NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Abl and Arg nonreceptor tyrosine kinases are widely expressed in mammals, where they contribute to the development of diverse organ and tissue systems. Deletion of abl or arg in mice reveals roles for the kinases in B and T lymphocyte development, neurulation, neuronal dendrite maintenance, synaptic plasticity, and osteoblast development. Double knockout abl-/-arg-/- mice die as embryos, indicating that Abl and Arg also perform essential and overlapping functions during embryonic development. Abl and Arg contain domains for protein-protein interactions (SH3, SH2, proline-rich sequences, PY sequences), cytoskeleton binding (filamentous actin and microtubule binding domains), nuclear translocation (nuclear localization and export sequences), and DNA binding. Although a full understanding of their molecular interactions is still forthcoming, it is clear that Abl and Arg provide many cell types with all-in-one multifunctional signaling tools that serve as links between the cell surface and downstream pathways to both the cytoskeleton and nucleus.
Introduction
The mammalian Abl nonreceptor tyrosine kinase family consists of Abl and Arg (Abl-related gene, also called Abl2). c-abl was identified as the cellular (c for cellular) homologue of v-abl (v for viral), the transforming gene of the Abelson murine leukemia virus (AMulV), and encodes the nonreceptor tyrosine kinase Abl.1-3 c-abl (hereafter abl) was later shown to be involved in human leukemias due to a chromosomal translocation resulting in fusion of N-terminal sequences of Bcr to abl, causing formation of the Philadelphia chromosome and Bcr-abl oncogenes.2 Bcr-abl tyrosine kinases are constitutively active and transform lymphoid cells in culture and in mice.4 Because of the involvement of abl in malignancy, a search for related genes was performed (actually using c-DNA probes to v-abl) and the abl-related gene (arg) was identified.5,6
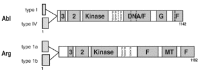
Abl and Arg share extensive sequence and structural similarity (fig. 1). Both have two predominant alternatively spliced forms, in mice designated Abl type I and IV and Arg type 1a and 1b, each containing one of two different first exons and resulting in distinct N-terminal peptide sequences.5,7,8 The biological functions of each isoform remains unclear, although a few reports have documented differences in requirements for type I and type IV Abl in mediating differentiation or survival in response to lipopolysaccharide (LPS) treatment of lymphoid cell lines.9,10 However, either Abl isoform can complement the lymphocyte defects (see below) of abl mutant mice when expressed as transgenes.11 Following the N-terminal domain, Abl and Arg contain Src homology 3 (SH3), Src homology 2 (SH2) protein interaction domains and tyrosine kinase domains. They contain three conserved PXXP motifs C-terminal to the kinase domain that serve as potential binding sites for SH3 domain-containing proteins.12 The near 90% identity in their N-terminal halves suggests that Abl and Arg have common interactors or substrates, and may share some cellular functions.
Abl and Arg also contain cytoskeleton-binding domains in their C-terminal halves, features unique among nonreceptor tyrosine kinases. These consist of filamentous (F)-actin binding domains in Abl and Arg13-15 (M. Krishnaswami and A.J. Koleske, unpublished data), and a microtubule (MT) binding domain in Arg.16 These domains localize Abl and Arg within the cell, and allow them to physically regulate actin and microtubule organization. Finally, Abl has three nuclear localization sequences (NLS)17,18 and a nuclear export sequence (NES)19 that are absent in Arg. These domains allow Abl to shuttle between the cytoplasm and the nucleus in response to various stimuli. A DNA binding domain identified in Abl68 currently has unknown physiological function.
One look at the lengthy list of functional domains present in Abl and Arg suggests that their molecular interactors are numerous and their physiological functions complicated. Add to that the wide tissue expression pattern of Abl and Arg (see below), and it becomes clear that understanding their roles in development would not be easy. This has proven true. Since the generation of abl mutant mice nearly 15 years ago, how Abl kinases contribute to mammalian development has been and continues to be the subject of intense study. Mice lacking the second mammalian Abl family kinase member, Arg, were subsequently engineered and analysis of their phenotypes gives insight into the unique roles the kinases play during development. Examination of these mutant mice demonstrates that Abl and Arg participate in a wide and seemingly unrelated variety of developmental processes, and that Abl and Arg possess both shared and unique functions.
In this chapter, I review the developmental processes that require Abl and Arg function in mammals (mostly mice), which include lymphocyte development, neuronal development, and bone development. The diversity of these processes makes it impossible to thoroughly discuss every aspect of the signaling that regulates them. Therefore, in considering how Abl and Arg may participate in these molecular pathways, I attempted to focus on data obtained from experiments using primary tissues or cells, with the assumption that these results provide the best insight into the normal functions of the kinases. From this survey, it becomes clear that Abl and Arg are multifunctional tools with many features cells find useful. Cells of many types employ these functions for their specific cellular tasks. In this way, Abl and Arg are recruited to participate in signaling to transduce information from a wide variety of cell surface receptors to both the cytoskeleton and the nucleus.
