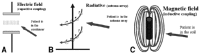NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Hyperthermia is a medical heat-treatment, widely used in various medical fields and has a well-recognized effect in oncology. It is an ancient treatment. However, when making hyperthermia we are limited by numerous biological, physical/technical and physiological problems. The word hyperthermia means increased temperature by heating of tumors. This relatively simple, physical-physiological method has a phoenix-like history with some bright successes and many deep disappointments. Why is this enigma? What do we have in hand? Answers lie in the applied techniques.
Introduction
Cancer and its treatment have been one of the greatest challenges in the medical science for centuries. Nowadays, enormous economic and human resources are involved in this field, but according to the epidemic data the solution shall still be awaited for. Sure, the cancer is not the first and probably not the last one among the diseases which despite of the exceptional human efforts have not had any cure for a long time. The development of the medical knowledge in most of the cases follows some critical situations and crises, preparing the medical science to avoid the next crisis of the same nature.
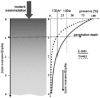
The medieval medicine insisted basically on the ancient rule of “no cure”1 for cancer, but the diagnostics and categorization had been further developed.2 However, the treatment of patients suffering in cancer was put in practice only in the second half of the 19th century. Definitely, the main idea was always to concentrate on the drastic elimination of the tumor by surgical way, but they improved the therapies by drugs and diets as well.3 At the beginning of the 20th century the basis of the modern oncology had been established by a distinguished branch of scientists: Schleiden, Schwann, Virchow, Halstedt, Wertheim, Billroth and others.4 Nowadays, the oncology became one of the most interdisciplinary research fields: including the biology, biophysics, biochemistry, genetics, environmental sciences, epidemiology, immunology, microbiology, pathology, physiology, pharmacology, psychology, virology, etc. The modern oncology applies highly effective methods and treatments, but their side-effects and, in consequence, the impairment of the quality of life are also remarkable. In general, patients are treated with chemo- and radiotherapy to their toxicity limits in order to achieve maximal tumor destruction. However, these treatments are often not enough. In general, the tolerable toxic level limits the applications: the actually expected tumor destruction would request higher doses than it is tolerable as regards the accepted level of side effects (fig. 1A). The applied therapies might drastically reduce the actual demand of the further tumor destruction, but unfortunately the acceptable toxic tolerance is also reduced and the therapeutic gap is reestablished in most of the cases (fig. 1B). The gap between the toxic tolerance and the desirable destruction has to be bridged by a method, such as the hyperthermia: based on physical and physiological effects, its stress has no chemical origin or serious toxicity. Hyperthermia is an ideal combination therapy. It has low toxicity, mild side effects, and has been shown to provide synergies with many of the traditional treatment modalities.

Figure 1
A) There is a therapeutic gap between the toxic tolerance and the desired destruction. B) Treatments modify but do not eliminate the gap. Rationale of hyperthermia: surmount the therapeutic gap.
Besides the limited toxicity, the developed resistance against the actually applied treatments could also limit the efficacy of the methods. While the first treatment is able to suppress the tumor under its detectability, but it is not the sure outcome, as some malignant cells remained behind keep the possibility of relapse. The observed and hopeful complete remission in most of the cases makes only a temporary success. Parallel with this a more serious problem arises than this: some of the nonvanished malignant cells might become resistant to the actual treatment, so its next application could not be that successful as it was before, and in the successive steps we lose this treatment facility. Hyperthermia can be helpful in these cases as well because it may resensitize the malignant cells and enables them to be destructed.
Heat therapy (Hyperthermia) is an aboriginal, traditional healing method. Even the first known, more than 5000 years old, written medical report from the ancient Egypt mentions hyperthermia.5 The use of hyperthermia for cancer therapy was first documented by Hypocrites for the treatment of breast tumor.4 His approach of course was mainly supported by the Greek philosophy, where the fire (heat) had the highest level of abilities and freedom. Hyperthermia was also mentioned throughout the Middle Ages,6 but due to the strict Galenus' school and the inadequate heating techniques, the treatment has never became a standard in the oncology practice.
Among the first modern curative applications in oncology, Busch7 and Coley8 were successful at the end of the 19th century with artificial fever generated by infection and toxins, respectively. These systemic applications soon were followed by local and regional heating.9-11 The leading German surgeon in that time, Bauer KH opinion in his monograph “Das Krebsproblem” about the oncologic hyperthermia is typical: ”All of these methods impress the patient very much, they do not impress their cancer at all.” However, very early, in 1912, a controlled Phase II clinical study was published on 100 patients showing the benefit of the thermo-radiation therapy.12
At the end of the last century, energy delivery by electromagnetic fields became possible; nevertheless, its use for hyperthermia only began about 30 years ago. The first symposium on oncological hyperthermia was held in Washington DC, USA in 1975; and the second one in Essen, Germany in 1977. Both conferences were supported by the local scientific communities. We may reckon with the born of the modern oncological hyperthermia from this time on as a strong candidate and a member of the acknowledged tumor therapies.
Hyperthermia today, like many early-stage therapies, lacks adequate treatment experience and long-range, comprehensive statistics that could help us optimize its use for all indications. Nowadays, the lack of acceptance of the oncological hyperthermia has not only statistical reasons; but the technical solutions are not adequate enough and the quality assurance, the control and standardization of the method itself have not been solved yet satisfactorily. Nevertheless, we will present a wealth of information about the mechanisms and effects of hyperthermia from the scientific literature and our own experience in the hope of proving hyperthermia's worth for further research.
Many of the researchers evaluating the capabilities of oncological hyperthermia share the opinion of the editorial comment of European Journal of Cancer in 2001: the biological effects are impressive, but physically the heat delivery is problematic. The hectic results are repulsive for the medical community. The opinion, to blame the “physics” (means technical insufficiency) for inadequate treatments is general in the field of oncological hyperthermia, formulating: “The biology is with us, the physics are against us”.13 In the latest oncological hyperthermia consensus meeting the physics was less problematic. However, in accordance with the many complex physiological effects a modification was proposed: “The biology and the physics are with us, but the physiology is against us”.14
The present situation apparently supports the above opinions. The opinion, to blame the “physics” (means technical insufficiency) for inadequate treatments is general in the field of oncological hyperthermia. Is the modern technique unable to meet the demands, indeed?
There is a definite group of physicians who submit that hyperthermia has a strong curative force in oncology; however, another group exists believing the opposite. Sure, both the positive and negative believers are not helpful to clarify the situation. We need interdisciplinary scientific analyses and hypotheses to go ahead with the topic.
The state of oncological hyperthermia today is similar to that of radiology at its infancy. When ionizing radiation was first discovered, many hypothesized its usefulness in oncology, yet its exact techniques, dose, contraindications, limits, and the conditions of optimal treatment were determined only several decades later. This is a natural process: every beginning shows these “symptoms”. However, the baby normally has to leave behind the teething-brash after a definite period. To remain a baby afterwards is abnormal. We think oncological hyperthermia has to get beyond the babyhood, it has to grow up in more definite manner; it has to cast off the infantile period!
Our present paper tries to explain the problem of the technical solutions. We would like to promote the harmony of the disciplines, and this interdisciplinary approach might be “with us” to win the war against the cancerous diseases.
Characterization Demand
Hyperthermia by its definition is the overheating of the selected tissues. Heat dosing and treatment standardization at hyperthermia are still significant problems. Technically, the dosing and control of the deep heat transfer is very difficult, requesting the same reproducible heat dose for each treatment within the target tissue. A “success parameter” has to take into account the efficiency of focusing, the heat conduction within the body and many other physiologic conditions before reliable protocols can be worked out.
Temperature as a Control Parameter
There is a considerable discussion over the relevant treatment parameters, controls, and treatment optimization. Discussions are primarily centered on the role of temperature and the effects initialized by temperature. Various technical solutions are in use at oncological hyperthermia. The reached temperature as a control parameter is compared. At the clinical results the technical differences are ignored, therefore subjective comparison gains ground. In most of the hyperthermia publications the temperature and the heat as the definite concepts are equally and interchangeably used. By the definition, hyperthermia has to have a heating dose, which is, in most of the medical practices, immediately identified by the temperature. Furthermore, the missing consensus in the explanation of the underlying mechanisms hampers perspicacity. Consequently, the selection of the proper technical quality control parameter is determined only by the actual technical solution and not by the desired effects. Discussions are primarily centered on the role of temperature and the effects initialized by temperature.
However, it is physically incorrect. The temperature and heat are two different and definitely not exchangeable quantities in physics. The heat is a kind of energy, which may be generally characterized by the specific absorption rate (SAR) (integrative view) and, microscopically, by the selective energy depletion mechanisms. This physical quantity is an extensive thermodynamical parameter; which means that the thermal energy is proportional to the mass/ volume/part of the targeted material. The temperature is an intensive thermodynamic parameter; which characterizes the actual state irrespective of its mass/volume/part. If we apply the temperature as a characteristic feature of volume, than every sub-volume has the same temperature, and the volume is characterized in quasi-equilibrium.
In most of the real cases we pump heat (energy) into the targeted system to change its chemical bonds and reactions. This is the case by the hyperthermia as well; our definite aim is to destroy the malignant cells/tissue. If the energy transfer is correct, than all the pumped in energy is devoted to make this job: changes the chemical bonds and destroys the actual biochemical processes in malignant tissue. If we were able to destroy the malignancy by changing only a single chemical bond, then our job would be simple: to deliver the corresponding braking energy (resonant frequency), break the bond, and no any temperature change would be requested. But the situation is not that simple. By pumping in a specific energy the temperature increases, and we get an average energization without targeting any specified structures.
To show the difference let us show a simple example: the body temperature of healthy humans is fairly constant (deviation is less than ± 1°C, approx. 0.3%), while our energy consumption (heat equivalent) varies in a wide range individually (deviation can be far more than 100%), even the same individual can have very hectic energy intake depending on the complex conditions with no mentionable change in his body temperature.
However, a phase transition (sudden structural/chemical change) at definite temperature could solve the contradiction in hyperthermia, this might justify the temperature concept. Indeed, a cellular phase transition observed around 42.5°C,15 and also the surprisingly accurate fit of the Arrhenius plot to experimental results16,17 supports well the idea. However, Arrhenius kink changes by the chemotherapies,18 and furthermore, the phase-transition temperature strongly depends on the heating dynamism (slow and rapid)19 and on the preheating conditions.20 Other clinical observations also lead to recent doubts about this concept.21 Interesting also that the thermal enhancement ratio (TER) increases linearly with the activation energy,18 so the step down heating based on the phase-transition concept20 is broken. The healthy living state is in a dynamic equilibrium (homeostasis). The malignant tissue is not in equilibrium, its progress is permanent. The physiology deeply changes the conditions and modifies the situation.22 The physiological conditions are so effective that the drastically increased forwarded power (from 450 W to 900 W) in fact does not improve the measured temperature, and varies considerably patient by patient.23 Due to the physiological factors, the heat-treatment success depends on the dynamism of the heat delivery.19 Measurements show that the tumor temperature is not significant when applying pretreatments and/or incubations.22
The isothermal curves seem to be unscientific dreams in the living tissue, because its heat flow is uncontrolled, and it is not a homogeneous media regarding any of its relevant thermodynamical parameters. Disturbing factors in the various stage of malignancies are mainly the blood-flow, the possible liquids and the heat-conductivity differences. Additionally, we expect the main energy-consumption is taken on the chemical effects. This is manifested dominantly in the cellular distortions and does not increase the temperature.
The doubts increase when we observe that the uniform and high temperature in the tumor (near the physiological threshold of 42°C) caused by extreme whole body hyperthermia (WBH) does not show better results than the locoregional heating with lower temperature average and with very heterogeneous temperature distribution. Application of lower temperatures (mild WBH) for treatments of longer periods (keeping the dose) also showed surprisingly good efficacy.24 The delivered heat dose25 (absorbed energy) or applied field26 (electromagnetic influence) could determine the treatment efficacy.
The incompleteness of the temperature only idea is well shown also by the necessity of two other characteristic parameters: the time (how long we are able to keep the given temperature, generally defined as a proportion of the actual treatment time) and the information on the temperature distribution (or quantities describing which portion of the tumor was over the given temperature, or measurement of the minimum and maximum temperature in the lesion) as well. When we talk about time-dependence, it means dynamism and nonequilibrium concept. By involving the time factor, the dynamics and the nonequilibrium (dynamic equilibrium, homeostasis) are automatically involved. The time by power is the delivered energy; but the time by temperature—as it is used in the hyperthermia practice—has not any physical meaning! Unfortunately, the temperature as an intensive thermostatic parameter is not enough for the proper control, even it may mislead the user.
Recently, numerous scientific theories have also started to concentrate on the significance of thermally induced nonthermal effects,27 such as heat-shock protein (HSP) production.28 The relevance of tumor temperature, heat dose, nonequilibrium, and nonthermal effects is apparent, and leads us to the conclusion that clinical outcome cannot be determined purely on the basis of any of these factors alone.
Thermodynamical Approach
Heat put into a system (ΔQ) plus work done on a system (ΔW) is equal to the increase in internal energy of the system (ΔU), (first law of thermodynamics29):

