ATP binding cassette (ABC)-containing drug efflux transporters play important roles in regulating intracellular drug concentrations that determine cell sensitivity to chemotherapeutic agents. Of particular relevance to cancer chemotherapy are the transporters P-glycoprotein (Pgp) encoded by multidrug resistance 1 gene, multidrug resistance protein (MRP), and breast cancer resistance protein (BCRP). More than 80% of currently used antitumor agents can be transported by these three transporters, and overexpression of these transporters renders multidrug resistance to a broad spectrum of antitumor agents. Elevated expression of these transporters is frequently found in breast cancers and correlations with elevated expression of Pgp or MRP1 to chemotherapeutic outcomes have been observed in some cases, suggesting that these transporters may contribute to chemoresistance in breast cancers. However, attempts to modulate the activities of these transporters using reversal agents have met with limited success. Future studies should focus on better understanding of the upregulation mechanisms of ABC transporter genes in breast cancers, and of the pharmacologic mechanisms of transporter-reversal agent interactions. These studies may lead to novel strategies for improving chemotherapeutic efficacies through targeted interventions of these ABC transporters.
Introduction
Breast cancer is a major health threat to women worldwide. One in every ten new cancers diagnosed each year is female breast cancer. It is also the principal cause of cancer-related death in women.1 In United States alone, breast cancer is estimated to account for 32% (215,900) of all new cancer cases among women in year 2004, making breast cancer the leader among the 10 top cancer types.2 Primary breast cancers without distant spread are highly curable with local or regional treatment. However, most women with primary breast cancer have subclinical metastases and eventually develop distant metastases that complicates the curability of the disease.
Over the past several decades, breast cancer survival rates have significantly improved.3 While many factors are credited, including the development of early detection methods, this improvement can be attributed to the development of new treatment modalities and new drugs. Regimens based on anthracyclines (doxorubicin, daunomycin, and epirubicin)4 and taxanes (paclitaxel and docetaxel)5,6 are the most frequently used combination therapy for breast cancers. However, the response rates remain suboptimal. Moreover, few effective therapeutic regimens are available to treat those had been exposed to anthracyclines or failed to anthracycline treatments.7,8 These observations underscore the importance of multidrug resistance in breast cancer chemotherapy.
One important strategy by which cancer cells acquire drug resistance is the overexpressing drug transporters through which intracellular drug contents reduce to sublethal levels. Of particular importance are ATP-binding cassette (ABC) transporters.
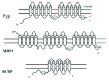
Forty-eight ABC proteins, grouped into seven subfamilies ranging from A to G, are encoded by the human genome (see http://nutrigene.4t.com/humanabc.htm), but only about a dozen are associated with resistance to chemotherapeutic agents. The first ABC transporter known to be associated with multidrug resistance to chemotherapeutic agents was identified about three decades ago as P-glycoprotein (MDR1/Pgp, ABCB1).9 The later realization that MDR1/Pgp alone could not account for all the MDR in many independently established multidrug resistance cells led to the discoveries of other drug resistance-related transporters, notably multidrug resistance (-acssociated) protein (MRP1, ABCC1)10 and breast cancer resistance protein (BCRP, ABCG2).11 These ABC transporters contain multiple transmembrane domains (TMD) and intracellularly localized nucleotide-binding domains (NBD) (fig. 1). These transporters function as efflux pumps by eliminating a diverse array of structurally dissimilar compounds. Because many antitumor agents used in current breast cancer chemotherapy are substrates of these ABC transporters and because these ABC transporters are frequently overexpressed in breast cancers, it is relevant to discuss their roles in breast cancer chemoresistance. Because of space limitation, this review can only briefly describes MDR1, MRP1 and BCRP, and evaluates their roles in breast cancer.
