NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
S-Modulin is a Ca2+-binding protein found in frog rod photoreceptors1,2 and its bovine homologue is known as recoverin.3,4 In the Ca2+-bound form, S-modulin inhibits rhodopsin phosphorylation5 through inhibition of rhodopsin kinase.6-9 Because rhodopsin phosphorylation is the quench mechanism of light-activated rhodopsin (R*),10,11 the inhibition of the phosphorylation by S-modulin probably contributes to increase the lifetime of R* to result in sustained hydrolysis of cGMP.5 The Ca2+ concentration decreases in the light in vertebrate photoreceptors,12–14 and this decrease is essential for light-adaptation.15,16 Thus, S-modulin is expected to regulate the lifetime of R* and thereby regulate the extent and the time course of hydrolysis of cGMP depending on the intensity of background light. With this mechanism, S-modulin is believed to regulate the waveform of a photoresponse and the efficiency of the light in the generation of a photoresponse.
Isolation
The S-modulin activity was first detected in an electrophysiological measurement of the cGMP phosphodiesterase (PDE) activity in a truncated preparation of a frog rod outer segment (ROS) which was internally perfused with a bathing solution.1 This measurement suggested the presence of a factor that regulates PDE activation in a Ca2+-dependent manner. In the measurement, it was also suggested that the factor binds to the disk membrane at high Ca2+ concentrations but becomes soluble at low Ca2+ concentrations. This characteristic of Ca2+-dependent binding to disk membranes of the factor was utilized in purification of S-modulin.
In our first attempt of purification of S-modulin,1,2 we isolated proteins that bind to disk membranes at high Ca2+ concentrations. S-Modulin, a 26-kDa protein, was purified after anion exchange column chromatography as the fraction that increases PDE activity in a Ca2+-dependent manner. Because the maximum PDE activity was constant irrespective of Ca2+ concentrations, S-modulin does not seem to increase the Vmax of PDE but rather regulates the efficiency of PDE activation.
The characteristic of Ca2+-dependent binding of S-modulin to disk membranes suggested that the binding to the membranes is due to exposure of a hydrophobic region(s) to the surface of S-modulin molecule upon Ca2+-binding. Similar kind of Ca2+-dependent structural changes are thought in a family of proteins that possess Ca2+-binding motifs, EF-hands. In these proteins, a phenyl-Sepharose column is a useful tool to purify them. In our subsequent purification, we used this column instead of disk membranes.17 Similar procedure is used in purification of recoverin.18
In the course of purification of S-modulin, we realized that another 26-kDa protein also binds to disk membranes in a Ca2+-dependent manner.2 Its chromatographical behavior on an anion exchange column is slightly different from that of S-modulin. Our studies showed that this protein, named s26, is a cone homologue of S-modulin.19 The molar abundance among rhodopsin, S-modulin and s26 in a bullfrog retina is approximately 100:7:5.
Function
The S-modulin activity was found as a Ca2+-dependent regulator of PDE activation.1 Because PDE is activated through the phototransduction cascade, the reaction that is regulated by S-modulin should be somewhere in the cascade.
When we measured the effect of S-modulin on rhodopsin phosphorylation, S-modulin inhibited the phosphorylation at high Ca2+ concentrations.5 Rhodopsin phosphorylation is a mechanism to quench light-activated rhodopsin (R*),10,11 and therefore, the inhibition of the phosphorylation will lead to the increase in the lifetime of R.*5 If this is the case, the hydrolysis of cGMP continues and a photoresponse develops for a relatively long period. Then, the photoresponse time course is rather slow and the response amplitude is large. Under steady illumination, the Ca2+ concentration decreases in the photoreceptor outer segment cytoplasm.12–14 At low Ca2+ concentrations, S-modulin does not inhibit the phosphorylation reaction on rhodopsin. Under steady illumination, therefore, the lifetime of R* seems to be relatively short and the photoresponse terminates more rapidly. Then the photoresponse terminates quickly and the response amplitude is small. This expected regulation of a photoresponse by S-modulin is consistent with the behavior of a photoresponse during dark- and light-adaptation, which is the reason why we believe that S-modulin is involved in the control in photoreceptor adaptation.
