NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Guanylate cyclase-activating proteins (GCAPs) control the activity of membrane-bound guanylate cyclases in vertebrate photoreceptor cells. They form a permanent complex with guanylate cyclase 1 (ROS-GC1) at low and high Ca2+ concentrations. Five different target regions of GCAP-1 have been identified in ROS-GC1 at rather distant sites. These findings could indicate a multipoint attachment site for GCAP-1 or, alternatively, the presence of transient binding sites with short contact to GCAP-1. In addition some data are consistent with the operation of one or more transducer units that represent regulatory regions without being direct binding sites. A permanent ROS-GC1/GCAP-1 complex is physiologically significant, since it allows a very short response time of cyclase activity when the intracellular Ca2+ concentration changes. Thereby, activation of cyclase participates in speeding up the recovery of the photoresponse after illumination and restores the circulating dark current.
Introduction
Light sensitivity of vertebrate photoreceptor cells is controlled by multiple feedback loops that include Ca2+-dependent and Ca2+-independent mechanisms 13 (see also other chapters in this book). A significant contribution to Ca2+-dependent feedback mechanisms is conferred by the Ca2+-sensitive regulation of two membrane-bound guanylate cyclases, which are synonymously termed ROS-GC1, retGC1, GC-E or ROS-GC2, retGC2, GC-F.4 Regulation of cyclases by Ca2+ is indirect and mediated by small EF-hand Ca2+-binding proteins which are named guanylate cyclase-activating proteins or GCAPs, of which three isoforms have been identified, GCAP-1, GCAP-2 and GCAP-3.5,6 So far most work on the expression profile of GCAPs and on their regulatory properties has been done with GCAP-1 and GCAP-2. For example rods of transgenic mice that do not express GCAP-1 and GCAP-2 (GCAPs−/−) show a higher light sensitivity than rods from wild-type mice.7 Rod cells from mutant mice also show a slowed time course of their flash response and slower kinetics of the recovery phase of a flash response. Guanylate cyclase activity of transgenic GCAPs−/− mice are normal, but any Ca2+-dependent regulation is absent. These results are consistent with previous biochemical observations that GCAPs have a strong effect on regulation of cyclase activity over the physiologically relevant range of free [Ca2+] from 600 nM in the dark-adapted state (low guanylate cylase activity) and 50 nM after illumination (high guanylate cyclase activity).4–6
GCAPs belong to a family of neuronal calcium sensor proteins (NCS) that show a wide distribution among species and within the nervous system. NCS proteins can be grouped into five subfamilies named recoverins, VILIPs, frequenins, K chips and GCAPs.8,9 In contrast to calmodulin that is ubiquitously expressed and can regulate a large variety of proteins NCS proteins seem to have a specialized function and target to a small set of proteins. This article discusses aspects concerning target recognition and protein-protein interactions of GCAPs when they regulate cyclases.
Complex of Guanylate Cyclase and GCAP
When guanylate cyclase activities are assayed in vitro, GCAP-1 and GCAP-2 activate ROS-GC1 severalfold at low free [Ca2+] and inhibit the enzyme at higher [Ca2+]3 1μM. ROSGC2 is activated by GCAP-2 and not by GCAP-1,10 which indicates that GCAPs and cyclases differ in their regulatory features. The physiological meaning of this different targeting, however, is unclear. GCAPs form a direct complex with cyclases and results from different experimental approaches are consistent with this model (see below). One method to demonstrate a direct interaction is chemical crosslinking. Proteins of interest in an existing complex react with bifunctional small organic molecules resulting in one or more crosslinked products of higher molecular mass. Most crosslinkers react with free amino or sulfhydryl groups in proteins. The presence of two (or more) proteins in a crosslinked product can then be detected by immunoblotting. Crosslinking of GCAP-1 and ROS-GC1 had been demonstrated by this approach.11,12
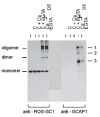
Crosslinking of GCAP-1
A typical result of a crosslinking experiment is shown in Fig. 1. The homobifunctional, water-insoluble crosslinker disuccinimidyl suberate (DSS) was incubated with a suspension of rod outer segments containing ROS-GC1 and endogeneous GCAP-1. Proteins were separated by SDS-PAGE, electroblotted and detected by specific antibodies against GCAP-1 and ROS-GC1. The results show several features of a cyclase/GCAP complex:
- ROS-GC1 forms a dimer at 230 kDa and an oligomer that barely enters the gel.
