NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
This review focuses on the biological rationale and the advantages for combining hyperthermia radiotherapy and chemotherapy. Other clinical aspects such as sequence of administration, effects on drug uptake and methods used to improve the efficacy of HT are also discussed. The actual applications and effects of HT on brain are presented. Furthermore, the preliminary clinical results obtained, by our group, on malignant brain tumors using this triple combination of cure are illustrated. The survival curves of brain tumors in total and of glioblastomas, treated with HT, were compared to the single standard application of conformational radiotherapy. The life quality and survival results are favorable to HT, however the number of patients treated is limited. In every case we suggest its use for a better clinical control of the disease.
Radiotherapy and Hyperthermia Interaction
Hyperthermia Killing Curves (Arrhenius Relationship)
Heat cell killing occurs exponentially as function of time and dose and its shape is not dissimilar from those obtained for x-rays (fig. 1A). The data in vitro are consistent with results in vivo and they show that a relatively small changes in temperature can have a large effect on cell killing.1-3
Another way to describe the kinetics of tumor cell killing is to use the Arrhenius relationship. This analysis relates time and temperature and it is the basis for the calculation of thermal activation energy.2 Arrhenius graph (Fig. 1B) shows that the reciprocal of D0 values plotted versus reciprocal of the absolute temperature 1/T results in a straight line and it permits to calculate the activation energy required to obtain thermal damage. The dramatic change of the slope of the curve occurring over 43°C, called the break point, means that the activation energy is different below and above this point, reflecting a different mechanism of cell killing.4 Above 43°C the activation energy for heat toxicity is similar to that for protein denaturation suggesting that the target is a protein (Chromosomal proteins, nuclear matrix repair enzymes, membrane components);5-7 below the break point thermotolerance can develop gradually during the heating suggesting a cell adaptability (see Thermotolerance paragraph). The Arrhenius plot can be modified by different factors such as pH, step-down heating, some chemotherapeutic drugs, ATP status, cell cycle phase, Bioflavonoids and CoX2 inhibitors.2,3,5,8-10
Hypoxia, pH
Blood perfusion in large solid tumors is generally poorer than that in normal tissue.11 The vascular beds in tumors are chaotic and poorly organized resulting in temporal and spatial unbalanced blood supply.12 Therefore, many regions within tumors result hypoxic/acidic and resistant to radiation and chemotherapy. The chronic or transient deficiency in tumor perfusion can generate state of chronic or acute hypoxia.12 Chronic hypoxia develops when cancer growth outstrips its blood supply, reaching a critical mass > 1-2 mm3 (106 cells) and a distance from host nutritive vessel > of 100-200 μm (fig. 2). Regions of transient hypoxia can develop within tumor mass following a temporary interruption of blood flow. Transient perfusional hypoxia is caused by various mechanisms. The most plausible ones seem to be:
- Transient stop of tumor blood flow or supply by platelets plug.15,16 In our opinion, this intravascular thrombosis deserves to be taken into much higher account than usually done.16 In fact, the majority of cancer patients has coagulation abnormalities associated to hypoxia.16,17 Recently, it has been demonstrated that hypoxia not only induces VEGF but also stimulates endothelial cells to over express tissue factor (TF) and Plasminogen activator inhibitor (PAI-I). These factors induce endothelium to become prothrombotic and cause fibrin formation and platelet activation. Furthermore, VEGF binds to fibrinogen and fibrin by stimulating endothelial cell proliferation.17 Fibrin has been demonstrated to be essential to supporting endothelial cells spreading and migration.15 The haemostatic system becomes, in a certain sense, a regulator of angiogenesis and can partially explain acute hypoxia and its regional appearance and disappearance. In conclusion hypoxia becomes a self perpetuating mechanism able to trigger angiogenesis, intratumoral fluid accumulation and thrombosis (fig. 3).16-18
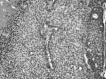
Figure 2
Photomicrograph of a section of mammary tumor, developed by a 5 month old female transgenic MMTV-neu (erbB2) mouse. T: tumor cord; N: necrosis; Hyp: hypoxic, perinecrotic, rim of tumor cells; C: capillary. Hematoxylin & Eosin. 40x objective.
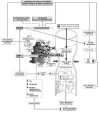
Figure 3
In this figure the structural and functional effects of Hypoxia, HIF-1 and VEGF on tumor microcirculation, cancer metabolism and therapies, are illustrated. (Modified with permission from: Baronzio et al. Anticancer Res 1994; 14:1145-1154.)
As just described, the two types of hypoxia have different origin and coexist together in a well-perfused zone of the tumor mass, causing a functional disturbance of macro and microflow.11 A more realistic vision shows that these two situations change continuously, because tumor blood flow is time fluctuating. Furthermore in this low flow regions associated but occurring independently of hypoxia an acidic environment is present.13,14,18,19 Hypoxia alone is the single strongest prognostic indicator of radiotherapy outcome. In fact, it has been observed that larger tumor have a greater proportion of hypoxic cells and respond less to radiotherapy.12,14,19 The precise nature by which hypoxia exerts its adverse effects on cells radiosensitivity is not completely understood.1 It is believed that oxygen enhance the efficacy of radiotherapy by inducing free radicals production in the tumor area causing DNA damage.2,18-20
Oxygen Enhancement Ratio (OER)
To better understanding the importance of oxygen on radiotherapy, survival curves of oxygenated and hypoxic cells treated with radiotherapy are generally compared. The ratio of hypoxic to aerated dose needed to achieve the same biologic effect is called Oxygen enhancement ratio (OER) and is defined by the Equation 1.19 OER = Dose in hypoxic conditions/Dose in aerated conditions (to achieve same cell survival fraction) (Eq. 1)
Gerweck et al,1 using the same methodology, compared the biological effect of hyperthermia on hypoxic and aerobic cells. The OER found by these authors and other confirmed that hypoxic cells sensitivity to hyperthermia were equal or greater than oxygenated cells (OER ≈; ≤ 1 for HT; OER ≥ 2.5 -3 for RT),19,20 as shown in Figure 4. Acute and chronic hypoxic cells, submitted to heat, behave similarly. In fact, acute hypoxic cells have an OER near 1, whereas chronically hypoxic cells have an OER slightly less.21 Furthermore, cells cultured at lower pH have a low extracellular pH, that is 0.2,0.4 units lower than blood.22,23 These cells are more responsive to HT as shown in Figure 5, however the effect seems not completely dependent from hypoxia. In fact cancer cells chronically deprived of nutrients, peculiarly of serum, are extremely sensitive to heat as demonstrated by Hahn.24
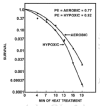
Figure 4
OER curves of Chinese hamster ovary cells (CHO) treated with hyperthermia. Hypoxia was induced 10-20 min prior to treatment (Suit an Gerweck, Cancer Res 1979; 39:2290-2298. Reprinted with permission from ref. .)
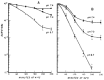
Figure 5
CHO cells cultured at different pH under aerobic conditions and heated during the midportion of pH exposure conditions. (Suit an Gerweck, Cancer Res 1979; 39:2290-2298. Reprinted with permission from ref. .)
Cells Cycle Stage
Beyond hypoxia, the reasons for combining hyperthermia and ionizing radiation are based on the following considerations:
- Heat can interact with radiation and potentiates its cellular action by retarding the repair of radiation induced DNA damage;25
Thermal Enhancement Ratio (TER) - Therapeutic Gain Factor - Heat Radiotherapy Sequence
The Radiosensitization effect of hyperthermia was quantified using the thermal enhancement ratio (TER). TER represents the ratio between the minimal dose of radiation to induce a biological effect and the dose required when radiotherapy and hyperthermia are used combined, [Eq. 2].1,2 TER = Radiation dose alone/Radiation dose with heat (Eq. 2) (to achieve same end point) TGF = TER (Tumor)/TER (Normal Tissues) (Eq. 3)
A supplementary way to express the biologic effect of heat and radiation is the therapeutic gain factor (TGF) that is defined as the ratio of the TER in tumor to the TER in normal tissues, [Eq. 3].2 However, this comparison is not obtained easily, because the tumor and normal tissues are not equally heated. In fact, a greater heat washout is present in normal tissue compared to tumor tissue.2,26 A practical aspect in order to obtain the maximum effect by combining radiation and hyperthermia is the time and the sequence of their application. Studies by Overgaard27 have shown that an advantageous clinical TER was obtained when HT and RT are delivered concomitantly; however, for inherent clinical difficultness to operate synchronously; a satisfactory TER is obtainable delivering HT and RT within a short period. Some investigators use a time interval of 2 - 4 hs between radiation and heat to obtain a satisfactory TER (fig. 6).27 Interaction between RT and HT can be additive or super-additive. RT survival curves are influenced by HT addition. Their behaviour has been studied on cells cultures and in animal experiments evidencing that temperature is critical for the determination of their sequence and clinical uselfuness. Brief exposure to temperatures of about 45°C can steepen the x ray survival curve reducing the D0. The predominant effect in this case is radiosensitization and the change of x-rays survival curve is minimal. For temperature more clinically obtainable (≤ 43°C) (not involved in cell killing) the principal effect is the removal of shoulder effect from X rays survival curve: this result proves that heat treatment after irradiation is the best sequence. The different effect obtained by higher (≥ 45°C) or lower temperatures (≤ 43°C) may reflect different critical target or induced thermotolerance. Recently Song et al26,28 have explained another enhancing factor that justifies the effectiveness of HT and RT combination. These Authors26,28 in accordance with others25,29 have reported that there is abundant evidence that oxygenation improves after moderate heating (42°C). The improvement in blood flow and thereby of oxygen content may increase the effectiveness of radiotherapy and chemotherapy. In every case, the clinical evidences concerning this moderate blood flow enhancement are not sure and need further investigations.26
Clinical Thermal Dosimetry: CEM
One of major impediments to use clinically hyperthermia is the lack of a standardized thermal dosimetry. This is not a simple task, it is due to different factors. The two most important ones are: a non invasive method of temperature determination and the incapacity to have a 3D dimensional dose. Actually temperature measurements are invasive and still limited to few points measurement within a tumor. The hyperthermic treatment is usually delivered as a multiple sequenced treatment and its biological effect is time temperature dependent. This implies that time temperature relationship varies in the same patient and from patient to patient. Thus a clinical useful unit of thermal dose could take into consideration the two variables. With the goal of reaching a uniform target temperature-time combination and a clinical method of utility, Sapareto and Dewey3 proposed the use of CEM 43°C (Cumulative equivalent minutes) as a formulation for comparing and normalizing thermal data from various HT treatments (Time- temperature combination) [Eqs. 4 and 5].2,6 CEM 43°C = tR (43-T) (Eq.4) CEM 43°C = StR (43-Tavg) (Eq.5)
Equation 4 CEM 43°C = cumulative equivalent minutes at 43°C (temperature suggested for normalization), t = time of treatment in minutes, T = average temperature during desired interval of heating, R is a constant = 0.5 when above break point and = 0.25 when below break point. Equation 4, for complex time-temperature sequence the heating profile is divided into intervals of time and CEM is calculated summing the average temperature Tavg for the entire heating regimen.