Tissue Expression Patterns of Abl and Arg
Overlapping patterns of Abl and Arg expression are detected in many cell types and tissues, although the level of Abl expression may be quite different from that of Arg in a given tissue. In addition, their expression levels can vary substantially from tissue to tissue, and may change during the course of normal development. Abl is expressed in a wide range of mouse tissues, including the spleen, thymus and brain.20-22 Abl can be isolated in purified synaptosomes from mouse brain.23 Of adult human tissues, Abl is found in small mucous glands and ductules, gastric crypts, endocervix, ovarian follicles, breast myoepithelium, prostatic acini, renal tubules, transitional epithelium, skin adnexae, myeloid cells and osteoblasts.24 In contrast to data obtained from mouse, no Abl is detected in human cerebral cortical neurons, lymph nodes or spleen.24 Human fetal tissues show a similar expression pattern to the adult, with additional expression in squamous mucosa, sex cords, adrenal cortex and medulla, small intestinal and colonic epithelium, skeletal, cardiac and smooth muscle, capillary endothelial cells and endocardial intima. Strong expression is also observed in osteoblasts and their associated neovasculature in sites of endochondral ossification.24
Arg expression is highest in the brain, with significant levels also found in spleen, thymus and muscle tissue.21,25 Arg is particularly concentrated in synapse-rich brain regions, and localizes to dendritic spines and to purified synaptosomes in adult mice.21,26 Embryonic mice have high levels of both Abl and Arg in the developing neuroepithelium.21 The variety of tissues expressing Abl and Arg suggests that many cellular processes and signaling pathways utilize these kinases to carry out their functions.
Deletion of abl and arg in Mice Reveals Essential Roles for the Kinases
Two c-abl mutant alleles were designed and utilized to engineer mice lacking Abl function. abl2 (hereafter abl-) is a true null allele, and no Abl protein is detected in abl2 homozygous (abl-/-) mice.27 A second allele, designated ablm1, eliminates the C-terminal one-third of Abl containing its DNA-binding and cytoskeleton-binding domains, but retains the N-terminal portion containing the SH3, SH2 and kinase domains, 3 proline-rich regions adjacent to the kinase domain, and NLS sequences.28-30 Approximately 75% of abl-/- and ablm1/m1 mice are runted and die within the first two weeks after birth. The remaining 25% of abl-/- and ablm1/m1 mutants are highly susceptible to infections throughout life, such as pneumonia, gastroenteritis and infections of the nasal passages and ears.27,28 The cause of increased susceptibility to infection in these mice remains to be demonstrated.
Homozygous mutant mice expressing a null allele of arg are strikingly healthy and robust compared to abl mutant mice.21 Although arg-/- pups are runted at 3 weeks of age, by 6 weeks they are similar in weight to wild type littermates. arg-/- mice are born at expected Mendelian ratios, and survive to healthy adulthood.21 Loss of Arg does not result in immune deficiency as seen in abl mutants.
Intercrosses of abl and arg mutants reveal overlapping roles for Abl and Arg during development.21 While abl+/-arg+/- mice are born and survive in expected Mendelian ratios, only 60% of abl+/-arg-/- mice survive to adulthood. abl-/-arg+/- mice die embryonically at 15.5 days post-coitum (dpc) and display pericardial sac and peritoneal hemorrhaging. abl-/-arg-/- mice are most severely affected and die as embryos at 10.5 dpc. Internal bleeding in the pericardial sac is also observed in these embryos, and likely results in their early death.21 However, the cellular and molecular defects that result in death of abl-/-arg-/- and abl-/-arg+/- embryos are yet unknown. Immunostaining revealed Abl expression in cardiac muscles of human fetuses,24 suggesting that heart development or function may require Abl and Arg. Alternatively, deletion of Abl and Arg may slightly compromise the development of several systems, which when combined fail to support the life of the embryo.
Lymphocyte Development in abl-/- and ablm1/m1 Mice
B and T Cell Development Require Abl
The bone marrow and thymus are the major sites for early development of the immune system's B and T lymphocytes, respectively. These organs provide both soluble and cell-anchored stromal signals that direct lymphocyte development, and as such, their unique environments are critical to the development of the immune system. Once they have completed their developmental programs there, naïve B and T lymphocytes leave the bone marrow and thymus, homing to peripheral lymphoid organs including the spleen and lymph nodes, where they complete their maturation and may become activated upon encountering antigen. As B and T cells work closely together to protect the body from invasion, both must properly develop and migrate in order for these cells to be available throughout the body to recognize and eliminate foreign antigens.
The increased susceptibility to infection of the abl-/- and ablm1/m1 mice suggested that these mutants have defects in immune development or function. Indeed, similar (but not identical) phenotypes of reduced total B and T cell numbers are observed in abl-/- and ablm1/m1 mutants.27,28,31 Consistent with this finding, abl-/- and ablm1/m1 mutant mice display a reduced size and cellularity of the thymus. Atrophy of the spleen is also observed, but is less severe than reductions in the thymus. In ablm1/m1 mice, spleens exhibit an abnormal short, squatty shape with rounded edges, instead of the normal elongated knife-blade shape.28
Lymphocytes express specific combinations of immunoglobulin and other cell surface receptors as they progress through development. The combination of receptors serves as a means to identify populations of cells in specific developmental stages. For B cells, the developmental program progresses though pro-B, pre-B, and immature B stages before reaching the mature B stage capable of responding to antigens (Table 1). When examined by flow cytometry for stage-specific markers, adult ablm1/m1 mice exhibit reductions in bone marrow-derived early B cell classes, including pro-B, pre-B and immature B cells (Table 1).28,31 On average, total B cells from bone marrow are reduced to 67% of control B cell numbers, with more severe reductions in pre-B cells relative to pro-B cells.31 In general, B cell depletion is less severe in spleen compared to bone marrow, and ablm1/m1 mutants exhibit at worst a 19% reduction in splenic B cells. Interestingly, circulating mature B cells in peripheral blood are nearly normal in ablm1/m1 mice, suggesting that the defect in early B cell development may be overcome in later stages of development.