- Three bands are visible in the molecular range above 100 kDa that were immunoreactive for a GCAP-1 antibody in the presence of crosslinker but were absent in control incubations without crosslinker. One of these bands (see arrows indicating band 1) comigrate with the oligomer ROS-GC1 band which indicates a crosslinked product of ROS-GC1 and GCAP-1.
- Crosslinking was observed in the presence and absence of Ca2+, which indicates that the complex of cyclase and GCAP-1 exists under high and low [Ca2+]. In fact, crosslinking was more efficient at high [Ca2+] as can be seen in the more intense labelling of band 1 in the presence of Ca2+ by the anti-GCAP-1 antibody.
- Crosslinking was performed by a water-insoluble substance, which points to a ROS-GC1/GCAP-1 contact site in the vicinity of the membrane.
Interaction of Guanylate Cyclases and GCAPs at Low and High [Ca2+]
The existence of a guanylate cyclase/GCAP complex at low and high [Ca2+] as revealed by chemical crosslinking was also inferred from other experiments. Ca2+-loaded GCAP-2 and to a lesser degree also Ca2+-loaded GCAP-1 inhibit ROSGC activity, leading to a lower basal activty at micromolar free [Ca2+].13,14 Under these conditions ROS-GC1 activity is lower than in washed membrane preparations where no GCAP is present. Furthermore, Ca2+-loaded GCAP-1 and GCAP-2 can compete with Ca2+-insensitive mutants of GCAPs that activate ROS-GC constitutively in a Ca2+-independent way.13,15,16 Co-immunoprecipitation studies had shown that GCAP-1, ROS-GC1 and tubulin form a complex at high and low [Ca2+].17 Thus it seems that dissociation of GCAP from the guanylate cyclase/GCAP complex is not triggered by a change in [Ca2+]. However, the complex is not very tight, since GCAPs can easily be washed off from rod outer segment membranes. This is different from the regulatory features of some calmodulin/target complexes that cannot be disrupted by complexing Ca2+. Examples are phosphorylase b kinase and the inducible form of nitric oxide synthase (NOS-II), which show strong binding to Ca2+-calmodulin and Ca2+-free calmodulin (apocalmodulin).18
In summary the current working hypothesis is that GCAPs exist in a permanent complex with ROS-GCs. A change in the intracelluar [Ca2+] triggers a conformational change in GCAP leading to a rearrangement within the complex. In consequence ROSGC is transformed into a state of higher catalytic activity. A first step to decipher this switch mechanism has been to identify regions in ROS-GC1 that directly interact with GCAP-1 or represent critical sites for regulation. Since all published work on this specific matter so far was done on ROS-GC1 and GCAP-1, I restrict the following discussion to this protein pair.
