NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Ca2+plays a key role in intracellular signal transduction in neurons but in excess it can lead to cell death. Thus its entry into cells is highly regulated by both extrinsic and intrinsic mechanisms. Little is known of the regulation of Ca2+ entry into retinal neurons. Here we describe the role of divalent cations and polyamines as intrinsic modulators of Ca2+ entry into retinal bipolar cells. Cone-dominant (small) bipolar cells of the white bass retina were studied using whole cell patch clamp techniques. With biophysical and pharmacological tools it was determined that these cells expressed a Ca2+ current similar to an L-type current. This current was very susceptible to blockage by divalent cations including Ca2+. In addition, when tested with the polyamines, spermine, spermidine and putrescine, only spermine effectively inhibited the current. When the dose-response curve was fit with the Hill function we found an EC50 of 28 mM and a Hill coefficient of about 2. Our results indicate that divalent cations and the polyamine, spermine, are effective modulators of calcium entry into cone-dominated bipolar cells. The in vivo regulation of the concentrations of these molecules provides an exquisitely sensitive mechanism for regulating Ca2+ entry into bipolar cells under different conditions.
Introduction
Transient changes in cytosolic Ca2+ play a pivotal role in intracellular signaling in neurons. Ca2+ modulates pathways such as dendritic signal integration, neurotransmitter release, synaptic plasticity, and ligand-gated ion channel function. The primary mechanism for increasing intracellular Ca2+ levels is by Ca2+ entry through voltage-activated Ca2+ channels.1 Neuronal Ca2+ channel activity is regulated as a means of controlling the amount of Ca2+ entering the cell. For example, neurotransmitters and neuromodulators as well as intrinsic agents such as divalent cations and polyamines have been shown to modulate the flow of Ca2+ through various types of Ca2+ channels.2,3 At present, our understanding is incomplete of the mechanism(s) of Ca2+ entry into retinal neurons and, in particular, the regulation of this entry.
In the retina, bipolar cells are second-order neurons that transfer information from the distal to the proximal retina. In the distal retina, they make synaptic contact with photoreceptor cells and horizontal cells and in the inner retina they are in contact with amacrine cells and ganglion cells.4 In some species they are also in contact with interplexiform cells.5 As with all neurons, Ca2+ entry into bipolar cells plays an important role in intracellular signal transduction processes including neurotransmitter release. This entry is regulated by a number of processes including the actions of neurotransmitters and neuromodulators. As mentioned above, in other neuronal systems, mechanisms intrinsic to the cell also have been shown to modulate Ca2+ channel activity6 but little is known about this in retinal bipolar cells. In fact, such intrinsic mechanisms have not been studied in detail in retinal neurons other than in photoreceptor cells.
In an effort to better understand intrinsic mechanisms that might regulate Ca2+ entry into bipolar cells, we studied Ca2+ currents found in isolated teleost bipolar cells. Here we report on divalent cations and the polyamine spermine as a mechanism for the bipolar cell to regulate its Ca2+ current and thus, the amount of Ca2+ entry into these cells.
Methods
Preparation
This study was conducted on bipolar cells isolated from the retina of the white bass (Morone chrysops). We have described the isolation procedure in detail in the past.7 In brief, eyes were removed from the fish following euthanasia in accordance with the guidelines set out by the Association for Research in Vision and Ophthalmology for the Use of Animal in Research. Retinas were removed from the eyes and placed in an enzyme (papain) solution. Following incubation, the retinas were then triturated and plated out into cell culture dishes. The cells were then used after between one to seven days in culture. Two types of bipolar cells result from the trituration procedure. Bipolar cells were identified by their characteristic morphology7 (see below).