Sign Δ means a small change. During this small change the system does not alter (quasi-static approach). In a more rigorous description a differential calculus has to be used. Eq. (1) shows that the internal energy U is determined by the energy exchange. This exchange has various forms, all the available interactions (denote their number by n) have to be calculated. These terms can be easily defined by the pair products of intensive (Yi) and extensive (Xi) parameters:

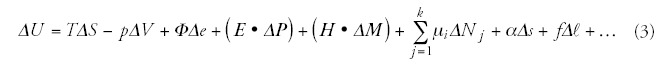
Definitely, if there are not any structural, chemical etc. interactions in the system, only the heat is absorbed, than (1) has no ΔL term:


Have we any reason why hyperthermia uses intensive thermodynamic parameter (temperature) regarding the quasi-equilibrium of the treatment? No, of course, we have not. It is only the historical “bad habit”. This bad characterization is mostly responsible for the contradictory results, for the loss of comparability of the results, for the blame of physics/physiology!
Bio-Heat Equation
The thermodynamical energy balance defines the actual heat exchange: the energy change in time (∂U/∂t), its flow in space is: grad(IU)= Σ(∂IU/∂xi), and its sources qU=qe+qm+qb, where IU is the energy current density, qe, qm and qb are the energy source/sink from electric, metabolic and blood perfusion origins, respectively. The well known balance equation30 is:

As it is well known from reference 31, the current density of the heat flow is proportional to the temperature gradient (heat diffusion):

By substituting (7) into (6) and by using (4) we obtain the so-called bio-heat (Pennes-) equation32 which serves for the description of tissue heating and internal energy transport. It has the form of
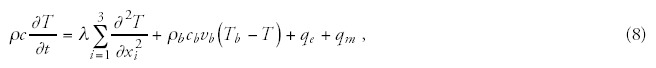
The qm term is determined by the metabolic rate (M) and the mass density: qm = ρM. The metabolic rate grows by the temperature gain ΔT: qm∼1.1ΔT;33 therefore, in the case of 6°C increase the amount of growth will be 1.8-times. The metabolic heat production of tumor depends on the doubling time of its volume (ref. 34 and fig. 2), so it is determined by the speed of the development.
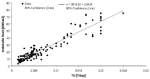
Figure 2
Metabolic heat production of breast cancer lesions (tumor size: 0.6-4.0 cm), shown as a function of the reciprocal value of volume doubling time. The rapid procedures (large 1/t values) are more intensive heat producers.
Defining qe is more complicated, because it needs an approximation of the energy absorbed from the electromagnetic fields. In real situation the bio-electromagnetic interactions are more complex, and the measurement of the absorbed energy is necessary. The energy absorption is characterized by the specific absorption rate (SAR, [W/kg]), identifying the absorbed energy in unit mass:

The simplest case is the current flow through the given tissue, when the well-known Joule-heat is developed. This value (taking into account the well-known Ohm-law) is:

The solution of the partial differential equation (bio-heat equation) (8) should be reasonably given numerically because of the following arguments:
- The exact geometry of tissues is unknown;
- The equation parameters are not available in their exact form, the values change by tissues, tumors and individuals, as well as those are nonhomogenic in the tumor-tissue;
- A certain part of equation parameters—for example perfusion rate, metabolic thermal power, electric thermal power—can be expressed as a function of temperature, therefore the equation may be nonlinear as well;
- The treating electric field and for this reason the electric thermal power can be expressed as a function of position, and because of the skin effect it depends also on the temperature;
- The parameters of the transitional zone between the tumorus and healthy tissues are unknown; therefore the exact definition of boundary conditions is almost impossible.
The unanimity conditions of the bio-heat equation (8) are the initial data and boundary values. The initial data can be easily specified by the actual initial body temperature (To), assuming its homogeneity in the malignant area and its surrounding. (Note: sometimes it is not the case because of the higher tumor temperature due to its increased metabolic rate.) However, the boundary conditions (which are essential to solve (8) at least numerically) are more complicated to determine. Three types of boundary conditions may be considered:
- The boundary surface of the targeted area has a constant temperature. This assumption is supported by the observation that the functioning of tissue is normal outside the area, thus the increase in temperature augments the perfusion rate and—as a result—keeps the healthy tissue section on constant temperature.
- A boundary layer is formed on the surface of the targeted area. This could be physiologically constructed by a transient tissue between the malignant and healthy areas. The considerable increase of perfusion in the healthy tissue section close to the boundary surface presents an other problem, as the extent of this should be known as well.
- The transition of conductive heat-flux density and temperature is continuous on the boundary surface of target area. The consideration of this boundary condition means that the bio-heat equation (8) shall be solved both for the target area and for the area outside thereof, and the two solutions shall be matched at the boundary of the two areas in consideration. This is a very complicated task, mainly in the case where the surroundings can not be considered as infinitely large. Therefore, a newer boundary condition is needed.
These conditions—with the additional problem of knowing the SAR distribution in-situ—clearly show that the solution of bio-heat equation does not realistic for practical use. However, note the actual driving forces in the equation, which are always the temperature changes (gradients in space and time), and no assumption of the homogenic temperature could be valid. (In the case of homogenic temperature the bio-heat equation (8) does not exists.)
Control-Parameters
Classical Approach
The first standard ever for the nonionizing radiation35 declared the maximal radiation limit Pmax = 10 mW/cm2 as a danger-threshold for humans, supposing 0.1°C increase of human body temperature by this energy absorption. Based on this concept any bio-application—in most of the cases—is controlled by the actual equilibrium temperature (considering the normal body temperature as a basis); and this is the starting point of the classical hyperthermia control as well. However, there are doubts in the scientific community on the issue if only the equilibrium temperature determines every biologically relevant processes. Nowadays, the delivered heat (absorbed energy dose) or applied field (electromagnetic influence) are also considered as important effects. However, serious arguments could be presented to support the presence of the thermally induced but basically nonthermal effects.27 These involve the thermally and nonthermally generated chaperone proteins, which are heat-shock proteins (HSP)28 for the most part. HSP proteins modify the stress tolerance to electromagnetic interactions as well.36-38
Definitely, the most commonly used control parameter is the temperature. However, the measurement of the temperature in the malignant area is not a simple task: it is rather nonhomogeneous site by site, even on different size scales (in cellular level the metabolic rate, in cluster level the necrotization, in tissue level the vasolidation makes the distribution inhomogeneous). In this manner the temperature measurement in a definite point may not give general information; it depends greatly on the actual sensor position, the actual tumor status and physiology. Consequently, the usual contact temperature measurement can give realistic control if the average of some of them is observed. This disadvantage comes from the problems of the invasivity (possible infections, ulcers, metastases, pain, discomfort, etc.) and the problem of the electromagnetic sensitivity of the sensors themselves. However, some noninvasive methods have been developed as well, but all of them have some serious problems by restringing the wide range of reliable applications. The most popular noninvasive temperature measuring methods and their problems are:
- Infrared image—It measures only the surface temperatures.
- MR-image—Time-shifts are measured, which first of all depend on the chemical bonds. The calibration made on the phantom, where the chemical changes are reversible and different from the living target tissue, and where our primary goal is to change the chemical bonds into irreversible one. Without the proper calibration, the temperature from the time-shifts is not calculable.
- Thermo-radiometry>—(passive radar techniques). It is not accurate enough and only the multi-frequency application can give information about the depth distribution. Technically, these devices with satisfactory number of frequencies do not exist yet.
- Impedance measurements—It gives overall information on the tumor, no vertical resolution available and the lateral resolution is also not satisfactory, and measures only the averages.
The development of science and technics is rapid, and we expect to have more accurate and reliable noninvasive temperature measurements in the future. However, the question remains: do we force the optimal way for the control? Due to the missing phase transition, due to the principally incomplete approach, due to the problematic measurements we do not support the temperature as an overall control parameter. In fact, the search for the qualitative extensive parametrization is not a surprise in the medical treatment characterization. All the medical treatments are quantitatively characterized by extensive (never by intensive) thermodynamic parameters. An example only from the oncology: the radiotherapy uses the gray [Gy] (=10-2rad), which is the absorbed energy pro mass (energy-density, [J/kg]); the chemotherapy uses the chemo-dose, mass of the active agent pro mass of the target/[body-mass/body-surface]); surgery uses the volume and/or the mass; etc. Except the hyperthermia there is not any other medical application, which uses intensive parameter to control any processes! It is definitely because of the dynamical actions: the medical processes can not be characterized in quasi-thermostatic way.
Proposed Characterization
In spite of its inadequate character, the temperature became gradually the base of the quality assurance and treatment control. To change it and to choose a heat-dose (energy dependent) characterization are desirable. To find an extensive parameter to characterize the quantity of the effect like in other treatment modalities is mandatory.
To measure the prompt effects during the treatment is really a quantitative factor, but to measure the conditions for the effective distortions afterwards, needs assumptions. The changes supposed to make the postponed actions (ischemia/hypoxia, stress factors, acidosis, etc.) are also measurable in situ. The temperature concept had oversimplified the situation: the suitably high temperature makes the jobs, prompt and postponed effects are expected, and it is supposed that the requested thermal dose has got into the target. As we see from (3), the temperature-dependent term (TΔS) in the internal energy (ΔU) is the only one from many. The main factor of the real desired action: to have cellular distortion and chemical reactions. If the bio-system undergoes chemical reactions then the nontemperature parts of the internal energy become important.27,30
We have to characterize the heat delivery, the treatment-effect in-situ in tumor-areas, as well as the actual quality of the hyperthermia treatments in order to keep proper control and to carry out the possible comparison of the various treatment techniques. There are various possibilities for the accomplishment of the relevant characterizations where biological/physiological parameter has to be applied for the characterization of treatment status. The actually used in-situ (or immediate off-situ) application of MRI check could be one of the possibilities in the selection of a qualitative parameter (first of all the spectroscopic one) without any force in regard to an unrealistic temperature approximation. However, the solution is simpler, as the physiologically and physically well studied electrolyte environment of the tissue offers an optimal possibility. The actual electrolytes in the tissue (mainly, the extracellular matrix (ECM)) depends on the metabolic rate, on the chemical reactions and on the structural changes as well. (Sure, it is also temperature-dependent under these extensives.) The ionic density and the structural changes can be followed well by the complex impedance (its imaginary and real parts), which is—in this meaning—a perfect candidate in the control of the energy processes.
Electric bio-impedance is a well known method and a simple electrical measurement technique. 39 It measures the tissue's specific electric field distribution. The measurement uses the special frequency dispersion of the actual tissue. As early as 1940 both the whole-body electrolyte status40 and the local changes (ECG)41 were studied by the method. Nowadays, it is widely applied, and received the FDA approval for breast tumor diagnostics.
Which effects could be used for characterizing?
- The prompt necrotic effect is trivially measurable by the impedance. During the capacitive hyperthermia most of the RF-current flows into the extracellular electrolyte, the cells are electronically capsulated (shielded) by their membrane by more than 1,000,000 V/m field strength. When the membrane is damaged, this (till that excluded) area becomes the part of the conductive process and the conductivity drastically changes.42 (Before this, the impedance grows because the cells swell and the extracellular volume shrinks).42 The cellular volume and the forming edema are also measurable in situ.43 The destructive phase of hyperthermia is well measurable, the necrotic and even the mitochondrial damage, as well as the cellular histolysis are observable.42 The process of the observed characteristic cellular response: cellular swelling, progressive membrane damage, cellular shrinkage and subsequent progressive histolysis. At the measurements, the histological changes44 and the induced coagulative necrosis in human xenografts45 are reflected. Special self-similar structure and the hierarchical circuit are also assumed,46 which well agrees with the fractal physiology approaches and the connected dynamism (noises).47
- The impedance measures selectively: differentiates between the cancerous and healthy tissue, and is able to distinguish the extra- and intracellular electrolyte as well. It is clinically proven that the cancerous and healthy tissue of the hepatic tumors are significantly different,49 as well as the VX-2 carcinoma can be also measured.50 Well applicable is the impedance method for breast cancer biopsies,51 for breast cancer diagnostics and prophylactics.52,53 Also clinically advanced lung cancer could be followed by the impedance observations,54 as well as, it has been successfully tried out in the skin-cancer diagnosis.55 There are commercially available devices for the impedance tomography for mamographical use.56 The impedance spectroscopy is effective in early tumor stages57 and single-cell characteristics58 too. The method is cell-selective.59
Recently, scientists have started to realize that hyperthermia induced temperature gradients (driving force of the dynamic effects) could have significant biological role. A new branch of hyperthermia known as extracellular hyperthermia74 or electro-hyperthermia26 has been developed around this concept. Although this new technique recognizes the benefits of increased tissue temperature and its biological consequences it also argues that nonequilibrium thermal effects are partially responsible for the observed clinical deviations from the purely temperature-based treatment theory. The applied impedance control facilitates to keep in hand the treatment and its effects, to characterize the actual phase of the process and to control the technology.
Technical Challenges
In the early days, simple heat diffusion was applied by using hot water or wax baths and heated objects.75 Today, focused and unfocused energy delivery are applied by using electromagnetic fields.
To heat up the malignant tissues is a relatively simple demand but doing it selectively (focused on the malignancy only) up to the desired temperature (over 42°C) is not a simple technical task. This complication was the basis of the editorial: the physics are against us.13 The main technical problems:
- Deepness—achieving a deep energy delivery,
- Focusing—proper focusing (selection) on the malignant area,
- Reproducibility—conducting the treatment in the reproducible way,
- Control—having proper control of the process by keeping the parameters of the treatment,
- Personalization—making an in-situ tuning to fit to the actual situation to the best.
Regarding the technical and biophysical side of hyperthermia there are some problems. The most important ones are as follows:
- The heat shock protein (HSP) concentration of tumorous tissues is high, as a matter of coarse, without any external influence and is further increased by the different inventions (apart form their modes).
- The heat effect not suitably provided or focused may increase the oxygen supply of tumour, and therefore may intitiate its quick growth. The misfocused heat input involves the risk of necrocytosis of healthy tissues, and therefore may step up the formation of metastasis.
- Technically, the reproduction and stability of heat input is difficult to control, therefore there is not any such technical “success parameter” the maintenance and monitoring of which can be considered as a reliable indicator of the success of treatment. The treatment of complex metamorphosis requires complex parameter ensemble, in this way, it is not to be expected that the succesful treatment process can be controlled by one or two parameters.
- Technically, the main problem is the appropriate selection of the heat input, as well as, the selection of heat input technics and the formation of actual applicators are of vital importance. The applied eletromagnetic effect may cause in itself HSP increase and undesired tolerance formation without any heat input and temperature increase,76 and may have some effect on the reactions of immune system as well.77
Overview of the Existing Methods
Because of the huge difficulties in the proper technical realization, numerous and quite different technical solutions have been developed. Some categorizing points of the available techniques are collected in Table 1.
Table 1
Categorizing of hyperthermia methods.
The form of heat (energy) delivery has also considerable varieties (Tables 2, 3) applied in practice. Their invasivity and selectivity are categorized in Tables 4 and 5. Sure, the most modern heating technics are connected to the electromagnetic interactions. Their increasing number of applications could be observed in different categories of frequency (Table 6).
Table 2
Heat delivery methods for hyperthermia.
Table 3
Energy production categories for hyperthermia.
Table 4
Invasivity of hyperthermia methods.
Table 5
Selectivity of hyperthermia methods.
Table 6
Applied frequency ranges for hyperthermia.
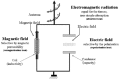
Electromagnetic Heating Processes
Basic Effects
The electromagnetic fields (magnetic induction B and electric field strength E) interact with the material and change its state. The interacting electromagnetic field is described by the Maxwellian equations adapted in the actual medium:
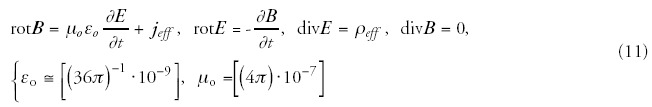
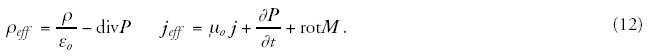
Assuming that the polarization depends only on the external electric field and the magnetization by the external magnetic field:


In the case of radiofrequency currents the energy source (10) of bio-heat equation (8) mainly derives from the dielectric loss, so:

The temperature gain by the absorbed energy (if the physiologic modifications are neglected) is shown in Figure 3.
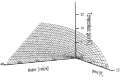
Figure 3
Result of a model calculation of the temperature gain in homogeneous media with stabilized surface temperature (constant environment).
The complex amplitude of the energy delivery (energy radiation, e.g., Poynting vector (S) expressed in W/m2) can be calculated as

The electromagnetic energy absorbed by the treated part and converted into heat equals to the surface (A) integral of the vector's average value calculated for the boundary surface of the treated part. Therefore,

The schematic structure of the complex conditions of the treated bio-matter is shown in Figure 4. The multilayered structure is the most common from the point of view of the chosen locoregional treatment. The layers represent the various tissues, e.g., skin epidermis, skin capillary bed, fatty tissue, muscle tissue, tumor tissue, etc. It may include nonlayered structures like blood-vessels, bone structures, lymph nodes, etc. The tissue (and the transmitter material, which is in most of the cases air or water) is characterized from electromagnetic point of view by the complex impedance function, which depends on the basic material constants, such as, on the dielectric permittivity (ε), the magnetic permeability (μ) and the conduction (σ) of the tissue. All the other parameters—like the attenuation constant (α), the phase-constant (β), the propagation constant (γ), the penetration depth (δ) and the actual wavelength in the tissue (λ), as well as the reflection ratio (ι)—can be calculated79 from these basic material constants ε, μ and σ (f is the frequency of the applied treatment):
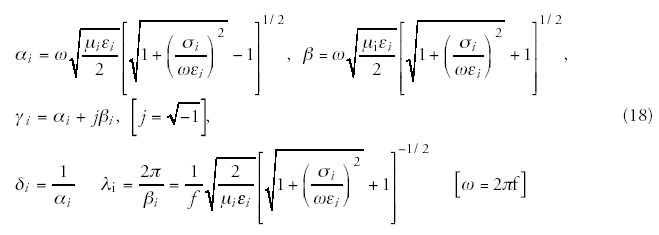
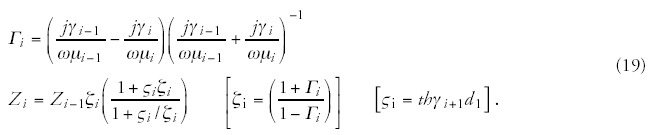
The interaction description is complicated enough, but it is definitely much more complex in the reality, where the perpendicularity, homogeneity, radiation condition, source geometry etc. might modify the results. This is the reason why at the calculation of real cases only numerical approximations can be considered. A minor possible simplification is that in the natural situation (without artificially added materials) the bio-material has no magnetic properties, as its relative magnetic permeability equals to one with high accuracy.
We have further complications when trying to keep in hand both the rather complex bioelectric interactions and the highly sophisticated mathematical apparatus: if our aim is to accomplish a deep heating condition we face many problems physically and physiologically as well. The main problematic points are:
- The incident energy has a frequency-dependent exponential decay in the depth (fig. 5). The penetration is defined by the depth where the field intensity decreases by 1/e-part (about 36%) of the incident beam. (The absorbed energy is even less: about 14% of the incident energy.) The penetration of the field into the targeted material depends significantly on the applied antenna arrangement. The planar waves at the applied—relatively low—frequency generated by capacitive coupled antennas penetrate into the bio-systems by 14-20 cm79,81 depending on the actual electric conductivity of the bio-tissue. The penetration depth for the planar waves is shown on Figure 6.
- The incident energy beam reflects on the surfaces and internal boundaries as well. Generally, we must note that the reflection at the air-skin boundary is very high (it is more than 0.8 at 100 MHz and about 0.6 at 10 GHz82).
- The subcutaneous capillary bed tries to balance the local homeostasis, cools the skin by intensive blood perfusion. Also the deeper layers are blood-perfunded delivering rapidly the heat away from the targeted area.
- The physical/electrodynamical parameters guide the heat absorption, which could prefer other selectivity than the desired one (e.g., the bone treatment could be problematic). Hot-spots/ layers/areas could be generated in unwanted locations involving risks for the healthy tissue. The hot-spot formation depends on many physical and physiological factors, such as:
- The local RF-current density increases by the decreased cross section of flow.
- The reflected waves at the internal tissue boundaries are summed.
- The tissues are nonhomogenic, their conduction and permitivity may change drastically by places.
- The body cavities could serve as hollow-space resonators caused by standing waves and their local field maxima.
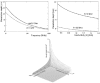
Figure 6
Penetration depth of planar waves into the tissue versus the frequency and the conductivity. The depth is approximated from simplified conditions of the imperfect conductor. (The shaded area represents the most common values for muscle tissue.)
The dangerous energy absorption and the consequent hot-spot on the surface are created not only by the maximal incident energy but by the low relative dielectric constant (large voltage drop) and the possible lateral currents (fig. 7).
Technical Solutions
The electromagnetic, “deep-thermal” (not convective and not conductive) heating can be considered as significantly better than the convective and conductive methods. While the convective and conductive methods are limited by the thermodynamic heat conduction, the efficacy of the electromagnetic methods is mainly determined by the extensive interactions determined microscopically. The utilized energy can be uncoupled at any essential position of oscillatory circuit producing the given frequency. Therefore, this fact determines the radiation (Poynting's vector), the magnetic (inductive) and electric (capacitive) treatment techniques (fig. 8). The techniques are basically different in their dominating electromagnetic effects, and also differ in their actual technical solution (fig. 9).
Main characteristics of the methods are collected in Figure 10.