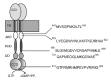
Target Regions in ROS-GC1
Three different groups have identified a total of 5 different sites in ROS-GC1 that are thought to represent target regions of GCAP-1. These sites are located in rather distant regions in the cytoplasmic part of the enzyme, two are in the juxtamembrane domain (JMD), two are in the kinase homolgy domain (KHD) and one is in the cyclase catalytic domain (CCD) (Fig. 2). Lange et al19 identified two regions in the juxtamembrane domain (445M-456L; 503L-522I) by screening a synthetic peptide library and by site-directed mutagenesis of hypothetic regulatory regions. The screening of the peptide library was restricted to the ROS-GC1 domain between amino acids 437R-552G. The library was screened with peptides overlapping by 10 out of 12 amino acids. This region was chosen because in a prior study, deletion- and hybrid-analysis of ROS-GC1 had revealed that this domain was pivotal in the GCAP modulation of the cyclase.12 GCAP-1 regulatory regions identified in the study by Lange et al gave a hint to explain the molecular causes of the rod dystrophy Leber's congenital amaurosis (LCA1) where the patients are born blind or soon become blind after birth.20 In these patients there is a point mutation in the ROS-GC1 gene between the region 503L-522I. Biochemical studies on heterologously expressed ROS-GC1 mutants show that this LCA mutant has completely lost its sensitivity to modulation by GCAP-1.21
Krylov and Hurley11 found two regions in KHD, 680S-699E and 724Q-738A by a different experimental strategy. They first stabilized ROS-GC1/GCAP-1 complexes by homobifunctional crosslinking of sulfhydryl groups in spatially proximate cysteines. These complexes were digested with trypsin in a second step and then analyzed by mass spectrometry to find crosslinked peptides. Candidate regions in ROS-GC1 and GCAP-1 were identified by assigning peaks in the mass spectrum. Synthetic peptides of candidate regions were then used in peptide competition studies.
Sokal et al22 identified a region in the catalytic domain, 966G-983G, which corresponds to 910G-927G in the mature protein (Fig. 2). In this study the authors screened the whole cytoplasmic domain with peptides of 15 amino acids in length with 8 amino acids overlapping by a similar competition assay as Lange et al.19 Interestingly, the region in the CCD shows a 2–3-fold higher affinity for GCAP-2 than for GCAP-1 as revealed by peptide titration experiments. Thus, the identified region in this study may represent the GCAP-2 site in ROS-GC1. This interpretation is consistent with the results of earlier studies, which demonstrated by the use of deletion and chimeric mutants that the GCAP-2 modulated domain in ROS-GC1 resides at its C-terminus.23,24
Apparent Affinities of Target Regions in ROS-GC1
Peptide competition experiments were used in all three studies to estimate apparent IC50 values (concentration of peptide at which inhibition is halfmaximal). All reported values are within the same order of magnitude, the two peptides in the JMD showed an IC50 between 100 and 260 μM, the two peptides in the KHD had an IC50 between 30 and 80 μM and the peptide of the CCD had an IC50 of 290 μM. If the identified regions represent true binding sites, then each site alone would bind GCAP-1 only with rather low affinity. Activation of ROS-GC1 by GCAP-1 is half-maximal at 0.8 μM in enzymatic assays.19,23 The apparent dissociation constant of GCAP-1 and ROS-GC1 then is expected to be 2 0.8 μM. All reported IC50 values are two to three orders of magnitude higher. Although the three studies do not rely solely on peptide competition studies and include site-directed mutagenesis, peptide-affinity column chromatography and crosslinking, they do not give a conclusive answer about right or wrong target sites so far. However, a large cytoplasmic fragment (aa7331054) of ROS-GC1 that includes three of the identified target regions (two in the KHD and one in the CCD) exhibits a small, but measurable cyclase activity. Interestingly this fragment is not stimulated by GCAP-1, but can still be activated by GCAP-2 at low [Ca2+].25 In addition this fragment can be activated by S100b, a small Ca2+-binding protein that stimulates ROS-GC1 activity severalfold at micromolar [Ca2+]. These experiments show that many properties regarding structural integrity are retained in the ROS-GC1 fragment aa733–1054, but that necessary regions for GCAP-1 recognition are not present.