General Descriptions of MDR1/Pgp, MRP1, and BRCP
MDR1/Pgp
The MDR1-encoded Pgp is responsible for multidrug resistance in cultured cells exposed to antitumor agents, including doxorubicin, vincristine, and taxanes, etoposide, teniposide, Actinomycin D.12-14 Many of these agents are used to treat breast cancers. Although structurally dissimilar, they are generally hydrophobic and therefore readily to interact with cytoplasmic membrane. It is believed that these agents enter the cells through passive diffusion and subsequently evicted by Pgp through a drug concentration gradient across the membrane. How Pgp transports such structurally diverse substrates has been a challenging topic to structural biologists and pharmacologists alike. While X-ray crystallographic information of Pgp is not available, crystallographic determinations of a bacterial homolog of multidrug transporter MsbA have been instrumental in elucidating the transport mechanism of Pgp.15 This information, together with biochemical studies which have identified several drug binding sites on various TMD of Pgp,14 suggest that the initial event of Pgp-mediated drug transport is substrate binding, resulting in conformational changes that bring the two NBD into cross proximity to facilitate ATP binding. Mutation analyses have demonstrated that both NBD are required for transporter activity. Nucleotide binding and subsequent ATP hydrolysis provide the needed energy for releasing the substrate outward through the multi-TMD forming pore.14,16 However, much of the complex dynamic and vectorial processes involved in the Pgp-mediated drug transport remains to be learned.
Humans have two MDR genes, MDR1 and MDR2. Only MDR1-encoded Pgp functions as drug transporter. MDR2-encoded Pgp functions as a phospholipid transporter. MDR1/Pgp is expressed in many normal tissues, including liver, kidney, small intestine, colon, adrenal gland, and blood-brain barrier, whereas MDR2/Pgp is expressed mainly in the liver (Table 1). Mice have three mdr genes, two of which (mdr1a and mdr1b) are drug transporters, whereas the third (mdr2) has a similar function as human MDR1. The endogenous substrates for MDR1, mdr1a, mdr1b are not known. Mice without mdr1a (-/-) or both mdr1a (-/-) mdr1b(-/-) alleles generated by knockout strategies are viable and fertile, suggesting that mdr1a and mdr1b are not essential for cell viability.17,18 However, these animals exhibit elevated sensitive to cytotoxic effects upon challenged by cytotoxic agents. While the endogenous substrates for P-gp remains unknown, it is generally accepted that animals utilize this efflux pump to prevent xenotoxins from entering the body (intestine, colon) and to remove cytotoxic compounds once inside the body (liver, kidney, bone marrow, and brain).
Table 1
Properties of some ABC transporters.
MDR1/Pgp-mediated transport can be inhibited by the so-called MDR-reversal agents or Pgp blockers. Some inhibitors have been in clinical applications in attempts to block MDR in chemotherapy-resistant tumors that express elevated levels of Pgp. Agents, e.g., calcium channel blocker verapamil and immunosuppressive agent cyclosporin A, are themselves Pgp substrates. They act as competitive inhibitors to Pgp-mediated transport. Other inhibitors, e.g., PSC-833, GF120918, and LY335979 have been used in various stages of clinical trials.13 The development of clinically applicable reversal agents are an important avenue in combating MDR in cancer chemotherapy.