Thermotolerance
This phenomenon refers to a development of resistance following prior hyperthermia treatment. 4 Chronic heating (multiple fractions) at low temperatures, below the break point, (40-42 °C), allows the development of thermotolerance. Thermotolerance occurs in almost mammalian cells in vitro and in vivo, and it is characterized by an appearance of a resistance plateau in the survival curve. Mammalian cells respond to environmental stress by activating heat shock transcription factors (HSF1) that regulate increased synthesis of heat shock proteins (HSPs). Heat shock proteins (HSPs) comprise several different families of proteins that are induced in response to a wide variety of physiological and environmental insults. Two of them (HSP 70 and HSP27) have been demonstrated to inactivate apoptosis. Hyperthermia has a killing effect on tumor cells or by inducing necrosis (Thermal ablation) or programmed cell death (apoptosis), depending on the temperature used [Apoptosis for temperature ≤ 42.5°C; necrosis for temperature ≥ 45°C].4 Recently the demonstrated importance covered by HSPs and Prostaglandins (PGs) in the inactivation of apoptosis opens new therapeutically opportunities in sensitizing tumor cells to hyperthermia.9,10 Furthermore, other factors have been found to modify thermotolerance ant heat response such as pH, nutrients and oxygen status. Thermotolerance can also modify the degree of thermosensitization to radiation and chemotherapeutic drugs.2,30 Although the nature of thermotolerance has yet to be clarified, its understanding and manipulation is critical in association with radiation, in a fractionated treatment schedule.
Chemotherapy and Hyperthermia Interaction
Introduction Biological Aspects
Several in vitro and in vivo animal studies have demonstrated that the combination of hyperthermia and cytostatic drugs can be employed synergistically.30 However not all chemotherapeutic agents behave similarly at elevated temperature. Studies conducted by Urano et al31 in vivo on animal tumor systems, demonstrated that some drugs, such as cisplatinum and bleomycin, have an increase in the activation energy in range of temperatures between 40 °C and 45°C. For these drugs a gain in cytotoxicity at elevated temperature is obtained, for other drugs this effect has not been demonstrated.30-33 This result has permitted to classify the interaction between anticancer drugs and hyperthermia as supraddictive, additive and independent (Table 1).33,34
Table 1
Activity of antitumor agents, vascular targeting agents, natural substances and various drugs in presence of hyperthermia, hypoxia and pHe.
Additive means that drugs increase linearly their cytotoxic activity with increasing temperature i.e., 5FU, methotrexate, vincristine. Supraddictive means that drugs have a threshold behavior: no increase of cytotoxicity at lower temperature, marked increase above a distinct threshold temperature (i.e., bleomycin, BCNU, CDDP, Mitomycin). Independent behavior means no effect of temperature on drug activity (i.e., Ara-C, topotecan). As outlined by Dahl and Mella,32 the increased effect of some drugs at temperature ≤ 42°C may be related to altered drug pharmacokinetic or pharmacodynamics parameters.
Pharmacokinetics Parameters (Drug Uptake, pH, and Metabolism)
The aqueous solubility of some drugs (i.e., BCNU, CCNU) increases with increasing temperature, whereas others (i.e., nitosureas, methotrexate, cisplatin and chlorambucil) tend to be reduced at higher temperature. Generally the drug uptake of alkilating agents, cisplatin, doxorubicin, bleomycin, 5FU increases with the temperature, whereas the uptake of methotrexate seems not to be affected. These data have been obtained in vitro and in vivo.33,35
As known by pharmacology, the drugs behave like a weak base or acid, according to the pH of tumor environment.36 Ionization decreases lipid solubility and diffusibility across cancer cells membranes. The effects of acidic TIF on various anticancer drugs have been studied and a remarkable influence on their activity has been found.34,36-38 CCNU, cyclophosphamide, bleomycin, ifosfamide, melphalan and cisplatin have shown an increase activity in acidic environment and an additive effect in presence of heat (Table 1).31
Hyperthermia may alter hepatic - renal metabolism and excretion. For example, during WBHT a slight decrease in renal elimination of carboplatin associated to moderate increase in its nephrotoxicity has been detected.39,40 This example outlines an important consideration on the different effects exerted by local hyperthermia (LHT) and WBHT. In the first case, the single organ is treated and the metabolism and drug distribution can interest that single organ, whereas for WBHT the long lasting exposure and the influence exerted on different organs (especially by circulation) may influence the pharmacokinetics of different drugs used synchronously.
Pharmacodynamics Parameters
The mechanisms responsible for the effect of potentiation of cell killing by hyperthermia have not been completely explained.35 In the case of melphalan it has been ascribed to an increase influx leading to higher intracellular accumulation and alkylation's; in the case of cisplatinum and derivatives to an a enhanced formation of DNA-platinum adducts.41 Mitomycin C is a quinone that under hypoxic and low pH conditions is subjected to one or two electron reduction. This reduction leads to the formation of unstable reactive molecules (free radicals) which determine irreversible DNA interstrand cross-links. Mitomycin C can be classed as hypoxia activated drugs and has a clinical relevance as their use in many clinical trials.42,43 Starting from these studies, a new class of molecules, the bioreductive drugs, which exert their anticancer activity in hypoxic and acidic environment, have been created.42,43 Tirazapamine (TPZ) is one of these new molecules. It exerts its effects by producing, like Mitomycin, free radicals and removing hydrogen atoms from macromolecules. This effect produces a damaging of DNA through the formation of both single - double strand breaks. TPZ is selectively metabolized by hypoxic cells.44 However, TPZ alone does not exert any antitumor effect, whereas combined with radiotherapy and HT, in experimental tumors and clinical trials, has shown to be highly effective.45
Drug Heat Sequence
Studies in cell culture have shown that the maximal cytotoxicity was reached when the drug was scheduled simultaneously with HT. When drugs were delivered 2 to 3 hs post HT the supraddictive effect was lost. The effects of timing and sequence between drugs and HT have been evaluated in vivo models. These studies have observed the maximum efficacy when drugs were delivered just before heating.24,32,33 However not all drugs behave similarly; for example the antimetabolite gemcitabicine must be delivered 24 hs before heat application to obtain the maximum effect as demonstrated in vitro in rat model. VP-16etoposide decreases its activity when combined with local HT, whereas it shows a different behavior when combined with WBH.32
Trimodality Therapy
Herman et al33 proposed in the late 1988 to combine HT RT and chemotherapy (Trimodality therapy) in order to obtain a better local control of the disease. These and other Authors31,33,34 reported that the combination of anticancer drugs, such as cisplatin, radiation and heat was better than the two separate modalities. These authors in a recent review have summarized many combinations of this trimodality treatment and they concluded that chemotherapy is to be delivered following a right schedule to obtain the maximum effect.33,34 Herman et al suggest to obtain a long lasting local control of tumor by using the maximal tolerated radiation dose followed by adjuvant chemotherapy and hyperthermia. Radiation was used at the maximum dose because it is the single most effective method of treatment.33,34 Clinical examples of trimodality association are treatments of Head & neck tumors,46 esophageal47 and brain tumors.48
Effects of Hyperthermia on Drugs Uptake and Targeting
Different anatomical and physiological barriers may limit drug penetration into tumor tissue. 11,12 One of the main barriers is interstitial penetration and transport. In solid tumor, interstitial fluid pressure (IFP) is significantly increased compared to normal tissue, and it is responsible for an outward pressure gradient that reduces tumor drug penetration.11,14,17 Another barrier is cellular targeting. The goal of cellular targeting is to increase exposure of tumor to drug improving response reducing antineoplastic agents toxicity. Hyperthermia may improve drug concentration in target tissue by different mechanisms and can be utilized with new drug delivery approaches for better targeting anticancer drugs.