Model of GCAP-1 Mediated Activation of ROS-GC1
The conflicting results on target regions in ROS-GC1 could indicate a multipoint attachment site for GCAP-1.19 Taking into account that all peptides are rather weak inhibitors in competition assays it is reasonable that several regions in ROS-GC1 are needed to form together a site of higher affinity for GCAP-1. These sites could well be located in distant regions of the molecule. The different results could also reflect the existence of several transient binding sites. These sites might have a short contact with GCAP-1, when GCAP-1 changes its conformational state due to binding or release of Ca2+. It is also possible that some regions are important for the switch from a low activity state to a high activity state in the cyclase as this has been proposed for the region 445M-456L in the JMD, that was assigned as transducer unit.19 Such a site must not necessarily be a direct binding site, but it could represent an important regulatory unit within the cytoplasmic part of the cyclase that is necessary to transmit the Ca2+-signal from GCAP-1 to the catalytic center of the cyclase.
Physiological Significance of a Permanent ROS-GC1/GCAP-1 Complex
It is somehow surprising that GCAP-1 and ROS-GC1 form a complex that is not disrupted by a change in free [Ca2+]. Why does GCAP-1 bind to ROS-GC1 in both its Ca2+-loaded and its Ca2+-free form? When a photoreceptor cell is excited by light, the cytoplasmic [Ca2+] decreases and the guanylyl cyclase activity increases with a time constant of approx. 200 msec.26 Therefore, the activation of ROS-GC1 participates significantly in speeding up the recovery of the photoresponse after saturating light flashes and restores the circulating dark current.1 A permanent complex of GCAP-1 and ROS-GC1 would immediately respond to changes in cytoplasmic [Ca2+] after illumination. No additional (time-consuming) diffusion step is necessary. Dissociation of Ca2+ from the EF-hand Ca2+-binding sites in GCAP-1 would then induce a conformational change in GCAP-1 that is directly transmitted to the cyclase catalytic domain. If ROS-GC1 and the built-in Ca2+-sensor GCAP-1 operate in the time frame of a photoresponse, the Ca2+-sensing of GCAP-1 must also fulfill at least two requirements: 1. the dissociation of Ca2+ from the EF-hand Ca2+-binding sites must be fast enough (less than 200 msec) and 2. the conformational change in GCAP-1 must be sufficient to trigger a several-fold increase in the catalytic turnover rate of the cyclase.
Affinities of GCAP-1 for Ca2+
According to a systematic study by Renner et al 27 relative affinities of Ca2+ among different groups of Ca2+-binding proteins can be predicted. If EF-hands contain a Glu at the ninth position, they release Ca2+ with slower dissociation rates than EF-hands that contain either an amino acid with a shorter side chain (Asp) or an uncharged side chain (Gln). Ca2+-buffer proteins like parvalbumin have slower dissociation rates of Ca2+ than Ca2+-trigger like calmodulin or troponin C (for review ref. 28). All three functional EF-hands in GCAP-1 (and also in GCAP-2 and GCAP-3) contain either an Asp or a Thr/Ser at the ninth position. GCAPs fit therefore perfectly into the group of Ca2+ trigger proteins with rapid off kinetics (mean residence time of Ca2+ would be a few milliceconds). A recent study reported an intermediate affinity of EF-hand 3 for Ca2+ in GCAP-1.29 With an apparent KD of 2.9 μM and the assumption that the on rate of Ca2+ to all three EF-hands is under control of diffusion 30 (k1=2 × 108 M1s1; KD=1/k1) the authors obtained an offrate of k1 = 5.8 × 103 s1, which would result in a time constant of ˜2 ms. Thus, dissociation of Ca2+ from EF-hand 3 is fast enough to trigger a conformational change in GCAP-1 and subsequent activation of ROS-GC1.
GCAP-1 probably undergoes a large conformational change upon binding Ca2+ ions. This change has been previously monitored by different methods, e.g., tryptophan fluorescence spectroscopy, Ca2+-induced shifts in electrophoretic mobility and limited proteolysis.30,32 This conformational change could be sensed by ROS-GC1 at one or more of the defined sequence motifs (see above) and then transmitted to the catalytic center to increase the turnover rate of cGMP synthesis.