The thymi of newborn ablm1/m1 mice contain on average only one-third the number of cells found in those of control littermates, although the relative proportions of immature and mature T cells appear to be normal in these mutants. In contrast to the reduction in thymic T cells, the number of circulating mature T cells in peripheral blood is only slightly reduced, similar to the effect seen in B cells.28 Splenic T cells are normal or elevated in ablm1/m1 mice. Thus, T cell development is less severely affected by the ablm1> mutation than B cell development.
Like ablm1/m1 mutants, abl-/- mice exhibit reductions in both B and T cell numbers.27 Early B cell classes are reduced in abl-/- bone marrow, while mature B cells are present at normal levels relative to animal body weight. However, in contrast to ablm1/m1 animals, reductions in total peripheral lymphocyte numbers to 73% of wild type levels are observed in 3 to 5 week old abl-/- mice. Deficiencies in both B and T cell populations contribute to this reduction; however, the specific populations of B or T cells affected has not been determined. Thus, very similar lymphocytic phenotypes are observed in two different abl mutants, providing solid evidence that Abl function contributes to the development of B and T lymphocytes.
Table 1
Average percentage of B cells in ablm1/m1 relative to control mice.
Does Arg contribute to lymphocyte development? Unlike abl mutants, no defects are observed in arg-/- lymphocyte numbers from bone marrow, thymus, spleen or peripheral blood.21 A recent report raises the possibility that Arg cannot functionally compensate for Abl during early B cell development because its expression is low during pro-B and pre-B cell stages when defects are most severe in abl mutants, and only later increases in mature B cells.32 However, because abl-/-arg-/- mice die as embryos before significant lymphopoeisis take place, whether Arg and Abl serve redundant functions in lymphocyte development remains unknown.
How Does Abl Regulate Lymphocyte Development?
The stromal environments of the thymus and bone marrow provide soluble and membrane- anchored signals that direct lymphocyte development. Therefore, it is possible that mutation or deletion of abl results in an abnormal stromal environment that fails to support proper lymphocyte development. This hypothesis is supported by the observation that bone marrow transfer from abl-/- mice to irradiated syngeneic wild type recipients fails to recapitulate the lymphopenic phenotype of abl-/- donors.27 In addition, long-term lymphoid bone marrow cultures containing normal numbers of lymphocytes and precursors, can be established from ablm1/m1 bone marrow in vitro,31 suggesting that the microenvironment in which lymphocytes develop in vivo is defective in abl mutants.
However, evidence also supports a cell autonomous role for Abl in regulating lymphocyte development. When ablm1/m1 pro-B cells are cultured on a wild type stromal cell layer, fewer pro-B cells remain after six days in culture compared to wild type cells,31 suggesting that intrinsic defects in ablm1/m1 lymphocytes prevent their proliferation in a wild type environment. Furthermore, the phenotype of depleted pro-B and pre-B cells can be reconstituted in normal irradiated syngeneic hosts after bone marrow transfer from ablm1/m1 mice,28,31 further supporting the idea that defects in ablm1/m1 lymphocytes are cell autonomous. The opposite experiment, to transplant wild type bone marrow into irradiated abl mutants, has not been performed. Together, these data are consistent with defects in both the stromal environment and the lymphocytes themselves contributing to lymphopenia in ablm1 mutants.
Interleukin-7, B Cell Receptor, and T Cell Receptor Signaling through Abl
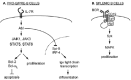
Several studies suggest that Abl helps to mediate interleukin-7 (IL-7) signaling, providing an attractive mechanism to explain many of the observed lymphopoeisis defects in abl mutant mice (fig. 2A). IL-7 is a stromal cell-derived cytokine critical for early bone marrow B cell development in mice. Similar to abl mutants, mice lacking IL-7 either through genetic deletion or treatment with anti-IL-7 antibodies display defects in early lymphopoeisis (pro-B cell stage)33,34 and consequent lymphopenia.35 IL-7 stimulates proliferation of pro-B cells in vitro and in vivo.36-38 This proliferative response to IL-7 is reduced in ablm1/m1 bone marrow colony formation cultures relative to wild type cultures, although IL-7-induced proliferation of ablm1/m1 cells in liquid culture is normal.31 These observations suggest that IL-7-stimulated pro-B cell proliferation in bone marrow requires Abl function.
