NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
α1-Microglobulin is one of the three original members of the lipocalin superfamily. It has been found in mammals, birds, amphibians and fish and is distributed in plasma and extravascular compartments of all organs. α1-Microglobulin has a free cysteine side-chain located in a flexible loop, giving the protein reductase and dehydrogenase properties with a broad biological substrate specificity. Three lysyl residues located around the opening of the lipocalin pocket carry yellow-brown modifications originating from the binding and degradation of heme and kynurenin, the latter a tryptophan metabolite. We have suggested that α1-microglobulin is involved in defending tissues against oxidation by heme, kynurenin and reactive oxygen species.
Introduction
α1-Microglobulin (α1m) was discovered in human urine 40 years ago and was named in the tradition of plasma proteins, reflecting its small size (26 kDa) and its electrophoretic migration slightly behind albumin.1 The protein was characterized by several research groups and given the alternative names protein HC, “human complex-forming protein, heterogeneous in charge”2 and α1-microglycoprotein.3 α1M, retinol-binding protein (RBP) and β-lactoglobulin were the three original members when the lipocalin family was defined in 1985.4
The physiological role of α1m has only recently been clarified. Immunoregulatory (mainly suppressive) properties of α1m were identified (see below), but did not seem distinct or strong enough to constitute the major function of the molecule in vivo. However, several very recent reports have suggested that α1m may play a biological role as an anti-oxidant with oxidant-scavenging and enzymatic reductase properties. In this review, we will describe the structural features of α1m, its distribution among tissues and species and the anti-oxidation and immunoregulatory properties of this lipocalin.
Structure
Peptide Chain
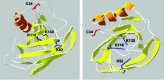
The full sequence of human α1m was first reported by Kaumeyer et al.5 The protein was found to consist of 183 amino acid residues. Since then, ten additional α1m cDNAs and/or proteins have been detected, isolated and/or sequenced, from other mammals,6-17 birds,18 amphibians, 19 and fish20-22 (Table 1). The length of the peptide chain of α1m differs slightly among species, due mainly to variations in the C-terminus. Alignment comparisons of the different deduced amino acid sequences show that the percentage of identity varies from approximately 75-80% between rodents or ferungulates and man, down to approximately 45% between fish and mammals. A free cysteine side-chain at position 34 is conserved. This group has been shown to be involved in redox reactions, in complex formation with other plasma proteins and in binding to a yellow-brown chromophore (see below). Computerised 3D models23 based on the known X-ray crystallographic structures of other lipocalins suggest that Cys34 is solvent exposed and located near the opening of the lipocalin pocket (fig. 1). Complement factor C8γ, another lipocalin, also carries an unpaired Cys in position 34 that is involved in the formation of the active C8 complex.24 In yet another lipocalin, prostaglandin D synthase, a free Cys65 has been shown to be of importance for the catalytic activity of the enzyme.25
Table 1
α1M and bikunin are encoded by a single gene and co-expressed as a precursor-protein. This genetic construction has been found in many species.
Carbohydrates
Human α1m is substituted with oligosaccharides in three positions, two sialylated complex-type, probably diantennary carbohydrates linked to Asn17 and Asn96 and one more simple oligosaccharide linked to Thr5.26-28 The carbohydrate content of α1m proteins from different species varies greatly, though, ranging from no glycosylation at all in Xenopus leavis19 over a spectrum of different glycosylation patterns. However, one glycosylation site, corresponding to Asn96 in man, is conserved in mammals, suggesting that this specific carbohydrate may be functionally important.
Chromophore
α1M is charge- and size-heterogeneous. Tightly bound, heterogeneous, brown-coloured prosthetic groups have been proposed as responsible for the heterogeneity.29 The heterogeneity and brown colour are universal properties of α1m from all species and at least parts of the brown materials are attached intracellularly to the protein.20,30 Several structurally different chromophores attached to multiple residues may explain the observed charge and size heterogeneity of the protein. Covalently linked coloured substances have been localized to Cys34,29 and Lys92, Lys118 and Lys130, the latter with molecular masses between 100 and 300 Da.31 Molecular modelling suggests that all four residues are located at the entrance of the lipocalin cavity (fig. 1). Recently, the tryptophan metabolite kynurenine was found covalently attached to lysyl residues in α1m from urine of hemodialysis patients and appears to be the source of the brown colour of the protein in this case.32
Lipocalin Ligand
Available data suggest that many different ligands can be fitted into the pocket of α1m. Several hydrophobic substances, including retinol, have been extracted from α1m, but in molar quantities never more than approximately 1/1000 of the protein.33 A 282 Da lipophilic substance was copurified with the peptides containing Lys92, Lys118 and Lys130.31 Recently, several papers demonstrated that heme binds specifically to α1m in plasma, urine and other tissue fluids.34-36 The affinity constant (Ka) was approximately 1-2 x 106 M-1, and the binding evolutionarily conserved.36 A physiological role as a heme-scavenger was therefore proposed for α1m (see below).