Solutions
At the beginning of each experiment the tissue culture medium in a dish was replaced with white bass Ringer's containing the following (in mM): 130 NaCl, 2.5 CaCl2, 2.8 KCl,1 MgCl2,10 glucose and 8.4 HEPES. The pH was adjusted to 7.4 using NaOH. The recording pipette solution contained (in mM): 120 cesium chloride, 4 NaCl, 11 EGTA, 1 MgCl2, 8.4 HEPES and 20 tetraethylammonium. For the divalent free solution, both magnesium chloride (MgCl2) and calcium chloride (CaCl2) were omitted and EDTA and EGTA were added at 5 mM each. In the Na free solution, NaCl was replaced with an equimolar amount of choline chloride.
Solutions were applied from a microperfusion system (DAD12, ALA Scientific Instruments, Westbury, NY). Drugs could be rapidly applied and the solutions completely bathed the cell under study. Unless otherwise indicated, cells were perfused with the divalent free solution prior to test agent application. The test agents were dissolved in the divalent-free media.
Recording Procedures
Whole cell recordings were made with micropipettes that had been pulled on a two-stage puller (Narishige Instruments, Tokyo, Japan) and were used unpolished. The electrode tip resistance when measured in the bath solution was from 5 to 7 MΩ. Electrode capacitance and series resistance were compensated for electronically. Electrical potentials were recorded using an Axopatch 200A amplifier (Axon Instruments, Union City, California). A personal computer interfaced to a Digidata 1200 data acquisition system driven by the pClamp suite of programs (Axon Instruments) controlled data collection and analysis. The recordings shown are representative of three or more samples.
Results
In isolation, bipolar cells were identified by their characteristic shape. There are two types of bipolar cells found in the white bass retina following isolation.7 One has been termed a large bipolar and the other a small bipolar. The large bipolar corresponds to the mixed rod cone bipolar found in the teleost retina8 which is characterized by a relatively large cell body with several apical processes opposite of which is a long thin axon that ends in a relatively large oval terminal. Small bipolar cells have a small oval or tear-shaped soma and on the apical end, contain several small, fine dendritic processes. A long thin axon emanates from opposite the apical end to terminate in a small axonal terminal. These likely correspond to cone-dominated teleost bipolar cells. Our studies here were confined to the small bipolar cell type.
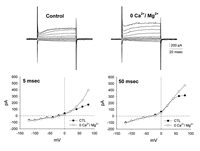
The complete membrane properties of bipolar cells in the bass retina have been previously described7 so we won't go into the details here. In Figure 1, in the upper left corner, is illustrated a control voltage clamp recording from a small bipolar cell. The current response to voltage commands is characterized by a slowly activating inward current that is characteristic of an inwardly rectifying H-type K current.9 Depolarizing responses are characterized by a slowly activating outward K current (IKV). Also present in these cells, but not visible in the control recordings because ofthe large K currents, is a small inward Ca2+ current which is elicited at voltage levels more positive than the holding potential.7
Since it was our desire to study the Ca2+ current, we tried to bring out the current by taking advantage of the fact that in a zero divalent cation solution, Ca2+ channels are highly permeable to monovalent ions and will conduct Na ions readily, generating a measurable inward current.10 It also took Ca2+ and Mg2+ away as possible modulators of the channel allowing us to study their action in isolation. So, voltage clamp recordings in a divalent free extracellular solution were made and revealed small voltage-dependent changes in the bipolar cell currents. This is illustrated in the upper right panel of Figure 1. In the lower half of the figure are shown current-voltage relationships measured 5 milliseconds after the onset of the voltage command (left) and 50 milliseconds following the onset of the voltage command (right). What was seen was a change in the outward currents evoked by the voltage steps. They increased in magnitude over control values at more depolarized levels. However, no inward current appeared in the voltage range typical of Ca2+ currents. It seemed likely that this was due to masking by K currents which were still relatively large.