If Pgp plays a role in cancer chemotherapy, its expression in tumor cells most likely is elevated. Indeed, Pgp expression levels are frequently elevated in many types of cancer. Understanding the upregulation mechanisms is of importance for modulating its expression. Most of our understanding on MDR1 regulation mechanisms are from cultured cell studies.19 Upregulation of MDR1 in cultured cells can be at the transcriptional and/or posttranscriptional levels. Transcriptional regulation involves a host of basal transcriptional factors, e.g., NF-Y, SP1, Egr1, and ets-1. Moreover, MDR1 expression can be induced by various stress conditions, including UV, inflammation, carcinogens, hypoxia, and chemotherapeutic agents. We have demonstrated that induction of MDR1 by the carcinogen (2-acetylaminofluorene) is mediated by DNA sequence located at -6092 bp which contains a NF-kappaB binding site, through upstream signaling via phosphoinositide 3-kinase- Rac1-and NAD(P)H oxidase-AKT pathway.20 Upregulation of MDR1 expression by chemotherapeutics is in part by posttranscriptional (enhanced mRNA stability). Posttranslational regulation phosphorylation is associated with enhanced MDR activity. Pgp phosphorylation can be regulated by PKCα which in turn is regulated by wild-type p53, a tumor suppressor protein.21
MRP
MRP1 was first identified in doxorubicin-resistant cells that did not express elevated levels of Pgp.10 Like Pgp1, MRP1 contains two intracellularly localized NBD. However, unlike Pgp1, it contains 17 TMD14,23,24 (fig. 1). The function of the five extra TMD is not clear, they are apparently not essential for catalytic function, because deleting this domain did not compromise its activity.22 Overexpression of MRP1 conferred resistance to a spectrum of antitumor agents that is similar, but not identical, to that of Pgp1. For example, while taxanes are good substrate for MDR1 Pgp but are poor for MRP1. Additionally, unlike Pgp1, MRP1-mediated efflux requires cofactors, glutathione (GSH), glucuronic acid or sulfate. Mice lacking mrp1 are viable and fertile but have a deficient imflammatory response to its mediator leucotriene LTC4, which is an endogenous substrate of MRP1.25
Since the discovery of MRP1, eight related sequences have been identified, i.e, MRP2 to MRP9.23,24 MRP1 and MRP2 have similar substrate selectivity but the tissue expression profiles are quite different: MRP1 expression is rather ubiquitous, whereas MRP2 expression is restricted to liver and kidney. Hepatic MRP2 is involved in the hepatobiliary extrusion of bilirubin glucuronide and defected MRP2 is associated with Dubin-Johnson syndrom.26
While the structural organization of MRP3 is similar to those of MRP1 and MRP2, namely, it also possesses 17 TMD, but the substrate specificity of MRP3 is quite different from those of MRP1 and MRP2. MRP3-mediated transport does not require intracellular GSH. Etoposide appears to be transported by MRP3 in unmodified form,27 whereas vincristine and doxorubicin which are transported by MRP1 and MRP2 through GSH conjugates are not transported by MRP3. It is important to note that cancer cells overexpress MRP3 are not resistant to anthracyclines which are important antitumor agents in breast cancer treatment.
MRP4 and MRP5 contain 12 TMD rather than 17 TMD, making them structurally more like MDR1 than does MRP1. MRP428 and MRP5 transport cyclic nucleotides and nucleotide analogs which are not transported by MRP1, MRP2, or MRP3. The roles of MRP4 and MRP5 in breast cancer chemoresistance are not known. MRP6, MRP7, MRP8, and MRP9 are newly cloned MRP gene family members. The substrate specificities and pharmacologic properties of these ABC transporters remain to be determined.
Like MDR1, expression of MRP1 in cultured cells can be induced by a variety of cytotoxic agents including prooxidants, heavy metals, antitumor agents, and nitric oxides. Because MRP1-mediated efflux requires GSH, intracellular GSH levels may play important roles in regulating the expression of MRP1. Biosynthesis of GSH is regulated by the rate-limiting enzyme γ-glutamylcysteine synthetase (γ-GCS), which consists of one heavy catalytic subunit (γ-GCSh) and one light (regulatory) subunit (γ-GCSl). Our laboratory has demonstrated that expression of γ-GCSh can be induced by many cytotoxic agents that also upregulate MRP1.29 Moreover, expression of MRP1 and γ-GCSh is frequently upregualted in colorectal cancers.30 These observations suggest that both genes may be regulated by the same mechanisms. Transcriptional regulation of γ-GCSh gene expression is mediated by an oxidative stress response element (ORE) located at -3802 bp which interacts with the leucine zipper transcription factor complex Nrf2/Maf.31 However, no ORE element has yet been identified in the promoter of MRP1. Expression of MRP3 can also be induced by prooxidants.32 While MDR1, MPR1 and MRP3, like γ-GCSh, may be considered as a stress inducible ABC transporters, but because many different signaling pathways can be associated with stress-induced gene expression, regulation mechanisms of these genes may not be the same.