Synthesis
Cosynthesis with Bikunin
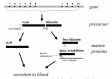
The gene for α1m is called AMBP (Alpha-1-Microglobulin-Bikunin Precursor gene) because it also encodes bikunin, another plasma protein5 (fig. 2). Bikunin37 is a common subunit of a group of protein/carbohydrate complexes that constitute the inter-α-inhibitor family. Its members are plasma and tissue proteins that have proteinase inhibitor activity38 and serve as structural components of extracellular matrix.39 Curiously, transcription of the AMBP gene, which takes place mainly in the liver, produces an mRNA that is translated into a precursor protein consisting of a 19 amino acid residue signal peptide and the α1m and bikunin proteins connected by a linker tripeptide. The linker tripeptide and the last amino acid in α1m, an arginine, constitute a basic cleavage site, R-V-R-R, that is recognized by furin and other subtilisin-like proprotein convertases (SPCs).40 Before secretion, the α1m- and bikunin-components are separated by proteolytic cleavage in the late phase of post-translational processing in the Golgi-system.41 The bikunin-part is modified by attachment of a glycosaminoglycan (chondroitin sulfate chain) to Ser1042,43 and the bikunin molecules are linked to larger subunits, so-called heavy chains, via N-acetylgalactosamine residues in the glycosaminoglycan.44,45 Both α1m and bikunin then leave the hepatocyte and enter the blood. No connection has been found between α1m and bikunin after they leave the hepatocyte and the reason for the cosynthesis of α1m and bikunin is not understood. Moreover, it has been shown in different expression systems that both α1m and bikunin can be expressed alone.20,46-49 In spite of this, the α1m/bikunin genetic construction is conserved in all species where α1m has been found.
Gene
The AMBP gene from man50 and mouse51 has been cloned. Of its ten exons, the first six code for α1m (fig.2). The AMBP gene has been mapped to the 9q32-33 region in man52 and to chromosome 4 in mouse,53 sites in both species where other lipocalin genes are clustered (reviewed in ref. 54). Intron F, which separates the α1m-exons from the bikunin-exons, contains retroposons and other repeated structures, suggesting that it is a recombinatorial hot-spot.51 This could have provided the basis for a fusion between an ancestral lipocalin gene (α1m) with an ancestral Kunitz inhibitor gene (bikunin).
Expression and Distribution
Liver, Blood and Kidney
Early quantitative tissue distribution studies revealed liver, blood plasma, and kidney as major sites of α1m localization.55 This pattern reflects the major phases of the metabolism of the protein. (1) The liver is a major site of synthesis of α1m in adult tissues in all species studied.56-59 (2) α1m is then secreted to the blood, where the protein exists in free form as well as in a variety of high molecular weight complexes (see below). Both free α1m and the complexed forms are very rapidly equilibrated between the intra- and extra-vascular compartments and their half-lifes in blood are 2-3 min.60,61 (3) Free monomeric α1m passes relatively freely through the glomerular membranes out into the primary urine, where it is reabsorbed by the proximal tubular cells and catabolized.62 The binding of α1m to the tubular cells is mediated by the multiligand receptor megalin, a member of the LDL-receptor gene family.63 Megalin also binds albumin and the lipocalins retinol-bindning protein (RBP) and major urinary protein (MUP).63
Expression
The liver is the predominant site of synthesis of α1m. AMBP gene expression in liver is tightly regulated by a unique set of cis-elements and transcription factors known as hepatocyte nuclear factors (HNF 1-4).64-66 Several secondary and minor locations of α1m expression have been reported. Thus, α1m mRNA has been detected in adult kidney,12,21 pancreas,67,68 stomach6 and blood cells.21 Traditionally, the expression of α1m has been considered as constitutive, but lately this view been challenged. Thus, induction of α1m-bikunin expression in kidneys by oxalate was recently demonstrated,69 suggesting a regulatory role of α1m in oxalate-containing kidney stone formation. Moreover, hemoglobin and pro-oxidants up-regulated the α1m-expression in hepatoma and blood cell lines,70 supporting the view that α1m is involved in anti-oxidation and heme-protection (see below and fig. 3).
Plasma
In human plasma, approximately 50% of α1m forms a one-to-one complex with monomeric IgA by a reduction-resistant bond between the penultimate cysteine in the a-chain and Cys34 of α1m.71-73 Approximately 7% is linked to albumin2,74 and 1% to prothrombin by a disulfide bond.74 High molecular weight (HMW) forms of α1m have been found in all species examined. In rat serum, α1m is found covalently linked by a disulfide link to fibronectin75 and by a reduction-resistant bond to the proteinase inhibitor α1-inhibitor-3, a homologue of human α2-macroglobulin.76 α1M complexes have also been detected in plaice serum.20 Thus, the complex-forming ability of α1m is conserved from fish to man, although the identity of the complex partners is not conserved. It is not known in what compartment any of the α1m-complexes are formed.