Further support for this hypothesis comes from studies of the activated v-Abl kinase expressed AMulV-transformed cells. AMulV specifically transforms lymphoid precursors, including pro-B and pre-B cells, rendering their growth IL-7 independent.39 Pre-B cells expressing v-Abl exhibit constitutively activated Janus protein kinase 1 (JAK1), JAK3, STAT5 and STAT6, critical elements of IL-7 signal transduction in pre-B cells.40,41 Conversely, IL-7 treatment can reconstitute some v-Abl-mediated activities when v-Abl is acutely inactivated in transformed pre-B cells.42 These results demonstrate that an activated Abl kinase deregulates and activates IL-7 signaling, leading to IL-7 independent, uncontrolled proliferation. Like expression of v-Abl, transgenic or exogenous IL-7 administration contributes to B lymphoid transformation.43,44 It will be of interest to determine whether exogenous administration or transgenic IL-7 can overcome the lymphopenia of abl mutants.
Both ablm1/m1 and IL-7-/- B cells also exhibit increased apoptosis, which may contribute to the observed reduction in pro-B and pre-B cells in mutant bone marrow.45,46 Interestingly, whereas IL-7 withdrawal from wild type progenitor B-cell lines results in G1 arrest, it promotes apoptosis in ablm1/m1 B-cell lines.45,47 Furthermore, as IL-7 and v-Abl have been shown to positively regulate the levels of antiapoptotic proteins Bcl-2 and Bcl-xL,42 loss of Abl signaling may result in reduced levels of Bcl-2 and/or Bcl-xL leading to increased apoptosis. Thus, Abl may mediate pro-survival signaling from the IL-7 receptor in bone marrow pro-B and pre-B cells.
Once B cells complete their development in the bone marrow, some will migrate to the spleen, where their survival and maintenance requires signals from the B cell receptor (BCR).48 Interestingly, a recent study reports that abl-/- splenic B cells are deficient in their proliferative response to BCR stimulation, suggesting that depletion of B cells in the spleen of abl mutants may result from specific defects in the signaling pathway activated by BCR stimulation.49 Abl associates with and phosphorylates the BCR coreceptor CD19, which serves to activate downstream components of BCR signaling by recruiting effector proteins such as Syk and Btk.49,50 These data suggest that Abl and CD19 act in a similar signaling pathway that modulates BCR-mediated splenic B cell proliferation (fig.2B).
What about T cells? ablm1/m1 and abl-/- mice display reductions in T cell numbers in the thymus and peripheral blood, respectively.27,28 Treatment of chronic myelogenous leukemia (CML), a disease caused by expression of the activated Bcr-abl protein in myeloid cells, with the specific Abl and Arg kinase inhibitor STI-571 (imatinib mesylate or Gleevec), has recently prompted investigation of the effects of Abl inhibition on T cell proliferation. Even though T cells from CML patients do not express the STI-571 target Bcr-abl, their proliferation is inhibited in a dose-dependent manner by STI-571,51,52 suggesting that Abl is required for the normal proliferation of T cells. In particular, T cell receptor (TCR)-mediated T cell proliferation is blocked by STI-571.53,54 Importantly, recent work demonstrates that TCR stimulation activates Abl to phosphorylate Zap70 and the linker for activation of T cells (LAT), effectors of TCR signaling that lead to transcription and eventual production of the cytokine IL-2.53 Thus, Abl can phosphorylate two downstream effectors of the TCR to stimulate a T cell proliferation pathway.
Immune Function in abl Mutants
The defects in B and T cell development may contribute to the susceptibility of ablm1/m1 and abl-/- mice to infections. Surprisingly, although in vitro responses of ablm1/m1 B and T cells to mitogens is reduced in several instances, the primary immune response of ablm1/m1 mice to sheep red blood cells (SRBCs) is normal.55 In this assay, mice are immunized with SRBCs and then tested to see if they produced antibodies to SRBC antigens. Thus, one unexplored possibility is that ablm1/m1 mice can produce antibodies, but defects in lymphocyte migration prevent the full and effective immune response. A new study finds that Abl, the Abl interactor protein-1 (Abi-1), and WAVE2, a member of the Wiscott-Aldrich syndrome family, form a complex that promotes actin polymerization in Jurkat T cells.56 As actin polymerization drives cell migration, deletion of Abl in T cells might compromise their ability to migrate to sites of infection.
Neuronal Development in abl-/- and arg-/- Mice
Neurulation and Dendrite Maintenance Require Abl and Arg
The entire central nervous system (CNS) develops from the neural tube, which forms when a sheet of ectodermal cells folds and fuses to become a fluid-filled tube in a process called neurulation. All of the specialized structures of the brain develop from this tube. Once this occurs, wiring the nervous system involves the formation of appropriate connections between neurons, a process that begins with the proliferation and migration of the neurons. From their final positions in the brain, neurons extend one axon and several dendrites, specialized compartments from which neurons send signals out and receive signals from other cells, respectively. Axons and dendrites make connections with each other at junctions called synapses, contact areas between a presynaptic axon from one neuron, and a postsynaptic dendrite from another neuron. Neurons are highly interconnected, and each neuron may have thousands of synapses. Because synapses are the basis of information transfer in the brain, the size and structure of axons and dendrites on which synapses form are important determinants of brain function.