Dimerization of ROS-GC1
Efficient catalysis of GTP to cGMP in ROS-GC1 requires the formation of a correct dimer interface. Thus, the catalytically active form of ROS-GC1 is a dimer. The dimerization domain DD that is located between the KHD and CCD forms a coiled-coil domain and is critical for a correct dimerization of the whole protein25,33,35 (Fig. 2). Mutations in the DD in the ROS-GC1 gene are linked to a cone-rod dystrophy (CORD6) and cause a severe distortion of dimer formation and GC activity.25 Concomitant with an impaired dimer formation is a decrease in basal, i.e., unstimulated cyclase activity. However some CORD6-related mutations cause a hypersensitivity to activation by GCAP-1.25,35 It seems that binding of GCAP-1 can compensate for some loss of catalytic efficiency when the dimer formation is distorted. Binding of GCAP-1 seems to control formation of a correct dimer interface in the CCD without interaction with the DD. It is however unclear how GCAP-1 can control this step, whether it binds to the CCD directly or whether it triggers the correct conformational change by binding to a distant site. To resolve this point conflicting results on target and binding sites as outlined above need to be clarified by further experiments.
Conclusion
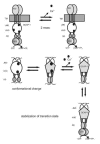
In summary the following sequence of events is plausible when ROS-GC1 is activated by GCAP-1 (Fig. 3). In the unstimulated state Ca2+-loaded GCAP-1 binds to a site in ROS-GC1 that is close to the membrane (see interpretation of crosslinking experiment in Fig. 1), which could be 503L-522I in the JMD and/or 680S-E699 and 724Q-A738 in the KHD. Decrease of cytoplasmic [Ca2+] is sensed by GCAP-1 first on its third EF-hand, which rapidly loses its Ca2+ ion and induces a large conformational change in GCAP-1 around EF-hand 3. This change leads first to a reorientation of the DD coiled-coil helices,35 which influences the conformation of the CCD monomers in the catalytic center. GCAP-1 might be bound to several noncontiguous binding sites even when it undergoes a conformational change after dissociation of Ca2+. The affinity to every single binding site probably changes and Ca2+-free GCAP-1 might be transferred to a different location where it can stabilize the active state of ROS-GC1 probably by stabilization of the transition state during catalysis, but this has to be tested experimentally. GCAP-2 probably binds to different sites in ROS-GC1. It is therefore reasonable to speculate that a different sequence of events takes place when GCAP-2 activates ROS-GC1.
Acknowledgement
I thank D. Höppner-Heitmann for excellent technical assistance. Work from this laboratory was supported by grants from the Deutsche Forschungsgemeinschaft (DFG).
References
- 1.
- Pugh E N Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr Opinion Neurobiol. 1999;9:410–18. [PubMed: 10448166]
- 2.
- Fain GL, Matthews HR, Cornwall MC. et al. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. [PubMed: 11152756]
- 3.
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. [PubMed: 11520918]
- 4.
- Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230:97–106. [PubMed: 11952100]
- 5.
- Palczewski K, Polans AS, Baehr W. et al. Ca2+binding proteins in the retina: structure, function, and the etiology of human visual diseases. BioEssays. 2000;22:337–350. [PubMed: 10723031]
- 6.
- Dizhoor AM, Hurley JB. Regulation of photoreceptor membrane guanylyl cyclases by guanylyl cyclase activator proteins. Methods. 1999;19:521–531. [PubMed: 10581151]
- 7.
- Mendez A, Burns ME, Sokal I. et al. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci U S A. 2001;98:9948–9953. [PMC free article: PMC55558] [PubMed: 11493703]
- 8.
- Braunewell KH, Gundelfinger ED. Intracellular neuronal calcium sensor proteins: a family of EF-hand calciumbinding proteins in search of a function. Cell Tissue Res. 1999;295:1–12. [PubMed: 9931348]
- 9.
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article: PMC1221537] [PubMed: 11115393]
- 10.
- Haeseleer F, Sokal I, Li N. et al. Molecular characterization of a third member of the guanylyl cyclase-activating protein subfamily. J Biol Chem. 1999;274:6526–6535. [PubMed: 10037746]
- 11.