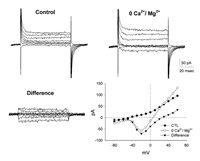
We next blocked the inward and outward K currents with 20 mM TEA in addition to cesium replacing K ions in the intracellular recording solution. This revealed a reduction in the K currents observed. The effect is illustrated in Figure 2. In the upper left corner is shown a control recording; the Ca2+ current was still not very prominent. We went on and tried the intracellular TEA in the presence of zero divalent Ringer's. The result of this manipulation is shown the upper right corner of the figure. In the zero divalent solution, both the inward and outward currents have increased in magnitude. The difference between the control currents (TEA and cesium in the pipette) and the zero Ca2+, zero Mg2+/control currents revealed the true inward current. In the lower right corner of Figure 2 is a current-voltage relationship illustrating the three recording conditions. One can see that the inward current was activated at around 50 mVs and reached a peak at about 20 mVs. Hence forth, all future recordings were made with TEA in addition to cesium in the recording pipette. Also, the I-V curves illustrate that the magnitude of the current was relatively small. We found this to be the case in all small bipolar cells from which we recorded.
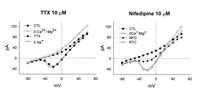
The I-V relationship and sustained time course of the currents that we recorded were similar to that of a high voltage-activated, L-type Ca2+ current, although the peak current occurred at a more hyperpolarized level than is characteristic for most L-type currents. However, we recorded this current in a zero Ca2+ medium. Thus, this current may be carried by Na ions flowing through Ca2+ channels.10 We next determined that in fact, it was the case that Na was flowing into these cells through voltage-activated Ca2+ channels and we further characterized the channel. Figure 3 (left) illustrates the effects of the Na channel blocker TTX on the current. Recordings were done in control conditions, zero Ca2+/Mg2+ solution and in the presence of TTX (10 mM). The I-V relationship shows the current in zero Ca2+/Mg2+ solution (open triangles) and the results of the application of TTX (solid squares). The TTX had no effect on the current measured, indicating that the current was not flowing through voltage-dependent Na channels. We then subjected the cells to a solution containing zero Na (see methods). Under these conditions, no current was observed to flow into the cell over the activation range for the Ca2+ channels (open diamonds). In a second set of experiments, we found that the Ca2+ channel blocker, nifedipine (10 μM), substantially inhibited the current. This is shown in Figure 3 to the right. The application of nifedipine resulted in an I-V curve in which the current was reduced by about 80% (solid triangles). Washing the cell with zero Ca2+/Mg2+ media resulted in a return to control as illustrated by the curve plotted with the open triangles. The L-type Ca2+ channel blocker diltiazam had a similar effect (not shown). The results from Figure 3 illustrate that indeed we are observing Na ions flowing through the bipolar cell Ca2+ channels in a zero Ca2+/Mg2+ solution and that these channels are likely to be similar to L-type channels. Consequently, we are able to measure the bipolar cell Ca2+ current in an indirect fashion and examine the modulatory actions of Ca2+ and Mg2+ in isolation.
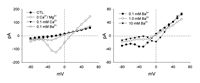
We next investigated the susceptibility of the current to block by divalent cations. Surprisingly, we found that as little as 0.1 mM Ca2+ in the extracellular media would substantially reduce the current. This is shown in Figure 4 (left). In this I-V plot, the current carried by Na through the Ca2+ channels is illustrated by the trace with the open circles. The trace with the solid triangles illustrates the current flowing through these channels when 0.1 mM Ca2+ is present in the bathing media. The current has been reduced by about 90% (the degree of block remained unchanged in 2.5 mM Ca2+). In a similar fashion, the divalent cation Ba2+ is also able to inhibit the current, but not to the same degree as Ca2+. This is illustrated in Figure 4, right, by the trace with the open triangles. Interestingly, increases in the concentration of Ba2+ did not improve the block. Rather, there was a depolarizing shift in the activation voltage and an increase in the peak current observed. Increasing the Ba2+ concentration from 0.1 mM to 1.0 mM produced a reduction in the size of the observed current. However, further increasing the concentration of Ba2+ to 10 mM caused a subsequent increase in the size of the current as well as a depolarizing shift in the activation range.