Figure 10
Basic electromagnetic methods: A) inductive (magnetic), B) capacitive (electric), C) antenna-array (radiative).
Radiative Coupling
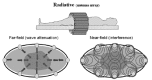
The radiative coupling by antenna array has two distinguishing categories in accordance with the relation of the wavelength (frequency) and the source-target distance (fig. 11). If the target distance measured from the source is smaller than the applied wavelength, then the wave can not be considered as radiaton, it acts by the change of the fields (E and B), and the Poynting vector (S) has no definite role. The two radiative methods are mainly different in their penetration depth (the far –field is more shallow) and the focusing ability (the interference can be modified by the phase-frequency characteristics, the attenuation only by the orientation of the beam). Basic disadvantage of the microwave treatments of higher frequency and shorter wavelength lies in its low penetration depth. (From this respect it slightly differs from the diffusion heating.) Other disadvantage is the possibility of destruction during the heating process, namely, similarly to the ionization radiation this method has harmful influence on the healthy tissues because of the high specific energy input and the selective energy absorption regarding the hydrogen oxide molecules. As the usage of kitchen microwave oven has also demonstrated, the open microwave source may entrain serious health problems.
A part of the near-field treatments uses significantly higher frequencies (up to the limit, which determines the wavelength and the target-distance), and uses interference focusing. The applied antennas (dipoles) work in the capacitive regime, however their optimal tuning is far away from the direct capacitive coupling; an array is harmonized to reach the interferences in the desired depth and place. Due to its higher frequency the penetration depth significantly decreases, which is partly compensated by the additional effects of the multi-antenna array.
The SAR values depend on the situation (far- or near-field), and are reduced considerably in near cases. The empirical ratio83 observed:
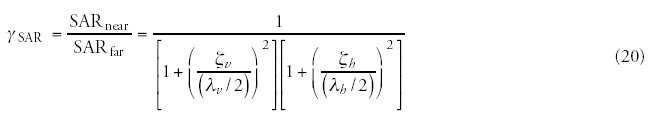
Radio-Frequency Waves in Near-Field Radiation
The radio-wave treatments belong to the lower frequency group of nonionizing radiations, covering the electromagnetic range approximately from 1 MHz to 30 MHz. With the reduction of frequency the risky and uncertain effects typical of microwave decrease, while the penetration depth significantly increases, and the specific delivered energy shows more homogenous distribution.
Magnetic Field
A possibility of energy decoupling induced fundamentally by magnetic field may signify deep energy absorption. The penetration depth of magnetic field is extremely high in bio-systems, because of the very week interaction between the magnetic field and bio-material. For this reason, the energy absorbed by the tissues—from the energy transported by the magnetic field—manifests itself in the Eddy current or induced current part, namely, it is proportional to the conductivity of material. In this manner the decoupling with magnetic field is weak, its control is difficult and its selectivity can be assured only as a function of conductivity. The magnetic coupling can be enhanced significantly, if a magnetic material is placed into the targeted area. Generally, nano- or microparticle suspensions, seed, grain or rod magnetic pieces are inserted invasively. In more sophisticated cases the magnetic material's Curie-temperature (ferro-/paramagnetic phase transition) is set to a desired temperature in order to control the heat-delivery and fix the maximal temperature of the magnetic material (the ferromagnetic material absorbs, the paramagnetic does not absorb the magnetic field).
Electric Field
The energy depletion is effectuated dominantly by electric field anyway. Its advantage, namely, the strong interaction with the highly organized bio-matter (which is typically good dielectrics) also could be a disadvantage, though the penetration depth decreases as compared to the magnetic field. Consequently, the capacitive arrangement offers other advantages (the absorbed energy can be significantly increased and extra selectivity factors can be included in the system) and sets other problems (low penetration and possible surface or subcutaneous burn).
Next, we are going to discuss the case when the treated, layered part (for example a kernel and its pellicle and surface interface) is between the electrodes of the condenser supplied by radio-frequency power supply (fig. 12, a simplified model of fig. 4). Now, we are going to determine the electric power transformed into heat in the treated area with some simplifying assumptions.
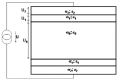
Figure 12
Principle of dielectric heating (Layers represent the actual structure).
(1) The dielectric materials pursuant to (fig. 12) are homogenous, namely, the εr relative permeability and the σ electric conductivity are constant by layer (2). The problem is symmetric from geometric and electric point of view, namely, the thickness of the appropriate layers and their electric parameters are identical; the treated area is spherical and is to be found in the geometrical centre of the arrangement. (3). The extension of the area where the treated part can be found is large as compared to the extension of the treated part. This later assumption assures that a homogenous field is produced even in the tissue part including the treated area. Its advantage is that the field to be matched in accordance with the boundary condition can be determined by using an arrangement not including the treated area. It should be noted that this restriction is not too strong as the dipole field induced by the treated area is weak, and—as we will see—decreases in inverse ratio to the third power of the distance. The absorbed power which is transformed to heat in bio-matter can be calculated as follows:
Figure 13
Physical model of dielectric heating.
As in the examined model the space charge equals to zero, and the living material is paramagnetic from magnetic point of view. We suppose that the living material between the electrodes can be considered homogenous—in the first approach—with linear behaviour at the given field strength. As the field strength is known, the electric thermal power can be calculated:
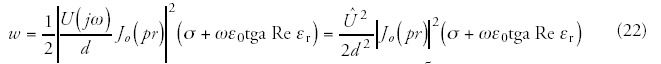
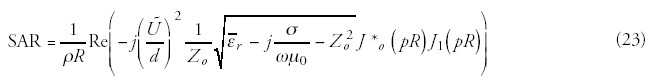
Comparison of the Methods
The typical characteristics of these methods are summarized in Table 7. Note that these values are only orienting, covering the mainstream of the electromagnetic heating; however, the actual applications could be rather different. In the followings, due to its dominant presence and practical importance, only the near-field applications are discussed.
Table 7
Basic electromagnetic methods: a) inductive (magnetic), b) capacitive (electric), c) antenna array (radiative).
A significant difference in application of the various methods lies in the focusing mechanisms. The artificial focusing (radiative and magnetic approaches) directs the energy absorption into the area where the malignancy is located, and tries to heat up the area homogeneously, which is in most of the cases not homogeneous at all. In the case of multi-centered liaisons the covered area includes both the healthy and malignant areas (fig. 14). In the capacitive case the actual differences in complex impedance and conductivity determine the area of the heat absorption irrespective of its size or multiplicity (fig. 15). The advantage is the self-supporting nature, and it has a positive feedback by the growing temperature42 when the selective differences between the malignant and healthy areas become even more pronounced.
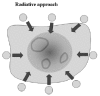
Figure 14
Focusing arrangement by artificial focusing (radiative and magnetic approaches).

Figure 15
Focusing arrangement by capacitive arrangement (self-selective technics).
New Directions in Electromagnetic Oncologic Hyperthermia
Our main aim—namely to destroy selectively the malignant cells on microscopic level— needs a modification in our fixed ideas, because the applied hyperthermic effects are not selective enough, and their demand to the high temperatures introduces a new risk of toxicity: the deep burn. Recently, scientists have started to realize that the thermostatical thinking (the temperature has to be raised up as homogeneously as possible in the malignant area) has to be replaced by a more adequate thermodynamical one (the properly conducted temperature differences could be effective in destruction). Now we see that the hyperthermia-induced temperature gradients could have significant biological effects. A new branch of hyperthermia known as extracellular hyperthermia74 or electro-hyperthermia26 has been developed around this concept. Although this new technique recognizes the benefits of increased tissue temperature and its biological consequences, it also argues that nonequilibrium thermal effects are partially responsible for the observed clinical deviations from the purely temperature based treatment theory.
Electro-hyperthermia is based on a capacitively coupled energy transfer applied at a frequency that is primarily absorbed in the extracellular matrix due to its inability to penetrate the cell membrane.86 Although these temperature gradients typically relax within a few milliseconds, a constant energy delivery can maintain this gradient for extended periods of time, and may initiate special transports through the cellular membrane. The gradient-constructed heat flow might be significantly large and damage the first step of the membrane. This heat flow can produce extra ionic currents through the membrane, which depolarizes and therefore destabilizes the membrane. This requires ATP, resulting in further heat production at the membrane. The membrane permeability of water is much higher than in the case of ions, therefore, it is the main transported component in the thermodynamic coupling. Therefore, this thermal flux builds up an intracellular pressure, which is about 30% above the normal.74 Since malignant cells typically have relatively rigid membranes due to increased phospholipids concentrations,87 an increase in pressure will selectively destroy the malignant cells before it affects the healthy ones. These effects allow the application of hyperthermia not only in a more effective way, but also for the areas which have been mostly contraindicated for this method: the lung, liver, pancreas and brain treatments. Its preliminary clinical trials show the reality of the expectations: the malignancies of the liver,88 brain89 and pancreas90 are exceptionally well respond to such type of treatment.
Cellular Effects
Some biophysical effects on cellular level might modify the technical applications. These microscopic effects mainly depend on the electric field, and in low frequency ranges may considerably influence the actual hyperthermia effects.74
Absorption in Cellular Level
The effective electric field acts by the high polarization (large dielectric constant) of the bio-systems. From dielectric point of view, the simplest approach is to study the cellular structure in its three rather different parts (fig. 16),86 as calculated, the frequency-dispersion energy absorption of the characteristic cellular structures (fig. 17).