Unusual forms of HMW α1m have been found in plasma from patients with various pathologies; α1m has a tendency to bind to mutated forms of coagulation factors that include a free Cys residue. The conserved unpaired Cys34 of α1m is probably involved in the formation of these complexes. Thus, circulating complexes have been described between α1m and factor IX Zutphen,77 factor XII Tenri78 and several protein C mutants.79 In all mutants the unusual free, unpaired cysteine residue is located in an N-terminal Gla-domain (γ-carboxy glutamic acid-domain). The Gla-domains are believed to mediate the binding of the coagulation factors to membrane surfaces. It was shown by molecular modelling of α1m and the protein C Gla-domain that electrostatic and hydrophobic interactions may attract the two proteins to each other and orient them in such a way that formation of a disulfide bond between the two free cysteines is favoured.22
Determination of the α1m concentration in human plasma or serum is complicated by the presence of complex forms of the protein (see above). Consequently, reports on normal α1m-concentrations in human plasma/serum have varied. Several investigators have measured free α1m and IgA-α1m separately in normal serum.64,80-82 For example, DeMars et al82 found a mean concentration of 33 mg/L for free α1m and 248 mg/L for IgA-α1m, corresponding to a molar ratio of approximately 1:1 (∼1 μM of each). Without distinguishing between free and complexed α1m, the values for “total” α1m in rat, guinea pig and plaice serum were determined to be 9-16 mg/L, 26 mg/L and 20 mg/L, respectively.8,12,20
Other Tissues
Besides its predominant localization in plasma and liver and kidney cells, α1m has been identified in the perivascular connective tissue of most organs67,83,84 and is especially abundant in epidermis of skin35,85 and epithelium of the gut.61,86,87 It is often colocalized with elastin and collagen83,85 and was reported to bind to collagen in vitro.88 A distribution of matrix-α1m at various interfaces between the cells of the body and the external environment (blood/tissue, air/tissue, intestinal lumen/villi), as well as at the interface between maternal blood and fetal tissues in placenta,89 is suggestive of a protective role of α1m in vivo (see below). A similar distribution was seen in human90 and mouse fetuses.15 In addition, fetal α1m was seen in the cytoplasm of many epithelial cells that do not express the protein post-term.15,90
Anti-Oxidant Properties
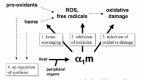
Recent reports suggest that α1m is involved in the defense against oxidative tissue damage (oxidative stress). The proposed anti-oxidant mechanisms are summarized in Figure 3. Pro-oxidants are constantly introduced to the human body via the environment (air, food, etc) but are also produced endogenously as metabolites in the normal homeostasis.91 Increased amounts of pro-oxidants are seen during inflammation, and oxidative stress is considered to be a major factor in the development of many conditions such as atherosclerosis, rheumatoid arthritis, ischemia/reperfusion injury, and diabetes. The pro-oxidants undergo reactions forming reactive oxygen species (ROS) and free radicals. These react with proteins, DNA and other molecules of human tissues by oxidation. These oxidation reactions are often harmful and may destroy the function of the target molecules (oxidative damage). Heme, the prosthetic group of heme-proteins, is a prominent example of an endogenous pro-oxidant and is harmful when released into the extracellular environment.
Thus, α1m uses three major mechanisms to achieve anti-oxidation: (1) scavenging heme and other pro-oxidants, (2) inhibiting oxidation reactions, and (3) enzymatic reduction of harmful oxidation products. Additionally, a fourth mechanism enhance its anti-oxidant action: heme- and pro-oxidant-induced up-regulation of the synthesis of α1m70 (see above).
Heme-Scavenging
Both α1m and its IgA-complex bind to the heme group.34-36 The exposure of α1m to erythrocyte membranes or purified hemoglobin leads to the binding of heme and the formation of a truncated form of the protein (t-α1m) that lacks the C-terminal tetrapeptide LIPR and has heme-degrading properties. A pronounced yellow-brown colour was formed by incubating t-α1m with heme, suggesting that at least some of the α1m-chromophores may be heme degradation products.34 The t-α1m form is present in urine and thus is formed in vivo.34,69,92 In chronic venous ulcers, an inflammatory condition where free heme and iron released after hemolysis are considered to be pathogenic factors, α1m was colocalized with heme and t-α1m was continuously formed.35 Based on these findings, a role as an extracellular heme-scavenger was proposed for α1m. As mentioned above, the tryptophan metabolite kynurenine is attached to lysyl residues in α1m from the urine of hemodialysis patients.32 Kynurenine metabolites are formed in tryptophan metabolism and are pro-oxidants, i.e., may induce oxidative stress.93-95 The binding of heme and kynurenin and the transformation of these substances to chromophores may be two examples of a pro-oxidant scavenging mechanism of α1m.
Inhibition of Oxidation and Enzymatic Reductase Activity
α1M inhibited the heme- and ROS-induced oxidation of collagen, low-density lipoproteins (LDL), membrane lipids and whole cells.96 α1M also removed preformed oxidation products present on collagen and LDL. This suggests that α1m may act as an oxidation repair factor. A possible mechanism for this may be the enzymatic reductase/dehydrogenase properties recently described for α1m.97 Thus, the protein was capable of reducing heme proteins, free iron and the synthetic compound nitroblue tetrazolium (NBT) using the electron donors ascorbate and NADH/NADPH as cofactors. The thiol group of Cys34 and the three lysyl residues of K92, K118 and K130 were found in the active site, suggesting that the chromophore or chromophore formation is linked to the reductase activities.
Immunoregulatory Properties
Due to its immunoregulatory properties, α1m is categorized as an immunocalin.98 It inhibits central events of the immune response in vitro. Thus, the antigen-induced cell division of peripheral blood lymphocytes was inhibited by α1m.99,100 The effects were species independent, i.e., similar effects on human cells were obtained with human, rat, rabbit or guinea pig α1m.14 It was also shown that the antigen-induced interleukin-2 (IL-2) production by mouse T helper cell hybridomas was inhibited by human α1m.101 Furthermore, inflammatory responses of blood cells were inhibited by α1m; these included migration98 and chemotaxis102 of neutrophil granulocytes and the production of free radicals and IL-1β by peripheral lymphocytes/monocytes.88 Finally, at low serum concentrations, a strong direct mitogenic effect by α1m on resting guinea pig and human lymphocytes was seen.100,103,104 At the high serum concentrations (10-20%) used to measure the inhibition of antigen-stimulated lymphocytes, no direct stimulation of the cell division was seen. It is possible that the immunoregulatory effects of α1m are related to its anti-oxidant and reductase activities, as ROS have been shown to be involved as (positive) factors in cell signalling during lymphocyte activation (reviewed in refs. 105, 106).