The results of Figure 4 (right) can be explained in terms of an anomalous molefraction effect between Na and Ba2+ ions9 in a multiion pore: in a divalent free solution, Na ions flow freely through the pore. At low concentration, Ba2+ is entering the Ca2+ channel but likely binding, thus producing a small block of the Na currents. At higher concentrations the repulsion between Ba2+ ions drives the current across the channels, which is now carried by Ba2+ ions. Anomalous molar fraction effects have been described in L- and N-type Ca2+ channels11,12 and in fact accounts for why Na can flow through the channel in the absence of divalents.13 The shift in the activation range has been previously observed and is probably related to surface charge effects.
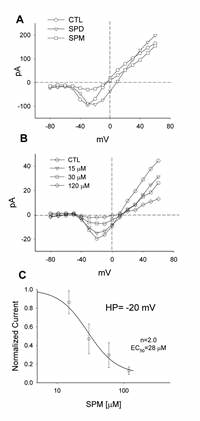
Intracellular polyamines at endogenous concentrations have been shown in past studies to block or modulate K channels,14–16 glutamate-activated channels17 and nicotinic acetylcholine activated ion channels18 in a voltage-dependent fashion. Polyamines applied extracellularly can also block K currents19 and L-type Ca2+ currents.20 So, we next investigated the effects of polyamine application. Figure 5A shows the effect of the polyamine spermine in zero Ca2+/Mg2+ media. The closed circles represent the control Na current. Application of spermine reduced the current by about 80%. We went on and tested other polyamines. Extracellular application of 200 μM putrescine had no effect on the divalent free currents (not shown). The polyamine spermidine (200 μM) did not show a blocking action although it tended to broaden the I-V relationship somewhat.
We found the block by spermine to be concentration dependent. This is illustrated in Figure 5B. Here the control I-V relationship of the Na current flowing through Ca2+ channels is compared to the currents measured with the application of 15, 30 and 120 mM of spermine. As can be seen from the I-V traces, as the concentration of the applied spermine increased, the peak of the I-V curve systematically became smaller. This dose-response relationship is plotted in Figure 5C. The response curve was then fit with the Hill function. From this we obtained an EC50 of 28 μM and a Hill-coefficient (n) of ˜2.0.
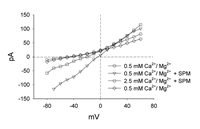
An unexpected effect was observed with the application of very high concentrations of spermine. This is illustrated in Figure 6. Rather than increasing the block of the current through the channel, a linear current developed. In the presence of a very low concentration of Ca2+ and Mg2+ (0.5 mM) the current through the Ca2+ channel carried by Na was blocked. In this same solution, with the application of 2 mM spermine, a large inward current was seen. When 2 mM of spermine was applied in the presence of a normal concentration of Ca2+ and Mg2+ (2.5 mM) the inward current was reduced by more than two-fold. The high concentration of spermine also eliminated the voltage-sensitive characteristics of the channel for unknown reasons. This may be due to surface charge effects or mass action simply driving the spermine through the channel. When the cells were returned to the control Ca2+/Mg2+ solution in the absence of spermine, the inward current was once again blocked. This indicates that the cell remains viable and this current was not due to some kind of toxic effect by the spermine. Results from this experiment suggest that at very high concentrations, spermine is able to flow through the Ca2+ channel without binding and producing a block. This is analogous to the block of rod photoreceptor cGMP-gated channels by spermine.21
Conclusion
Intrinsic mechanisms play an important role in altering an ion channel's ability to conduct current and thus regulate ion entry into a cell. In this study we have shown that divalent cations and the polyamine spermine play a central role in the modulation of Ca2+ channel conductance in teleost, cone-dominated bipolar cells (small bipolars).