BCRP
The ABC transporter BCRP was first cloned from the doxorubicin-resistant MCF-7 breast cancer cell line,11 but it is not implied that the expression is associated with breast cancer. This tranporter encodes only 655 amino acids, about one half of the sizes of MDR1 and MRP transporter (fig. 1). It is likely that two half-molecules form a homodimer to function as a drug transporter.33-35 Cell lines selected for resistance to many antitumor agents, including mitoxantrone, topotecan, doxorubicin, SN-38 exhibit MDR phenotype and overexpressed BCRP, suggesting an important role of BCRP in MDR development. BCRP-mediated transport apparently does not require GSH cofactor. Like mdr1a(-/-), mdr1b(-/-), and mrp(-/-) mice, bcrp(-/-) animals are fertile with no apparent phenotypic alterations as compared with those in the wild-type animals, suggesting that murine bcrp is not essential for normal animal physiology.36 The fact that these individual knockout animals fail to display normal physiological abnormality also suggest that there are functional redundancy among these transporters.
Roles of Pgp, MRP1, and BCRP in Breast Cancer Chemotherapy
For an ABC transporter to play a role in reducing cancer chemoresistance, its expression levels should be inversely correlated with the chemosensitivity of antitumor agents that are known to be substrates of the transporter. In addition, an enhanced response to chemotherapy should be observed when inhibitors or reversal agents are used. These criteria are discussed here in the context of Pgp, MRP1 and BCRP in breast cancer chemotherapy. A review describing similar issues has recently been published.37
Expression of MDR1/Pgp in nonneoplastic breast tissue and in breast cancer tissues has been extensively investigated at mRNA and protein levels. MDR1 mRNA levels were mainly determined by using RT-PCR method and proteins levels were by immunohistochemical (IHC), flow cytometry, and western blot analyses. Agreement between IHC analyses and RT-PCR results were found in many studies, although inconsistent results were also found, perhaps because the levels of MDR1/Pgp regulation (transcriptional vs. prostranslcriptional regulation) vary in different patient population. The disparate results may also reflect differences in analytic methodologies, including tissue sampling (IHC analysis detects expression in tumor cells whereas RT-PCR may use a heterogeneous pool of cell types), the use of different probes (RT-PCR can be gene-specific, whereas some antibodies used for IHC can cross-react both MDR1- and MDR2-encoded Pgp). It is therefore, careful evaluation of experimental designs is needed before results can be compared.38
Many studies aimed at determining the correlation between expression levels of MDR1/Pgp in various tumor types and responses to chemotherapy with antitumor agents that are substrates of MDR1/Pgp have been published. For breast cancer treatment, while some studies showed positive correlations between reduced Pgp expression levels and improved response rates39,40 whereas other failed to find such a correlation.40
Expression of MRP1 is frequently observed in breast cancer even before chemotherapy, and chemotherapy has been reported to increased MRP1 expression.41 A correlation between MRP1 expression and patient survival rates after chemotherapy has been noted in some studies.41,42 whereas other reports showed no correlation between MRP1 expression and prognosis.43-45
Evaluation of BCRP expression in human cancers has most been performed in leukemia. And several studies have also been published for the expression of BCRP in breast cancers, mostly determined by using RT-PCR method. While levels of BCRP in AML patients are variable in some studies and the expression levels are increased in associated with relapsed/reflactory,46 whereas other studies did not show correlation.47 In breast cancer, expression levels of BCRP are low.48 The role of BCRP in the chemoresistance of breast cancer remains to be investigated.