The above possible regulation mechanism by S-modulin has been tested in many ways, but the results are still controversial. When recoverin (bovine homologue of S-modulin) was introduced into a salamander ROS with a patch pipette,20 the photoresponse was altered as one would expect. The time-to-peak of a photoresponse was lengthened and fractional amplitude of a photoresponse increased.
The effect of ATP on a photoresponse was examined in a truncated preparation of an ROS.21 ATP was introduced into the cytoplasm of an ROS through the truncated end of the preparation. ATP reduced the time-to-peak, duration and amplitude of the flash response. These effects can be explained by quench of R* with phosphorylation. These ATP effects were detected within a few seconds after a light flash at high Ca2+ concentrations, but the time was shortened to about 0.5 sec at low Ca2+ concentrations. The results showed that the ATP-sensitive step is Ca2+-sensitive. If the ATP-sensitive step is R* phosphorylation, which is very plausible, then the result indicated that the phosphorylation is rapid at low Ca2+ concentrations. These observations are consistent with the postulated function of S-modulin.
The cytoplasmic Ca2+ concentration in an ROS can be clamped by exposing the ROS to a 0 Ca2+/0 Na solution. When it was clamped at the dark high level, the duration of a saturating photoresponse was prolonged.22 The result indicated that the cytoplasmic Ca2+ concentration decreases in the light and this decrease shortens the duration of a saturating photoresponse. The prolongation effect was dependent on how long the cytoplasmic Ca2+ concentration was kept at the dark level after a light flash.22 The longer the time was, the larger the effect was. However, the effect was observed in a limited time period after a light flash with a time constant of about 0.5 sec. At about 2 sec after the flash, the clamp effect was saturated. The result showed that only a very brief period after a light flash is Ca2+sensitive. The responsible reaction would most possibly be R* phosphorylation.
In the study of the recoverin knockout rods, the kinetics of the response were found to be faster and the amplitude was found to be smaller than in control rods.23 When recoverin was internally dialyzed into the truncated ROS, recoverin prolonged the recovery phase of a bright flash response.24 These observations are consistent with the postulated function of S-modulin, but the effect was not so large. So far, biochemical attempt to detect the S-modulin effect on R* phosphorylation in intact rod photoreceptors has not been successful.25
As summarized in the earlier chapters in this book, Ca2+ seems to regulate photoreceptor adaptation in many ways. From quantitative studies estimating relative contribution of these regulations, the effect of S-modulin has been suggested to be small at weak background light and becomes large at strong background light.26 The relative contribution of S-modulin to light-adaptation has yet to be determined.27
Mechanism of Inhibition of Rhodopsin Phosphorylation
There are at least two possible mechanisms that account for the inhibition of rhodopsin phosphorylation by S-modulin. One possibility is the binding of Ca2+-bound form of S-modulin (Ca2+/S-modulin) to R* to interfere the access of rhodopsin kinase to R*. The other possibility is the binding of Ca2+/S-modulin to rhodopsin kinase to inhibit the kinase activity.5
To identify the target of S-modulin, a reagent was used to link Ca2+/S-modulin and its target molecule.28 A 60-kDa protein was linked to S-modulin in a Ca2+-dependent manner. In contrast, rhodopsin was not linked at all. The result suggested that Ca2+/S-modulin binds to rhodopsin kinase to inhibit rhodopsin phosphorylation.
All the other studies from other laboratories reached the same conclusion. With affinity chromatography, Ca2+/recoverin has been shown to bind to rhodopsin kinase.6,7 The inhibition of the phosphorylation by Ca2+/recoverin does not depend on the ratio of R* : recoverin,8 which excludes the possibility that recoverin binds to R*. In addition, it has been shown that β-adrenergic receptor kinase, which can phosphorylate R*, is not inhibited by recoverin,9 which indicated that there is a specific interaction between Ca2+/S-modulin with rhodopsin kinase.
