NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Recent developments in thermal ablation and perfusion hyperthermia have expanded the treatment options of patients with certain cancer. Initially thermal ablation was applied to liver tumor, later its application has been extending for treating focal malignancies confined in other organs such as: breast, kidney, adrenal glands, pancreas, bone, and lung. Metastases to localized organs, such liver, lung, and pleura are a common event. The inoperable tumors (primary or metastatic) are generally treated by systemic chemotherapy, however the toxicity is very high. Some clinicians have developed regional therapies to reduce this toxicity. Perfusional therapy method permits a higher concentration of antineoplastic agents in the tumor target. Furthermore the combination of hyperthermia with appropriate antineoplastic agents has demonstrated to increase the effect of the single therapy and further to reduce the toxicity. Lung, pleura and liver perfusion in combination with hyperthermia, will briefly be described here. This review is not exhaustive, its purpose is to illustrate the applications that we hope will become routine in cancer therapy in the near future.
Introduction
Image-guided ablation therapy, in with radiofrequency (RFA/HiTT), microwave, high-intensity focused ultrasound (HIFU), laser (LiTT) etc. has gained increasing attention by researchers as a tool for treating focal malignancies.1,2 As outlined by Goldberg,3 the methods offer many advantages compared to surgical resection, such as: reduction of morbidity and mortality, low cost and ability to perform ablative procedure on an outpatient basis. Initially RFA was applied only to tumors confined to the liver, later its application has been extended to tumors at different sites in the body (see Table 1). Some inclusion criteria are common to all patients treated with thermal ablation and are listed in Table 2. Even complications following treatment may be common or specific and confined to the organ treated (see Table 3). Specific criteria of inclusion and complications for singular treated tumors will be discussed later.
Table 1
RFA Primitives and metastatic human solid tumors treated.
Table 2
Patient inclusion criteria.
Table 3
Common complications following RFA.
Technical Aspects
Thermal tumor destruction can be obtained by using radiofrequency, microwave, laser or ultrasound under the continuous and direct visualization by US and MRI.1,2 The aims of these therapies are similar: coagulation of the tumor by sparing adjacent healthy tissue. Clinically this last aim is not completely attainable; in fact, tumor margins of normal parenchyma, comprised between 0.5-1 cm are often included during the treatment together.1,2 At first glance this can be thought negative, but practically it has an important consequence in reducing the risk of tumor regrowth. Cancer cells at the periphery of the tumor mass are generally more active and proliferating; this is due to metabolic favourable conditions. These cells, which are not killed by radiotherapy/and or chemotherapy and are infiltrating normal parenchyma, can restart tumor growth. The temperatures reached by the two techniques are generally > 45°C, in general 90 to 100°C and the treatment lasts between 15-30 minutes. In this range of heat, coagulative necrosis is developed and tumor cells death begins to occur 4 to 5 minutes at 60°C and more, rapidly at increasing temperature.2,4 Performing thermal ablation, the borders of the tumor are defined using non-invasive ultrasound or magnetic resonance imaging (MRI). Hence, a needle is inserted into the tumor mass for delivering heat (fig. 1).
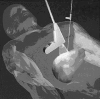
Figure 1
Schematic drawing of thermal ablation with RF-needle technique.
The radiofrequency technique will be briefly described.
Radiofrequency Ablation (RFA)
With radiofrequency ablation (RFA) a high frequency alternating current (100 to 500kHz) is generally delivered to tissue via an electrode tip inserted percutaneously or with minimal surgery. The electromagnetic energy transmitted is converted to heat by generating ionic agitation. As ions try to change direction and follow the alternating current, localized ‘frictional energy’ is created in the area surrounding the electrode tip. The heat generated is at its maximum near the electrode tip and dissipates rapidly with increasing distance.
Four RFA devices, which work on the same principles, are available at the time worldwide. The four devices currently in use (RITA medical System; Radionics Inc. Tyco Healthcare, Radio therapeutics Inc., and Elektrotom 106 HiTT Berchtold Medical Electronics) differ each other for the following aspects:
- Power of the generator.
- The gauge and geometry of the needle.
- The electrical parameter monitored.
- Cooling needle tip.5
The various characteristics of the four devices are illustrated in Figure 2 and Table 4. The needles are generally constituted by an insulated shaft and a distal conducting tip. RITA device consists of a 50 W alternating current generator and a 15-gauge electrode. The needle electrode has a movable hub and 8 retracting curved electrodes that are deployed from the tip of the needle after its positioning into the tumor. Each tips of the needle contain a thermocouple that can register the temperature in the heated area. Radionics needle consists of a straight needle with an internal channel. Inside this channel a saline solution circulates for cooling. Some holes are present for permitting the leakage of saline solution.2,6,7 In this case the saline solution is used to increases the tissue conductivity.8,9 The Radiotherapeutic device is similar to RITA device. The movable hub can deploy 10 needles creating a more spherical heat distribution. This device differently than RITA device, does not have the temperature surveillance at the tips of needles.2,5 The Electrotom 106 HiTT system consists of a single or dual insulated RF needle system with a non-insulated tip where the RF energy applied ranges from 20 to 50 W. At the proximal end, the needle has an electric connector for the RF cable and a luer lock for the rinsing solution. This rinsing solution (isotonic sodium chloride) cools the heated area at the tip to prevent the occurrence of vapour or charring tissue, which could lead to a bad conductivity.
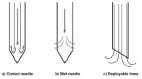
Figure 2
Schematic images of three different RF needle applicators.
Table 4
Characteristics of various RFA devices.
The tumor masses treated are generally of the order of less than 3 cm of diameter. RITA and Radiotherapeutic systems, that have expandable multiple arrays, can reach tumor masses of <5 cm.2,4 With local perfusion of saline solution also with single needle systems tumors larger than 3 cm can be treated. But, with increasing tumor diameter the rate of complete responses is decreasing rapidly. A relation between tumor diameter treated and the achievable local control has been studied when applied to liver tumors (see Table 5).