Influence of HT on Interstitial Fluid Pressure (IFP) and Microvascular Permeability
Elevated Interstitial fluid pressure in the tumor mass may limit the delivery and distribution of therapeutic agents.11,15 Jain and coworkers49 have demonstrated that HT induces a significant decrease in IFP ameliorating in this sense the drug uptake. Furthermore, HT increases drug extravasation and uptake by altering tumor microvascular permeability as demonstrated by Nilsen50 and Lefor.51
Influence of HT on Drug Accumulation and Resistance
Hahn et al30 have also shown an increased cellular uptake of chemotherapeutic drugs, such as adriamycin at elevated temperatures. This increased uptake can partially explaining the decreased resistance in presence of HT, as demonstrated in preclinical and clinical studies on mitomycin-C, cisplatin, BCNU and anthracyclines.52
Influence of HT on Drug Retention
As previous reported, the better time of drug administration is concomitantly or just prior HT. In fact HT determines for the first 20-25 minutes a vasodilatation, that is more evident in the tumor microcirculation compared to the normal counterpart. After this vasodilatation a decreased perfusion/or a blood flow stopping happens. This events can obtain an initial enhancement of drug retention in tumors followed by their entrapping in the tumor area.
Interaction of HT with Drug Delivery Technologies (Liposomes, Magnetic Drugs, Nanoparticles, Degradable Microspheres) for Improving Drug Targeting
Drug targeting is a method for distributing drug in the body in such way that its major fraction interacts exclusively with the target tissue (tumor cells) at the cellular level. HT can enhance drug delivery and efficacy when combined with appropriate drug delivering methodologies, such as:
- Liposomes: Liposomes are small unilamellar lipid vesicles designed to have specific temperature-dependent phase transitions point. Many drugs such as methotrexate and doxorubicin can be included in these lipid vesicles and released at the point of phase transition temperature, avoiding systemic side-effects and increasing tumour cell killing.53,54 Maekawa et al55 have demonstrated a survival prolongation in rats receiving temperaturesensitive liposomes containing bleomycin compared to groups receiving hyperthermia or bleomycin alone, or a combination of both.
- Magnetic drugs: Recently a new class of liposomes (magnetic cationic liposomes [MCL])has been developed. These liposomes consist in cationic liposomes containing 10-nm of magnetite nanoparticles obtained by sonication. Preliminary experimental results on hamster osteosarcoma in association with Hyperthermia (obtained by the application of a magnetic field with a frequency of 118 KHz), has shown a complete regression in the group using this methodology compared to a control group.56
- Nanoparticles: are particulate system with a size between 500 nm and 1 mm. They are used since 1970 to carry vaccine or anticancer drugs.57 Kong et al58 have investigated their behavior during HT and have demonstrated that their extravasation was temperature dependent and lasting 6 h post heat application.
- Degradable starch microspheres: Starch microspherex DSM (Spherex, Pharmacia, Sweden) are particles of cross-linked starch, measuring 20-70 μm, degraded by amylases, and able to block transiently and reversibly microcirculation. This method can achieve a larger increase in intratumoral temperature when administered before hyperthermia (for a decreased washout effect of heat), or can trap drugs within the heated area if used after drug administration.35
Methods for Enhancing Thermal Sensitivity
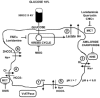
The special nature of tumor blood flow and its consequences hypoxia and intra tumoral pH reduction, previous described, does not simply produce a special hostile microenvironment but offer a method to enhance thermal sensitivity. In other words, the most important aspect of heat deposition into a tumor mass is to obtain intratumor temperature sufficient high (≥ 42.5°C) and homogeneous to achieve the maximum tumor kill. Clinically this energy distribution can be influenced by many pathophysiological factors such as: (a) the depth of tumor mass; (b) its 3D conformation; (c) the quantity of body fat; (d) the interstitial pH (pHe)or the intracellular pH(pHi); (e) the tumor membranes composition; (f ) the tumor tissue perfusion, (g) the time of exposure to heat. Although some of them (tumor mass depth, 3D conformation and body fat composition) are not modifiable, tumor perfusion, oxygenation, extracellular pH (pHe) and membranes composition can be manipulated to improve clinically Hyperthermia response (fig. 7).59 - 61
Metabolic and Micromilieau Modification
Extracellular acidification of tumors has demonstrated to enhance the effect of hyperthermia, to inhibit thermotolerance in cultured tumor cells and to enhance the cytotoxicity of certain chemotherapeutic drugs.1,25-27 Tumors generally exhibit high levels of glycolysis even under aerobic conditions. The excessive production of lactate from pyruvate and the decreased wash out in the tumor microenvironment carry to tumor extracellular acidification. However, this acidification would not achieve if the tumor exhibit a high level of oxidative metabolism, which competes with lactate for the available pyruvate.62,63 For a sensitization to heat, reduction of intracellular pH (pHi) is more important than the reduction of extracellular pH (pHe) as reviewed by Ward and Jain.64 However, Ward and Jain64 outlined that the effect of glucose load is not to be ascribed to a reduction of pH but to a decreased tumor perfusion and consequently to a deprivation of nutrients. Earlier experiments have demonstrated that i.v or i.p injection of glucose is able to reduce intratumoral pH,26,64 but the possible competition of lactated for pyruvate has prodded some researchers to use various metabolic inhibitors to enhance the effect of glucose load and tumor intracellular acidification further (fig. 8).62,63,65,66 When metabolic inhibitors such as: substitutes of glucose (D-Glucose, 5-thio-d-glucose), metabolic inhibitors (lonidamide, metaiodobenzylguanidine [MIBG]), or inhibitors of Na* / H* and HCO3-/ Cl- antiporters (cariporide, 4,4,-diisothiocyanatostilbene -2,2,disulfonic acid [DIDS], were added to the combined treatment of heat and chemotherapy, the tumor growth was more delayed than the singular combined treatment.26,62,63,65,66
Tumor Blood Flow (TBF) Modulation
The goal of TBF modulation is to make tumor sufficiently hypoxic or underfed so to determine an increased thermal sensitivity. This biologic effect can be obtained or by stopping TBF or by modifying the tumor / stroma vasculature.
TBF Reduction or Deprivation
With clamping or chemoembolization the tumor becomes totally or partially hypoxic and very sensitive to heat.26,59
Drugs Able to Modify Tumor Microcirculation
TBF/stroma microcirculatory modification can underfed tumor indirectly (Steal Phenomenon) or directly destroying tumor vasculature (VTAs). Steal phenomenon is a biologic effect obtained by vasodilatators. They divert blood flow towards normal tissues decreasing, so, TBF. This diversion occurs because tumor vasculature lacks of innervation and metabolic adaptability.11,12,59
Vasodilatators
Hydralazine
It is an arteriolar vasodilatator currently used as anthyipertensive agents. Its association with hyperthermia has been tested on C3H/Tif mammary carcinoma with a clear enhancement of heat damage. This effect was independent of the hydralazine doses used, as well as the time and temperature of heating. The hypoxia, that follows for a steal phenomena, is the major responsible for the consequent enhanced response to heat.67
Calcium Blocking Agents (Verapamil, Flunarizine)
They are vasodilator drugs able to alter smooth muscle contraction by blocking entry of Ca2+ into myocytes. They can increase experimentally tumor blood flow and intratumoral drug retention68 however the experimental conditions were not clinically relevant. In fact, in many studies, tumor blood flow decreased probably for a steal phenomenon, demonstrating that tumor vasculature heterogeneity is not simply predictable.59
Serotonin and its Analogues
5-hydroxytryptamine [5-HT] (Serotonin) stimulates endothelial cells to release nitric oxyde (NO). NO causes the relaxation of endothelial smooth muscle cells producing a vasodilatation. In fact, Jirtle59 has demonstrated that the decline in tumor blood flow produced by Serotonin is secondary to a vasodilatation in surrounding normal tissues and to a local blood pressure decrease.