Cell Receptor
α1M has been shown to bind to the surface of various white blood cells, including human peripheral B and T lymphocytes, human NK cells,60 the human histiocytic cell-line U937,107 mouse peripheral B and T lymphocytes,104 and mouse T helper cell hybridomas.101 The binding is species independent, specific for α1m, saturable and trypsin-sensitive, suggesting that a protein that recognizes a conserved part of α1m is present on the surface of white blood cells. The α1m-receptor on blood cells has not yet been identified, however.
Concluding Remarks
Above, we have reviewed the current knowledge about the lipocalin α1-microglobulin, its structural features, its unusual synthesis as a diprotein, its tissue expression and distribution, and we have particularly highlighted recent elucidation of its anti-oxidant properties and how these may be employed in tissue protection.
Acknowledgements
The text has been revised by Linda Lögdberg, PhD.
References
- 1.
- Ekström B, Peterson PA, Berggård I. A urinary and plasma α1-glycoprotein of low molecular weight: Isolation and some properties. Biochem Biophys Res Commun. 1975;65(4):1427–33. [PubMed: 79416]
- 2.
- Tejler L, Grubb AO. A complex-forming glycoprotein heterogeneous in charge and present in human plasma, urine and cerebrospinal fluid. Biochim Biphys Acta. 1976;439(1):82–94. [PubMed: 952962]
- 3.
- Seon BK, Pressman D. Unique human glycoprotein, α1-microglycoprotein: Isolation from the urine of a cancer patient and its characterization. Biochemistry. 1978;17(14):2815–21. [PubMed: 80228]
- 4.
- Pervaiz S, Brew K. Homology of β-lactoglobulin, serum retinol-binding protein, and protein HC. Science. 1985;228(4697):335–7. [PubMed: 2580349]
- 5.
- Kaumeyer JF, Polazzi JO, Kotick MP. The mRNA for a proteinase inhibitor related to the HI-30 domain of inter-a-trypsin inhibitor also encodes α1-microglobulin (protein HC). Nucleic Acids Res. 1986;14(20):7839–50. [PMC free article: PMC311818] [PubMed: 2430261]
- 6.
- Tavakkol A. Molecular cloning of porcine α1-microglobulin/HI-30 reveals developmental and tissue-specific expression of two variant messenger ribonucleic acids. Biochim Biophys Acta. 1991;1088(1):47–56. [PubMed: 1703444]
- 7.
- Lindqvist A, Åkerström B. Bovine α1-microglobulin/bikunin. Isolation and characterization of liver cDNA and urinary α1-microglobulin. Biochim Biophys Acta. 1996;1306(1):98–106. [PubMed: 8611630]
- 8.
- Åkerström B, Berggård I. Guinea-pig α1-microglobulin. Isolation and properties in comparison with human α1-microglobulin. Eur J Biochem. 1979;101(1):215–23. [PubMed: 92407]
- 9.
- Yoshida K, Suzuki Y, Yamamoto K. et al. Guinea pig α1-microglobulin/bikunin: cDNA sequencing, tissue expression and expression during acute phase. Comp Biochem Physiol B Biochem Mol Biol. 1999;122(2):165–72. [PubMed: 10327606]
- 10.
- Vincent C, Bouic P, Revillard JP. Characterization of rat α1-microglobulin. Biochem Biophys Res Commun. 1983;116(1):180–8. [PubMed: 6196025]
- 11.
- Slota A, Sjöquist M, Wolgast M. et al. Bikunin in rat plasma, lymph and bile. Biol Chem Hoppe Seyler. 1994;375(2):127–33. [PubMed: 8192857]
- 12.
- Kastern W, Björck L, Åkerström B. Developmental and tissue-specific expression of α1-microglobulin mRNA in the rat. J Biol Chem. 1998;261(32):15070–4. [PubMed: 2429963]
- 13.
- Lindqvist A, Bratt T, Altieri M. et al. Rat α1-microglobulin: Coexpression in liver with the light chain of inter-a-trypsin inhibitor. Biochim Biophys Acta. 1992;1130(1):63–7. [PubMed: 1371936]
- 14.
- Åkerström B, Lögdberg L, Babiker-Mohamed H. et al. Structural relationship between α1-microglobulin from man, guinea-pig, rat and rabbit. Eur J Biochem. 1987;170(1-2):143–8. [PubMed: 2446872]
- 15.
- Sanchez D, Martinez S, Lindqvist A. et al. Expression of the AMBP gene transcript and its two protein products, α1-microglobulin and bikunin, in mouse embryogenesis. Mech Dev. 2002;117(1-2):293–8. [PubMed: 12204273]
- 16.
- Chan P, Salier J-P. Mouse α1-microglobulin/bikunin precursor: cDNA analysis, gene evolution and physical assignment of the gene next to the orosomucoid locus. Biochim Biophys Acta. 1993;1174(2):195–200. [PubMed: 7689339]
- 17.
- Ide H, Itoh H, Nawa Y. Sequencing of cDNAs encoding α1-microglobulin/bikunin of Mongolian gerbil and Syrian golden hamster in comparison with man and other species. Biochim Biophys Acta. 1994;1209(2):286–92. [PubMed: 7529051]
- 18.
- Åkerström B. Immunological analysis of α1-microglobulin in different mammalian and chicken serum. α1-Microglobulin is 5-8 kilodaltons larger in primates. J Biol Chem. 1985;260(8):4839–44. [PubMed: 2580828]
- 19.