Table 5
Tumor diameter-tumor control.
Multiple needle applicators for radiofrequency or microwave ablation can increase the coagulation zone enabling a higher rate of complete necrosis. If the multiple needle array is connected as a bipolar module to each other, the densities of the electric fields will be increased resulting in larger coagulation zones. Bipolar needle applicators significantly reduce treatment time and may lead to a reduction in local tumor recurrence.
Liver Cancer
The first experimental treatments with thermal ablation have been tested in patients with primary or metastasized liver tumors (this kind of treatment is elsewhere described in the book). Other tumors than liver tumors have been treated with RFA and MWA devices. Further indications for RFA applicators are tumors of the kidney, adrenal gland, pancreas, breast, thyroid, parathyroid, lymphoma, bone, and osteoma. A brief description of the different tumors treated with RFA follows.
Renal Cell Carcinoma
For this kind of tumor the mainstay treatment is surgery, however when renal conservation is desired RFA provides an opportunity.10 Conservation refers in primis on patients with unresectable tumors, singular kidney, non functioning kidney or bilateral recurrent tumors (von Hippel Lindau syndrome).11 Renal tumors with a component in the renal sinus cannot be completely treated as demonstrated by Gervais.12 Complications are generally not severe and include hemorrhage, ureteral stricture, and thermal injury to psoas muscle. No deaths were reported.12 The results of thermal ablation depend at least from two factors: tumor dimension (≤ 3 cm),13 tumor location14,15 and the power of the generator.16 Rohde et al17 treated with RFA and cryoablation metastatic renal cell carcinoma. They concluded that for patients with low performance status this technique may become an elective and effective method of treatment. For a review of various clinical studies conducted until 2004 see Hines-Peralta18 and Ogan.19
Breast Cancer
The advances on imaging devices have contributed to discover breast tumor masses at its beginning, with the consequence of reducing radical surgical interventions. The standard treatment has been radical mastectomy although actually a less surgical radical approach is used (lumpectomy) followed by radiation therapy. Notwithstanding these earlier interventions a non surgical approach with a less cosmetic defect is desirable. RFA is an ideal treatment at least for masses less than 1.5 cm, with negative sentinel lymph node and it is a useful preoperative antitumoral treatment in advanced breast carcinoma with or without chemotherapy.20,21 In animal models, the approach with chemotherapy, specifically with intratumoral doxorubicin determines and increases response in tumor destruction, compared to RFA alone.22 Studies with other methods (cryotherapy, laser hyperthermia, microwave hyperthermia) have been conducted to eradicate breast tumor. The purpose of these studies is similar as the initial clinical results to the RFA one.23-26
Lung Tumors
Many patients with primary or secondary lung neoplasia are not surgical candidates. Radiotherapy or conformational radiotherapy is a treatment option not devoid of side effects. RFA is gaining acceptance as treatment modality, however heat distribution in lung tissue is irregular and not uniform. The reasons responsible for this no homogeneous heat diffusion are the presence of air filling zones and the large nearby vessels acting as heat sinks. To target the lesion to be treated with RFA, computed tomography or ultrasound are used. Akebosshi et al27 have reported up to 46% of complete response in the treatment of primary lung tumors with minimal toxicity. Patient's pulmonary functions are preserved and the application of other therapies such as radiation or chemotherapy can be avoided. The major complications consist in pleural effusions and pneumothorax. Herrera et al treated 18 patients with inoperable primary lung tumors. They reported similar results and complications.28 Diameter of the lesions greater than 5 cm seems critical as assumed by studies on liver tumors. A recent trial undertaken on 27 patients with non small cell lung cancer (NSCLC) or metastases to lung has clearly demonstrated that the size of the tumor is the major discriminator regarding patient survival and the necrosis obtained by RFA application.29
In another trial not only primary and metastatic tumors were treated but also bone metastases to the ribs or sternum, in order to obtain pain relief. Authors report that this last type of treatment is feasible and avoid of serious complications. Pain palliation is obtainable with this technique and an association to RFA and radiation therapy for larger masses can be used.30 A recent study by Steinke31 on 14 centers around the world, reports that, on 493 RFA procedures performed two deaths occurred, pneumothorax in 30% and 10% of pleural effusion. In this author opinion pulmonary hemorrage is however more frequent than assumed and reached the 5.9 percent.32 Studies in Italy by Gadaleta et al33 confirm the utility of RFA for primary and metastatic lung neoplasm, and in agreement with other authors, they report the following complications after post RFA procedure: 46% patients had moderate fever, 37% pain, 29% pleural effusion and cough, 16% pneumothorx and 12.5% dyspnea. Some authors have also demonstrated the possible use of RFA in combination with surgery.
From these preliminary studies it is possible to conclude that pulmonary RFA appears to be safe, minimally invasive, with negligible mortality, little morbidity, short hospital stay and gain in quality of life.31,32,34
Bone Tumors
Different malignant tumors such as from the breast, prostate, kidney and lung metastasize to bone. Bone metastases are lytic or plastic and associated with intermittent or constant pain. Sometimes fractures and sensimotoric disorders are associated. RFA has been used to treat spinal metastatic lesions, osteoid osteomas, and lytic vertebral metastases.35-37 The results of these groups are at its beginning. In every case the study of Callstrom et al37 reported, beyond a decrease in the pain degree, an objective decrease in analgesics medication. More randomized trials are in every case necessary, as outlined by Wood.38 The only conclusions, in agreement with animal experiments,39 that can be retained are: RFA application on bone metastases is a safe and potentially successful therapy. Pain relief seems fast and persistent, as demonstrated by Poggi et al.40
Miscellaneous Tumors Treated with RFA
RFA application on different other tumors are in progress and can be only pointed out. Wood41,42 treated with his group adrenal and splenic metastases. In all the two cases an effective treatment was reproted. Pascella43 et al in Italy applied the technique of RFA to thyroid tissue. The treatment caused a decrease in dysphonia and symptoms from tumor-associated compression. However, the authors outlined leave also that it is hazardous to attempt a complete necrosis of the lesions because of proximity of vital surrounding structures. Resection was considered the gold standard of treatment for pancreatic tumor, unlikely most patients are at far advanced stage and cannot be cured. Different chemotherapeutic regimens, not avoid of severe toxicity are used. Actually application of RFA to primitive and metastatic pancreatic tumors has been performed and found a useful tool.44,45 Ghezzi et al demonstrated that RFA ablation of uterine fibroids during laparoscopy is feasible and safe.46 Furthermore, RFA potentially provides a safer option for removing prostate tumors and for treating brain tumors.