- Krylov DM, Hurley JB. Identification of proximate regions in a complex of retinal guanylyl cyclase 1 and guanylyl cyclaseactivating protein1 by a novel mass spectrometry-based method. J Biol Chem. 2001;276:30648–30654. [PubMed: 11387342]
- 12.
- Duda T, Goraczniak R, Surgucheva I. et al. Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry. 1996;35:8478–8482. [PubMed: 8679607]
- 13.
- Dizhoor AM, Hurley JB. Inactivation of EF-hands makes GCAP-2 (p24) a constitutive activator of photoreceptor guanylyl cyclase by preventing a Ca2+-induced activator-to-inhibitor transition. J Biol Chem. 1996;271:19346–19350. [PubMed: 8702620]
- 14.
- Otto-Bruc A, Buczylko J, Surgucheva I. et al. Functional reconstitution of photoreceptor guanylate cyclase with native and mutant forms of guanylate cyclase-activating protein 1. Biochemistry. 1997;36:4295–4302. [PubMed: 9100025]
- 15.
- Dizhoor AM, Boikov SG, Olshevskaya EV. Constitutive activation of photoreceptor guanylate cyclase by Y99C mutant of GCAP-1. J Biol Chem. 1998;273:17311–17314. [PubMed: 9651312]
- 16.
- Sokal I, Li N, Surgucheva I. et al. GCAP-1 (Y99C) mutant is constitutively active in autosomal dominant cone dystrophy. Mol Cell. 1998;2:129–133. [PubMed: 9702199]
- 17.
- Schrem A, Lange C, Beyermann M. et al. Identification of a domain in guanylyl cyclase-activating protein 1 that interacts with a complex of guanylyl cyclase and tubulin in photoreceptors. J Biol Chem. 1999;274:6244–6249. [PubMed: 10037711]
- 18.
- Jurado LA, Chockalingam PS, Jarret HW. Apocalmodulin. Physiol Rev. 1999;79:661–682. [PubMed: 10390515]
- 19.
- Lange C, Duda T, Beyermann M. et al. Regions in vertebrate photoreceptor guanylyl cyclase ROS-GC1 involved in Ca2+dependent regulation by guanylyl cyclase-activating protein GCAP-1. FEBS Lett. 1999;460:27–31. [PubMed: 10571055]
- 20.
- Perrault I, Rozet JM, Calvas P. et al. Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat Genet. 1996;14:461–464. [PubMed: 8944027]
- 21.
- Duda T, Venkataraman V, Goraczniak R. et al. Functional consequences of a rod outer segment membrane guanylate cyclase (ROS-GC1) gene mutation linked with Leber's congenital amaurosis. Biochemistry. 1999;38:509–515. [PubMed: 9888789]
- 22.
- Sokal I, Haeseleer F, Arendt A. et al. Identification of a guanylyl cyclase-activating protein-binding site within the catalytic domain of retinal guanylyl cyclase 1. Biochemistry. 1999;38:1387–1393. [PubMed: 9931003]
- 23.
- Krishnan A, Goraczniak RM, Duda T. et al. Third calcium-modulated rod outer segment membrane guanylate cyclase transduction mechanism. Mol Cell Biochem. 1998;178:251–259. [PubMed: 9546607]
- 24.
- Goraczniak RM, Duda T, Sharma RK. Calcium-modulated signaling site in type 2 rod outer segment membrane guanylate cyclase (ROSGC2). Biochem Biophys Res Commun. 1998;245:447–453. [PubMed: 9571173]
- 25.
- Duda T, Venkataraman V, Jankowska A. et al. Impairment of the rod outer segment membrane guanylate cyclase dimerization in a cone-rod dystrophy results in defective calcium signaling. Biochemistry. 2000;39:12522–12533. [PubMed: 11027131]
- 26.
- Calvert PD, Ho TW, LeFebvre YM. et al. Onset of feedback reactions underlying vertebrate rod photoreceptor light adaptation. J Gen Physiol. 1998;111:39–51. [PMC free article: PMC1887766] [PubMed: 9417133]
- 27.