Site of the Interaction of S-Modulin to Rhodopsin Kinase
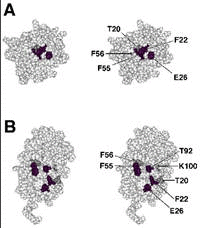
The S-modulin site interacting with rhodopsin kinase has been identified.29 In this study, firstly the S-modulin peptides that show an S-modulin effect (inhibition of R* phosphorylation) were identified. Because S-modulin family proteins so far examined all inhibited R* phosphorylation, the interaction site of S-modulin should be conserved among the proteins of the family. The conserved amino acids in the effective peptides were determined and they were mutated with one mutation in each of S-modulin mutant proteins. S-Modulin changes its conformation upon Ca2+-binding.30-32 In the mutants generated, some of them were not able to change the protein conformation upon Ca2+-binding. These amino acids are therefore essential in the Ca2+-dependent conformational change of S-modulin.
With careful determination, several amino acids were identified as the interaction site with rhodopsin kinase (Fig. 1). These amino acids are buried in the inactive, Ca2+-free form of S-modulin molecule, but they are exposed to the surface of the molecule in the active, Ca2+-bound form of S-modulin. These amino acids are located at the surface of a groove.
So far many S-modulin-like proteins were found. All of the family proteins examined inhibited rhodopsin phosphorylation in a Ca2+-dependent manner.33 The result suggests that all of the family members have similar sites interacting with rhodopsin kinase and that the mechanism of the actions of these proteins is similar to that of the S-modulin-rhodopsin kinase system.
Structure
Visinin is the protein first described in the S-modulin family,34 although its function was not known at the time of the finding. S-Modulin, recoverin and visinin contain four potential Ca2+-binding motifs known as EF-hands.3,4 However, only EF2 and 3 (numbered from the N-terminus) are functional.35 The N-terminus glycine is modified with a lipid, mainly myristic acid.36 This myristoylation is essential for the Ca2+-dependent binding of recoverin to ROS membranes,37-39 but not for the function of S-modulin. Non-myristoylated recoverin can also inhibit R* phosphorylation at high Ca2+ concentrations.39,40 The N-terminal myristoylation has been indicated to be essential for cooperative Ca2+-binding to recoverin.40,41 The first Ca2+-binding to EF3 probably induces the second Ca2+-binding to EF2 in a cooperative manner.42
The Ca2+ binding of myristoylated (native) S-modulin to a membrane preparation was observed only in the membranes derived from ROS.17 Hypotonically washed ROS membranes, proteolysed ROS membranes, phospholipase A2-treated ROS membranes,phospholipase C-treated ROS membranes, and vesicles made from chloroform/methanol-extracted ROS membranes were all good substrates of the binding of Ca2+/S-modulin. However, bovine brain extracts1,3,5,8,10 or vesicles made from purified phospholipids (PA, PC, PE, PS) or sphyngomyelin did not allow the binding (but see ref. 37 for the case of recoverin). The positive charges near the C-terminus have been suggested to be involved in the binding.43
Localization
Immunohistochemical studies showed that S-modulin and recoverin are present uniformly throughout the photoreceptors.3,19 S-Modulin and recoverin are postulated to act on rhodopsin kinase, and therefore it is reasonable that they are present in ROS. The uniform distribution may indicate that S-modulin and recoverin have other roles in other part of a photoreceptor. Because an S-modulin homologue, frequenin,44 is believed to function in the transmitter release at the neuromuscular junction, S-modulin and recoverin may have similar function at the synaptic terminal of a photoreceptor.
Acknowledgement
This work was supported by Research for the Future Program of the Japan Society for the Promotion of Science under the Project “Cell Signaling” JSPS-RFTF97L00301.
References
- 1.
- Kawamura S, Murakami M. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature. 1991;349:420–423. [PubMed: 1846944]
- 2.
- Kawamura S. Lightsensitivity modulating protein in frog rods. Photochem Photobiol. 1992;56:1173–1180. [PubMed: 1337215]
- 3.
- Dizhoor AM, Ray S, Kumar S. et al. Recoverin: a calcium sensitive activator of retinal guanylate cyclase. Science. 1991;251:915–918. [PubMed: 1672047]
- 4.
- Kawamura S, Hisatomi O, Kayada S. et al. Recoverin has S-modulin activity in frog rods. J Biol Chem. 1993;268:14579–14582. [PubMed: 8392055]
- 5.
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cGMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. [PubMed: 8386803]
- 6.
- Gorodovikova EN, Philippov PD. The presence of a calcium-sensitive p26-containing complex in bovine retina rod cells. FEBS Lett. 1993;335:277–279. [PubMed: 7902818]
- 7.