Abl and Arg are highly expressed in neurons of the developing and adult mouse brain.21,26,57 Abl has been localized to synapses 23,26 and to axonal growth cones.57 Compared to other tissues, Arg is most highly expressed in the brain, where it is concentrated in synapses and neuronal processes.21,26 For example, Arg is found in the synapse-rich molecular layers of the cerebellum and the hippocampus, but not in the cell body-containing granular layers of these structures.21arg-/- mice exhibit multiple behavioral abnormalities, including motor deficits and sensorineural deafness,21 suggesting that Arg may regulate synaptic transmission. The expression levels of Abl and Arg are developmentally regulated. During embryogenesis, Abl is about 5-fold more abundant than Arg in whole brain extracts. Postnatally, Arg becomes about 8-fold more abundant than Abl at postnatal day 21 (P21), and 5-fold more abundant than Abl in the six-week-old brain (E.M.Y. Moresco and A.J. Koleske, unpublished data). Their differential expression levels may indicate different roles for Abl and Arg over the course of brain development.
The generation of abl-/-arg-/- double knockout mice revealed overlapping roles for Abl and Arg in embryonic central nervous system (CNS) development.21 While single knockout abl-/- or arg-/- mice live to adulthood and display no gross brain abnormalities, abl-/-arg-/- embryos die before embryonic day 11 and exhibit several defects in neurulation. Closure of the neural tube is delayed in abl-/-arg-/- embryos. Then, as the neural tube of these mice proceeds towards closure, the neuroepithelium buckles into the lumen of the neural tube. Abl and Arg colocalize with each other and with actin filaments at the apical surface of the developing neuroepithelium, and this actin network is disrupted in abl-/-arg-/- embryos.21 It remains unclear whether the actin cytoskeletal defects cause the failure in neural tube closure, or whether they are the result of other unknown defects in these embryos. Accumulating evidence suggests that Abl and Arg regulate actin cytoskeletal organization,58,59 supporting the hypothesis that actin defects in the neuroepithelium cause the failure in neurulation.
Our recent studies analyzed the structure of cortical neurons in abl-/-, arg-/- and conditional brain-specific (bs)-abl-/-arg-/- double knockout mice, and found that dendrite arbor maintenance is deficient in these mutants.60 Cortical dendrites initially develop normally in arg-/- and bs-abl-/-arg-/- mice, but by early adulthood mutant dendrites are reduced in size and complexity relative to wild type dendrites. Interestingly, adult bs-abl-/-arg-/- dendrites are more severely affected than either single mutant, suggesting that Abl and Arg have overlapping functions in dendrite maintenance. Using primary cortical neurons from mutant mice, we demonstrated that Arg is required for neurite branching stimulated by integrin-dependent cell adhesion to the ligands Semaphorin7A (Sema7A) or laminin-1. Together, the data suggest that Abl and Arg regulate dendrite branch maintenance in response to adhesive cues.
The downstream effectors of Abl and Arg signaling in dendrite maintenance are currently unknown. However, one good candidate is the 190kD GTPase activating protein for RhoA (p190RhoGAP), a neuronal substrate of Arg during postnatal periods.61 p190RhoGAP inhibits the RhoA GTPase, which has been shown to negatively regulate dendrite branching in other systems.62 In the future, examining the dendrites of sema7a and p190rhogap mutants, and crossing these mutations into abl-/- or arg-/- background, should provide insight into whether these proteins function in similar pathways to regulate dendrite morphology.
Regulation of Synapse Structure and Function by Abl and Arg
Emerging evidence suggests that Abl family kinases regulate synaptic structure and function. Abl family kinases regulate the assembly of postsynaptic components at the neuromuscular junction (NMJ).63 Abl and Arg are required for the agrin-induced clustering of acetylcholine receptors (AchRs) on the postsynaptic membrane, where they form a complex with and phosphorylate the muscle-specific receptor tyrosine kinase (MuSK). Thus, Abl and Arg regulate neurotransmitter receptor distribution in the postsynaptic compartment of the NMJ.
In the CNS, Abl and Arg also localize to both presynaptic terminals and dendritic spines, the postsynaptic compartments of excitatory synapses.26 Electrophysiological studies in the mouse hippocampus reveal that Abl and Arg modulate the efficiency of neurotransmitter release from the presynaptic terminal.26 Paired-pulse facilitation (PPF), a transient form of presynaptic plasticity, is reduced in abl-/- and arg-/- hippocampal slices, and in wild type slices treated acutely with STI-571. Interestingly, treatment of abl-/- or arg-/- slices with STI-571 did not further reduce PPF, indicating that Abl and Arg have unique roles in the synapse and both are required for PPF. These data suggest that Abl and Arg kinase activity support optimal neurotransmitter release from the presynaptic terminal.
The mechanisms by which Abl and Arg regulate synaptic function are currently unknown. It is likely that they mediate interactions between cell surface receptors and the cytoskeleton in synapses, but it is unclear with which cell surface receptors they interface. Cell adhesion receptors are likely candidates because of their established roles in the formation, maintenance and remodeling of synaptic contacts. Investigating the synaptic partners of Abl and Arg will be an important area for future research.