The fact that ABC transporter expression levels and resistance to chemotherapy are positively correlated in some, but not all, breast cancers may reflect differences in analytic methods, patient population, or the chemotherapeutic drugs used. The use of reversal agents for combating MDR1/Pgp-related clinical drug resistance began soon after the discovery of Pgp inhibitors in many types of cancer, including breast cancer. Verapamil was one of the very early discovered MDR1 reversal agent used in clinical trials. From a pool of four studies, verapamil appears to resensitize 15% of advanced breast cancer patients refractory to anthracycline-containing regimens.18 However, this may not be beneficial because the response rate of the same patients to alternative second-line chemotherapy could achieve a better response. Reversal agents such as quinidine and biricodar in clinical trials have not shown evidence of benefits. These results suggest that expression levels of MDR1/Pgp1 levels are not readily for prognostic evaluation of drug sensitivities and thus for pharmacologic intervention for improving chemotherapeutic efficacies remain to be further developed.
The difficulties associated with clinical trials using reversal agents may be explained as follows: Reliably assessing the contribution of the overexpressed ABC transporter to drug resistance is difficult, even the transporters are overexpressed in the tumors. Not all the Pgp- tumor respond to chemotherapeutics; and not all the Pgp+ tumors are resistant to chemotherapy. Aside from the technical aspects in measuring expression levels, no studies have convincingly shown that high levels of transporter expression translate into high transporter activities. Another difficulty is that there is no direct evidence showing that reversal agents indeed downregulate the transporter activity at tumor sites. A third consideration is that multiple ABC transporters can pump the same antitumor substrate, and in many cases, overexpression of multiple transporters is found in tumors. Thus, inhibition of one or a few ABC transporters may not be sufficient to bring down drug resistance to therapeutic achievable levels. The functional redundancy of ABC transporter family proteins may then encourage the development of reversal agents that can simultaneously inhibit multiple transporters. For instance, some Pgp inhibitors such as cyclosporin A and PSC 833 also inhibit the function of MRP, albeit less effective, and another Pgp inhibitor GF129018 can also suppress the function of BCRP. Last but not the least, in clinical settings where combination chemotherapy is often used, multiple mechanisms may contribute to the overall response to chemotherapeutic agents. Inhibition of drug transport alone may be insufficient to overcome the overall drug resistance.
Conclusion
The discovery of ABC transporters associated with MDR phenotype in cultured cells revealed an important mechanism bywhich cancer cells acquire resistance to many chemotherapeutic agents. Much has been learned about how expression of these transporters, notably MDR1, MRP1, and BCRP, in cultured cells confer resistance to antitumor agents. This knowledge holds great implications for clinical drug resistance. Overexpression of Pgp and MRP1 in some breast cancers has been correlated with chemoresistance in clinical setting in some studies but not in others. The disappointing results of clinical applications in using reversal agents suggest that more investigation is needed for translational gains in breast cancer chemotherapy. Future studies should focus on the molecular basis of how the expression of these transporters is regulated in normal breast cells and in their malignant counterparts. These studies may lead to novel strategies of controlling MDR through gene regulation. Another area of research may involve developing strategies for modulating transporter activities through better understanding pharmacodynamic and pharmacogenetic behaviors of reversal agents. In combination of advancing imaging systems, suppression of transporter activities in tumor sites can be measured. These studies may eventually lead to effective evaluation on the roles of these ABC transporters in breast cancer chemoresistance and the development of strategies of circumventing it.
Acknowledgements
Research in author's laboratory was supported in part by grants CA72404 and CA79085 from the National Cancer Institute.
References
- 1.
- Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. [PMC free article: PMC1064079] [PubMed: 15535852]
- 2.
- Jemal A, Tiwari RC, Murray T. et al. Cancer statistics, 2004. CA cancer J Clin. 2004;54:8–29. [PubMed: 14974761]
- 3.
- Giordano SH, Buzdar AU, Smith TL. et al. Is breast cancer survival improving? Cancer. 2004;100:44–52. [PubMed: 14692023]
- 4.
- Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. [PubMed: 9753714]
- 5.
- Piccart M. The roles of taxenes in the adjuvant treatment of early breast cancers. Breast Cancer Res Treat. 2003;79:S25–S34. [PubMed: 12868803]
- 6.
- Nabholtz JMA. Docetaxel-anthracycline combinations in metastatic brease cancers. Breast Cancer Res Treat. 2003;79:S3–S9. [PubMed: 12868800]
- 7.
