: 2670246]
- 42.
- Lewis JM, Baskaran R, Taagepera S. et al. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic- nuclear transport. Proc Natl Acad Sci USA. 1996;93(26):15174–15179. [PMC free article: PMC26376] [PubMed: 8986783]
- 43.
- Wang B, Mysliwiec T, Krainc D. et al. Identification of ArgBP1, an Arg protein tyrosine kinase binding protein that is the human homologue of a CNS-specific Xenopus gene. Oncogene. 1996;12(9):1921–1929. [PubMed: 8649853]
- 44.
- Wang Y, Miller AL, Mooseker MS. et al. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin- binding domains to bundle F-actin. Proc Natl Acad Sci USA. 2001;98(26):14865–14870. [PMC free article: PMC64950] [PubMed: 11752434]
- 45.
- Van EttenRA. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 1999;9(5):179–186. [PubMed: 10322452]
- 46.
- Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. [PubMed: 12374288]
- 47.
- Truong T, Sun G, Doorly M. et al. Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proc Natl Acad Sci USA. 2003;100(18):10281–10286. [PMC free article: PMC193552] [PubMed: 12928501]
- 48.
- Hernandez SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr Biol. 2004;14(8):691–696. [PubMed: 15084284]
- 49.
- Woodring PJ, Hunter T, Wang JY. Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J Biol Chem. 2001;276(29):27104–27110. [PubMed: 11309382]
- 50.
- Lewis JM, Schwartz MA. Integrins regulate the association and phosphorylation of paxillin by c-Abl. J Biol Chem. 1998;273(23):14225–14230. [PubMed: 9603926]
- 51.
- Brasher BB, Van EttenRA. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J Biol Chem. 2000;275(45):35631–35637. [PubMed: 10964922]
- 52.
- Tanis KQ, Veach D, Duewel HS. et al. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol Cell Biol. 2003;23(11):3884–3896. [PMC free article: PMC155218] [PubMed: 12748290]
- 53.
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(Pt 8):1409–1416. [PubMed: 12640026]
- 54.
- Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–113. [PubMed: 9889179]
- 55.
- Plattner R, Kadlec L, DeMali KA. et al. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13(18):2400–2411. [PMC free article: PMC317022] [PubMed: 10500097]
- 56.
- Plattner R, Koleske AJ, Kazlauskas A. et al. Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol. 2004;24(6):2573–2583. [PMC free article: PMC355852] [PubMed: 14993293]
- 57.
- Zhang X, Chattopadhyay A, Ji QS. et al. Focal adhesion kinase promotes phospholipase C-gamma1 activity. Proc Natl Acad Sci USA. 1999;96(16):9021–9026. [PMC free article: PMC17725] [PubMed: 10430888]
- 58.
- Plattner R, Irvin BJ, Guo S. et al. A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat Cell Biol. 2003;5(4):309–319. [PubMed: 12652307]
- 59.
- Clegg DO, Wingerd KL, Hikita ST. et al. Integrins in the development, function and dysfunction of the nervous system. Front Biosci. 2003;8:d723–750. [PubMed: 12700040]
- 60.
- Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20(44):6459–6472. [PubMed: 11607845]
- 61.
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84(4):1315–1339. [PubMed: 15383653]
- 62.
- Kwiatkowski AV, Gertler FB, Loureiro JJ. Function and regulation of Ena/VASP proteins. Trends Cell Biol. 2003;13(7):386–392. [PubMed: 12837609]
- 63.
- Howe AK, Hogan BP, Juliano RL. Regulation of vasodilator-stimulated phosphoprotein phosphorylation and interaction with Abl by protein kinase A and cell adhesion. J Biol Chem. 2002;277(41):38121–38126. [PubMed: 12087107]
- 64.
- Renshaw MW, Lewis JM, Schwartz MA. The c-Abl tyrosine kinase contributes to the transient activation of MAP kinase in cells plated on fibronectin. Oncogene. 2000;19(28):3216–3219. [PubMed: 10918577]
- 65.
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11(1):48–53. [PubMed: 11163150]
- 66.
- Juliano RL, Reddig P, Alahari S. et al. Integrin regulation of cell signalling and motility. Biochem Soc Trans. 2004;32(Pt3):443–446. [PubMed: 15157156]
- 67.
- Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem. 2004;91(1):41–46. [PubMed: 14689580]
- 68.
- Sini P, Cannas A, Koleske AJ. et al. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat Cell Biol. 2004;6(3):268–274. [PubMed: 15039778]
- 69.
- Wisniewski D, Strife A, Wojciechowicz D. et al. A 62-kilodalton tyrosine phosphoprotein constitutively present in primary chronic phase chronic myelogenous leukemia enriched lineage negative blast populations. Leukemia. 1994;8(4):688–693. [PubMed: 8152267]
- 70.
- Carpino N, Wisniewski D, Strife A. et al. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88(2):197–204. [PubMed: 9008160]
- 71.
- Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88(2):205–211. [PubMed: 9008161]
- 72.
- Campellone KG, Rankin S, Pawson T. et al. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J Cell Biol. 2004;164(3):407–416. [PMC free article: PMC2172230] [PubMed: 14757753]
- 73.
- Rivera GM, Briceno CA, Takeshima F. et al. Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr Biol. 2004;14(1):11–22. [PubMed: 14711409]
- 74.
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. [PubMed: 1643657]
- 75.
- Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35(2):239–246. [PubMed: 12415742]
- 76.
- Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10(12):719–722. [PubMed: 10873807]
- 77.
- Ren XD, Kiosses WB, Sieg DJ. et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–3678. [PubMed: 11017882]
- 78.
- Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15(5):2635–2645. [PMC free article: PMC230493] [PubMed: 7537852]
- 79.
- Lin YH, Park ZY, Lin D. et al. Regulation of cell migration and survival by focal adhesion targeting of Lasp-1. J Cell Biol. 2004;165(3):421–432. [PMC free article: PMC2172195] [PubMed: 15138294]
- 80.
- Tybulewicz VL, Crawford CE, Jackson PK. et al. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65(7):1153–1163. [PubMed: 2065352]
- 81.
- Li B, Boast S, de los Santos K. et al. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat Genet. 2000;24(3):304–308. [PubMed: 10700189]
- 82.
- Koleske AJ, Gifford AM, Scott ML. et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21(6):1259–1272. [PubMed: 9883720]
- 83.
- D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4(3):183–194. [PubMed: 12180273]
- 84.
- Quiroz JA, Singh J, Gould TD. et al. Emerging experimental therapeutics for bipolar disorder: clues from the molecular pathophysiology. Mol Psychiatry. 2004;9(8):756–76. [PubMed: 15136795]
- 85.
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10(10):981–991. [PubMed: 11007549]
Publication Details
Author Information and Affiliations
Authors
Keith Quincy Tanis* and Martin Alexander Schwartz.Affiliations
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Tanis KQ, Schwartz MA. Regulation of Cell Adhesion Responses by Abl Family Kinases. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.