Bone Development in abl-/- Mice
Consistent with the high expression level of Abl in osteoblasts of human fetuses,24 abl-/- mice have osteoporosis, displaying thinner bone volume and bone mineral content than wild type mice.64 Thinning of bone volume may be due to increased bone absorption by osteoclasts or reduced bone deposition by osteoblasts. Therefore, proper development and function of osteoclasts and osteoblasts is critical for bone development and maintenance. Osteoclastogenesis and osteoclast function are normal in abl-/- mice, but by several measures, osteoblastogenesis is delayed or defective during early maturation.64 This leads to a dramatically reduced rate of bone mineral deposition in abl-/- mice.
Bone development is probably the least understood of all abl mutant phenotypes. There have been no further studies on Abl regulation of osteoblast development, or any reports of whether Arg may contribute to this process. Li et al64 speculate that Abl may participate in extracellular matrix (ECM)-integrin signaling in osteoblasts, a critical pathway in osteoblast differentiation. This hypothesis is supported by the finding that Abl kinase activity increases upon fibroblast adhesion to the ECM protein fibronectin,65 and by our work demonstrating that Abl mediates integrin signaling in neurons.60
Conclusion
The study of abl and arg mutant mice has certainly advanced our understanding of the normal functions of Abl and Arg. We now know that Abl and Arg participate in a wide variety of developmental processes. However, we have yet to fully understand the molecular mechanisms the kinases use to regulate these processes. In addition, the molecular basis of overlapping versus distinct functions of Abl and Arg in specific processes is unknown. Many mutant phenotypes occur with variable penetrance and severity. This has been noted for several aspects of lymphocyte development,27,28,31,55 as well as bone development,64 but its cause also remains unknown. Future work will undoubtedly shed light on these questions.
There seems to be no rhyme or reason to the group of tissue systems in which Abl and Arg function during development, except that in each developmental event, Abl and Arg have been utilized by a specialized cell type for a specific signaling need. For Abl and Arg, these range from pathways promoting cell proliferation and survival to cytoskeletal regulation. Clearly, with the versatility of a Swiss Army knife, Abl and Arg are perfectly suited for this multi-tasking.
References
- 1.
- Goff SP, Gilboa E, Witte ON. et al. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980;22(3):777–785. [PubMed: 6257398]
- 2.
- Shtivelman E, Lifshitz B, Gale RP. et al. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315(6020):550–554. [PubMed: 2989692]
- 3.
- Abelson HT, Rabstein LS. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970;30(8):2213–2222. [PubMed: 4318922]
- 4.
- Van EttenRA. Studying the pathogenesis of BCR-ABL+ leukemia in mice. Oncogene. 2002;21(56):8643–8651. [PubMed: 12476310]
- 5.
- Kruh GD, Perego R, Miki T. et al. The complete coding sequence of arg defines the Abelson subfamily of cytoplasmic tyrosine kinases. Proc Natl Acad Sci USA. 1990;87(15):5802–5806. [PMC free article: PMC54416] [PubMed: 2198571]
- 6.
- Kruh GD, King CR, Kraus MH. et al. A novel human gene closely related to the abl proto-oncogene. Science. 1986;234(4783):1545–1548. [PubMed: 3787260]
- 7.
- Shtivelman E, Lifshitz B, Gale RP. et al. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986;47(2):277–284. [PubMed: 3021337]
- 8.
- Ben-Neriah Y, Bernards A, Paskind M. et al. Alternative 5' exons in c-abl mRNA. Cell. 1986;44(4):577–586. [PubMed: 3512096]
- 9.
- Daniel R, Chung SW, Eisenstein TK. et al. Specific association of Type I c-Abl with Ran GTPase in lipopolysaccharide-mediated differentiation. Oncogene. 2001;20(21):2618–2625. [PubMed: 11420673]
- 10.
- Daniel R, Wong PM, Chung SW. Isoform-specific functions of c-abl: type I is necessary for differentiation, and type IV is inhibitory to apoptosis. Cell Growth Differ. 1996;7(9):1141–1148. [PubMed: 8877095]
- 11.
- Hardin JD, Boast S, Mendelsohn M. et al. Transgenes encoding both type I and type IV c-abl proteins rescue the lethality of c-abl mutant mice. Oncogene. 1996;12(12):2669–2677. [PubMed: 8700526]
- 12.
- Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. [PubMed: 12374288]
- 13.
- McWhirter JR, Wang JY. An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 1993;12(4):1533–1546. [PMC free article: PMC413366] [PubMed: 8467803]
- 14.
- Van EttenRA, Jackson PK, Baltimore D. et al. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J Cell Biol. 1994;124(3):325–340. [PMC free article: PMC2119935] [PubMed: 8294516]
- 15.
- Wang Y, Miller AL, Mooseker MS. et al. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin. Proc Natl Acad Sci USA. 2001;98(26):14865–14870. [PMC free article: PMC64950] [PubMed: 11752434]
- 16.
- Miller AL, Wang Y, Mooseker MS. et al. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J Cell Biol. 2004;165(3):407–419. [PMC free article: PMC2172189] [PubMed: 15138293]
- 17.
- Wen ST, Jackson PK, Van EttenRA. The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 1996;15(7):1583–1595. [PMC free article: PMC450068] [PubMed: 8612582]
- 18.