- Chen CK, Inglese J, Lefkowitz RJ. et al. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. [PubMed: 7629115]
- 8.
- Klenchin VA, Calvert PD, Bownds MD. Inhibition of rhodopsin kinase by recoverin. J Biol Chem. 1995;270:16147–16152. [PubMed: 7608179]
- 9.
- Sanada K, Shimizu F, Kameyama K. et al. Calciumbound recoverin targets rhodopsin kinase to membranes to inhibit rhodopsin phosphorylation. FEBS Lett. 1996;384:227–230. [PubMed: 8617359]
- 10.
- Chen J, Makino CL, Peachey NS. et al. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science. 1995;267:374–377. [PubMed: 7824934]
- 11.
- Chen CK, Burns ME, Spencer M. et al. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci USA. 1999;96:3718–3722. [PMC free article: PMC22360] [PubMed: 10097103]
- 12.
- McCarthy ST, Younger JP, Owen WG. Dynamic, spatially nonuniform calcium regulation in frog rods exposed to light. J Neurophysiol. 1996;76:1991–2004. [PubMed: 8890309]
- 13.
- Sampath AP, Matthews HR, Cornwall MC. et al. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J Gen Physiol. 1998;111:53–64. [PMC free article: PMC1887770] [PubMed: 9417134]
- 14.
- GrayKeller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. [PubMed: 7524559]
- 15.
- Matthews HR, Murphy R L W, Fain GL. et al. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988;334:67–69. [PubMed: 2455234]
- 16.
- Nakatani K, Yau KW. Calcium and light adaptation in retinal rods and cones. Nature. 1988;334:69–71. [PubMed: 3386743]
- 17.
- Kawamura S, Takamatsu K, Kitamura K. Purification and characterization of S-modulin, a calcium-dependent regulator on cGMP phosphodiesterase in frog rod photoreceptors. Biochem Biophys Res Commun. 1992;186:411–417. [PubMed: 1321610]
- 18.
- Polans AS, Buczytko J, Crabb J. et al. A photoreceptor calcium binding protein is recognized by autoantibodies obtained from patients with cancer-associated retinopathy. J Cell Biol. 1991;112:981–989. [PMC free article: PMC2288874] [PubMed: 1999465]
- 19.
- Kawamura S, Kuwata O, Yamada M. et al. Photoreceptor protein s26, a cone homologue of S-modulin in frog retina. J Biol Chem. 1996;271:21359–21364. [PubMed: 8702916]
- 20.
- GrayKeller MP, Polans AS, Palczewski K. et al. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron. 1993;10:523–531. [PubMed: 8461139]
- 21.
- Sagoo MS, Lagnado L. Gprotein deactivation is rate-limiting for shutoff of the phototransduction cascade. Nature. 1997;389:392–394. [PubMed: 9311782]
- 22.
- Matthews HR. Actions of Ca2+ on an early stage in phototransduction revealed by the dynamic fall in Ca2+ concentration during the bright flash response. J Gen Physiol. 1997;109:141–146. [PMC free article: PMC2220062] [PubMed: 9041444]
- 23.
- Dodd RL, Makino CL, Chen J. et al. Visual transduction in transgenic mouse lacking recoverin. Invest Ophthalmol Vis Sci. 1995;36:S6–41.
- 24.
- Erickson MA, Lagnado L, Zozulya S. et al. The effect of recombinant recoverin on the photoresponse of truncated rod photoreceptors. Proc Natl Acad Sci USA. 1998;95:6474–6479. [PMC free article: PMC27811] [PubMed: 9600991]
- 25.
- Otto-Bruc AE, Fariss RN, Van Hooser JP. et al. Phosphorylation of photolyzed rhodopsin is calcium-insensitive in retina permeabilizesd by alpha-toxin. Proc Natl Acd Sci U S A. 1998;95:15014–15019. [PMC free article: PMC24567] [PubMed: 9844007]
- 26.
- Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. [PubMed: 8820871]
- 27.
- Mendez A, Burns ME, Sokal et al. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci USA. 2001;98:9948–9953. [PMC free article: PMC55558] [PubMed: 11493703]
- 28.
- Sato N, Kawamura S. Molecular mechanism of S-modulin action: Binding target and effect of ATP. J Biochem. 1997;122:1139–1145. [PubMed: 9498557]
- 29.