- Kawahara A, Hikosaka A, Sasado T. et al. Thyroid hormone-dependent repression of α1-microglobulin/ bikunin precursor (AMBP) gene expression during amphibian metamorphosis. Dev Genes Evol. 1997;206(6):355–62. [PubMed: 27747396]
- 20.
- Lindqvist A, Åkerström B. Isolation of plaice (Pleuronectes platessa) α1-microglobulin: Conservation of structure and chromophore. Biochim Biophys Acta. 1999;1430(2):222–33. [PubMed: 10082950]
- 21.
- Leaver MJ, Wright J, George SG. Conservation of the tandem arrangement of α1-microglobulin/ bikunin mRNA: Cloning of a cDNA from plaice (Pleuronectes platessa). Comp Biochem Physiol Biochem Mol Biol. 1994;108(3):275–81. [PubMed: 7521726]
- 22.
- Hanley S, Powell R. Sequence of a cDNA clone encoding the Atlantic salmon α1-microglobulin/ bikunin protein. Gene. 1994;147(2):297–8. [PubMed: 7523247]
- 23.
- Villoutreix B, Åkerström B, Lindqvist A. Structural model of human α1-microglobulin: proposed scheme for the interaction with the Gla domain of anticoagulant protein C. Blood Coagul Fibrinolysis. 2000;11(3):261–75. [PubMed: 10870807]
- 24.
- Schreck SF, Parker C, Plumb ME. et al. Human complement protein C8γ Biochim Biophys Acta. 2000;1482(1-2):199–208. [PubMed: 11058761]
- 25.
- Urade Y, Tanaka T, Eguchi N. et al. Structural and functional significance of cysteine residues of glutathione-independent prostaglandin D synthase. Identification of Cys65 as an essential thiol. J Biol Chem. 1995;270(3):1422–8. [PubMed: 7836410]
- 26.
- Ekström B, Lundblad A, Svensson S. Structural studies on the carbohydrate portion of human α1-microglobulin. Eur J Biochem. 1981;114(3):663–6. [PubMed: 6165582]
- 27.
- Escribano J, Lopez-Otin C, Hjerpe A. et al. Location and characterization of the three carbohydrate prosthetic groups of human protein HC. FEBS Lett. 1990;266(1-2):167–70. [PubMed: 1694784]
- 28.
- Amoresano A, Minchiotti L, Cosulich ME. et al. Structural characterization of the oligosaccharide chains of human α1-microglobulin from urine and amniotic fluid. Eur J Biochem. 2000;267(7):2105–12. [PubMed: 10727951]
- 29.
- Escribano J, Grubb A, Calero M. et al. The protein HC chromophore is linked to the cysteine residue at position 34 of the polypeptide chain by a reduction-resistant bond and causes the charge heterogeneity of protein HC. J Biol Chem. 1991;266(24):15758–63. [PubMed: 1714898]
- 30.
- Åkerström B, Bratt T, Enghild JJ. Formation of the α1-microglobulin chromophore in mammalian and insect cells: A novel post-translational mechanism? FEBS Lett. 1995;362(1):50–4. [PubMed: 7535251]
- 31.
- Berggård T, Cohen A, Persson P. et al. α1-microglobulin chromophores are located to three lysine residues semiburied in the lipocalin pocket and associated with a novel lipophilic compound. Protein Sci. 1999;8(12):2611–20. [PMC free article: PMC2144230] [PubMed: 10631976]
- 32.
- Sala A, Campagnoli M, Perani E. et al. Human α1-microglobulin is covalently bound to kynurenine-derived chromophores. J Biol Chem. 2004;279(49):51033–41. [PubMed: 15452109]
- 33.
- Escribano J, Grubb A, Mendez E. Identification of retinol as one of the protein HC chromophores. Biochem Biophys Res Commun. 1988;155(3):1424–9. [PubMed: 2460097]
- 34.
- Allhorn M, Berggård T, Nordberg J. et al. Processing of the lipocalin α1-microglobulin by hemoglobin induces heme-binding and heme-degradation properties. Blood. 2002;99(6):1894–901. [PubMed: 11877257]
- 35.
- Allhorn M, Lundqvist K, Schmidtchen A. et al. Heme-scavenging role of α1-microglobulin in chronic ulcers. J Invest Dermatol. 2003;121(3):640–6. [PubMed: 12925227]
- 36.
- Larsson J, Allhorn M, Åkerström B. The lipocalin α1-microglobulin binds heme in different species. Arch Biochem Biophys. 2004;432(2):196–204. [PubMed: 15542058]
- 37.
- Blom A, Fries E. Bikunin-not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32(2):125–37. [PubMed: 10687949]
- 38.
- Salier J-P, Rouet P, Raguenez G. et al. The inter-a-inhibitor family: From structure to regulation. Biochem J. 1996;315(Pt 1):1–9. [PMC free article: PMC1217155] [PubMed: 8670091]
- 39.
- Chen L, Mao SJT, Larsen WJ. Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-a-trypsin inhibitor family. J Biol Chem. 1992;267(17):12380–6. [PubMed: 1376324]
- 40.
- Molloy SS, Bresnahan PA, Leppla S. et al. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267(23):16396–402. [PubMed: 1644824]
- 41.
- Bratt T, Olsson H, Sjöberg EM. et al. Cleavage of the α1-microglobulin-bikunin precursor is localized to the Golgi apparatus of rat liver cells. Biochim Biophys Acta. 1993;1157(2):147–54. [PubMed: 7685189]
- 42.