Perfusional Treatment
Introduction
The metastatic process is a multistep event and represents the most dreadful aspect of cancer. At the moment of diagnosis, cancers are relatively far advanced in their natural history and the presence of metastases is a common event. In fact, approximately 30% of patients will have detectable metastases at the moment of clinical diagnosis and a further 30% of patients will have occult metastases. Metastases can be disseminated and they can interest different organs at the same time, or can be localized to a specific organ.47 In the case of localized disease, surgery is the treatment of choice, however recurrence and prognosis depend on many criteria such as: resectability, patients clinical situation, and number of metastases.
After resection, recurrence is common suggesting that micrometastatic foci are present already at the moment of diagnosis. Chemotherapy is an ideal setting but only a few patients will be cured by it but in the majority systemic chemotherapy fails. Many physiological barriers and pharmacokinetics parameters48-50 contribute to decrease its efficacy (see Table 6).
Table 6
Physical and pharmacokinetic barriers to drug delivery.
Systemic chemotherapy compared to regional chemotherapy has limited benefits. Regional chemotherapy consists in the isolation of an anatomical region and in treating this by using chemotherapy at high doses with absence or minimum systemic toxicity.51 Typical indications of regional chemotherapy are limbs, lung, liver, pleura, and pancreas. The method is common to all organs and consists in different sequential steps. The first step is the surgical isolation of the organ; the second is to keep the organ perfused. Oversimplifying the perfusion is kept by a circuit that consists of an out and inflow placed catheters, tubing, a roller pump, a reservoir, a heat exchanger and an oxygenator.52 A typical device with its circuitry is described in Figure 3A,B. Hyperthermia is applied to increase the sensitivity of tumor cells to antineoplastic agents to kill even more tumor cells and lowering the recurrence.53,54 The biological and physiological reasons for the combination of chemotherapy with hyperthermia are described elsewhere in this book. Aside from limbs, for which isolated chemohyperthermia is now an accepted treatment, the organs actually treated with perfusion chemohyperthermia are: lung, pleura and liver.
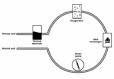
Figure 3A
Example of circuit scheme of isolated perfusion.

Figure 3B
Example of device (Perfomer Rand, Modena, Italy) for regional therapy in which venous reservoir, oxygenator, heat exchanger, roller pump and temperature detectors are included. (Reprinted with permission from Rand, Modena, Italy.)
Clinical Applications
Isolated Lung Perfusion Chemohyperthermia (ILUPH)
The lung is the most common site of metastatic involvement beside of lymph nodes for all cancer types. Lung metastases occur in 50% of patients with cancer diagnosis.55 Retroprospective studies have demonstrated that surgical removal, with an aggressive approach in selected patients, is the treatment of choice. However some technical and clinical limitations exist. Patients with unresectable lung metastases are candidates for Isolated Lung Perfusion Chemotherapy.56 This implies, as described above, a more efficient drug delivery to lung tissue. Different animals studies have been conducted demonstrating a superiority of perfusion technique compared to systemic chemotherapy. These authors determined that this kind of chemotherapy delivery method is reproducible and safe.56-59 The perfusion technique of these authors included the isolation of the two lungs Jacobs in the 1961 ameliorated the technique isolating the single lung. In the dogs he demonstrated the feasibility and the safety of the surgical technique.60 Pierpont59 and Jacobs60 demonstrated also, by using radioisotopes that no leakage of the drug was present outside the pulmonary circulation. In animal studies the method of Jacobs is still used today. Clinical studies were initiated by Creech52 and have been continued by different authors.53 The Johnston and Ratto groups61,62 used cisplatinum and demonstrated an high concentration of the drug inside the diseased lung. Other authors used or are using other antineoplastic agents such as: melphalan,56 Doxorubicin63 and TNF-α.64
Technical Aspects
Different post-mortem studies have demonstrated that primitive lung tumors and lung metastases are supplied by bronchial arterial circulation.65,66 Furthermore primary lung tumors show differences depending on the tumor zone supplied. The inner part is generally supplied by bronchial artery whereas its peripheral part is supplied by pulmonary arteries.66 Because pulmonary arterial circulation, in a normal person, drains exclusively through the pulmonary veins, this permits a complete isolation of the lungs and a selective perfusion.66,67 At the moment we can distinguish three methods of regional lung perfusion (RLP) with: (1) inflow Pulmonary artery occlusion;68 (2) Bronchial Artery infusion;69 (3) no atrial appendage cannulation.70 The first method consists of an unilateral perfusion with occlusion of the right or left pulmonary artery, using a balloon-tipped cardiac catheter, inserted percutaneously and radiographically positioned. After the occlusion of the pulmonary arteries chemotherapy is infused distal to the balloon. The second technique consists of the infusion of antineoplastic agents through the bronchial artery and is performed by isolating the aorta from which bronchial arteries arose using a double-balloon catheter. Compared to the 1st technique this method permits to treat both lungs simultaneously. The third method is a technique of single isolated lung perfusion that is performed by cannulating the pulmonary artery and vein, instead of the left atrial appendage. The bronchial arteries were clipped temporarily to decrease leakage and toxicity of chemotherapeutic drugs. Another procedure is the ‘Isolated Thoracic Perfusion’ (ITP) which is the most used actually. ITP consists of the insertion of two balloon catheters under X-ray control , one in the aorta, just above the celiac axis, and the other in to the inferior vena cava below the right atrium. In order to reduce the perfusion volume, Esmarch bandages are placed around the roots of both arms.71