Figure 16
Cellular structure by its dielectric properties.
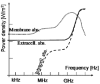
Figure 17
Frequency-dependence of the absorbed energy for the cellular parts.
Changes of the Membrane Potential
The cell-membrane potential as a function of temperature may be calculated from the Goldman-Hodgkin-Katz (GHK) equation.91
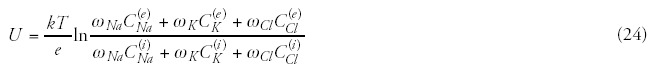
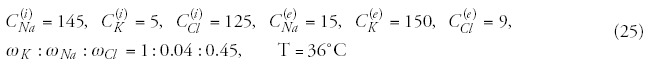
Consequently, the membrane potential increases, and the cell is hyperpolarized under increasing temperature.
Temperature Gradient on the Membrane
When the frequency is low enough to the selective energy-absorption (see fig. 17), then the temperature gradient has essential role in the membrane destruction. The actual energy balance is: ρiciV(dT/dt) = Iq + Pm, where ρici is the specific heat capacity of the cell, V is the cell-volume, Pm is the metabolic heat-power. The Iq heat-current contains a metabolic and an electric term: Iq = Iqm + Iqe, where Iqm = -Pm ≈ const., so Iqe = ρiciV(dT/dt). Hence the heat-current density is jqe = ρici(V/A)(dT/dt), where A is the surface area of the cell. Its numerical value at 1K/s heating rate equals to jqe = 140(W/m2), which is significant.74 The temperature difference between the two sides of cell membrane can be calculated by the expression ΔT = (jq/α) ≈ 0.007K. Consequently, the actual gradient on the 7 nm thick membrane amounts to 106 K/m, which is a remarkable large value! It is well above the natural heat flow induced by metabolism, which is only jqcell≅ 1.5·10-3(W/m2), thus causes negligible (ΔT ≅ 7.5·10-8K) temperature difference at the cell membrane.
Membrane Damage by Constrained Ion-Currents
According to the Onsager's nonequilibrium thermodynamic approach, all the various transport processes are tightly coupled, so in our present case, heat-transport is accompanied by electric current and mass transport. The Onsager-relation for heat and electric transport coupling takes the form of
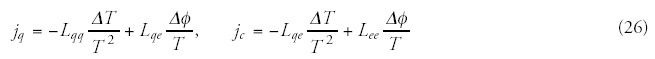
Membrane Damage by Increasing Pressure
According to the Onsager's theorem, the heat flow is coupled to the volume (mass) transport as well. (The entire complex phenomenon is simplified on the intensive pairs only.) The relevant Onsager-relations are:
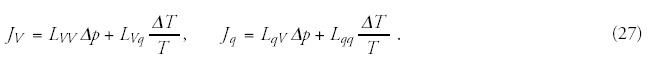
The membrane permeability is much higher for water than for ions, so the main transported component in this coupling is water. The dynamic equilibrium hydrostatic pressure can be determined by the pressure equality on both sides of the membrane,30 so:


This means that the ΔT = 0.01K temperature difference generates Δp = 1.32 bar = 1.32·105 Pa pressure, which is a large value. Consequently, the lateral tensile stress rises due to the lateral dilatation generated by the radial mechanical pressure from the electric field, and the hydrostatic pressure difference tries to balance it. The lateral tensile stress calculated from the above nonequilibrium transports using D = 10 μm cell-diameter and ξ = 10nm membrane thickness results in σny = (DΔp/4x) = 1.32(10 μm/410 nm) ≈ 3·107 Pa = 30 MPa, which is a huge value! Comparing this value to the regular conditions: the induced scalar pressure (from the Maxwell stress-tensor78) is σr = εE2 ≈ 3·103 Pa, which is too small to be relevant and negligible in relation to the transport pressure. The maximal tolerable lateral tensile stress amounts to σmax = (2 - 20)·105 Pa,94 which is smaller by two order of magnitudes than the temperature induced stress. The increase of the osmotic pressure also contributes as an additional factor to the damage of cellular membrane. The increase of the ECM temperature boosts the electro-chemical potentials in the ECM electrolyte. The chemical potentials are: μ = μi0 + V ip + RTlnγci + ZiFU, where Vi is the molar-volume, μi0 is the initial chemical potential, p is the pressure, γ is the activity coefficient, ci is the concentration and Zi is the ionizing state of the i-th component. F is the Faraday number and U is the membrane potential. With the increasing jNa, both jK and jCl decrease, therefore, in stationary membrane state the intracellular concentrations Ck(i) (k = Na+, K+, Cl-) also grow. This process increases the osmotic pressure in the intracellular liquid, while decreases it in ECM.
The above theoretical considerations can be used in many applications. Unfortunately, the complications in the analytical way force us to use different numerical calculations. The numerically calculated results for kernels are in good correspondence with the observations, and allow explaining some seed stimulation effects. Also the nondirected effect of the power lines in the plough-lands could be studied in this frame.
Summary
In our opinion, the hyperthermia (definitely, it is a heat-dose treatment) is a temperature-dependent but not temperaturedetermined process. The temperature concept is not bad as long as the physiological factors (blood flow/vascularization, metabolism, chaperone-protein production, dissemination, apoptotic action, etc.) are included, and the tissue can be regarded as homogenic, semi-isolated from the surroundings. Unfortunately, these conditions are not common, so sometimes the temperature gives statistically significant, sometimes random results. This is the reason why some trials check the patients before, and divide them on the “heatable” and “not-heatable” groups,95 and randomize for trial only the previous group. For this group the anyway scientifically incorrect and assumed equivalence of the heat dose to the temperature is an acceptable approach. On the other hand, this preselection excludes a large number of patients receiving hyperthermia; however, this treatment could be a help for them as well. The exclusion was made on an insufficient characterization of the method.
A relevant characterization of oncological hyperthermia for quality guidelines has to be started from the aims: it is to destroy the malignant cells! This demand contains some more precise requests: selectivity and blockage of further proliferation and dissemination. Distortion could be promptly direct (the cells become necrotic during the treatment) or indirect (tune killing conditions; the cells become necrotic or apoptotic after the treatment). The demands actually do not contain any temperature request.
Hyperthermia is an emerging effective treatment method in oncology. It has shown significant improvements in tumor response rates and patient morbidity in combination with other treatment methods, such as surgery, chemotherapy, radiation therapy and gene therapy or applied as a monotherapy. Nevertheless, hyperthermia is still in its infancy. The thermal dose quantification is likely to remain of practical importance. We have to characterize the hyperthermia by thermal dose and not by temperature. Thermal dose changes many energetic processes in the tissue, and in their physiology. Most of the changes (structural and chemical changes) are out of modification of the temperature, only the entropy changes. The nonequilibrium thermodynamics describes how the absorbed heat could excite various (e.g., diffusional, electric, chemical, etc.) processes. The dynamism of the absorption determines the dynamism of the processes (e.g., reaction rates, diffusion dynamism, etc.) and, by these, drives the efficacy of the processes as well. These phenomena (which are anyway in the focus of the tumor destruction) are completely out of the possibility of temperature characterization. Hyperthermia suffers from a lack of standards and a lack of scientific consensus about its effects on malignant and healthy tissues. In order that hyperthermia shall gain widespread approval and clinical use, the technique requires extensive further research and standardization. For this we need an open mind and have to outgrow the dogmatic habits.
The hyperthermia is an interdisciplinary approach. We have to use the historic roots, the scientific achievements on other areas. The hyperthermic oncology is in the wave of the demands: nontoxic, excellent in any combinations with other treatments, with minor contraindications.
Hyperthermia has been considered a remarkably developing form of tumorous tissue overheating and tumour treatment. This method is based on the higher heat sensitivity of tumorous tissues and the totality of physiological processes resulting from the effect of heat.
Conclusions and Perspectives
What/who is against the oncologic hyperthermia? Nothing/nobody from outside. We are against ourselves by constraining the temperature concept over all. The physics, biology and physiology are with us if we make a correct approach. We are convinced that the perspectives of hyperthermia in oncology are very bright and promising. What we have in hand is a practically nontoxic effect with huge potential and advantages. However, we have to clarify the technical issues to make this capable method technically comparable, and provide for a control which is safe enough in terms of modern medical demands.
References
- 1.
- Jones S, Henry W. HippocratesCambridge: Harvard University Press,1959 .
- 2.
- Vesalius A. Dehumani corporis fabrica libri septem. p. 1543.
- 3.
- Warren JC. Surgical observations on Tumors with cases and operations 1837. (Ref Pollay M The first American book on tumors Thesis University of Madison Wisconsin 1955)
- 4.
- Seegenschmiedt MH, Vernon CC. A Historical perspective on hyperthermia in oncologyIn: Seegenschmiedt MH, Fessenden P, Vernon CC, eds.Thermoradiotherapy and Thermochemotherapy. Vol 1Berlin Heidelberg: Springer/Verlag,1995 .
- 5.
- Smith E. Egyptian surgical papyrus dated around 3000 B.C. (cited by: van der Zee J: Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. [PubMed: 12181239]
- 6.
- Medieval literature—1. Medieval Turkish Surgical manuscript from Charaf ed-Din, 1465 (Paris, Bibliotheque Nationale), 2. Armamentarium chirurgicum of Johann Schultes, Amsterdam 1672 (Paris, Bibliotheque de Faculte Medicine), cited by: Seegenschmiedt MH, Vernon CC. A historical perspective on hyperthermia in oncologyIn: Seegenschmiedt MH, Fessenden P, Vernon CC, eds.Thermoradiotherapy and Thermochemotherapy. Vol 1Berlin Heidelberg: Springer/Verlag,1995 .
- 7.
- Busch W. Uber den Einfluss welche heftigere Erysipeln zuweilig auf organisierte Neubildungenausuben, Vrh. Naturhist. Preuss Rhein Westphal. 1866;23:28–30.
- 8.
- Coley WB. The treatment of malignant tumors by repeated inoculationsof erysipelas, with a report of ten original cases. Am J Med Sci. 1893;105:488–511. [PubMed: 1984929]
- 9.
- Westermark F. Uber die Behandlung des ulcerierenden Cervixcarcinoms mittels konstanter Warme. Zentralbl Gynaekol. 1898;22:1335–1337.
- 10.
- Westermark N. The effect of heat on rat tumors. Skand Arch Physiol. 1927;52:257–322.
- 11.
- Overgaard K. Uber Warmeterapie bosartiger Tumoren. Acta Radiol [Ther.] (Stockholm). 1934;15:89–99.
- 12.
- Muller C. Therapeutische Erfahrungen an 100 mit kombination von Rontgenstrahlen un Hochfrequenz, resp. Diathermie behandeleten bosartigen Neubildungen. Munchener Medizinische Wochenschrift. 1912;28:1546–1549.
- 13.
- Nielsen OS, Horsman M, Overgaard J. A future of hyperthermia in cancer treatment? Eur J Canc 2001371587–1589.(Editorial comment) [PubMed: 11527682]
- 14.
- Osinsky S, Ganul V, Protsyk V. et al. Local and regional hyperthermia in combined treatment of malignant tumors: 20 years experience in Ukraine. Awaji Japan: The Kadota Fund International Forum. 2004
- 15.
- Dewey WC, Hopwood LE, Sapareto SA. et al. Cellular response to combination of hyperthermia and radiation. Radiology. 1977;123:463–474. [PubMed: 322205]
- 16.
- Lindholm C-E. Hyperthermia and Radiotherapy. Ph.D. Sweden: Thesis, Lund University, Malmo. 1992
- 17.
- Hafstrom L, Rudenstam CM, Blomquist E. et al. Regional hyperthermic perfusion with melphalan after surgery for recurrent malignant melanoma of the extremities. J Clin Oncol. 1991;9:2091–2094. [PubMed: 1960549]
- 18.
- Urano M. Thermochemotherapy: From in vitro and in vivo experiments to potential clinical applicationIn: Urano M, Douple E, eds.Hyperthermia and OncologyUtrecht-Tokyo: VSP19944169–204.
- 19.
- Hasegawa T, Gu Y-H, Takahashi T. et al. Enhancement of hyperthermic effects using rapid heatinIn: Kosaka M, Sugahara T, Schmidt KL, Simon E, eds. Thermotherapy for Neoplasia, Inflammation, and PainTokyo-Berlin: Springer Verlag, 2001439–444.
- 20.
- Lindegaard JC. Thermosensitization induced by step-down heating. Int J Hyperthermia. 1992;8:561–582. [PubMed: 1402135]
- 21.
- Wust P, Hildebrandt B, Sreenivasa G. et al. Hyperthermia in combined treatment of cancer. The Lancet Oncol. 2002;3:487–497. [PubMed: 12147435]
- 22.
- Hayashi S, Kano E, Hatashita M. et al. Fundamental aspects of hyperthermia on cellular and molecular levelIn: Kosaka M, Sugahara T, Schmidt KL, Simon E, eds. Thermotherapy for Neoplasia, Inflammation, and PainTokyo-Berlin: Springer Verlag, 2001335–345.
- 23.
- Karasawa K, Muta N, Nakagawa K. et al. Thermoradiotherapy in the treatment of locally advanced Nonsmall cell lung cancer. Int J Rad Oncol Biol Phys. 1994;30:1171–1177. [PubMed: 7961027]
- 24.
- Kraybill W, Olenki T. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumors: Correlation with mouse models Int J Hyperthermia 2002183 (253-266 and Burd R Dziedzic ST Tumor cell apoptosis lymphocyte recruitment and tumor vascular changes are induced by low temperature long duration (feverlike) whole body hyperthermiia J Cellular Physiology 1998 177137-147)
- 25.
- Field SB. Biological aspects of hyperthermia. Physics and Technology of HyperthermiaIn: Field SB, Franconi C, eds.NATO ASI Series, E: Applied Sciences, No.127Dordrecht/Boston: Martinus Nijhoff Publ,198719–53.
- 26.
- Szasz A, Szasz O, Szasz N. Electrohyperthermia: A new paradigm in cancer therapy. Wissenschaft and Forschung, Deutsche Zeitschrift für Onkologie. 2001;33:91–99.
- 27.
- de PomaraiD, Daniels C, David H. et al. Nonthermal heat-shock response to microwaves. Nature. 2000;405:417–418. [PubMed: 10839528]
- 28.
- Bukau B, Horwich AL. The HSP70 and HSP60 chaperone machines. Cell. 1998;92:351–366. [PubMed: 9476895]
- 29.
- Feynman PR, Leighton RB, Sands M. The feynman lectures on physics Reading and Caltech, MA and CAUSA: Addison-Wesley Publ Co.,1963 .
- 30.
- Katchalsky A, Curran PF. Nonequilibrium thermodynamics in biophysicsCambridge: Harvard University Press,1967 .
- 31.
- Lupis CHP. Chemical Thermodynamics of MaterialsNewYork, Amsterdam, Oxford, North Holland:1983 .
- 32.
- Pennes HH. J Applied Physiology. 1948;1:93–122. [PubMed: 18887578]