Vascular Targeting Agents (VTAs)
Recently in a variety of transplanted and spontaneous murine solid tumors VTAs have demonstrated to induce tumor necrosis, if the reduction of tumor blood flow is kept for sufficient period. Despite these effects tumor inhibition has been always short, suggesting their use in combination with other therapies. Hyperthermia and radiotherapy are the most likely to benefit from combination with VTAs. The rationale for using VTAs in association with HT is founded on the blood flow reduction induced by VTAs and on the increased degree of hypoxia that follows, with the consequent enhancement of heat application effectiveness. With RT a benefit should be possible because the use of VTAs after RT can cause an extensive necrosis of tumor central core leaving an outer shell of viable and oxygenated cells, very responsive to radiotherapy. Horsman and coll. have studied the response of C3H mouse mammary carcinoma to heat in combination with various VTAs. They have demonstrated a linear relationship between the time of heating and tumor growth time delay.69 DMXXA: 5,6- dimethylxanthenone-4-acetic acid and Flavone acetic acids (FAA) have shown a greater effect compared to Combretastatin-A4 (CA4DP). Their effect was maximal when heating was starting after drug administration.69
Flavone Acetic
Flavone Acetic [FAA] is a synthetic bioflavonoid, able to induce in vivo a rapid and marked reduction in tumor blood flow. FAA exerts in addition to tumor blood flow decline a drop in platelets numbers and in the blood clotting time.70 Hill et al71 in a series of experiments have concluded that the antitumor activity of FAA involves besides tumor vasculature also a tumor-host factor. This factor has been identified as an immune mechanism and it consists in an increased local production of TNF-α and Natural killer activity.71,72 This involvement of TNF-α is supported by the fact that tumor shutdown by FAA is inhibited by antibodies to TNF-α.73-75 Different animal studies, in vivo, have demonstrated that the association between FAA and localized hyperthermia determines an important reduction of tumor blood flow.73 Furthermore, FAA increases the effect of hydralazine and the cytotoxicity of Mitomycin C as demonstrated by Yamamoto70 and Takeuchi.76 Notwithstanding these positive results in animal experiments,77 FAA has demonstrated to be of any clinical utility as shown by Kerr.78 This Author,78 in a phase 2 trial, reported that the administration of FAA by 6h intravenous infusion to 19 patients with advanced colorectal carcinoma and 15 patients with advanced malignant melanoma, was associated with no toxicity, but in contrast with the results predicted from the phase I study, the clinical response was generally mild in either disease conditions.
5,6- Dimethylxanthenone-4-acetic Acid [DMXXA]
It is a new investigational flavonoid with an antivascular activity greater than FAA. DMXXA has been studied in a wide variety of tumors including transplanted and spontaneous rodent tumors and human tumor xenografts in nude mice. The reduction of Tumor blood flow is obtained without significant effect on normal tissue.79 The mechanism of action, like FAA, seems to be indirect and to involve the in situ production of vascular mediators such as nitric oxide (NO), Serotonin, von Willebrand's factor and TNF-α. These substances have been studied and found elevated in s.c murine Colon 38 carcinomas growing in normal or Tumor necrosis factor receptor -1 knockout mice. The finding that nitric oxide (NO), Serotonin and TNF-α are released after DMXXA suggests that these substances contribute to the antivascular effect of the drug.80 Different clinical trials are in progress, also in association with Hyperthermia.80
Combretastatin-A4 [CA4DP]
It is an agent member of a family of chemicals obtained from the bark of South African willow. It is a prodrug, that binds to tubulin microtubules of proliferating capillary endothelial cells, partially sparing normal nonproliferating endothelial cells.81 This mechanism of action determines a prolonged and extensive shutdown of blood flow carring to necrosis the central mass of the tumor but lets a rim of viable cells adjacent to normal tissue.79,81
These effects have been demonstrated in a variety of tumor models including transplanted and spontaneous rodent tumors.79,81,82 The persistence of viable cells permits a rapid tumor regrowth. Various strategies have been attempted in order to delay tumor regrowth such as drug readministration. This strategy has been demonstrated to improve tumor response but was unable to completely eliminate clinically the rim of viable tumor cells. A different approach to overcome this factor is to use CA4DP in a combined modality with conventional anticancer therapy. Recent studies have demonstrated that the combination of this drug with radiation and hyperthermia is feasible. The concept of combining these therapies together is double. In fact the cells let viable by CA4AP are generally at tumor periphery and well oxygenated, hence responsive to radiation.82-84 The best sequence of CA4DP administration in this case is after radiotherapy. In the case of hyperthermia, studies done by Horsman and Murata69 indicate that better results were obtainable when CA4DP were administered before hyperthermia. An interesting aspect is that the enhancement occurred at mild temperature from 40.5°C to 41°. These temperatures are easily obtainable in humans. Such combinations merit to be clinically tested.
TNFα-, IL-1α
TNFα and IL-1α are a cytokines with pleiotropic biologic activities that have demonstrated in vivo an antivascular activity. Both can decrease tumor blood supply and are able to induce hypoxia, as demonstrated by Kluge.85 An enhanced antitumor effect has been obtained combining Interleukin 1-α with hyperthermia.86 Furthermore TNFα has been demonstrated to be induced by antivascular agent such as DMXAA and FAA partially explaining their activity.78,83
Role of Biological Membranes in Heat Response
Heat has demonstrated to induce morphological and physiological alterations on cell membranes. 87,88 Membranes consist of a lipid bilayer composed of phospholipids and cholesterol with proteins embedded in this bilayer. Its lipid component can deeply modify their normal function of barrier.60 The weight ratio of Cholesterol-phospholipids/ protein and the degree of saturation can increase or decrease the membrane fluidity and consequently the membrane permeability to different ions.88 After hyperthermia an increased inward accumulation of Ca2+ may occur. Calcium is biologically very active and take part in many cellular process such as apoptosis. Beyond intracellular calcium increase, hyperthermia can also alter the rate of diffusion of other ions and the function of membrane kinase enzymes. Different Authors have outlined that cellular membranes composition can alter the hyperthermia response and a reciprocal interaction between the two is present. Many substances belonging to drugs or natural substances can alter biological membranes and sensitise tumour cells to hyperthermia.
Natural Substances
Anaesthetics (lidocaine, procaine, and dibucaine) have shown to interact with membranes and to increase thermal sensitization depending on concentration and temperature.30 Alcohols, such as ethanol, have been shown to be thermal sensitizers and to have effects similar to the heat. In fact alcohols increase membrane fluidity and affect protein structure and function.87,88 Other factors acting directly or indirectly on membranes such as calcium antagonist drugs (verapamil),89 COX2 Prostaglandin Inhibitors,10 bleomycin,90 Polyamines-aldehydes60 and essential fatty acids (EFAs)92 have been demonstrated to be useful in conjunction with heat. Our group93 suggested the use of EFAs of marine origin for treating Hepatocarcinoma in association with Radiofrequency Hyperthermia. Kokura et al, on experimental basis, have confirmed this suggestion and proved that EFAs administration carries to a gain of 30% in association with HT.92 Among natural substances, retinoids95 Vit.E and bioflavonoids94,96 differently exert a thermal sensitization. According to Prasad94 α-Tocopheryl succinate represents the most active form of Vit. E. It association with heat is justified by its capacity to reduce currently temperature used to obtain the same biological effect. Trans retinoic acid sensitises colon adenocarcinoma cell line HT29 to HT decreasing spreading activity of these cells.95 Quercetin sensitises cells suppressing thermotolerance development by inhibiting the synthesis of HSP70;96,97 furthermore recent studies have shown an enhancing effect at low pH tumoral environment.
Thermo-Chemo- Radiotherapy for Malignant Brain Tumors
Introduction
Glioblastoma is relatively frequent and represents the most malignant form of primary brain tumors. Despite the major advances in treatment such as gamma knife and radiosurgery the prognosis is poor and generally is fatal within 1 to 2 years after the onset of symptoms.98,99 The resistance of gliomas to treatment with radiation and antineoplastic drugs may result in part from the effects of the extensive, severe hypoxia that is present in these tumors.100,101 Beyond hypoxia, malignant glioma tends to infiltrate and to be replicative, so, hyperthermia represents a method to overcome hypoxia and to permit a greater local control.
Effects of Hyperthermia on Gliomas: In Vitro and in Vivo Studies
Different studies in vitro have demonstrated that heat at 41-44°C is effective and cytotoxic against glioma cells in association with RT.102 Fuse et al have recently tried to understand the direct effect of heat on human glioblastoma cell line A172.103 The cells were treated for 1 h at 43-44°C in the growing phase. Heat treatment induced cell death in a time and temperature dependent manners. Using Hoechst 33342, the authors have demonstrated morphological nuclear changes that are consistent with apoptosis, such as accumulation of p53 protein, bax proteins and mRNA.103
Tanaka et al104 have studied the combined effects of heat and chemotherapy on human glioblastoma cell line (SKMG1) and on 3 rat malignant brain tumor cell lines (T9,EB 679, TR 481). Treatments included heat alone (42°C for 1 h), drug alone (42°C for 1h), and HT (42°C for 1 h) before, after or concomitantly to Nimustine (ACNU), Cisplatin or Aclarubin (ACR) exposures. The greatest cytotoxic effect was obtained using simultaneously HT plus ACNU, ACR or cisplatin.
Different works have been performed in animals in vivo. Kobayashi105 and Tanaka106 obtained a significant prolongation of survival on two different animal models using magnetic induction heating (45°C for 1h) through a ferromagnetic implant. Furthermore, Tanaka group106 demonstrated a longest survival when implanted HT was used combined with Chemotherapy (ACNU).