- Van EttenRA, Jackson P, Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989;58(4):669–678. [PubMed: 2670246]
- 19.
- Taagepera S, McDonald D, Loeb JE. et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci USA. 1998;95(13):7457–7462. [PMC free article: PMC22649] [PubMed: 9636171]
- 20.
- Muller R, Slamon DJ, Tremblay JM. et al. Differential expression of cellular oncogenes during preand postnatal development of the mouse. Nature. 1982;299(5884):640–644. [PubMed: 6289129]
- 21.
- Koleske AJ, Gifford AM, Scott ML. et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21(6):1259–1272. [PubMed: 9883720]
- 22.
- Renshaw MW, Capozza MA, Wang JY. Differential expression of type-specific c-abl mRNAs in mouse tissues and cell lines. Mol Cell Biol. 1988;8(10):4547–4551. [PMC free article: PMC365533] [PubMed: 2460747]
- 23.
- Courtney KD, Grove M, Vandongen H. et al. Localization and phosphorylation of Abl-interactor proteins, Abi-1 and Abi-2, in the developing nervous system. Mol Cell Neurosci. 2000;16(3):244–257. [PubMed: 10995551]
- 24.
- O'Neill AJ, Cotter TG, Russell JM. et al. Abl expression in human fetal and adult tissues, tumours, and tumour microvessels. J Pathol. 1997;183(3):325–329. [PubMed: 9422989]
- 25.
- Perego R, Ron D, Kruh GD. Arg encodes a widely expressed 145 kDa protein-tyrosine kinase. Oncogene. 1991;6(10):1899–1902. [PubMed: 1923513]
- 26.
- Moresco EM, Scheetz AJ, Bornmann WG. et al. Abl family nonreceptor tyrosine kinases modulate short-term synaptic plasticity. J Neurophysiol. 2003;89(3):1678–1687. [PubMed: 12626632]
- 27.
- Tybulewicz VL, Crawford CE, Jackson PK. et al. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65(7):1153–1163. [PubMed: 2065352]
- 28.
- Schwartzberg PL, Stall AM, Hardin JD. et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65(7):1165–1175. [PubMed: 2065353]
- 29.
- Schwartzberg PL, Goff SP, Robertson EJ. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989;246(4931):799–803. [PubMed: 2554496]
- 30.
- Schwartzberg PL, Robertson EJ, Goff SP. Targeted gene disruption of the endogenous c-abl locus by homologous recombination with DNA encoding a selectable fusion protein. Proc Natl Acad Sci USA. 1990;87(8):3210–3214. [PMC free article: PMC53865] [PubMed: 2183226]
- 31.
- Hardin JD, Boast S, Schwartzberg PL. et al. Bone marrow B lymphocyte development in c-abl-deficient mice. Cell Immunol. 1995;165(1):44–54. [PubMed: 7671324]
- 32.
- Bianchi C, Muradore I, Corizzato M. et al. The expression of the nonreceptor tyrosine kinases Arg and c-abl is differently modulated in B lymphoid cells at different stages of differentiation. FEBS Lett. 2002;527(1-3):216–222. [PubMed: 12220663]
- 33.
- Peschon JJ, Morrissey PJ, Grabstein KH. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–1960. [PMC free article: PMC2191751] [PubMed: 7964471]
- 34.
- Grabstein KH, Waldschmidt TJ, Finkelman FD. et al. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178(1):257–264. [PMC free article: PMC2191094] [PubMed: 8315381]
- 35.
- von Freeden-JeffryU, Vieira P, Lucian LA. et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–1526. [PMC free article: PMC2191954] [PubMed: 7699333]
- 36.
- Valenzona HO, Dhanoa S, Finkelman FD. et al. Exogenous interleukin 7 as a proliferative stimulant of early precursor B cells in mouse bone marrow: efficacy of IL-7 injection, IL-7 infusion and IL-7-anti-IL-7 antibody complexes. Cytokine. 1998;10(6):404–412. [PubMed: 9632525]
- 37.
- Namen AE, Lupton S, Hjerrild K. et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333(6173):571–573. [PubMed: 3259677]
- 38.
- Morrissey PJ, Conlon P, Charrier K. et al. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J Immunol. 1991;147(2):561–568. [PubMed: 1712810]
- 39.
- Rosenberg N. Abl-mediated transformation, immunoglobulin gene rearrangements and arrest of B lymphocyte differentiation. Semin Cancer Biol. 1994;5(2):95–102. [PubMed: 8061334]
- 40.
- Darnell JEJr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. [PubMed: 8197455]
- 41.
- Danial NN, Pernis A, Rothman PB. Jak-STAT signaling induced by the v-abl oncogene. Science. 1995;269(5232):1875–1877. [PubMed: 7569929]
- 42.
- Banerjee A, Rothman P. IL-7 reconstitutes multiple aspects of v-Abl-mediated signaling. J Immunol. 1998;161(9):4611–4617. [PubMed: 9794389]
- 43.
- Fisher AG, Burdet C, Bunce C. et al. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int Immunol. 1995;7(3):415–423. [PubMed: 7794821]
- 44.