Bipolar Cell Ca2+ Channels
Previous studies have demonstrated several types of Ca2+ channels in retinal bipolar cells. In rodents, both low voltage activated (LVA) and high voltage activated (HVA) currents are present.22,23 In lower vertebrates only HVA currents have been reported in bipolar cells of goldfish24,25 salamander26 and in ‘large’ (mixed rod-cone) bipolar cells of white bass,7 HVA and LVA Ca2+ currents are closely associated with neurotransmitter release in bipolar cell synaptic terminals.24,27,28
Consistent with this, HVA Ca2+ channels locate at higher densities in the synaptic terminal.24,25 Althought we did not record specifically from axon terminals, a presynaptic localization of Ca2+ channels is also likely in the case of the small bipolar cells in bass as can be inferred from our results in figures 1 and 2. As noted, the cells were clamped at the soma and not the synaptic terminal which here is very small in size (<3–4 μm). This is unlike the mixed rod-cone, Ab, bipolars in goldfish and carp that can have a terminal of ˜10 μm in diameter. In our studies, the currents through the Ca2+ channels were only evident (of sufficient magnitude) after blocking the K channels, suggesting that the channel blockage improved the capacity to adequately clamp the terminal at the end of the long and thin axonal process. While there is a high concentration of Ca2+ channels in the axon terminal, this is not to suggest that they solely contributed to the currents we recorded. It is likely that some of the Ca2+ channels underlying the Ca2+ currents, were located in other regions of the cell.
Despite the improved clamping conditions, we had to go to a divalent-free medium to enhance the magnitude of the currents flowing through the Ca2+ channels.10 Under these conditions the currents are carried by monovalent ions, predominantly Na. The currents are sustained in nature, and triggered at relatively depolarized levels. They are sensitive to nifedipine and not to TTX, consistent with the idea that although the currents are not carried by divalent ions, they flow through Ca2+ channels exhibiting anomalous mole fraction effects and increased permeability to Ba2+ ions. The I-V curve and the pharmacological sensitivity of the current suggest that it is flowing through channels that are, or closely resemble, L-type Ca2+ channels. This is consistent with results from other lower vertebrates.24,25
It is intriguing however that under normal conditions the magnitude of the current is negligible and cannot readily be measured. One possibility is that the small size of the synaptic terminal in these particular cells, if this is where the channels are predominantly located, results in a scaled down magnitude of the current. A second possibility is that conditions in culture may alter expression of the channels, or it may be possible that these are ‘silent’ currents that a regulatory agent, may modulate in amplitude25,29 and are only active under certain conditions. Lastly, they may simply be blocked in culture by the concentrations of divalents in the recording solution.
Ca2+ Channel Block
In cone-dominated bipolar cells, we found that the extracellular divalent cations Ca2+, Mg2+ and Ba2+ blocked the Ca2+ channels at physiological concentrations. In the absence of divalents, Na permeates the channel. This is due to the permeation properties of the channel. Energy profiles suggest that two Ca2+ ions are involved in the permeation process, whereas for Na, three ions are involved.13 Because of the energy profiles in the channel, it is easy for a Ca2+ ion to enter and drive out one or more Na ions, but once a Ca2+ ion is in the channel Na ions cannot do the same.13 A similar situation exists for Mg2+ and Ba2+, however, as illustrated by the “anomalous molefraction effect”, Ba2+ is less effective as a blocker.
We found that extracellular application of the polyamine spermine can reduce the magnitude of these currents at relatively low concentrations (EC50 = 28 μM). Polyamines are a group of amine containing compounds that are synthesized as an intermediate as part of the catabolism of arginine.30 Normally the intracellular concentration is highly regulated.31 Levels can rise under various conditions including in a circadian cycle32and interact with cellular processes in a variety of ways.3,31 As we showed here, in retinal bipolar cells they seem to interact with Ca2+ channels and alter the magnitude of the Ca2+ currents. Along these lines, in the case of certain disease states33 and cell lysis, local extracellular levels of spermine could rise to micromolar levels blocking entry of Ca2+, and perhaps exert a neuroprotective effect.