Side Effects
Ratto et al62 also studied the pulmonary function after 90 days from the perfusion. They demonstrated that forced vital capacity (FVC) decreased approximately 25% as the forced expired volume (FEV1). Furthermore a decreased of 20% in CO2 diffusion capacity was shown with no significant change in arterial PO2 and PCO2. Johnston reported that the use of doxorubicin (DOX) at 40 mg/m2 was safe and not associated with haematological, gastrointestinal or cardiac toxicity. Data at 8 weeks post perfusion showed no statistical significant difference on cardiac ejection fraction, on FEv1 and DLCO.61 All the authors have reported that drug leakage was minimal as well as the toxicity to normal tissues.53,61,62,71,72
Pleura Perfusion Treatment
Metastatic pleura effusion is frequently associated with the terminal stage of gastric, lung and breast cancer. The pleura is also the site of insurgence of rare primitive tumors such as mesothelioma. Mesothelioma diagnosis is generally difficult to obtain and its incidence is increasing in Western world. About 5% of patients have bilateral disease and 50% have distant metastases. Their survival at 5 years is less than 5% and their quality of life is poor. Today a standard treatment for mesothelioma does not exist.73 Many treatment programmes combining surgery, chemotherapy, radiotherapy and immunotherapy have been used with more or less success. Radiotherapy has more a role on palliation and the response to chemotherapy is very low (< 20%). The highest response rate regards doxorubicin, mitomycin and ifosfamide. Biological response modifiers such as IL-2 and interferon have been associated with an objective response for patient at early stage.74,75 Tumor localized to the pleura offers a local treatment with anticancer drugs. This treatment method has advantages: the tumor is directly exposed to a high drug concentration and the toxic side effects are limited. However as learned by peritoneal perfusion treatment, prerequisites for effective intracavitary chemotherapy are the absence of tumor outside the cavity and the surgical remove of all macroscopic tumor, as drug penetration by diffusion is limited to a few millimetres. Furthermore, the synergistic effect of heat and certain drugs like mitomicyn and cisplatin, have induced many groups to treat mesothelioma and metastases confined to pleura with intrathoracic chemohyperthermia.76,77
Technical Aspects
The treatment method consists in two phases: the surgical one (thoractomy) followed by intrapleural perfusion thermochemotherapy (PPTC).76,78 The PPTC is however also possible without thoractomy79 or performed under video-assisted thorascopic surgery (VATS).80 The group of Matsuzaki78 has demonstrated that a significant difference exists between the group treated with surgery followed by intrapleural perfusion thermochemotherapy and the group treated by surgery alone. The group treated only by (PPTC) without previous surgery had not advantage compared to group treated by surgery alone. Surgical procedure is done in lateral position, under general and thoracic epidural anesthesia and with a double—lumen endotracheal tube position. A posto lateral thoractomy through the fifth intercostals space is performed. Extrapleural dissection follows, subsequently the thickened pleural sheets are removed and separated by the underlying healthy tissue (pleura or lung parenchyma). After decortication if lung is damaged, a pneumectomy is performed. Small tumor nodules on diaphragm and pericardium are eliminated by coagulation, whereas larger masses are partially or completely resected.76,78,80 After cytoreduction pleural perfusion thermochemotherapy (PPTC) is carried out keeping .the patient in posterolateral position . A typical example of circuit used to treat pleura with (PPTC) is illustrated in Figure 4. Generally the lung, underlying the treated pleura, is kept collapsed or partially inflated as the controlateral lung is ventilated. The lung is kept collapsed to allow sufficient space between parietal and visceral pleura to adequate perfusion and to limit toxicity to lung. As shown from the circuit an inflow and outflow catheters are placed associated to silicone tubes for temperature measurement in the pleura and in the proximal oesophagus. The inflow outflow catheters are then connected to the roller pump the heat exchanger and the reservoir. Sometimes outflow liquid is filtered permitting the escalation of the total regional drug dose. The drugs used are cisplatin [CDDP] (50-80 mg/m2), adriamycin (15-25 mg/m2) or mitomycin [MMC] (0.7 mg/Kg; maximum dose 60 mg), sometimes MMC ands CDDP are associated. The drugs are diluted in a sterile perfusate (4L) that is propelled by the roller pump in a closed circuit at the rate of 200 cm3 min-1. The temperature is kept between 40-42°C for 60-90 min. Every 30 min during PPTC different blood samples are measured. The temperature is monitored every 10 min.76,78-80
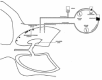
Figure 4
Methods for treating pleural cavity.
The second method of PPTC, not associated to thoracotomy, the so called video-assisted thorascopic surgery (VATS) is performed similarly, with the difference that no surgical resection of primary or secondary tumors is performed. This last method is however more palliative than the thoractomy combined with hyperthermia.80
Side Effects
All the authors have reported no major complications to lungs beside of wound infection or diaphragmatic prosthesis displacement. The overall procedure was completed without any death or toxicity.76,78-80
Liver Isolated Perfusion
Liver, lungs and lymph nodes are filter organs and therefore inclined to metastasization. The poor chemonsensitiviity of metastases, peculiarly those of colorectal origin has forced many researchers to use methods for increasing the time and the concentration of drugs. The need for decreasing or limiting the side effects for this important and delicate organ, carried Aigner et al in 1981 to perform liver isolation for perfusion of antineoplastic agents.81 Since 1981, modifications and technical improvements have been continuously introduced by Aigner's group and by other investigators.81-83 Liver metastases may be of different origin and their chemonsensitivity may vary according to the histological type and their response in presence of heat.