- 33.
- Matay G, Zombory L. Physiological effects of radiofrequency radiation and their application for medical biology[in Hungarian], Muegyetemi Kiado, Budapest,200080.
- 34.
- Gautherie M. Temperature and blood-flow patterns in breast cancer during natural evolution and following radiotherapyIn: Alan R Liss, ed.Biomedical ThermologyNew York,198221–24. [PubMed: 7167480]
- 35.
- ANSI C95.1-1966 (H. Schwan, Pennsylvania USA).
- 36.
- Lin H, Head M, Blank M. et al. Myc-Mediated transactivation of HSP70 expression following explosure to magnetic fields. J Cell Biochem. 1998;69:181–188. [PubMed: 9548565]
- 37.
- Goodman R, Blank M. Insights into electromagnetic interaction mechanisms. J Cellular Physiology. 192:16–22. [PubMed: 12115732]
- 38.
- Lin H, Blank M, Goodman R. A magnetic field-responsive domain in the human HSP70 promoter. J Cell Biochem. 1999;75:170–176. [PubMed: 10462715]
- 39.
- Scholz B, Anderson R. On Electrical impedance scanning—principles and simulations. Electromedica 68 - Onco. 2000:35–44.
- 40.
- Barnett A. Electrical method for studying water metabolism and transloction in body segments. Proc Soc Exp Biol Med. 1940;44:142–147.
- 41.
- Nyboer J, Bango S, Barnett A. et al. Radiocardiograms - the electrical impedance changes of the heart in relation to electrocardiorganms and heart sounds. J Clin Invest. 1940;19:963–966.
- 42.
- McRae DA, Esrick MA, Mueller SC. Noninvasive, in-vivo electrical impedance of EMT-6 tumors during hyperthermia: Correlation with morphology and tumour-growth delay. Int J Hyperthermia. 1997;13:1–20. [PubMed: 9024923]
- 43.
- Esrick MA, McRae DA. The effect of hyperthermia induced tissue conductivity changes on electrical impedance temperature mapping. Phys Med Biol. 1994;39:133–144. [PubMed: 7651992]
- 44.
- McRae DA, Esrick MA. The dielectric parameters of excised EMT-6 tumours and their change during hyperthermia. Phys Med Biol. 1992;37:2045–2058. [PubMed: 1438561]
- 45.
- McRae DA, Esrick MA, Mueller SC. Changes in the noninvasive, in vivo electrical impedance of the xenograpfts during the necrotic cell-response sequence. Int J Radiat Oncol Biol Phys. 1999;43:849–857. [PubMed: 10098441]
- 46.
- Dissado LA, Alison JM, Hill RM. et al. Dynamic scaling in the dielectric response of excised EMT-6 tumours undergoing hyperthermia. Phys Med Biol. 1995;40:1067–1084. [PubMed: 7659731]
- 47.
- Szendro P, Vincze G, Szasz A. Bio-response on white-noise excitation. Electro-and Magnetobiology. 2001;20:215–229.
- 48.
- Gersing E. Monitoring temperature induced changes in tissue during hyperthermia by impedance methodsIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987313–20. [PubMed: 10372145]
- 49.
- Haemmerich D, Staelin ST, Tsai JZ. et al. In vivo electrical conductivity of hepatic tumors. Physiol Meas. 2003;24:251–260. [PubMed: 12812412]
- 50.
- Smith SR, Foster KR, Wolf GL. IEEE Trans Biomed Eng BME. 1986;33:522–525. [PubMed: 3710509]
- 51.
- Jossinet J. The impedivity of freshly excised human breast tissue. Physiol Meas. 1998;19:61–75. [PubMed: 9522388]
- 52.
- Jossinet J, Schmitt M. A review parameters for the bioelectrical characterization of breast tissueIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987330–41. [PubMed: 10372147]
- 53.
- Chauveau N, Hamzaoui L, Rochaix P. et al. Ex vivo discrimination between normal and pathological tissues in human breast surgical biopsies using bioimpedance spectroscopyIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987342–50. [PubMed: 10372148]
- 54.
- Tosso S, Piccoli A, Gusella M. Nutrition. 2000. pp. 120–124. [PubMed: 10696635]
- 55.
- Glickman YA, Filo O, David M. et al. Electrical impedance scanning: A new approach to skin cancer diagnosis. Skin Res Techn. 2003;9(3):262. [PubMed: 12877689]
- 56.
- TransCan TS2000, Transcan Medical Ltd. Migdal Ha'Emek, Israel, distributed by Siemens AG, Germany.
- 57.
- Skourou C, Hoopes PJ, Strawbridge RR. et al. Feasibility studies of electrical impedance spectroscopy for early tumour detection in rats. Physiol Meas. 2004;25:335–346. [PubMed: 15005327]
- 58.
- Chillcott TC, Coster HGL. Electrical impedance tomography study of biological processes in a single cellIn: Riu P, Rosell J, Bragos R, Casas O, eds.The data Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 1999873269–286. [PubMed: 10372176]
- 59.
- McRae , Esrick MA. Deconvolved electrical impedance spectra track distick cell morphology changes. IEEE Trans Biomed Eng. 1996;43:607–618. [PubMed: 8987265]
- 60.
- Bioelectric impedance analysis in body composition measurement. National Institute of Health, USA: Technology Assessment Conference Statement. 1994:12–14. [PubMed: 8974099]
- 61.
- Bowen WD, Beck CA, Iverson SJ. Bioelectrical Impedance analysis as a means of estimating total body water in grey seals. Can J Zool. 1999;77:418–422.
- 62.
- Talluri T, Lietdke RJ, Evangelisti A. et al. Fat-free mass qualitative assessment with bioelectric impedance analysisIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987394–98. [PubMed: 10372155]
- 63.
- Goovaerts HG, Faes THJC, DeValk-DeRoo GW. et al. Estimation of extracellular volume by two frequency measurementIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987399–104. [PubMed: 10372156]
- 64.
- McRae DA, Esrick MA. Changes in electrical impedance of skeletal muscle measured during hyperthermia. Int J Hyperthermia. 1993;9:247–261. [PubMed: 8468508]
- 65.
- Shchepotin IB, McRae DA, Shabahang M. et al. Hyperthermia and verapamil inhibit the growth of human colon cancer xenografts in vivo through apoptosis. Anticancer Res. 1997;17:2213–2216. [PubMed: 9216690]
- 66.
- Casas O, Bragos R, Riu PJ. et al. In vivo and in situ ischemic tissue characterization using electrical impedance spectroscopyIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987351–58. [PubMed: 10372149]
- 67.
- Schafer M, Kirlum H-J, Schlegel C. et al. Dielectric properties of scaletal muscle during ischemia in the frequency-range from 50Hz to 200 MHzIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987359–64. [PubMed: 10372150]
- 68.
- Gheorghiu M, Gersing E, Gheorghiu E. Quantitative analysis of impedance spectra of organs during ischemia. In: Riu P, Rosell J, Bragos R, Casas O, eds. Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci. 1999;873:65–71. [PubMed: 10372151]
- 69.
- Osterman KS, Paulsen KD, Hoopes PJ. Application of linear circuit models to impedance spectra in irradiated muscleIn: Riu P, Rosell J, Bragos R, Casas O, eds.Electrical Bioimpedance Methods: Applications to Medicine and Biotechnology. Ann New York Acad Sci 199987321–29. [PubMed: 10372146]
- 70.
- Santini MT, Cametti C, Zimatore G. et al. A dielectric relaxation study on the effects of the antitumor drugs Lomidamineand Rhein on the membrane electrical properties of Erlich ascites tumour cells. Anticancer Res. 1995;15:29–36. [PubMed: 7733637]
- 71.
- Keese CR, Wegener J, Walker SR. et al. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci USA. 2004;101:1554–1559. [PMC free article: PMC341773] [PubMed: 14747654]
- 72.
- Avitall B, Mughal K, Hare J. et al. The effects of electrode-tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol. 1997;20:2899–2910. [PubMed: 9455749]
- 73.
- Schmidt D, Trubenbach J, Konig CW. et al. Radiofrequency ablation ex vivo: Comparison of the efficacy impedance control mode versus manual control mode by using internally cooled clustered electrode. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2003;175:967–972. [PubMed: 12847653]
- 74.
- Szasz A, Vincze Gy, Szasz O. et al. An energy analysis of extracellular hyperthermia. Magneto- and electro-biology. 2003;22:103–115.
- 75.
- Seegenschmiedt MH, Vernon CC. A Historical perspective on hyperthermia in oncologyIn: Seegenschmiedt MH, Fessenden P, Vernon CC, eds.Thermoradiotherapy and ThermochemotherapyBerlin: Clinical Applications, Springer Verlag,199523–46.
- 76.
- Blank M. Coupling of AC electric fields to cellular processes. First International Symposium on Nonthermal Medical/Biological Treatments Using Electromagnetic Fields and Ionized Gases. ElectroMed'99, Norfolk VA, USA, Symposium Record Abstracts. 1999:23.
- 77.
- Young RA. Stress proteins and immunology. Ann Rev Immunology. 1990;8:401–420. [PubMed: 2188668]
- 78.
- Jackson JD. Classical ElectrodynamicsNew York: John Wiley and Sons Inc.,1999 .
- 79.
- Rao NN. Elements of engineering electromagneticsLondon: Prentice Hall International,1994 .
- 80.
- Matay G, Zombory L. Physiological effects of radiofrequency radiation and their application for medical biology[in Hungarian], Muegyetemi Kiado, Budapest,200071.
- 81.
- Polk C, Postow E. Handbook of biological effects of electromagnetic fieldsNew York, London, Tokyo: CRC Press,199615.
- 82.
- Tremoliers J. Effects biologiques des champs electromametiques non ionisants. Electron Appl. 1978;7:71–77.
- 83.
- Chatterjee I, Hagmann M, Gandhi O. An empirical realtionship for electromagnetic energy absorption in man for near-field exposure condition. IEEE Trans On Microwave Theory and Techniques, MTT-29. 1981;11:1235–1238.
- 84.
- Chain C. A theoretical basis for microwave and RF field effects on excitable cellular membranes. IEEE Trans On Microwave Theory and Techniques, MTT-29. 1980;2:142–147.
- 85.
- Iskander M, Olson S, MacCalmont J. Near-field absorption characteristics of models in the resonance frequency range. IEEE Trans On Microwave Theory and Techniques, MTT-35. 1987;8:776–779.
- 86.
- Kotnik T, Miklavcic D. Theoretical evaluation of the distributed power dissipation in biological cells exposed to electric field. Bioelectromagnetics. 2000;21:385–394. [PubMed: 10899774]
- 87.
- Galeotti T, Borrello S, Minotti G. et al. Membrane alterations in cancer cells: The role of oxy radicalsIn: Bianchi G, Carafoli E, Scarpa A, eds.Membrane Pathology. Ann New York Acad Sci 1986488468–480. [PubMed: 3555261]
- 88.
- Hager ED, Dziambor H, Hohmann D. et al. Deep hyperthermia with radiofrequencies in patients with liver metastases from colorectal cancer. Anticancer Res. 1999;19:3403–3408. [PubMed: 10629627]
- 89.
- Hager D, Dziambor H, App EM. et al. ASCO 2003 Meeting, Chicago USA. 2003:470.
- 90.
- Dani A, Varkonyi A, Nyiro I. et al. Clinical experience of elecctro-hyperthermia for advanced pancreatic tumors. ESHO 2003 Conference, Munich. 2003:41.
- 91.
- Hodgkin AL, Huxley A. A quantitative description of membrane current and its application to conduction and excitation of nerve. J Physiol. 1952;117:500–544. [PMC free article: PMC1392413] [PubMed: 12991237]
- 92.
- Weiss TF. Cellular Biophysics Cambridge, MA, USA: A Bradford Book, The MIT Press, 1996 .
- 93.
- Spanner DC. Symp Soc Exptl Biol. 1954;8:76.
- 94.
- Sackmann E. Physical basis of self-organisation and function of membranes: Physics of vesiclesIn: Lipowsky, Sackmann E, Elsevier Science BV. eds.Handbook of Biological Physics 19951.
- 95.
- Vujaskovic Z, Jones EL, Oleson JR. et al. A Randomized trial of hyperthermia and radiation for superficial tumors. Awaji Yumebutai, Japan: Presentation and Abstracts for The Kadota Fund International Forum. 2004
- Physical Background and Technical Realizations of Hyperthermia - Madame Curie Bi...Physical Background and Technical Realizations of Hyperthermia - Madame Curie Bioscience Database
- The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway - Madame C...The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway - Madame Curie Bioscience Database
- RNA NETWORKS IN PROKARYOTES II: tRNA Processing and Small RNAs - Madame Curie Bi...RNA NETWORKS IN PROKARYOTES II: tRNA Processing and Small RNAs - Madame Curie Bioscience Database
- DNA Packaging by Bacteriophage P22 - Madame Curie Bioscience DatabaseDNA Packaging by Bacteriophage P22 - Madame Curie Bioscience Database
- Regulation of Insulin Action and Insulin Secretion by SNARE-Mediated Vesicle Exo...Regulation of Insulin Action and Insulin Secretion by SNARE-Mediated Vesicle Exocytosis - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...