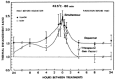
Shem and Dahl107 conducted an interesting work on animals in vivo using HT associated to chemotherapy on glioblastoma. This methodology is the biological basis for our approach of Trimodality treatment (RT +HT+ BCNU and glucose load) on glioblastoma and astrocytomas. They used Carmustine (BCNU) as chemotherapeutic agent for its demonstrated efficacy on gliomas.108,109 Furthermore, associated to HT, nitrosureas (BCNU) showed an interesting behavior that consisted in a higher cytotoxicity when used at ph 6.5-7.0 at 43°C compared to that at normal pH and temperature.110 Studies by Watanabe have also demonstrated that Nitrosureas had similar cytotoxicity under euoxic and hypoxic conditions.111 As previous, reported the majority of human tumors have a hypoxic core and gliomas are not an exception.101 Hypertonic glucose has demonstrated to induce pH extracellular change by increasing lactic acid production during HT increasing so the effect of Nitrosureas at high temperature. Moreover, this work has analyzed the sequence of administration of the various therapies.107 As shown in Figure 9, ACNU followed by HT + glucose is the best sequence. The Growth rate of tumors decreased after this sequence of therapy and the effect lasted for 2 weeks at least (fig. 9).107 Another interesting aspect of use of HT in gliomas is the thermotolerance behavior. In fact gliomas, unlike other cells, do not develop thermotolerance when treated for long time at mild temperature at 39-42°C for up to 48 hs. Thermotolerance develops at temperature above 42°C and its decay is temperature dependent.112
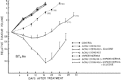
Figure 9
Growth curves of BT4 An tumors after ACNU 20 or 10 mg/kg combined with hyperthermia with or without hypertonic glucose 6 g/Kg i.p. 2 hours before treatment (Shem BC, Dahl O. J Neuroncology 1991; 10:247-251 with permission from ref. ).
Clinical Human Trials of HT + RT on Brain Tumors
Different brain heating methods have been used and may be classified as external or interstitial. All methods have advantages and disadvantages; however, the principal discriminator between them is the dimensional target control of the power deposition. Different clinical trials on human have been collected in two recent reviews.113-115 The authors conclude that HT is a feasible treatment and has an effective approach to brain tumors. Important selection criteria are tumor size (> 6 cm) and tumor location. The majority of studies have been conducted with interstitial hyperthermia as adjuvant to convectional radiotherapy114,116 and have demonstrated an increased survival. An example is the work of Sneed et al113 that have treated a considerable number of patients (112 pts) with a significant median survival increase for the groups treated with heat compared to non heat-treated patients.
Toxicity of HT
Seegenschmiedt115 in his review of 1995 affirmed that treatment toxicity is relatively low and long-term side effects are similar to that observed with RT alone. Ikeda et al117 studied the toxicity of radiofrequency interstitial HT in dog and found alteration of Blood Brain Barrier (BBB). Other authors outlined that the maximum tolerated heat dose of central Nervous system lies in the range of 40-60 min at 42-42.5°C or 10-30 min at 43°C.118 A recent review by Sharma and Hoopes119 has reported that HT produces specific alterations in the mammalian CNS that may have long term behavioral, physiological and pathological consequences. The morphological alterations for temperature in the range 40°C to 42 °C for 4 hs regard the axons, the nerve cells, the glial cells and the vascular endothelium.
Sneed randomized study had demonstrated that HT has an acceptable toxicity, in fact no grade 5 toxicity was found outside 4 patients on 112 (3.5%) with grade 2 and 7.113
In conclusion we may say that HT treatment of brain, once believed to be nontoxic, must be delivered under the observation of skillful staff to avoid serious side effects.
Experimental Studies
Conformational Radiotherapy Administration
Conformal radiotherapy (CFRT) or Linac radiosurgery is now a mainstay of treatment for patients with primary and metastatic brain cancers.120,121 It allows delivering a larger dose of radiation on the lesion sparing normal tissues surrounding the tumor area. Radiation oncologists using computerized tomography scans (CT) or Magnetic resonance (MRI) scans, or both, can determine the cancer size and shape in 3 dimensions (3D) (fig. 10). In this way precisely focused, high dose, radiation beams can be delivered to cancer mass (usually 3 cm or less in diameter) in a single or multiple treatment sessions. Brain cancers treated with these techniques are generally considered inoperable. Prior to radiation patients are fitted with a head frame, meantime CT and MRI scans are performed to determine treatment planning. After the acquisition of these informations, patients are positioned on a sliding bed around the linear accelerator circles (fig. 11). The linear accelerator directs arcs of radioactive photon beams to tumor.121 The pattern of the arc is computer - matched to the tumor shape using specific multileaf collimators.122
Figure 10
Example of RT isodose calculation for brain tumors.
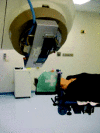
Figure 11
Example of patient's positioning before Conformational Radiotherapy treatment.
Hyperthermia Device Characteristics
Synchrotherm radiofrequency (RF) clinical hyperthermia unit was developed by DUER®, Vigevano, Italy. It consists of following components: (1) a RF generator (13.56MHz) (2) a pair of mobile plates or electrodes with independent superficial cooling system, (3) a heat exchanger, (4) a computerized control console. Special characteristic of this device compared to similar on commerce is the complete automatic coupling of power deposition with minimum human engagement as described in a previous work of our group.123
The choice of plates/electrodes available in diameters of 10,15,20,25,30 cm and the appropriate application on opposite sides of the brain to better focalize the power deposition depended principally from the volume and the depth from the skull of cancer mass. The thermal profiles to obtain a probable deposition of the energy were obtained by heating patterns produced in a static phantoms under various conditions. Cylindrical phantoms with diameters of 30 cm and various thicknesses were made of 4% agar gel containing 0.2% NaCl. The isotherms were monitored and reconstructed through computerizations of images obtained by a special film sensible to temperature (fig. 12).
A flexible vinyl sheet, forming a space filled with 0.4% NaCl solution, covered the surfaces of the electrodes. The saline solution circulated between the electrode and the heat exchangers. Differently to other cooling system, the two electrodes were independently controlled and were simply adaptable to the contour of the brain patients, thanks to their flexibility (fig. 13). These plates are coupled to opposite side of the patient ‘s brain and kept in place thanks to a girdle permitting a better contact over the irregularity of the skull contour.

Figure 13
Example of electrode contour adaptability.
Patients Profile and Treatment Schedule
Thirteen patients (4 female, 9 male; median age 37±11 years old) with astrocytomas of various degree, were treated combining chemotherapy, radiotherapy and hyperthermia with the following sequence. HT was applied after 2hs from CRT administration, and the patients used orally 120 mg of BCNU two hs prior HT. BCNU was administered once every cycle of HT, generally at the first application. A complete cycle of HT consisted in five applications, applied every 48 hs (Table 2). Four mg of e.v. dexamethasone was started 1/2h before HT associated to the hypertonic solution of glucose 10% 500cc that lasted for all the treatment period (60') (Table 2). Dexamethasone was preferred for its relatively little mineralcorticoid activity and fluid retention. Patients were treated orally with a standard dose of 100 mg of anticonvulsants to control and avoid seizures. They received a supplementation of bioflavonoids and omega three fatty acids, following the standard demonstrated to be effective by our group,123 to avoid radiation damage.
Table 2
Treatment schedule clinically used by our group for treating Glioblastoma.
The principal end point of this preliminary work has been the overall calculated survival, according the Kaplan-Meier method starting on the first day of Conformational Radiotherapy (CRT). The control group was constituted by 17 patients with Astrocytomas of III and IV stage (8 F; 9 M median age 41±14) treated with CRT alone.
The significance was posed as p < 0.05 and the follow up was achieved at 45 day interval with CT / MR or Positron emission tomography (PET) with 82 Rb and 18 F-FDG.
Results
The survival curves of the groups were compared, according to the Kaplan-Meier method and log rank test. Hyperthermia group survival was compared in total with the group of brain tumors consisting of glioblastoma, with the best response according to the standard therapy of our institution. The first survival curve (fig. 14) represents all patients treated with HT versus CRT. The significance between the two groups favors the HT treated group (p = 0.003). To avoid bias, the 2nd survival curve were compared, excluding from the HT group 4 pts, with low malignancy tumors. Even in this 2nd comparison (fig. 15) the HT group survival was evidently increased (p = 0.04). Four patients are actually alive and with more or less remarkable quality of life. Our patients did not suffer of side effects out of that induced by CRT.
Figure 14
Survival curves of patients affected by glioblastoma treated with CRT alone and patients with brain tumors of different histologies treated with HT + CRT.
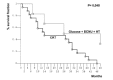
Figure 15
Survival curves of patients affected by glioblastoma treated with CRT alone and with CRT + HT.
Conclusions and Comments
Actually a strong biological base for associating Hyperthermia to Radiotherapy and to chemotherapy exists. On the other part, the confirmation of the potential addition of hyperthermia and radiotherapy in several phase III trials oblige the oncologists to take in greater consideration this association, and to consider the combination CRT, chemotherapy and glucose load an attractive approach at least for non responsive tumor such as glioblastomas.
Preliminary studies combining local hyperthermia chemotherapy and glucose load in the treatment of primary brain tumors have been demonstrated by our group feasible and useful. It has been demonstrated by the increased survival of these kind of patients and by the reduction of great intracranial tumor mass (fig. 16), however many aspects are to be clarified, for example the methodology and the validity of the treatment. In fact, for a definitive approval of this approach, future patients will be randomized and treated with different sequence of HT-CRT. In this preliminary study HT chemotherapy followed CRT however a different sequence such as HT chemotherapy before CRT must be proved and compared.
References
- 1.
- Suit HD, Gerweck LE. Potential for hyperthermia and radiation therapy. Cancer Res. 1979;39:2290–2298. [PubMed: 36224]
- 2.
- Hall EJ. HyperthermiaIn: Hall EJ, ed.Radiobiology for the RadiologistLippincott Williams Wilkins,2000495–520.
- 3.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. [PubMed: 6547421]
- 4.