Technical Aspects
Different surgical methods of liver isolation have been tested in both animals and patients.81-83 Actually with different variations the methods can be classified as:
- Surgical hepatic isolation.
- Percutaneous isolated hepatic perfusion.
Procedure A: Surgical Hepatic Isolation
As first step an external extracorporal veno-venous bypass is established by placing a catheter in the saphenous vein, advanced into the infrarenal inferior vena cava, and a second cannula inserted through the axillary vein until the superior vena cava. The second step is the shunting of portal venous blood flow by placing a venous cannula into the mesenteric vein and incorporating this into the veno-venous bypass. The third step is the position of inflow cannula in the gastruodenal artery followed by a clamping of the common hepatic artery and of suprahepatic artery. After this positioning, the liver is isolated and ready to be perfused. For a schematic vision of the perfusion circuit (see fig. 5 )81,83,84 The perfusate is kept for 1 h at temperatures between 39.5°C and 40°C. Generally temperature is measured in the liver parenchyma using a Swan-Ganz catheter inserted into the portal vein. Three kinds of chemotherapeutic drugs are used: (a) melphalan, (b) mitomycin-C and (c) Tumor necrosis factor (TNF-α). All in different combinations are used to treat colorectal metastases or metastases of other origin such as malignant melanoma.81,82,83,85-89
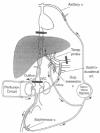
Figure 5
Isolated hepatic perfusion (IHP) circuit scheme. Reproduced with permission from: Grover A, Alexander HR. The past decade of experience with isolated Hepatic perfusion. The Oncologist 2004; 9:653-664.
Procedure B: Percutaneous Isolated Hepatic Perfusion
Surgical procedure is complex and cannot be repeated. This drawback limits the results of this technique, and has forced many researchers to develop a simpler and repetitive procedure. The developed method is a partial isolation of the liver (isolated hepatic perfusion (IHP)) by closing the inferior caval vein above and below the hepatic veins using a four-lumen/two-balloon catheter. This positioning permits to collect blood from hepatic veins diverting it to a charcoal filter before it returns to patient's systemic circulation. Drugs are administred via a percutaneously placed catheter in the femoral artery (see fig. 6).84,85
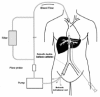
Figure 6
Percutaneous hepatic perfusion circuit scheme reproduced and modified with permission from: Grover A, Alexander HR. The past decade of experience with isolated hepatic perfusion. The Oncologist 2004; 9:653-664.
Since 1993 358 patients, with hepatic metastases, were treated with IHP. The results of these clinical trials have been recently reviewed by Groover and Alexander.85 On 15 trials reported, the majority have been conducted with melphalan alone or in association with cisplatin or TNF-α, 12 of them with metastases from colorectal cancer, 3 malignant melanoma and five Mixed histology.85-87,89 Some authors used mitomicyn-C for the treatment of colorectal metastases for the known synergistic effect with hyperthermia.88 A 41% of partial response has been obtained with this procedure and in 6-9% of complete response. Ocular melanoma has the greater percent of complete and partial response compared to liver metastases of other origin. The median survival time has been ≥ 10 months.
Side Effects
With the surgical procedure only a transient elevation of hepatic enzymes and bilirubin has been noted. However some fatal deaths for total liver failure and grade 4 leukopenia have been described. The mortality in the majority of the studies was of the order of 5%. An elevation of hepatic enzymes beyond 7 days after the perfusion procedure itself can be considered as melphalan related.85,86
Conclusions
Radio frequency thermoablation (RFA) applied under sonographic guidance has certain features which may make it a first choice in the treatment of certain hepatocellular carcinoma (HCC) and make it a promising method for the curative and palliative treatment of secondary tumors of the liver. Randomized controlled trials have been performed for HCC but not yet for secondary liver tumors like from colorectal or breast cancer. Actually, the various procedures of perfusion, herein described are at their beginning. To our opinion RFA procedures have a greater future compared to some isolated procedures. RFA techniques are easier to learn and to be performed. They can be performed by few qualified persons, whereas perfusion procedures need a skilled and coordinated team. For perfusion chemotherapy less invasive methods have to be developed like the percutaneous intraperitoneal hyperthermic chemoperfusion or the percutaneous isolated hepatic perfusion, which in turn has to be compared with local chemoembolisation. Further randomized controlled trials are warranted.
References
- 1.
- Sackenheim MMCD. Radiofrequency Ablation The Key to cancer treatment. JDMS. 2003;19:88–92.
- 2.
- Hanson PS, Soulen MC. Tumor ablation: a review of technique and outcomes. Applied Radiology. 2001
- 3.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal Ablation Therapy for focal malignancy. A unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR. 2000;174:323–331. [PubMed: 10658699]
- 4.
- Nazir B, Chaturvedi AK, Rao A. Image-guided radiofrequency ablation of tumors: current status. Ind J Imag. 2003;13:315–322.
- 5.
- Neeman Z, Wood BJ. Radiofrequency ablation beyond the liver: Techniques in vascular and Interventional radiology. 2002. pp. 156–163. [PMC free article: PMC2408945] [PubMed: 12524646]
- 6.
- Goldberg SN, Gazelle GS, Solbiati L. et al. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3(8):636–644. [PubMed: 8796727]
- 7.
- deBaere T, Denys A, Wood BJ. et al. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. Am J Roentgenol. 2001;176:187–192. [PubMed: 11133564]
- 8.
- Solbiati L, Goldberg N, Ierace T. et al. Hepatic metastases: Percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–373. [PubMed: 9356616]
- 9.
- Livraghi T, Goldberg SN, Monti F. et al. Saline-enhanced radiofrequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205–210. [PubMed: 8988212]
- 10.
- Fiske H. Radiofrequency ablation takes aim at kidney tumors. Radiology Today. 2002;19-22
- 11.