- Valero V, Holmes FA, Walters RS. et al. Phase II trial of docetaxel: A new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995;13:23886–2894. [PubMed: 8523051]
- 8.
- Nabholtz JM, Gelmon K, Bontenbal M. et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Onco. 1996;14:1858–1867. [PubMed: 8656254]
- 9.
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biophys Acta Biochim. 1975;445:152–162. [PubMed: 990323]
- 10.
- Cole SP, Bhardway JH, Gerlach JE. et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. [PubMed: 1360704]
- 11.
- Doyle LA, Yang W, Abruzzo LV. et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;5:15665–15670. [PMC free article: PMC28101] [PubMed: 9861027]
- 12.
- Deng L, Tatebe S, Lin-Lee YC. et al. MDR and MRP gene families as cellular determinant factors for resistance to clinical anticancer agents. Cancer Treat Res. 2002;112:49–66. [PubMed: 12481711]
- 13.
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv Drug Deliv Rev. 2003;55:3–29. [PubMed: 12535572]
- 14.
- Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. [PubMed: 12045106]
- 15.
- Chang G, Roth CB. Structure of MsbA from E. coli: A homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science. 2001;293:1793–1800. [PubMed: 11546864]
- 16.
- Higgins CF, Linton KJ. The ATP switch model for ABC transporter. Nature Struct Mol Biol. 2004;11:918–926. [PubMed: 15452563]
- 17.
- Schinkel AH, Smit JJM, Van Tellingen O. et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. [PubMed: 7910522]
- 18.
- Schinkel AH, Mayer U, Wagenaar E. et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci USA. 1997;94:4028–4033. [PMC free article: PMC20562] [PubMed: 9108099]
- 19.
- Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. [PubMed: 14576854]
- 20.
- Kuo MT, Liu Z, Wei Y. et al. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945–1954. [PubMed: 11960367]
- 21.
- Zhan M, Yu D, Lin J. et al. Transcriptional repression of protein kinase Cα via Sp1 by wild-type p53 is involved in inhibition of MDR1 P-glycoprotein phosphorylation. J Biol Chem. 2005;280:4825–4833. [PubMed: 15563462]
- 22.
- Bakos E, Evers R, Szakacs G. et al. Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem. 1998;273:32167–32175. [PubMed: 9822694]
- 23.
- Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. [PubMed: 14576857]
- 24.
- Heimeur A, Conseil G, Deeley RG. et al. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: Biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. [PubMed: 14965249]
- 25.
- Wijnholds J, Evers R, van Leusden MR. et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nature Med. 1997;3:1275–1279. [PubMed: 9359705]
- 26.
- Keppler D, Kartenbeck J. The canalicular conjugate export pump encoded by the cmrp/cmoat gene. Prog Liver Dis. 1996;14:55–67. [PubMed: 9055574]
- 27.
- Zelcer N, Saeki T, Reid G. et al. Characterization of drug transport by the human mulltidrug resistance protein 3 (ABCC3). J Biol Chem. 2001;276:46400–46407. [PubMed: 11581266]
- 28.
- Schuetz JD, Connelly MC, Sun D. et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Naure Med. 1999;5:1048–1051. [PubMed: 10470083]
- 29.
- Yamane Y, Furuichi M, Song R. et al. Expression of multidrug resistance protein/GS-X pump and gamma-glutamylcysteine synthetase genes is regulated by oxidative stress. J Biol Chem. 1998;273:31075–31085. [PubMed: 9813007]
- 30.
- Kuo MT, Bao JJ, Curley SA. et al. Frequent coordinated overexpression of the MRP/GS-X pump and gamma-glutamylcysteine synthetase genes in human colorectal cancers. Cancer Res. 1996;56:3642–3644. [PubMed: 8705999]
- 31.
- Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277:36544–36552. [PubMed: 12145307]
- 32.