The mechanism by which polyamines act as Ca2+ channel blockers is related to their binding within the pore. We found spermine to be the most effective polyamine blocker. Spermine has four positive charges whereas spermidine has three, 3 so spermidine may not bind as effectively. As mentioned, two Ca2+ ions seem to be involved in the permeation process,13 suggesting that there are two binding sites for Ca2+ within the channel. Spermine more effectively interacts with these binding sites to effect a block. In fact, we found a Hill coefficient of ˜2 for spermine block, suggesting that a spermine molecule is binding at each site. This mechanism appears to be the way by which the polyamine toxin FTX acts.2,34,35 Or, it may be that spermidine binds, but because of its smaller size is not a sterically effective blocker.36 It is interesting that at low concentrations spermine blocks the channel but at high concentrations permeates it. This suggests that the block is probably only weakly to moderately voltage dependent36 although we did not test this specifically. Permeation of the channel also indicates that the block may not be difficult to overcome under certain circumstances.
Functional Implications
Our results here suggest that divalent cations and polyamines are effective agents for modulating Ca2+ entry into bipolar cells. In vivo, at rest it is likely little or no Ca2+ would enter the cell. Upon depolarization, only small amounts would enter, assuming normal levels of extracellular cations and physiological levels of polyamines.31 Should these levels be decreased, more Ca2+ would flow into the cell. In photoreceptors, it has been shown that polyamines block photoreceptor cyclic nucleotide-gated channels from both sides of the membrane.21 From the outside it acts as a permeant blocker. Lu and Ding 21 suggest that the polyamine blockmay play a role in suppressing noise in the signal transduction cascade in that it would prevent indiscriminant entry of Ca2+ which has a major role in the phototransduction cascade. Polyamines may be acting similarly in preventing Ca2+ entry into bipolar cells under particular conditions or at particular membrane potential levels. This then might prevent ‘noise’ in synaptic transmission under certain circumstances such as low light levels.
Obviously the concentration of extracellular cations and polyamines will determine the level of block that occurs. Along these lines, it is interesting that the concentrations of polyamines at which we observed a block, is roughly 30–300-fold lower than that which was needed for block in NMDA-activated channels.2,37–39 This suggests that extracellular polyamine concentrations are highly regulated in the retina and may exist at lower levels than found in the CNS, otherwise these channels might be permanently blocked. Unfortunately we know little of how or under what circumstances retinal levels of divalents and polyamines might be regulated. In other systems a variety of mechanisms exist for up and downregulating cation levels (transport, intracellular release, sequestering, etc.)1,40–42 and polyamine levels (ie. synthesis, transport, degradation, etc).3,30,31 These mechanisms may be active in the retina or it may be possible that function is regulated by activity.17 In this instance, increased levels of activity could lead to a decrease of synaptic levels of divalents and polyamines, resulting in greater influx of Ca2+. It is possible that M¨ller cells provide such a mechanism for clearing divalents and polyamines from the synaptic cleft.43–45 Whatever the case, it is clear that divalent cations and polyamines are powerful intrinsic modulators of bipolar cell Ca2+ channel activity.
Acknowledgments
This work was supported by an NIH grant EY05972 (E.M.L.) from the National Eye Institute and an unrestricted grant to the Department of Ophthalmology and Visual Sciences, University of Utah from Research to Prevent Blindness, Inc. (RPB). E.M.L. is an RPB Lew R. Wasserman Merit awardee.
References
- 1.
- Bertolino M, Llinas RR. The central role of voltage-activated and receptor-operated calcium channels in neuronal cells. Annu Rev Pharmacol Toxicol. 1992;32:399–421. [PubMed: 1318672]
- 2.
- Scott RH, Sutton KG, Dolphin AC. Interactions of polyamines with neuronal ion channels. Trends Neurosci. 1993;16:153–60. [PubMed: 7682349]
- 3.
- Johnson TD. Modulation of channel function by polyamines. Trends Pharmacol Sci. 1996;17:22–7. [PubMed: 8789355]
- 4.
- Dowling JE. The Retina: An approachable part of the BrainCambridge: The Belknap Press of Harvard University Press,1987. [PMC free article: PMC373129]
- 5.
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–3. [PubMed: 804181]
- 6.