- Lepock JR. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int J Hyperthermia. 2003;19:252–266. [PubMed: 12745971]
- 5.
- Oleson JR. HyperthermiaIn: Mauch PM, Loffler JS, eds.Radiation Oncology - Technology and BiologyWB Saunders,1994276–299.
- 6.
- Dewhirst MW, Viglianti BL, Lora-Michels M. et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267–294. [PubMed: 12745972]
- 7.
- Dewey WC. Arrhenius relantionship from molecule and cell to the clinic. Int J Hyperthermia. 1994;10:457–483. [PubMed: 7963805]
- 8.
- Dewey WC, Hopwood LE, Sapareto SA. et al. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123:463–474. [PubMed: 322205]
- 9.
- Calderwood SK, Asea A. Targeting HSP70-induced thermotolerance for design of thermal sensitizers. Int J Hyperthermia. 2002;18:597–608. [PubMed: 12537758]
- 10.
- Asea A, Mallick R, Lechpammer S. et al. Cyclooxygenase inhibitors are potent sensitizers of prostate tumours to hyperthermia and radiation. Int J Hyperthermia. 2001;17:401–414. [PubMed: 11587078]
- 11.
- Jain RK. Determinants of tumor blood flow: A review. Cancer Research. 1988;48:2641–2658. [PubMed: 3282647]
- 12.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply and metabolic microenvironment of human tumors, a review. Cancer Res. 1989;49:6449–6465. [PubMed: 2684393]
- 13.
- Freitas I, Baronzio GF. Tumor hypoxia, reoxygenation and oxygenation strategies: Possible role in photodynamic therapy. J Photochem Photobiol B Biol. 1991;11:3–30. [PubMed: 1791492]
- 14.
- Gullino PM. The internal milieu of tumors. Prog Exp Tum Res. 1966;8:1–25. [PubMed: 5330588]
- 15.
- Vaupel P. Tumor blood flowIn: Molls M, Vaupel P, eds.Blood Perfusion and microenvironment of human tumorsBerlin Heidelberg New York: SpringerVerlag,200041–46.
- 16.
- Durand RE. Intermittent blood flow in solid tumours: An under-appreciated source of “drug resistance” Cancer and Metastasis reviews. 2001;20:57–61. [PubMed: 11831648]
- 17.
- Baronzio GF, Freitas I, Kwann H. Tumor microenvironment (hypoxia-interstitial fluid) and haemorheologic abnormalities. Seminars in Haemostasis and Thrombosis. 2003;29:489–497. [PubMed: 14631549]
- 18.
- Denko NC, Giaccia AJ. Tumor hypoxia, the physiological link between Trousseau's Syndrome (carcinoma -induced Coagulopaty) and metastasis. Cancer Res. 2001;61:795–798. [PubMed: 11221857]
- 19.
- Hall EJ. The oxygen effect and reoxygenationIn: Hall EJ, ed.Radiobiology for the radiologistLippincott Williams Wilkins,200091–111.
- 20.
- Gerwek LE, Richards B. Influence of pH on thermal sensitivity of cultured human glioblastoma cells. Cancer Res. 1981;41:4019–4024. [PubMed: 7459870]
- 21.
- Gerwek LE, Dahlberg WK, Greco B. Effect of pH on single or fractionated heat treatment at 42-43°C. Cancer Res. 1983;43:1163–1167. [PubMed: 6825089]
- 22.
- Asby BS, Cantab MB. pH studies in human malignant tumours. Lancet. 1996;2:312–315. [PubMed: 4161494]
- 23.
- Stubbs M, McSheehy PMJ, Griffiths JR. et al. Causes and consequences of tumor acidity and implications for treatment. Molecular Medecine Today. 2000;6:15–19. [PubMed: 10637570]
- 24.
- Hahn GM. Metabolic aspects of the role of hyperthermia in mammalian cell inactivation and their possible relevance to cancer treatment. Cancer Res. 1974;34:3117–3123. [PubMed: 4138162]
- 25.
- Kamping HH, Dikomey E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. [PubMed: 11304434]
- 26.
- Song CW, Park H, Griffin RJ. Theoretical and experimental basis of HyperthermiaIn: Kosaka M, Sugahara T, Schmidt KL, Simon E, eds.Thermotherapy for neoplasia, inflammation, and painSpringer Verlag Tokyo,2001394–407.
- 27.
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiation Oncology Biol Phys. 1989;16:535–549. [PubMed: 2646256]
- 28.
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiation Research. 2001;155:515–528. [PubMed: 11260653]
- 29.
- Bicher HI, Hetzel FW, Sandhu TS. et al. Effects of hyperthermia on normal and tumor microenvironment. Radiology. 1980;137:523–30. [PubMed: 7433686]
- 30.
- Hahn GM. Thermal Enhancement of the actions of anticancer agentsIn: Hahn GM, ed.Hyperthermia and CancerNew York, London: Plenum Press,198255–85.
- 31.
- Urano M, Kuroda M, Nishimura Y. Invited Review. For The clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 1999;18:79–107. [PubMed: 10323618]
- 32.
- Dahl O, Mella D. Hyperthermia and chemotherapeutic agentsIn: Field SB, Hand JW, eds.An introduction to the practical aspects of clinical hyperthermiaLondon, New York, Philadelphia: Taylor and Francis,1990108–142.
- 33.
- Herman TS, Teicher BA, Jochelson M. et al. Rationale for use of local hyperthermia with radiation therapy and selected anticancer drugs in locally advanced human malignancies. Int J Hyperthermia. 1988;4:143–158. [PubMed: 3283266]
- 34.
- Teicher BA, Herman ST. Trimodality therapy: Antitumor chemotherapy with hyperthermia and radiationIn: Mauch PM, Loeffler Jay S, eds.Radiation Oncology Technology and biologyPhiladelphia, London, Toronto, Montreal, Sydney, Tokyo: WB Saunders,1994316–347.
- 35.
- Raaphorst GP. Fundamental aspects of hyperthermic biologyIn: Field SB, Hand JW, eds.An introduction to the practical aspects of clinical hyperthermiaLondon, New York, Philadelphia: Taylor and Francis,1990108–142.
- 36.
- Gerweck L, Tumor PH. Implications for treatment and novel drug design. Seminars in Radiation Oncology. 1998;8:176–182. [PubMed: 9634494]
- 37.
- Douple EB, Strohbehn JW, de Sieyes DC. et al. Therapeutic potentiation of cis-dichlorodiammineplatinum(II) and radiation by interstitial microwave hyperthermia in a mouse tumor. National Cancer Institute Monographs. 1982;61:259–262.
- 38.
- Herman TS, Teicher BA. Summary of studies adding systemic chemotherapy to local hyperthermia and radiation. Int J Hyperthermia. 1994;10:443–449. [PubMed: 7930812]
- 39.
- Vanakoski J, Seppälä T. Heat exposure and drugs. ClinPharmacokinet. 1998;34:311–322. [PubMed: 9571303]
- 40.
- Gerke P, Filejski W, Robins HI. et al. Nephrotoxicity of ifofosfamide, carboplatin and etoposide (ICE) alone or combined with extracorporeal or radiant-heat-induced whole-body hyperthermia. J Cancer Res Clin Oncol. 2000;126:173–177. [PubMed: 10741912]
- 41.
- Bates DA, MacKillop WJ. Effect of hyperthermia on the uptake and cytotoxicity of melphalan in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys. 1998;16:187–191. [PubMed: 2912942]
- 42.
- Zaffaroni N, Villa R, Orlandi L. et al. Effect of hyperthermia on the formation and removal of DNA interstrand cross-links induced by melphalan in primary cultures of human malignant melanoma. Int J Hyperthermia. 1992;8:341–349. [PubMed: 1607739]
- 43.
- Rienbroek RC, van de Vaart PJ, Haveman J. et al. Hyperthermia enhances the cytotoxicity activity and platinum-DNA adduct formation of lobaplatin and oxiplatin in cultured SW 1573 cells. J Cancer res Clin Oncol. 1977;1213:6–12. [PubMed: 8996534]
- 44.
- Denny WA. The role of hypoxia - activated prodrugs in cancer therapy. Lancet Oncol. 2000;1:25–29. [PubMed: 11905684]
- 45.
- Masunaga S-I, Ono K, Nishimura Y. et al. Combined effects of tirapazamine and mild hyperthermia on anti-angiogenic agent (TNP-470) treated tumors-reference to the effect on intratumor quiescent cells. Int J Radiat Oncol Biol Phys. 2000;47:799–807. [PubMed: 10837967]
- 46.
- Arcangeli G, Cividalli A, Lovisolo G. et al. Effectiveness of Local Hyperthermia in association with radiotherapy or chemotherapyIn: Arcangeli G, Mauro F, eds.Comparison of multimodality treatments on multiple neck node metastases. Proceedings of the 1st meeting of European group of hyperthermia in radiation oncology, Milan, Masson Italia Editori 1980257–265.
- 47.
- Nozoe T, Saeki H, Ito S. et al. Preoperative hyperthermochemoradiotherapy for esophageal carcinoma. Surgery. 2002;131:s35–s38. [PubMed: 11821785]
- 48.
- Sneed PK, Stea B. Thermoradiotherapy for brain tumorsIn: Seegenschmiedt MH, Fessenden P, Vernon CC, eds.Thermoradiotherapy and ThermochemotherapyBerlin, Heidelberg, New York: Springer Verlag,1995159–175.