- Jacomides L, Ogan K, Watumill L. et al. Laparoscopic application of radio frequency energy enables in situ renal tumor ablation and partial nephrectomy. J Urol. 2003;169:49–53. [PubMed: 12478100]
- 12.
- Gervais DA, McGovern FJ, Arellano RS. et al. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424. [PubMed: 12563135]
- 13.
- Pavlovich CP, McClellan MW, Choyke PL. et al. Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol. 2002;167:10–15. [PMC free article: PMC4136670] [PubMed: 11743264]
- 14.
- Michaels MJ, Rhee HK, Mourtzinos AP. et al. Incomplete renal destruction using radio frequency interstitial ablation. J Urol. 2002;168:2406–2410. [PubMed: 12441927]
- 15.
- Corwin TS, Lindberg G, Traxer O. et al. Laparoscopic radiofrequency thermal ablation of renal tissue with and without hilar occlusion. J Urol. 2001;166:281–284. [PubMed: 11435886]
- 16.
- Gettman MT, Lotan Y, Corwin TS. et al. Radiofrequency coagulation of renal parenchyma: comparison of effects of energy generators on treatment efficacy. J Endourol. 2002;16:83–88. [PubMed: 11962560]
- 17.
- Rohde I, Alberts C, Mahnken A. et al. Regional thermoablation of local and metastatic renal cell carcinoma. Oncology reports. 2003;10:753–757. [PubMed: 12684654]
- 18.
- Hines-Peralta A, Goldberg SN. Review of radiofrequency ablation for renal cell carcinoma. Clin Cancer Res. 2004;10:6328s–6334s. [PubMed: 15448026]
- 19.
- Ogan K, Jacomides L, Dolmatch B. et al. Percutaneous radiofrequency ablation of renal tumors: technique, limitations, and morbidity. Urology. 2002;60:954–958. [PubMed: 12475648]
- 20.
- Fujimoto , Kobayashi K, Takahasashi M. et al. Clinical pilot studies on preoperative hyperthermic tumour ablation for advanced breast carcinoma using an 8 MHz radiofrequency heating. Int J Hyperthermia. 2003;19:13–22. [PubMed: 12519708]
- 21.
- BuraK WE, Agnese DM, Povoski SP. et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer. 2003;98:1369–1376. [PubMed: 14508822]
- 22.
- Goldberg SN, Saldinger PF, Gazelle et al. Percutaneous tumor ablation: increased necrosis with combined Radiofrequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology. 2001;220:420–427. [PubMed: 11477246]
- 23.
- Robinson D, Parel JM, Denham DB. et al. Interstitial Laser Hyperthermia model development for minimally invasive therapy of breast carcinoma. J Am Coll Surg. 1998;186: 284–292. [PubMed: 9510259]
- 24.
- Morin J, Traoré A, Guy D. et al. Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Can J Surg. 2004;47:347–351. [PMC free article: PMC3211947] [PubMed: 15540687]
- 25.
- Gardner RA, Vergas HI, Block JB. et al. Focused microwave phased array thermotherapy for primary breast cancer. Ann Surg Oncol. 2002;9:326–332. [PubMed: 11986183]
- 26.
- Akimov A, Seregin VE, Rusanov KV. et al. Nd:YAG interstitial laser thermometherapy in the treatment of breast cancer. Lasers Surg Med. 1998;22: 257–267. [PubMed: 9671991]
- 27.
- Akeboshi M, Yamakado K. Percutaneous radiofrequency ablation of lung neoplasms: initial therapeutic response. J Vasc Interv Radiol. 2004;15:463–470. [PubMed: 15126656]
- 28.
- Herrera L, Fernando HC, Perry Y. Radiofrequency ablation of pulmonary malignant tumors in non surgiucal candidates. J Thor Cardiovasc Sur. 2003;125:929–937. [PubMed: 12698158]
- 29.
- Lee JM, Jin GY, Golderg SN. et al. Percutaneously radiofrequency ablation for inoperable non-small cell lung cancer and metastases:preliminary report. Radiology. 2004;230:125–134. [PubMed: 14645875]
- 30.
- Dupuy DE, Mayo-Smith WW, Abbot GF. Clinical applications of radiofrequency tumor ablation in the thorax. Radiographics. 2002;22:s59–s69. [PubMed: 12376615]
- 31.
- Steinke K, Sewell PE, Dupuy D. et al. Pulmonary radiofrequency ablation---an international study survey. Anticancer Research. 2004;24:339–344. [PubMed: 15015618]
- 32.
- Steinke K, King J, Glenn Detal. Pulmonary hemorrage during Percutaneous radiofrequency ablation: a more frequent complications than assumed. Interactive Cardiovascular Thoracic Surgery. 2003;2:462–465. [PubMed: 17670096]
- 33.
- Gadaleta C, Mattioli V, Colucci G. et al. Radiofrequency ablation of 40 lung neoplasms: Preliminary results. Am J Roentgenol. 2004;183:361–368. [PubMed: 15269026]
- 34.
- van SonnenbergE, Shankar S, Morrison PR. et al. Radiofrequency ablation of thoracic lesions: part2, Initial clinical experience. Technical and multidisciplinary considerations in 30 patients. Am J Roentgenol. 2005;184:381–390. [PubMed: 15671350]
- 35.
- Groenemeyer DHW, Schirp S, Gevargez A. Image-guided Percutaneous thermal ablation of bone tumors. Acad Radiol. 2002;9:467–477. [PubMed: 11942662]
- 36.
- Groenemeyer DHW, Gevargez A, Schirp S. Image-giuided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002;8:33–39. [PubMed: 11898806]
- 37.
- Callstrom MR, Charboneau JW, Goetz MP. et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224:87–97. [PubMed: 12091666]
- 38.
- Wood JB. Feasibility of thermal ablation of lytic vertebral metastases with radiofrequency current. A commentary. Cancer J. 2002;8:26–28. [PMC free article: PMC2408949] [PubMed: 11895200]
- 39.