- Scott RH, Pearson HA, Dolphin AC. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36:485–520. [PubMed: 1658850]
- 7.
- Lasater EM. Membrane currents of retinal bipolar cells in culture. J Neurophysiol. 1988;60:1460–80. [PubMed: 3193166]
- 8.
- Ishida AT, Stell WK, Lightfoot DO. Rod and cone inputs to bipolar cells in goldfish retina. J Comp Neurol. 1980;191:315–335. [PubMed: 7410596]
- 9.
- Hille B. Ionic Channels of Excitable Membranes Second Ed. Sunderland, Massachusetts: Sinauer Associates Inc,1992 . [PMC free article: PMC50168]
- 10.
- Almers W, McCleskey EW. Nonselective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984;353:585–608. [PMC free article: PMC1193323] [PubMed: 6090646]
- 11.
- Friel DD, Tsien RW. Voltage-gated calcium channels: Direct observation of the anomalous mole fraction effect at the single-channel level. Proc Natl Acad Sci USA. 1989;86:5207–11. [PMC free article: PMC297587] [PubMed: 2544893]
- 12.
- Wakamori M, Strobeck M, Niidome T. et al. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J Neurophysiol. 1998;79:622–34. [PubMed: 9463426]
- 13.
- Corry B, Allen TW, Kuyucak S. et al. A model of calcium channels. Biochim Biophys Acta. 2000;1509:1–6. [PubMed: 11118513]
- 14.
- Wible BA, Taglialatela M, Ficker E. et al. Gating of inwardly rectifying K channels localized to a single negatively charged residue. Nature. 1994;371:246–249. [PubMed: 8078584]
- 15.
- Fakler B, Brandle U, Glowatzki E. et al. Strong voltage-dependent inward rectification of inward rectifier K channels is caused by intracellular spermine. Cell. 1995;80:149–54. [PubMed: 7813010]
- 16.
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–91. [PubMed: 9074760]
- 17.
- Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci. 1998;18:8175–85. [PMC free article: PMC6792845] [PubMed: 9763464]
- 18.
- Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci. 1998;18:4050–62. [PMC free article: PMC6792788] [PubMed: 9592086]
- 19.
- Solessio E, Rapp K, Perlman I. et al. Spermine mediates inward rectification in potassium channels of turtle retinal Muller cells. J Neurophysiol. 2001;85:1357–67. [PubMed: 11287460]
- 20.
- Herman MD, Reuveny E, Narahashi T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J Physiol. 1993;462:645–60. [PMC free article: PMC1175320] [PubMed: 8392576]
- 21.
- Lu Z, Ding L. Blockade of a retinal cGMP-gated channel by polyamines. J Gen Physiol. 1999;113:35–43. [PMC free article: PMC2222994] [PubMed: 9874686]
- 22.
- Pan ZH. Differential expression of high and two types of low voltage-ctivated calcium currents in rod and cone bipolar cells of the rat retina. J Neurophysiol. 2000;83:513–27. [PubMed: 10634892]
- 23.
- de la Villa P, Vaquero CF, Kaneko A. Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur J Neurosci. 1998;10:317–23. [PubMed: 9753140]
- 24.
- Tachibana M, Okada T, Arimura T. et al. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from retinal bipolar cells. Ann NY Acad Sci. 1993;707:359–61. [PubMed: 9137568]
- 25.
- Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol (Lond). 1992;447:235–56. [PMC free article: PMC1176034] [PubMed: 1317429]
- 26.
- Maguire G, Maple B, Lukasiewicz P. et al. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamader. Proc Natl Acad Sci U S A. 1989;86:10144–7. [PMC free article: PMC298663] [PubMed: 2557620]
- 27.
- von Gersdorff H, Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994;367:735–9. [PubMed: 7906397]
- 28.
- Pan ZH. Voltage-activated Ca2+ channels and ionotropic GABA receptors localized at axon terminals of mammalian retinal bipolar cells. Vis Neurosci. 2001;18:279–88. [PubMed: 11417802]
- 29.