- Renner M, Danielson MA, Falke JJ. Kinetic control of Ca(II) signaling: Tuning the ion dissociation rates of EF-hand Ca(II) binding sites. Proc Natl Acad Sci U S A. 1993;90:6493–6497. [PMC free article: PMC46958] [PubMed: 8341660]
- 28.
- Falke JJ, Drake SK, Hazard AL. et al. Molecular tuning of ion binding to calcium signaling proteins. Q Rev Biophys. 1994;27:219–290. [PubMed: 7899550]
- 29.
- Hwang JY, Schlesinger R, Koch KW. Calcium-dependent cysteine reactivities in the neuronal calcium sensor guanylate cyclase-activating protein 1. FEBS Lett. 2001;508:355–359. [PubMed: 11728451]
- 30.
- Sokal I, OttoBruc AE, Surgucheva I. et al. Conformational changes in guanylyl cyclase-activating protein 1 (GCAP-1) and its tryptophan mutants as a function of calcium concentration. J Biol Chem. 1999;274:19829–19837. [PubMed: 10391927]
- 31.
- Frins S, Bönigk W, Müller F. et al. Functional characterization of a guanylyl cyclase-activating protein from vertebrate rods. J Biol Chem. 1996;271:8022–8027. [PubMed: 8626484]
- 32.
- Rudnicka-Nawrot M, Surgucheva I, Hulmes JD. et al. Changes in biological activity and folding of guanylate cyclase-activating protein 1 as a function of calcium. Biochemistry. 1998;37:248–257. [PubMed: 9425045]
- 33.
- Yang RB, Garbers DL. Two-eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742. [PubMed: 9153227]
- 34.
- Liu Y, Ruoho AE, Rao VD. et al. Catalytic mechanism of the adenylyl and guanylyl cyclases: Modeling and mutational analysis. Proc Natl Acad Sci U S A. 1997;94:13414–13419. [PMC free article: PMC28319] [PubMed: 9391039]
- 35.
- Ramamurthy V, Tucker C, Wilkie S. et al. Interactions within the coiled-coil domain of RetGC1 guanlyly cyclase are optimized for regulation rather than for high affinity. J Biol Chem. 2001;276:26218–26229. [PubMed: 11306565]
- Introduction
- Complex of Guanylate Cyclase and GCAP
- Crosslinking of GCAP-1
- Interaction of Guanylate Cyclases and GCAPs at Low and High [Ca2+]
- Target Regions in ROS-GC1
- Apparent Affinities of Target Regions in ROS-GC1
- Model of GCAP-1 Mediated Activation of ROS-GC1
- Physiological Significance of a Permanent ROS-GC1/GCAP-1 Complex
- Affinities of GCAP-1 for Ca2+
- Dimerization of ROS-GC1
- Conclusion
- Acknowledgement
- References
- Target Recognition of Guanylate Cyclase by Guanylate Cyclase-Activating Proteins...Target Recognition of Guanylate Cyclase by Guanylate Cyclase-Activating Proteins - Madame Curie Bioscience Database
- Vascular Endothelial Growth Factor - Madame Curie Bioscience DatabaseVascular Endothelial Growth Factor - Madame Curie Bioscience Database
- The Biology of Telomeres in Hypotrichous Ciliates - Madame Curie Bioscience Data...The Biology of Telomeres in Hypotrichous Ciliates - Madame Curie Bioscience Database
- Homo sapiens small EDRK-rich factor 1A (SERF1A), transcript variant 1, mRNAHomo sapiens small EDRK-rich factor 1A (SERF1A), transcript variant 1, mRNAgi|1890270737|ref|NM_021967.4|Nucleotide
- Gossypium hirsutum isolate:1008001.06Gossypium hirsutum isolate:1008001.06Gossypium hirsutum isolate:1008001.06 RefSeq Raw sequence readsBioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...