- Sjöberg EM, Fries E. Biosynthesis of bikunin (urinary trypsin inhibitor) in rat hepatocytes. Biochem J. 1990;272(2):113–8.
- 43.
- Chirat F, Balduyck M, Mizon C. et al. A chondroitin-sulfate chain is located on serine-10 of the urinary trypsin inhibitor. Int J Biochem. 1991;23(11):1201–3. [PubMed: 1794445]
- 44.
- Enghild JJ, Salvesen G, Hefta SA. et al. Chondroitin 4-sulfate covalently cross-links the chains of the human blood protein preα-inhibitor. J Biol Chem. 1989;266(2):747–51. [PubMed: 1898736]
- 45.
- Morelle W, Capon C, Balduyck M. et al. Chondroitin sulphate covalently cross-links the three polypeptide chains of inter-α-trypsin inhibitor. Eur J Biochem. 1994;221(2):881–8. [PubMed: 7513643]
- 46.
- Bratt T, Cedervall T, Åkerström B. Processing and secretion of rat α1-microglobulin-bikunin expressed in eukaryotic cell lines. FEBS Lett. 1994;354(1):57–61. [PubMed: 7525349]
- 47.
- Thuveson M, Fries E. Intracellular proteolytic processing of the heavy chain of rat preα-inhibitor. The COOH-terminal propeptide is required for coupling to bikunin. J Biol Chem. 1999;274(10):6741–6. [PubMed: 10037773]
- 48.
- Wester L, Johansson MU, Åkerström B. Physicochemical and biochemical characterization of human α1-microglobulin expressed in baculovirus-infected insect cells. Protein Expr Purif. 1997;11(1):95–103. [PubMed: 9325144]
- 49.
- Falkenberg C, Wester L, Belting M. et al. Expression of a functional proteinase inhibitor capable of accepting xylose: Bikunin. Arch Biochem Biophys. 2001;387(1):99–106. [PubMed: 11368189]
- 50.
- Diarra-Mehrpour M, Bourguignon J, Sesboué R. et al. Structural analysis of the human inter-α-trypsin inhibitor light-chain gene. Eur J Biochem. 1990;191(1):131–9. [PubMed: 1696200]
- 51.
- Lindqvist A, Rouet P, Salier J-P. et al. The α1-microglobulin/bikunin gene: Characterization in mouse and evolution. Gene. 1999;234(2):329–36. [PubMed: 10395906]
- 52.
- Diarra-Mehrpour M, Bourguignon J, Sesboué R. et al. Human plasma inter-α-trypsin inhibitor is encoded by four genes on three chromosomes. Eur J Biochem. 1989;179(1):147–54. [PubMed: 2465147]
- 53.
- Salier J-P, Verga V, Doly J. et al. The genes for the inter-α -inhibitor family share a homologous organization in human and mouse. Mamm Genome. 1992;2(4):233–9. [PubMed: 1371941]
- 54.
- Salier J-P. Chromosomal location, exon/intron organization and evolution of lipocalin genes. Biochim Biophys Acta. 2000;1482(1-2):35–45. [PubMed: 11058744]
- 55.
- Åkerström B. Tissue distribution of guinea pig α1-microglobulin. Cell Mol Biol. 1983;29(6):489–95. [PubMed: 6197169]
- 56.
- Tejler L, Eriksson S, Grubb A. et al. Production of protein HC by human fetal liver explants. Biochim Biophys Acta. 1978;542(3):506–14. [PubMed: 687667]
- 57.
- Åkerström B. Synthesis of α1-microglobulin by guinea-pig liver. Eur J Biochem. 1983;133(1):235–9. [PubMed: 6189713]
- 58.
- Åkerström B, Landin B. Rat α1-microglobulin. Purification from urine and synthesis by hepatocyte monolayers. Eur J Biochem. 1985;146(2):353–8. [PubMed: 2578392]
- 59.
- Vincent C, Marceau M, Blangarin P. et al. Purification of α1-microglobulin produced by human hepatoma cell lines. Biochemical characterization and comparison with alpha 1-microglobulin synthesized by human hepatocytes. Eur J Biochem. 1987;165(3):699–704. [PubMed: 2439335]
- 60.
- Wester L, Fast J, Labuda T. et al. Carbohydrate groups of α1-microglobulin are important for secretion and tissue localization but not for immunological properties. Glycobiology. 2000;10(9):891–900. [PubMed: 10988251]
- 61.
- Larsson J, Wingårdh K, Berggård T. et al. Distribution of iodine 125-labeled α1-microglobulin in rats after intravenous injection. J Lab Clin Med. 2001;137(3):165–75. [PubMed: 11241026]
- 62.
- Strober W, Waldmann TA. The role of the kidney in the metabolism of plasma proteins. Nephron. 1974;13(1):35–66. [PubMed: 4607245]
- 63.
- Leheste JR, Rolinski B, Vorum H. et al. Megailin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155(4):1361–70. [PMC free article: PMC1867027] [PubMed: 10514418]
- 64.
- Rouet P, Raguenez G, Tronche F. et al. A potent enhancer made of clustered liver-specific elements in the transcription of control sequences of human α1-microglobulin/bikunin gene. J Biol Chem. 1992;267(29):20765–73. [PubMed: 1383209]
- 65.
- Rouet P, Raguenez G, Tronche F. et al. Hierarchy and positive/negative interplays of the hepatocyte nuclear factors HNF1, -3 and -4 in the liver-specific enhancer for the human α1-microglobulin/ bikunin precursor. Nucleic Acids Res. 1995;23(3):395–404. [PMC free article: PMC306689] [PubMed: 7533900]
- 66.