- Maguire G, Maple B, Lukasiewicz P. et al. γ-Aminobutyrate-type B receptor modulation of L-type calcium channel current at bipolar cell termimnals in the retina of the tiger salamander. Proc Natl Acad Sci U S A. 1989;86:10144–7. [PMC free article: PMC298663] [PubMed: 2557620]
- 30.
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–90. [PubMed: 6206782]
- 31.
- Morgan DM. Polyamines. An overview. Mol Biotechnol. 1999;11:229–50. [PubMed: 10503240]
- 32.
- Macaione S, Cangemi F, Fabiano C. et al. Putrescine, polyamines, and N1-acetylpolyamine levels in retina, visual cortex and cerebellum of free-running mice kept under continuous light or darkness. Ital J Biochem. 1993;42:151–64. [PubMed: 8407267]
- 33.
- Haghighi AP, Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J Neurosci. 1998;18:4050–62. [PMC free article: PMC6792788] [PubMed: 9592086]
- 34.
- Llinas R, Sugimori M, Lin JW. et al. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989;86:1689–93. [PMC free article: PMC286766] [PubMed: 2537980]
- 35.
- Williams K. Interactions of polyamines with ion channels [published erratum appears in Biochem J 1997 Sep 15;326(Pt 3):943] Biochem J. 1997;325:289–97. [PMC free article: PMC1218558] [PubMed: 9230104]
- 36.
- Oliver D, Baukrowitz T, Fakler B. Polyamines as gating molecules of inwardrectifier K+ channels. Eur J Biochem. 2000;267:5824–9. [PubMed: 10998040]
- 37.
- Rock DM, Macdonald RL. Polyamine regulation of N-methyl-D-aspartate receptor channels. Annu Rev Pharmacol Toxicol. 1995;35:463–82. [PubMed: 7598503]
- 38.
- Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13. [PubMed: 9067625]
- 39.
- Bahring R, Bowie D, Benveniste M. et al. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol (Lond). 1997;502:575–89. [PMC free article: PMC1159530] [PubMed: 9279810]
- 40.
- Llinas R, Moreno H. Local Ca2+ signaling in neurons. Cell Calcium. 1998;24:359–66. [PubMed: 10091005]
- 41.
- Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci. 1993;13:623–31. [PMC free article: PMC6576661] [PubMed: 8093901]
- 42.
- Bindokas VP, Yoshikawa M, Ishida AT. NaCa2+ exchanger-like immunoreactivity and regulation of intracellular Ca2+ levels in fish retinal ganglion cells. J Neurophysiol. 1994;72:47–55. [PubMed: 7965029]
- 43.
- Newman E, Reichenbach A. The Muller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–12. [PubMed: 8843598]
- 44.
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–8. [PMC free article: PMC2904245] [PubMed: 9592083]
- 45.
- Biedermann B, Skatchkov SN, Brunk I. et al. Spermine/spermidine is expressed by retinal glial (Muller) cells and controls distinct K channels of their membrane. Glia. 1998;23:209–20. [PubMed: 9633806]
- Regulation of Voltage-Sensitive Ca2+ Channels in Bipolar Cells by Divalent Catio...Regulation of Voltage-Sensitive Ca2+ Channels in Bipolar Cells by Divalent Cations and Polyamines - Madame Curie Bioscience Database
- DNA Primer Extension by Telomerase - Madame Curie Bioscience DatabaseDNA Primer Extension by Telomerase - Madame Curie Bioscience Database
- Caspase-Independent Cell Death Mechanisms - Madame Curie Bioscience DatabaseCaspase-Independent Cell Death Mechanisms - Madame Curie Bioscience Database
- Neurotrophins - Madame Curie Bioscience DatabaseNeurotrophins - Madame Curie Bioscience Database
- In Vitro Endothelialization: Its Contribution Towards an Ideal Vascular Replacem...In Vitro Endothelialization: Its Contribution Towards an Ideal Vascular Replacement - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...