- Lin-Lee YC, Tatebe S, Savaraj N. et al. Differential sensitivities of the MRP gene family and gamma-glutamylcysteine synthetase to prooxidants in human colorectal carcinoma cell lines with different p53 status. Biochem Pharmacol. 2001;61:555–563. [PubMed: 11239498]
- 33.
- Allen JD, Schinkel AH. Multidrug resistance and pharmacological protein mediated by the breast cancer resistance protein (BCRP/ABCG2). Mol Cancer Therap. 2002;1:427–434. [PubMed: 12477055]
- 34.
- Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22:7340–7358. [PubMed: 14576842]
- 35.
- Bates SE, Robey R, Miyake K. et al. The roles of half-transporters in multidrug resistance. J Bioenergetics and Biomem. 2001;33:503–517. [PubMed: 11804192]
- 36.
- Jonker JW, Buitelaar M, Wagenaar E. et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA. 2002;99:15649–15654. [PMC free article: PMC137771] [PubMed: 12429862]
- 37.
- Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in brease cancer. Endocrine-related Cancer. 2003;10:43–73. [PubMed: 12653670]
- 38.
- Beck WT, Grogan TM, Willman CL. et al. Methods to detect P-glycoprotein-associated multidrug resistance in patients' tumors: Consensus recommendations. Cancer Res. 1996;56:3010–3020. [PubMed: 8674056]
- 39.
- Verrelle P, Meissonnier F, Fonck Y. et al. Clinical relevance of immunohistochemical detection of multidrug resistance P-glycoprotein in breast carcinoma. J Nat Cancer Inst. 1991;83:111–116. [PubMed: 1671103]
- 40.
- Chevillard S, Pouillart P, Beldjord C. et al. Sequential assessment of multidrug resistance phenotype and measurement of S-phase fraction as predictive markers of breast cancer response to neoadjuvant chemotherapy. Cancer. 1996;77:292–300. [PubMed: 8625237]
- 41.
- Rudas M, Filipits M, Taucher S. et al. Expression of MRP1, LRP and Pgp in breast carcinoma patients treated with preoperative chemotherapy. Breast Cancer Res Treat. 2003;81:149–157. [PubMed: 14572157]
- 42.
- Filipits M, Malayeri R, Suchomel RW. et al. Expression of the multidrug resistance protein (MRP1) in breast cancer. Anticancer Res. 1999;19:5043–5049. [PubMed: 10697508]
- 43.
- Ferrero JM, Etienne MC, Formento JL. et al. Application of an original RT-PCR-ELISA multiplex assay for MDR1 and MRP, along with p53 determination in node-positive breast cancer patients. Br J Cancer. 2000;82:171–177. [PMC free article: PMC2363171] [PubMed: 10638986]
- 44.
- Faneyte IF, Kristel PM, van de Vijver MJ. Multidurg resistance associated genes MRP1, MRP2 and MRP3 in primary and anthracycline exposed breast cancer. Anticancer Res. 2004;24:2931–2939. [PubMed: 15517899]
- 45.
- Kanzaki A, Toi M, Nakayama K. et al. Expression of multidrug resistance-related transporters in human breast carcinoma. Jpn J Cancer Res. 2001;92:452–458. [PMC free article: PMC5926724] [PubMed: 11346468]
- 46.
- Van den Heuvel-Eibrink MM, Wiemer EA, Prins A. et al. Leukemia. 2002;16:833–839. [PubMed: 11986944]
- 47.
- Sargent JM, Williamson CJ, Maliepaard M. et al. Breast cancer resistance protein expression and resistance to daunorubicin in blast cells from patients with acute myeloid leukaemia. Brit J Haematol. 2001;115:257–262. [PubMed: 11703319]
- 48.
- Faneyte IF, Kristel PM, Maliepaard M. et al. Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res. 2002;8:1068–1074. [PubMed: 11948115]
Publication Details
Author Information and Affiliations
Authors
M Tien Kuo*.Affiliations
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Tien Kuo M. Roles of Multidrug Resistance Genes in Breast Cancer Chemoresistance. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.