- Rouet P, Raguenez G, Ruminy P. et al. An array of binding sites for hepatocyte nuclear factor 4 of high and low affinities modulates the liver-specific enhancer for the human α1-microglobulin/bikunin precursor. Biochem J. 1998;334(Pt 3):577–84. [PMC free article: PMC1219726] [PubMed: 9729465]
- 67.
- Berggård T, Oury TD, Thøgersen IB. et al. α1-microglobulin is found both in blood and in most tissues. J Histochem Cytochem. 1998;46(8):887–93. [PubMed: 9671439]
- 68.
- Itoh H, Tomita M, Kobayashi T. et al. Expression of inter-α-trypsin inhibitor light chain (bikunin) in human pancreas. J Biochem. 1996;120(2):271–5. [PubMed: 8889810]
- 69.
- Grewal JS, Tsai JY, Khan SR. Oxalate inducible AMBP gene and its regulatory mechanism in renal tubular epithelial cells. Biochem J. 2005 (Published ahead of print as manuscript no BJ20041465) [PMC free article: PMC1134990] [PubMed: 15533056]
- 70.
- Olsson MG, Olofsson T, Åkerström B. Up-regulation of α1-microglobulin by hemoglobin and pro-oxidants in hepatoma and blood cell lines. Submitted. [PubMed: 17320766]
- 71.
- Grubb A, Lopez C, Tejler L. et al. Isolation of human complex-forming glycoprotein, heterogeneous in charge (protein HC), and its IgA complex from plasma. Physiochemical and immunochemical properties, normal plasma concentration. J Biol Chem. 1983;258(23):14698–707. [PubMed: 6196366]
- 72.
- Grubb A, Mendez E, Fernandez-Luna JL. et al. The molecular organization of the protein HC-IgA complex (HC-IgA). J Biol Chem. 1986;261(30):14313–20. [PubMed: 2429955]
- 73.
- Calero M, Escribano J, Grubb A. et al. Location of a novel type of interpolypeptide chain linkage in the human protein HC-IgA complex (HC-IgA) and identification of a heterogeneous chromophore associated with the complex. J Biol Chem. 1994;269(1):384–9. [PubMed: 7506257]
- 74.
- Berggård T, Thelin N, Falkenberg C. et al. Prothrombin, albumin and immunoglobulin A form covalent complexes with α1-microglobulin in human plasma. Eur J Biochem. 1997;245(5):676–83. [PubMed: 9183005]
- 75.
- Falkenberg C, Enghild JJ, Thøgersen IB. et al. Isolation and characterization of a fibronectin-α1- microglobulin complex in rat plasma. Biochem J. 1994;301(Pt 3):745–51. [PMC free article: PMC1137050] [PubMed: 7519849]
- 76.
- Falkenberg C, Grubb A, Åkerström B. Isolation of rat serum α1-microglobulin. Identification of a complex with α1-inhibitor-3 a rat α2-macroglobulin homologue. J Biol Chem. 1990;265(27):16150–7. [PubMed: 1697852]
- 77.
- Wojcik EG, van der Berg M, van der Linden IK. et al. Factor IX Zutphen: a Cys18→Arg mutation results in formation of a heterodimer with α1-microglobulin and the inability to form a calcium-induced conformation. Biochem J. 1995;311(Pt 3):753–9. [PMC free article: PMC1136067] [PubMed: 7487929]
- 78.
- Kondo S, Tokunaga F, Kawano S. et al. Factor XII Tenri, a novel cross-reacting material negative factor XII deficiency, occurs through a proteasome-mediated degradation. Blood. 1999;93(12):4300–8. [PubMed: 10361128]
- 79.
- Wojcik EG, Simioni P, van der Berg M. et al. Mutations which introduce free cysteine residues in the Gla-domain of vitamin K dependent proteins result in the formation of complexes with α1-microglobulin. Thromb Haemost. 1996;75(1):70–5. [PubMed: 8713782]
- 80.
- Fernandez-Luna JL, Moneo I, Grubb A. et al. A sensitive and rapid enzyme-linked immunosorbent assay using monoclonal antibodies for simultaneous quantitation of free and IgA-complexed protein HC. J Immunol Methods. 1985;82(1):101–10. [PubMed: 2411818]
- 81.
- Vincent C, Revillard J-P. Differential measurement by ELISA of free and IgA bound α1-microglobulin in human serum without prior fractionation. J Immunol Methods. 1985;82(1):111–9. [PubMed: 2411819]
- 82.
- DeMars DD, Katzmann JA, Kimlinger TK. et al. Simultaneous measurement of total and IgA-conjugated α1-microglobulin by a combined immunoenzyme/immunoradiometric assay technique. Clin Chem. 1989;35(5):766–72. [PubMed: 2470534]
- 83.
- ødum L, Nielsen HW. Human protein HC (α1-microglobulin) and inter-alpha-trypsin inhibitor in connective tissue. Histochem J. 1994;26(10):799–803. [PubMed: 7533752]
- 84.
- ødum L, Nielsen HW. Bikunin and α1-microglobulin in human zona pellucida and connective tissue. Histochem J. 1997;29(3):199–203. [PubMed: 9472382]
- 85.
- Bouic P, Kanitakis J, Schmitt D. et al. α1-microglobulin: A new antigenic component of the epidermo-dermal junction in normal human skin. Br J Dermatol. 1985;112(1):35–41. [PubMed: 2578800]
- 86.