- 49.
- Leunig M, Goetz AE, Dellian M. et al. Interstitial fluid pressure in solid tumors following hyperthermia: Possible correlation with therapeutic response. Cancer Res. 1992;52:487–490. [PubMed: 1728421]
- 50.
- Nilsen NO. Endothelial changes and microvascular leakage due to hyperthermia in chick embryos. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46:165–174. [PubMed: 6147925]
- 51.
- Lefor AT, MaKohon S, Ackerman NB. The effects hyperthermia on vascular permeability in experimental liver metastasis. J Surg Oncol. 1985;28:297–300. [PubMed: 3982038]
- 52.
- Towle LR. Hyperthermia and Drug ResistanceIn: Urano, Douple eds.Hyperthermia and Oncology. VSP 1994491–113.
- 53.
- Drummond DC, Meyer O, Hong K. et al. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacological Reviews. 1999;51:692–743. [PubMed: 10581328]
- 54.
- Sandip BT, Udupa N, Rao BSS. et al. Thermosensitive liposomes and localised hyperthermia - An effective biomodality approach for tumor management. Indian Journal of Pharmacology. 2000;32:214–220.
- 55.
- Maekawa S, Sugimachi K, Kitamura Y. et al. Selective treatment of metastatic lymph nodes with combination of local hyperthermia and temperaturesensitive liposomes containing bleomycin. Cancer Treatment Reports. 1987;71:1053–1059. [PubMed: 2445481]
- 56.
- Matsuoka F, Shinkai M, Honda H. et al. Hyperthermia using magnetite cationic liposomes for hamster osteosarcoma. Biomagnetic Research and Technology. 2004;2:3. [PMC free article: PMC400754] [PubMed: 15040804]
- 57.
- Moghini SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory and practice. Pharmacology Reviews. 2001;53:283–318. [PubMed: 11356986]
- 58.
- Kong G, Braun RD, Dewhirst W. Characterization of the effect of hyperthermia on nanoparticles extravasation from tumor vasculature. Cancer Res. 2001;61:3027–3032. [PubMed: 11306483]
- 59.
- Jirtle RL. Chemical modification of tumor blood flow. Int J Hyperthermia. 1988;4:355–371. [PubMed: 3290350]
- 60.
- Yatvin MW, Cramp WA. Role of cellular membranes in hyperthermia: Some observations and theories reviewed. Int J Hyperthermia. 1993;9:165–185. [PubMed: 8468503]
- 61.
- Sensiterra GA, Lepock JR. Thermal destabilization of transmembrane proteins by local anesthetics. Int J Hyperthermia. 2000;16:1–17. [PubMed: 10669313]
- 62.
- Zhou R, Bansal N, Leeper DB. et al. Enhancement of hyperglycemia-induced acidification of human melanoma xenografts with inhibitors of respiration and ion transport. Acad Radiol. 2001;8:571–582. [PubMed: 11450957]
- 63.
- Zhou R, Bansal N, Leeper DB. et al. Intracellular acidification of human melanoma xenografts by the respiratory inhibitor m-iodobenzylguanidine plus hyperglycemia: A 31P magnetic resonance spectroscopy study. Cancer Res. 2000;60:3532–3536. [PubMed: 10910065]
- 64.
- Ward KA, Jain RK. Response of tumours to hyperglycaemia: Characterization, significance and role in hyperthermia. Int J Hyperthermia. 1988;4:223–250. [PubMed: 3290346]
- 65.
- Song CW, Lyons JC, Makepeace C. et al. Effects of HMA, an analog of amiloride, on thermosensitivity of tumors in vivo. Int J Radiation Oncology Biol Phys. 1994;30:133–139. [PubMed: 8083106]
- 66.
- Lyons JC, Kim GE, Song CW. Modification of intracellular pH and thermosensitivity. Radiat Res. 1992;129:79–87. [PubMed: 1728060]
- 67.
- Horsman MR, Christensen KL, Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989;5:123–136. [PubMed: 2926180]
- 68.
- Vaupel P, Menke H. Effects of various calcium antagonists on blood flow and red blood cell flux in malignant tumors. Prog Appl Microcirc. 1989;14:88–103.
- 69.
- Horsman MR, Murata R. Combination of vascular targeting agents with thermal or radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:1518–1523. [PubMed: 12459380]
- 70.
- Yanamoto M, Kusumoto T, Endo K. et al. Vasoacting agents flavone acetic acid and hydralazine given in combination enhance antitumor effects under condition of hyperthermia. Oncology. 1996;53:147–152. [PubMed: 8604241]
- 71.
- Hill SA, Williams KB, Denekamp J. Studies with a panel of tumors having a variable sensitivity to FAA, to investigate the mechanisms of action. Int J Radiat Oncol Biol Phys. 1991;60:379–384. [PubMed: 1677998]
- 72.
- Baguley BC, Calveley SB, Crowe KK. et al. Comparison of the effects of Flavone acetic acid, fostriecin, homoharringtonine and tumor necrosis factor alpha on colon 38 tumors in mice. Eur J cancer Clin Oncol. 1989;25:263–269. [PubMed: 2702981]
- 73.
- Chin L-M, Baguley BC. Induction of natural killer cell activity by the antitumor compound flavone acetic (NSC 347512). Eur J Cancer Clin Oncol. 1987;23:1047–1050. [PubMed: 3665989]
- 74.
- Mahadevan V, MaliK STA, Meager A. et al. Role of tumor necrosis factor in Flavone acetic acid induced tumor shutdown. Cancer Res. 1990;50:5537–5542. [PubMed: 2386959]
- 75.
- Horsman MR, Samponson LE, Chaplin DJ. et al. The in vivo interaction between flavone acetic acid and Hyperthermia. Int J Hyperthermia. 1996;12:779–789. [PubMed: 8950158]
- 76.
- Takeuchi H, Baba H, Maehara Y. et al. Flavone acetic acid increases the cytotoxicity of Mitomycin c when combined with hyperthermia. Cancer Chemother Pharmacol. 1996;38:1–8. [PubMed: 8603441]
- 77.
- Mc Bibby, Double JA. Flavone acetic acid - from laboratory to clinic and back. Anticancer Drugs. 1993;4:3–17. [PubMed: 8457711]
- 78.
- Kerr DJ, Maughan T, Newlands E. et al. Phase two trials of Flavone acetic acid in advanced malignant melanoma and colorectal cancer. Br J Cancer. 1989;60:104–106. [PMC free article: PMC2247346] [PubMed: 2803908]
- 79.
- Baguley BC, Wilson WR. Potential of DMXAA combination therapy for solid tumors. Expert Rev Anticancer Ther. 2002;2:593–603. [PubMed: 12382527]
- 80.
- Baguley BC. Small molecule cytokine inducers causing tumor necrosis. Current Opinion in Investigational Drugs. 2001;2:967–975. [PubMed: 11757800]
- 81.
- Nihei Y, Suzuki M, Okamo A. et al. Evaluation of antivascular and antimitotic effects of tubulin binding agents in solid tumour therapy. Jpn J Cancer Res. 1999;90:1387–1396. [PMC free article: PMC5926039] [PubMed: 10665658]
- 82.
- Grosios K, Holwell SE, McGown. et al. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer. 1999;81:1318–1327. [PMC free article: PMC2362967] [PubMed: 10604728]
- 83.
- Beauregard DA, Hill SA, Chaplin DJ. et al. The susceptibility of tumors to the antivascular drug combretastatin A4 phosphate correlates with vascular permeability. Cancer Res. 2001;61:6811–6815. [PubMed: 11559555]
- 84.
- Tozer GM, Kanthou C, Parkins CS. et al. The biology of the combretastatin as tumor vascular agents. International J Exp Pathol. 2002;83:21–38. [PMC free article: PMC2517662] [PubMed: 12059907]
- 85.
- Kluge M, Elger B, Engel T. et al. Acute effects of tumor necrosis α or lymphotoxin on global blood flow, Laser Doppler flux, and bioenergetic status of subcutaneous rodent tumors. Cancer Res. 1992;52:2161–2173. [PubMed: 1559220]
- 86.
- Song CW, Lin J-C, Lyons JC. Antitumor effect of interleukin 1-α in combination with hyperthermia. Cancer Res. 1993;53:324–328. [PubMed: 8417825]
- 87.
- Kim JH. Modification of thermal effects: Chemical modifiersIn: Urano M, Douple E, eds.Hyperthermia and OncologyThe Netherlands: VSP,1988199–119.
- 88.
- Marcocci L, MondovÌ B. Biochemical and ultrastructural changes in the hyperthermic treatment of tumor cells: An outline. Consensus On Hyperthermia for 1990s. Adv Exp Med Biol. 1990;267:99–120. [PubMed: 2088075]
- 89.
- Kameda K, Kondo T, Tanabe K. et al. The role of intracellular Ca2+ in apoptosis-induced hyperthermia and its enhancement by verapamil in U937 cells. Int J Radiat Oncol Biol Phys. 2001;49:1369–1379. [PubMed: 11286845]
- 90.
- Agostinelli E, Arancia G, Calcabrini A. et al. Hyperthermia-induced biochemical and ultrastructural modifications in cultured cells. Experimental Oncology. 1995;17:269–276.