- Rich BE, Campos-Torres J, Tepper RI. et al. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993;177(2):305–316. [PMC free article: PMC2190896] [PubMed: 7678850]
- 45.
- Dorsch M, Goff SP. Increased sensitivity to apoptotic stimuli in c-abl-deficient progenitor B-cell lines. Proc Natl Acad Sci USA. 1996;93(23):13131–13136. [PMC free article: PMC24058] [PubMed: 8917556]
- 46.
- Lu L, Osmond DG. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol Rev. 2000;175:158–174. [PubMed: 10933601]
- 47.
- Griffiths SD, Goodhead DT, Marsden SJ. et al. Interleukin 7-dependent B lymphocyte precursor cells are ultrasensitive to apoptosis. J Exp Med. 1994;179(6):1789–1797. [PMC free article: PMC2191526] [PubMed: 8195708]
- 48.
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. [PubMed: 9323135]
- 49.
- Zipfel PA, Grove M, Blackburn K. et al. The c-Abl tyrosine kinase is regulated downstream of the B cell antigen receptor and interacts with CD19. J Immunol. 2000;165(12):6872–6879. [PubMed: 11120811]
- 50.
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. [PubMed: 9143696]
- 51.
- Cwynarski K, Laylor R, Macchiarulo E. et al. Imatinib inhibits the activation and proliferation of normal T lymphocytes in vitro. Leukemia. 2004;18(8):1332–1339. [PubMed: 15190258]
- 52.
- Dietz AB, Souan L, Knutson GJ. et al. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104(4):1094–1099. [PubMed: 15100154]
- 53.
- Zipfel PA, Zhang W, Quiroz M. et al. Requirement for Abl kinases in T cell receptor signaling. Curr Biol. 2004;14(14):1222–1231. [PubMed: 15268851]
- 54.
- Seggewiss R, Lore K, Greiner E. et al. Imatinib inhibits T-cell receptor mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105(6):2473–9. [PubMed: 15572591]
- 55.
- Hardin JD, Boast S, Schwartzberg PL. et al. Abnormal peripheral lymphocyte function in c-abl mutant mice. Cell Immunol. 1996;172(1):100–107. [PubMed: 8806812]
- 56.
- Leng Y, Zhang J, Badour K. et al. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc Natl Acad Sci USA. 2005;102(4):1098–1103. [PMC free article: PMC545868] [PubMed: 15657136]
- 57.
- Zukerberg LR, Patrick GN, Nikolic M. et al. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000;26(3):633–646. [PubMed: 10896159]
- 58.
- Woodring PJ, Hunter T, Wang JY. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116(Pt 13):2613–2626. [PubMed: 12775773]
- 59.
- Hernandez SE, Krishnaswami M, Miller AL. et al. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14(1):36–44. [PubMed: 14729179]
- 60.
- Moresco EMY, Donaldson S, Williamson A. et al. Integrin-mediated dendrite maintenance requires Abl family kinases J Neuroscisubmitted. [PMC free article: PMC6725048] [PubMed: 15987940]
- 61.
- Hernandez SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr Biol. 2004;14(8):691–696. [PubMed: 15084284]
- 62.
- Ruchhoeft ML, Ohnuma S, McNeill L. et al. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19(19):8454–8463. [PMC free article: PMC6783015] [PubMed: 10493746]
- 63.
- Finn AJ, Feng G, Pendergast AM. Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat Neurosci. 2003;6(7):717–723. [PubMed: 12796783]
- 64.
- Li B, Boast S, de los Santos K. et al. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat Genet. 2000;24(3):304–308. [PubMed: 10700189]
- 65.
- Lewis JM, Schwartz MA. Integrins regulate the association and phosphorylation of paxillin by c-Abl. J Biol Chem. 1998;273(23):14225–14230. [PubMed: 9603926]
- 66.
- Kurosaki T. Checks and balances on developing B cells. Nat Immunol. 2003;4(1):13–15. [PubMed: 12496972]
- 67.
- Muljo SA, Schlissel MS. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat Immunol. 2003;4(1):31–37. [PubMed: 12469118]
- 68.
- Miao YJ, Wang JY. Binding of A/T-rich DNA by three high mobility group-like domains in c-Abl tyrosine kinase. J Biol Chem. 1996;271(37):22823–22830. [PubMed: 8798460]
- Abl Family Kinases in Mammalian Development - Madame Curie Bioscience DatabaseAbl Family Kinases in Mammalian Development - Madame Curie Bioscience Database
- Cell-Cell Channels and Their Implications for Cell Theory - Madame Curie Bioscie...Cell-Cell Channels and Their Implications for Cell Theory - Madame Curie Bioscience Database
- Antibiotic Resistance in Bacteria Caused by Modified Nucleosides in 23S Ribosoma...Antibiotic Resistance in Bacteria Caused by Modified Nucleosides in 23S Ribosomal RNA - Madame Curie Bioscience Database
- Proteolytic Degradation of Αβ by Neprilysin and Other Peptidases - Madame Curie ...Proteolytic Degradation of Αβ by Neprilysin and Other Peptidases - Madame Curie Bioscience Database
- The Role of IL-10 in Autoimmune Pathology - Madame Curie Bioscience DatabaseThe Role of IL-10 in Autoimmune Pathology - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...