- Tachibanaki S, Nanda K, Sasaki K. et al. Amino acid residues of S-modulin responsible for interaction with rhodopsin kinase. J Biol Chem. 2000;275:3313–3319. [PubMed: 10652319]
- 30.
- Tanaka T, Ames JB, Harvey TS. et al. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. [PubMed: 7630423]
- 31.
- Ames JB, Ishima R, Tanaka T. et al. Molecular mechanics of calciummyristoyl switches. Nature. 1997;389:198–202. [PubMed: 9296500]
- 32.
- Johnson WC, Palczewski K, Gorczyca WA. et al. Calcium binding to recoverin: implications for secondary structure and membrane association. Biochimica et Biophysica Acta. 1997;1997;1342:164–174. [PubMed: 9392525]
- 33.
- De Castro E, Nef S, Fiumelli H. et al. Regulation of rhodopsin phosphorylation by a family of neuronal calcium sensors. Biochem Biophys Res Commun. 1995;216:133–140. [PubMed: 7488079]
- 34.
- Yamagata K, Goto K, Kuo CH. et al. Visinin: a novel calcium binding protein expressed in retinal cone cells. Neuron. 1990;4:469–476. [PubMed: 2317380]
- 35.
- Flaherty KM, Zozulya S, Stryer L. et al. Three-dimensional structure of recoverin, a calcium sensor in vision. Cell. 1993;75:709–716. [PubMed: 8242744]
- 36.
- Dizhoor MD, Ericsson LH, Johnson RS. et al. The NH2terminus of retinal recoverin is acylated by a small family of fatty acids. J Biol Chem. 1992;267:16033–16036. [PubMed: 1386601]
- 37.
- Zozulya S, Stryer L. Calcium-myristoyl switch. Proc Natl Acad Sci USA. 1992;89:11569–11573. [PMC free article: PMC50594] [PubMed: 1454850]
- 38.
- Dizhoor AM, Chen CK, Olshevskaya E. et al. Role of the acylated amino terminus of recoverin in Ca2+dependent membrane interaction. Science. 1993;259:829–832. [PubMed: 8430337]
- 39.
- Kawamura S, Cox JA, Nef P. Inhibition of rhodopsin phosphorylation by nonmyristoylated recombinant recoverin. Biochem Biophys Res Commun. 1994;203:121–127. [PubMed: 8074645]
- 40.
- Calvert PD, Klenchin VA, Bownds MD. Rhodopsin kinase inhibition by recoverin. J Biol Chem. 1995;270:24127–24129. [PubMed: 7592614]
- 41.
- Ames JB, Porumb T, Tanaka T. et al. Amino-terminal myristoylation induces cooperative calcium binding to recoverin. J Biol Chem. 1995;270:4526–4533. [PubMed: 7876221]
- 42.
- Matsuda S, Hisatomi O, Ishino T. et al. The role of calcium-binding sites in S-modulin function. J Biol Chem. 1998;273:20223–20227. [PubMed: 9685370]
- 43.
- Matsuda S, Hisatomi O, Tokunaga F. Role of carboxyl-terminal charges on S-modulin membrane affinity and inhibition of rhodopsin phosphorylation. Biochemistry. 1999;38:1310–1315. [PubMed: 9930992]
- 44.
- Pongs O, Lindemeier J, Zhu XR. et al. Frequenin—a novel calcium-binding protein that modulate synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. [PubMed: 8101711]
- S-Modulin - Madame Curie Bioscience DatabaseS-Modulin - Madame Curie Bioscience Database
- Development of Biological Potential - Madame Curie Bioscience DatabaseDevelopment of Biological Potential - Madame Curie Bioscience Database
- Neurons, Neurotrophins and Ceramide Signaling: Do Domains and Pores Contribute t...Neurons, Neurotrophins and Ceramide Signaling: Do Domains and Pores Contribute to the Dichotomy? - Madame Curie Bioscience Database
- Dopamine and Parkinson's Disease - Madame Curie Bioscience DatabaseDopamine and Parkinson's Disease - Madame Curie Bioscience Database
- Cytokines as Regulators of Coagulation - Madame Curie Bioscience DatabaseCytokines as Regulators of Coagulation - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...