- 91.
- Wachsberger PR, Burd R, Wahl ML. et al. Betulinic acid sensitization of low pH adapted human melanoma cells to hyperthermia. Int J Hyperthermia. 2002;18:153–164. [PubMed: 11911485]
- 92.
- Baronzio GF, Solbiati L, Ierace T. et al. Adjuvant therapy with essential fatty acids (EFAs) for primary liver tumors: Some hypotheses. Med Hypotheses. 1995;44:149–154. [PubMed: 7609665]
- 93.
- Kokura S, Yoshikawa T, Kaneko T. et al. Efficacy of hyperthermia and polyunsaturated fatty acids on experimental carcinoma. Cancer Res. 1997;57:2200–2202. [PubMed: 9187121]
- 94.
- Prasad K, Kumar B, Yan X-D. et al. α-tocopheryl succinate, the most effective form of Vit. E for adjuvant cancer treatment: A review. Journal of American College of Nutrition. 2003;22:108–117. [PubMed: 12672706]
- 95.
- Callari D, Sinatra F, Paravizzini GL. et al. All trans retionic acid sensitizes colon cancer cells to hyperthermia cytotoxic effects. International Journal of Oncology. 2003;23:181–188. [PubMed: 12792792]
- 96.
- Wachsberger PR, Burd R, Bhala SB. et al. Quercetin sensitizes cells to hyperthermia. Int J Hyperthermia. 2003;19:507–519. [PubMed: 12944166]
- 97.
- Middleton E, Kandaswami C, Theoharis C. et al. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed: 11121513]
- 98.
- Harsh IVthGF. Management of recurrent gliomasIn: Berger Michel S, Wilson Charles B, eds.The GliomasPhiladelphia, London, Toronto, Miami, Sydney, Tokyo: WB Saunders Publisher,1999649–659.
- 99.
- Shapiro WR, Shapiro JR, Walker RW. Central nervous systemIn: Abeloff MD, Armitage JO, Lichter AS, Niederbuber JE, eds.Clinical OncologyNew York, Edinburgh, London, Madrid, Melbourne, San Francisco, Tokyo: Churchill Livingstone,20001103–1192.
- 100.
- Knisely JPS, Rockwell S. Importance of hypoxia in the biology and treatment of brain tumors. Neuroimag Clin N Am. 2002;12:525–536. [PubMed: 12687909]
- 101.
- Brat DJ, Mapstone TB. Malignant glioma physiology: Cellular response to hypoxia and its role in tumor progression. Ann Intern Med. 2003;138:659–668. [PubMed: 12693889]
- 102.
- Gerweck LE, Richards B. Influence of pH on the thermal sensitivity of cultured human glioblastoma. Cancer Res. 1981;41:845–849. [PubMed: 7459870]
- 103.
- Fuse T, Yoon KW, Kato T. et al. Heat-induced apoptosis in human glioblastoma cell line A172. Neurosurgery. 1998;42:843–849. [PubMed: 9574649]
- 104.
- Tanaka T, Kobayashi T, Kida Y. et al. The effect of hyperthermia and antitumor drugs on brain tumor cells lines. Gan To Kagaku Ryoho. 1986;13:2993–2997. [PubMed: 3464229]
- 105.
- Kobayashi T, Kida Y. Magnetic induction hyperthermia for brain tumor using ferromagnetic implant with low Curie temperature. Experimental studies. J Neurooncol. 1986;4:175–181. [PubMed: 3783211]
- 106.
- Tanaka T, Kobayashi , Takahashi T. et al. Effect of combined treatment with magnetic induction hyperthermia and chemotherapy in a rabbit brain tumor model. Neurol Med Chir (Tokyo). 1989;29:377–381. [PubMed: 2477736]
- 107.
- Shem BC, Dahl O. Thermal enhancement of ACNU and potentiation of thermochemotherapy with ACNU by hypertonic glucose in the BT4 A rat glioma. Journal of Neuroncology. 1991;10:247–252. [PubMed: 1895166]
- 108.
- Raaphorst GP, Cheng ENG, Shahine B. Comparison of Radiosensitization by 41°C hyperthermia during low dose rate irradiation and during pulsed simulated low dose rate irradiation in human glioma cells. Int J Radiation Oncology Biol Phys. 1999;44:185–188. [PubMed: 10219813]
- 109.
- Papavero L, Loew F, Jaskshe H. Intracarotid infusion of ACNU and BCNU as adjuvant therapy of malignant gliomas. Acta Neurochir. 1987;85:128–137. [PubMed: 3035882]
- 110.
- Hahn GM, Shiu EC. Effect of pH and elevated temperature on the cytotoxicity of some chemotherapeutic agents on Chinese hamster cells in vitro. Cancer Res. 1983;43:5789–5791. [PubMed: 6196107]
- 111.
- Watanabe M, Tanaka R, Rondo H. et al. Effects of antineoplastic agents and hyperthermia on cytotoxicity toward chronically hypoxic glioma cells. Int J Hyperthermia. 1992;8:131–138. [PubMed: 1545159]
- 112.
- Raaphorst GP, Mao J, NG CE. Thermotolerance in human glioma cells. Int J Hyperthermia. 1995;11:523–529. [PubMed: 7594806]
- 113.
- Sneed PK, Stea B. Thermoradiotherapy for brain tumorsIn: Seegenschmiedt MH, Fessenden P, Vernon CC, eds.Thermoradiotherapy and ThermochemotherapyBerlin, Heidelberg, New York: Springer Verlag,1995159–175.
- 114.
- Takahashi H, Suda T, Motoyama H. et al. Radiofrequency interstitial hyperthermia on malignant brain tumors: Development of heating system. Experimental Oncology. 2000;22:186–190.
- 115.
- Seegenschmiedt MH, Feldmann HJ, Wust P. et al. Hyperthermia---Its actual role in radiation oncology. Part IV. Thermoradiotherapy for malignant brain tumors. Strhlentherapie und Onkologie. 1995;171:560–572. [PubMed: 8571175]
- 116.
- Sneed P, Stauffer PR, McDermott MW. et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiation Oncology Biol Phys. 1998;40:287–295. [PubMed: 9457811]
- 117.
- Ikeda N, Hayashida O, Kameda H. et al. Experimental study on thermal damage to dog normal brain. Int J Hyperthermia. 1994;10:553–561. [PubMed: 7963810]
- 118.
- Sminia P, Van Der Zee J, Wondergem J. et al. Effect of hyperthermia on central nervous system: A review. Int J Hyperthermia. 1994;10:1–30. [PubMed: 8144981]
- 119.
- Sharma HS, Hoopes PJ. Hyperthermia induced pathophysiology of the central nervous system. Int J Hyperthermia. 2003;19:325–354. [PubMed: 12745974]
- 120.
- Larson D, Shreve DC, Gutin P. RadiosurgeryIn: Berger Michel S, Wilson Charles B, eds.The GliomasPhiladelphia, London, Toronto, Miami, Sydney, Tokyo: WB Saunders Publisher,1999511–518.
- 121.
- Purdy JA. Three dimensional conformal radiation therapy: Physics, treatment planning, and clinical aspectsIn: Perez CA, Brady LW, Halperin EC, Schmit-Ulrich RK, eds.Principles and practice of Radiation Oncology. 4th edPhiladelphia, Baltimore, New York, London: Linpiccott Williams and Wilkins,2004283–313.
- 122.
- Gramaglia A, Loi GF, Mongioj V. et al. Increased survival in brain metastatic patients treated with Stereotactic radiotherapy, omega three fatty acids and bioflavonoids. Anticancer Res. 1999;19:5583–5586. [PubMed: 10697622]
- 123.
- Baronzio GF, Gramaglia A, Scorsetti M. et al. Biosynchrotherm: New capacitive hyperthermia device: Preliminary clinical reports. Abs XXIII meeting of International Clinical hyperthermia Society. Lyon, France. 2000
- Thermo-Chemo-Radiotherapy Association: Biological Rationale, Preliminary Observa...Thermo-Chemo-Radiotherapy Association: Biological Rationale, Preliminary Observations on Its Use on Malignant Brain Tumors - Madame Curie Bioscience Database
- IgA and Mucosal Homeostasis - Madame Curie Bioscience DatabaseIgA and Mucosal Homeostasis - Madame Curie Bioscience Database
- Epithelial-Mesenchymal Transformation in the Embryonic Heart - Madame Curie Bios...Epithelial-Mesenchymal Transformation in the Embryonic Heart - Madame Curie Bioscience Database
- Inferring Evolutionary History through Inter- and Intraspecific DNA Sequence Com...Inferring Evolutionary History through Inter- and Intraspecific DNA Sequence Comparison: The Drosophila janus and ocnus Genes - Madame Curie Bioscience Database
- SELECTIVE NEURODEGENERATION, NEUROPATHOLOGY AND SYMPTOM PROFILES IN HUNTINGTON'S...SELECTIVE NEURODEGENERATION, NEUROPATHOLOGY AND SYMPTOM PROFILES IN HUNTINGTON'S DISEASE - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...

![Figure 7. The interactions of hyperthermia with chemotherapy, radiotherapy are illustrated together the principal points of tumor microenvironment modulation [TBF, micromilieu, membranes, metabolism] for improving the therapeutic response to heat.](/books/NBK6001/bin/ch369f7.gif)