- Letcher FS, Goldring S. The effect of radiofrequency current and heat on peripheral nerve action potential in the cat. J Neurosurg. 1968;29:42–47. [PubMed: 5674091]
- 40.
- Poggi G, Melazzini M, Bernardo G. et al. Percutaneous ultrasound-guided radiofrequency thermal ablation of malignant osteolysis. Anticancer Res. 2003;23:4977–4983. [PubMed: 14981955]
- 41.
- Wood JB, Abraham J, Hzizda J. Radiofrequency ablation of adrenal tumors and adrenocortical cxarcinoma metastases. Cancer. 2003;97:54–60. [PMC free article: PMC2443414] [PubMed: 12548596]
- 42.
- Wood JB, Bates S. Radiofrequency thermal ablation of a splenic metastasis. J Vasc Interv Radiol. 2001;12:261–263. [PMC free article: PMC2375914] [PubMed: 11265893]
- 43.
- Pacella CM, Bizzari GC, Spiezia S. et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology. 2004;280:272–280. [PubMed: 15155898]
- 44.
- Elias D, Baton O, Sideris L. et al. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumors. Eur J Surg Oncol. 2004;30:85–87. [PubMed: 14736529]
- 45.
- Radiofrequency tissue ablation: an early Indian experience. Indian J Gastroeril. 2003;22:91–93. [PubMed: 12839380]
- 46.
- Ghezzi. et al. Am J Obstet Gynecol. 2005;192:768–773. [PubMed: 15746670]
- 47.
- Liotta L, Kohn EC. Invasion and metastases. In Cancer Medicine 6 Edition. BC Decker. Kufe, Pollock, Weichselbaum, Bast, Gansler, Holland, and Frei, editors.2003:151–159.
- 48.
- Munn LL. Aberrant vascular architecture in tumors and its importance in drug based therapies. Drug Discovery Today. 2003;8:396–403. [PubMed: 12706657]
- 49.
- Curti BD. Physical barriers to drug delivery in tumors. Critical Reviews in Oncology/Hematology. 1993;14:29–39. [PubMed: 8373539]
- 50.
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Ann Rev Biomed Eng. 1999;01:241–263. [PubMed: 11701489]
- 51.
- Aigner KR. Regional chemotherapy [editorial review article] Reg Cancer Treat. 1994;2:55–66.
- 52.
- Creech OJ, Krementz ET, Ryan RF. et al. Chemotherapy of cancer: Regional perfusion utilizying an extracorporeal circuit. Ann Surg. 1958;148:616–632. [PMC free article: PMC1450870] [PubMed: 13583933]
- 53.
- Weksler B, Burt M. Isolated lung perfusion with antineoplastic agents for pulmonary metastases. Chest Surgery Clinics of North America. 1998;8:157–183. [PubMed: 9515180]
- 54.
- Stehlin I. Hyperthermia perfusion with chemotherapy for cancer of extremities. Surg Gynecol Obstet. 1969;129:305–308. [PubMed: 5815841]
- 55.
- Van Schil PE. Surgical treatment for pulmonary metastases. Acta Clinica Belgica. 2002;57:333–339. [PubMed: 12723252]
- 56.
- Van Putte BP, Hendricks JMH, Romijin S. et al. Isolated lung perfusion for the treatment of pulmonary metastases current mini-review of work in progress. Surgical Oncology. 2003;12:187–193. [PubMed: 12957622]
- 57.
- Ellis JL, Ng B, Port J. et al. Isolated lung perfusion with carboplatin for metastatic sarcoma in the F344 rat. Surgical Forum. 1994;294:–295.
- 58.
- Weksler B, Lenert J, Ng B. et al. Isolated single lung perfusion with doxorubicin is effective in eradicating soft tissue sarcoma lung metastases in rat model. J Thorac Cardiovasc Surg. 1994;107: 50–54. [PubMed: 8283918]
- 59.
- Pierpont H, Blades B. Lung perfusion with chemotherapeutic agents. J Thorac Cardiovasc Surg. 1960;39:159–165. [PubMed: 14432855]
- 60.
- Jacobs JK, Flexner JM, Scott HWJ. Selective isolated perfusion of right or left lung. J Thorac Cardiovasc Surg. 1961;42:546–552. [PubMed: 14450680]
- 61.
- Johnston MR, Minchen RF, Dawson CA. Lung perfusion with chemotherapy in patients with unresectable metastatic sarcoma to the lung or diffuse bronchioalveolar carcinoma. J Thorac Cardiovasc Surg. 1995;110:368–373. [PubMed: 7637354]
- 62.
- Ratto GB, Toma S, Civalleri D. et al. Isolated lung perfusion with platinum in the treatment of pulmonary metastases from soft tissue sarcomas. J Thorac Cardiovasc Surg. 1996;112:614–622. [PubMed: 8800147]
- 63.
- Burt ME, Liu D, Abolhoda A. et al. Isolated lung perfusion for patients with unresectable metastases from sarcoma: a phase I trial. Ann Thorac Surg. 2000;69:1542–1549. [PubMed: 10881839]
- 64.
- Pass HI, Mew DJY, Kranda KC. et al. Isolated Lung perfusion with tumor necrosis factor for pulmonary metastases. Ann Thorac Surg. 1996;61:1609–1617. [PubMed: 8651757]
- 65.
- Miller BJ, Rosenbaum AS. The vascular supply to metastatic tumors of the lung. Surg Gynecol Obstet. 1967;125:1009–1115. [PubMed: 6075076]
- 66.
- Milne ENC. Circulation of primary and metastatic pulmonary neoplasm: a post-mortem microarteriographic study. AJR. 1967;100:603–619. [PubMed: 5230250]
- 67.
- Pass HI. Isolated lung perfusion for pulmonary metastasesIn: Lotze MT, Rubin TJ, eds.Regional therapy of advanced cancerNew York: Lippincott-Raven Press, Philadelphia,199763–73.
- 68.