- Bouic P, Vincent C, Revillard JP. Immunohistological localization of α1-microglobulin in normal rat tissues. J Histochem Cytochem. 1984;32(7):717–23. [PubMed: 6203960]
- 87.
- Bouic P, Vincent C, Revillard JP. Localization of α1-microglobulin (protein HC) in normal human tissues: An immunohistochemical study using monoclonal antibodies. Histochem J. 1984;16(12):1311–24. [PubMed: 6085075]
- 88.
- Santin M, Cannas M. Collagen-bound α1-microglobulin in normal and healed tissues and its effect on immunocompetent cells. Scand J Immunol. 1999;50(3):289–95. [PubMed: 10447938]
- 89.
- Berggård T, Enghild JJ, Badve S. et al. Histological distribution and biochemical properties of α1-microglobulin in human placenta. Am J Repr Immunol. 1999;41(1):52–60. [PubMed: 10097787]
- 90.
- Lögdberg L, Åkerström B, Badve S. Tissue distribution of the lipocalin α1-microglobulin in the developing human fetus. J Histochem Cytochem. 2000;48(11):1545–52. [PubMed: 11036097]
- 91.
- Halliwell B, Gutteridge JMC. Free radicals in Biology and Medicine Oxford: Oxford University Press.
- 92.
- Lopez C, Grubb A, Mendez E. Human protein HC displays variability in its carboxyterminal amino acid sequence. Febs Lett. 1982;144(2):349–53.
- 93.
- Ishii T, Iwahashi H, Sugata R. et al. Formation of hydroxanthommatin-derived radical in the oxidation of 3-hydroxykynurenine. Arch Biochem Biophys. 1992;294(2):616–22. [PubMed: 1314547]
- 94.
- Okuda S, Nishiyama N, Saito H. et al. Hydrogen-peroxide-mediated neuronal cell death induced by an endogeneous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci USA. 1996;93(1):12553–8. [PMC free article: PMC38030] [PubMed: 8901620]
- 95.
- Vazquez S, Garner B, Sheil MM. et al. Characterization of the major autooxidation products of 3-hydroxykynurenine under physiological conditions. Free Rad Res. 2000;32(1):11–23. [PubMed: 10625213]
- 96.
- Allhorn M, Larsson J, Olsson MG. et al. Oxidative modifications on collagen and low-density lipoprotein are inhibited and reduced by the lipocalin α1-microglobulin. Submitted.
- 97.
- Allhorn M, Klapyta A, Åkerström B. Redox properties of the lipocalin α1-microglobulin: Reduction of cytochrome c, hemoglobin, and free iron. Free Radic Biol Med. 2005;38(5):557–67. [PubMed: 15683711]
- 98.
- Lögdberg L, Wester L. Immunocalins: A lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta. 2000;1482(1-2):284–97. [PubMed: 11058769]
- 99.
- Lögdberg L, Åkerström B. Immunosuppressive properties of α1-microglobulin. Scand J Immunol. 1981;13(4):383–90. [PubMed: 6171029]
- 100.
- Lögdberg L, Åkerström B, Shevach E. α1-microglobulin is mitogenic for guinea pig lymphocytes. Scand J Immunol. 1986;24(5):575–81. [PubMed: 2431460]
- 101.
- Wester L, Michaëlsson E, Holmdahl R. et al. Receptor for α1-microglobulin on T lymphocytes: Inhibition of antigen-induced interleukin-2 production. Scand J Immunol. 1998;48(1):1–7. [PubMed: 9714404]
- 102.
- Mendez E, Fernandez-Luna JL, Grubb AO. et al. Human protein HC and its IgA complex are inhibitors of neutrophil chemotaxis. Proc Natl Acad Sci USA. 1986;88(5):1472–5. [PMC free article: PMC323098] [PubMed: 2419908]
- 103.
- Babiker-Mohamed H, Olsson MO, Boketoft Å. et al. α1-microglobulin is mitogenic to human peripheral blood lymphocytes. Regulation by both enhancing and suppressive serum factors. Immunobiology. 1990;180(2-3):221–34. [PubMed: 1693133]
- 104.
- Babiker-Mohamed H, Åkerström B, Lögdberg L. Mitogenic effect of α1-microglobulin on mouse lymphocytes. Evidence of T- and B-cell cooperation, B-cell proliferation, and a low-affinity receptor on mononuclear cells. Scand J immunol. 1990;32(1):37–44. [PubMed: 1696392]
- 105.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. [PubMed: 11773609]
- 106.
- Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signalling. Free Radic Biol Med. 2004;37(8):1144–51. [PubMed: 15451054]
- 107.
- Fernandez-Luna JL, Levya-Cobian F, Mollinedo F. Identification of the protein HC receptor. Febs Lett. 1988;236(2):471–4. [PubMed: 2457516]
- α1-Microglobulin - Madame Curie Bioscience Databaseα1-Microglobulin - Madame Curie Bioscience Database
- Macroautophagy in Mammalian Cells - Madame Curie Bioscience DatabaseMacroautophagy in Mammalian Cells - Madame Curie Bioscience Database
- Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience...Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience Database
- Inflammatory Myopathies: Dermatomyositis, Polymyositis and Inclusion Body Myosit...Inflammatory Myopathies: Dermatomyositis, Polymyositis and Inclusion Body Myositis - Madame Curie Bioscience Database
- Caspases as Targets for Drug Development - Madame Curie Bioscience DatabaseCaspases as Targets for Drug Development - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...