- Smyth NP, Blades B. Selective chemotherapy of lung during unilateral pulmonary arterial occlusion with a balloon-tipped catheter. J Thorac Cardiovasc Surg. 1960;40:653–666.
- 69.
- Cliffton EE, Mahajan DR. Technique for visualization and perfusion of bronchial arteries: suggested clinical and diagnostic applications. Cancer. 1963;16:444–452. [PubMed: 14021748]
- 70.
- Liu D, Burt M, Ginsberg RJ. Lung perfusion for treatment of metastatic sarcoma to the lungsIn: Markman M, ed.Current Clinical Oncology: Regional chemotherapy: clinical research and practiceNew Jersey: Humana Press Inc.,200087–99.
- 71.
- Guadagni S, Müller H, Valenti M. et al. Thoracic stop-flow perfusion in the treatment of refractory non small Cell lung cancer. J Chemotherapy. 2004;16s:40s–43s. [PubMed: 15675476]
- 72.
- Müller H, Guadagni S. Regional plus systemic chemotherapy: an effective treatment in recurrent non-small cell lung cancer. EJSO. 2001;27:190–195. [PubMed: 11289757]
- 73.
- American Thoracic Society Management of malignant pleural effusions. Am J Resp Care Med. 2000:1987–2001. [PubMed: 11069845]
- 74.
- Astoul PH, Viallat JR, Laurent JC. et al. Intrapleural recombinant Il-2 in passive immunotherapy for malignant pleural effusion. Chest. 1993;103:209–213. [PubMed: 8417880]
- 75.
- Rosso R, Rimoldi R, Salvatri F. Intrapleural beta interferon in the treatment of malignant pleural effusions. Oncology. 1988;45:253–256. [PubMed: 2453008]
- 76.
- Sugarbacher DJ, Jacklitsch MT, Soutter AD. et al. Multimodality therapy of malignant mesotheliomaIn: Roth JA, Ruckdeschel JC, Weisenburger TH, eds.Thoracic Oncology. 2nd edPhiladelphia: WB Saunders Co.,1996538–555.
- 77.
- Pass HI, Robinson BW, Testa J. et al. Emerging translational therapies for mesothelioma. Chest. 1999;116s:455s–460s. [PubMed: 10619507]
- 78.
- Monneuse O, Beaujard AC, Guibert B. et al. Long-term result of intrathoracic chemohyperthermia (ITCH) for the treatment of pleural malignancies. Br J Cancer. 2003;88:1839–1843. [PMC free article: PMC2741113] [PubMed: 12799624]
- 79.
- Matsuzaki Y, Shibata K, Yoshioka M. et al. Intrapleural perfusion hyperthermo-chemotherapy for malignant pleural dissemination and effusion. Ann Thorac Surg. 1995:127–131. [PubMed: 7818311]
- 80.
- Shigemura N, Akashi A, Mitsumori O. et al. Combined surgery of intrapleural perfusion hyperthermic chemotherapy and panpleuroonectomy for lung cancer with advanced pleural spread: a pilot study. Interactive Cardiovascular and Thoracic Surgery. 2003;2:671–675. [PubMed: 17670154]
- 81.
- Aigner KR. Isolated liver perfusionIn: Morris DL, McArdle CS, Onik GM, eds.Hepatic MetastasesOxford: Butterworth Heinemann,1996101–107.
- 82.
- Rothbarth J, Tollenar RAEM, Schellen JHM. et al. Isolated hepatic perfusion for the treatment of colorectal metastases confined to the liver: recent trends and perspectives. European J of Cancer. 2004;40:1812–1824. [PubMed: 15288282]
- 83.
- Oldhafer KJ, Lang H, Frerker M. et al. First experience and technical aspects of isolated liver perfusion for extensive liver metastases. Surgery. 1998;123:622–631. [PubMed: 9626312]
- 84.
- Carroll NM, Alexander HR. Isolation perfusion of the liver. Cancer J. 2002;8:181–193. [PubMed: 12004803]
- 85.
- Grover A, Alexander HR. The past decade of experience with isolated hepatic perfusion. The Oncologist. 2004;9:653–664. [PubMed: 15561809]
- 86.
- Vahrmeijer AL, vanDierendonck JK, Keizer HJ. et al. Increased local cytostatic drug exposure by isolated hepatic perfusion: a phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer. 2000;82:1536–1546. [PMC free article: PMC2363396] [PubMed: 10789721]
- 87.
- Lindenér P, Fjälling M, Hafström L. et al. Isolated hepatic perfusion with extracorporeal oxygenation using hyperthermia, tumor necrosis factor alpha and melphalan. European J of Surgical Oncology. 1999;25:179–185. [PubMed: 10218462]
- 88.
- Marinelli A, dE Brau LM, Beerman H. et al. Isolated liver perfusion with mitomycin-c in the treatment of colorectal cancer metastases to the liver. Jpn J Clin Oncol. 1996;26:341–350. [PubMed: 8895675]
- 89.
- Alexander HR, Libutti SK, Bartlett DL. et al. A phase I-II study of isolated hepating perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clinical Cancer Research. 2000;6:3062–3070. [PubMed: 10955785]
- Future Perspectives of Interstitial and Perfusional Hyperthermia - Madame Curie ...Future Perspectives of Interstitial and Perfusional Hyperthermia - Madame Curie Bioscience Database
- α1-Microglobulin - Madame Curie Bioscience Databaseα1-Microglobulin - Madame Curie Bioscience Database
- Macroautophagy in Mammalian Cells - Madame Curie Bioscience DatabaseMacroautophagy in Mammalian Cells - Madame Curie Bioscience Database
- Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience...Regulation of Phagocytosis by FcγRIIb and Phosphatases - Madame Curie Bioscience Database
- Inflammatory Myopathies: Dermatomyositis, Polymyositis and Inclusion Body Myosit...Inflammatory Myopathies: Dermatomyositis, Polymyositis and Inclusion Body Myositis - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...
