NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
TRAF2 and TRAF5 are closely related members of the TRAF family of proteins. They are important signal transducers for a wide range of TNF receptor superfamily members, in cluding TNFR1, TNFR2, CD40 and other lymphocyte costimulatory receptors, RANK/TRANCE-R, EDAR, LTβR, LMP-1 and IRE1. TRAF2 and TRAF5 therefore regulate diverse physiological roles, ranging from T and B cell signaling and inflammatory responses to organogenesis and cell survival. The major pathways mediated by TRAF2 and TRAF5 are the classical and alternative pathways of NF-κB activation, and MAPK and JNK activation. TRAF2 is heavily regulated by ubiquitin signals, and many of the signaling functions of TRAF2 are mediated through its RING domain and likely its own role as an E3 ubiquitin ligase.
Introduction
The focus of this chapter will be on TRAF2 and TRAF5, which are closely related in both structure and function. Since they play important roles in mediating signals induced by the TNF receptor superfamily, the physiological roles of TRAF2 and TRAF5 will be discussed in the context of the receptors that they associate with. In addition, TRAF2 has been reported to play roles in LMP-1 signaling and endoplasmic reticulum (ER) stress responses. These two signaling contexts will be discussed at the end of this chapter.
TNF-associated factor 2 (TRAF2), a 56kD protein, was discovered through yeast two hybrid screening for proteins interacting with the c-terminal region of human TNF receptor 2.1 Along with TRAF1, TRAF2 was one of the first members of the TRAF protein family to be identified. TRAF5 was later discovered through yeast two hybrid interaction, while screening for proteins binding to the cytoplasmic tail of CD40.2 Furthermore, TRAF5 was independently identified as a protein interacting with the lymphotoxin β receptor (LTβR).3
Like all TRAF family members, TRAF2 and TRAF5 are characterized by a highly conserved carboxy-terminal TRAF domain, which can be further subdivided into TRAF-N and TRAF-C domains. The TRAF domain mediates receptor binding, interactions with a number of adapter and signaling molecules, self association, and interactions with other TRAF proteins. TRAF2 can oligomerize with itself or with TRAF1 or TRAF6.1,4 TRAF5 also associates with itself, but is also known to hetero-oligermize with TRAF3.5 In addition to the conserved TRAF domain, TRAF2 and TRAF5 each contain an N-terminal ring finger domain followed by five zinc fingers and a coiled-coil domain.
TRAF5 is highly similar to TRAF2 both structurally and functionally. However, whereas TRAF2 is expressed ubiquitously, TRAF5 expression is only found at significant levels in lung, thymus, spleen, and kidney and at lower levels in brain and liver.1,2,6 This more restricted expression pattern may explain to some extent why deletion of TRAF2 leads to perinatal lethality whereas deletion of TRAF5 only leads to more specific defects in CD40 and CD27 mediated lymphocyte activation. On the other hand, double knockouts of TRAF2 and TRAF5 suggest some functional redundancy between these two molecules in the context of TNF induced NF-κB activation.6-8
Mechanisms of TRAF2/5 Mediated Signal Transduction
There have been many studies over the years that have examined TRAF2 signaling and regulation. TRAF5 has also been examined, albeit to a lesser extent, therefore, the focus of this section will be on TRAF2.
TRAF2/5 and NF-kB Activation
NF-κB is one of the primary pathways activated by TRAF2 and TRAF5. Since NF-κB activation is not significantly impaired in mouse embryonic fibroblasts derived from TRAF2 and TRAF5 single knockouts, but is significantly reduced in TRAF2/TRAF5 double knockouts, there is some functional redundancy between the two molecules in this context.8
Activation of most receptors, including TNFR1, result in the activation of the canonical NF-κB pathway. The canonical pathway generally depends on the activation of IKKβ and IKKγ/NEMO by upstream kinases, including the involvement of the TAK1 kinase complex.9 The IKK complex, consisting of IKKα, IKKβ and IKKγ/NEMO, then goes on to phosphorylate IκB, which targets the molecule for ubiquitination and proteasome-mediated degradation. As IκB normally binds and sequesters NF-κB in the cytoplasm, its degradation results in release and translocation of NF-κB to the nucleus. The canonical pathway results in the formation of primarily p65/RelA-p50 heterodimers.10-14
Early studies have indicated that an intact RING domain is important for TRAF2 functions, including activation of NF-κB and JNK. The RING presence of the domain also suggested a role for ubiquitination in TRAF2 function and regulation. Interestingly mutational analyses also indicated that the RING domain and fourth zinc finger are necessary for TRAF2 ubiquitination.15 Mutational analyses have also identified that the amino-terminal ring finger and two adjacent zinc fingers of TRAF2 are required for NF-κB activation.16 Like TRAF2, TRAF5 contains a similar RING domain.2
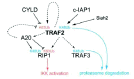
TRAF2 associates with the E2 ligase complex Ubc13-Uev1A to catalyze the synthesis of polyubiquitin chains through a lysine-63 (K63) linkage.15 K63 linkage poly-ubiquitin chains are found an TRAF2, TRAF6, RIP1 and NEMO, and are therefore important for signalling in TNFR family pathways. In TRAF2-deficient cells, K63 polyubiquitination of RIP1 is defective, indicating that TRAF2 is likely the E3 ligase involved in RIP1 ubiquitination.17 Alternatively, TRAF2 may be required for recruiting other E3 ligases, such as A20, to help processing and turnover (see Fig. 1).18,19 Since recruitment of the TAK1 kinase complex is dependent on ubiquitinated RIP1, TRAF2 mediated ubiquitination is likely critical in activating the canonical NF-κB pathway.17 It as also been shown that TNFR1 activation of the IKK complex and NF-κB activation requires both RIP1 and TRAF2, where RIP1 is responsible for IKK activation and TRAF2 is necessary for recruitment of IKK to the complex.11,20 Interestingly, while overexpression of TRAF2 RING domain mutants incapable of auto-ubiquitination suggested that the RING domain is not necessary for IKK activation, complete deletion the RING domain prevented IKK activation.15 Furthermore, TRAF2 is also known to complex with proteins such as TANK and the kinase T2K/TBK1, which have also been shown to play a role in NF-κB activation.21,22
Activation of NF-κB may also occur through an alternative pathway. This pathway is primarily found in B-cells, however, it can be present in other cell types as well. The noncanonical or alternative pathway depends on activation of NIK and IKKα. IKKα activation leads to NFκB2/p100 processing to p52 and the formation of p52/RelB-p50 heterodimers.23-27
TRAF2 has been implicated in both activation and negative regulation of the noncanonical NF-κB pathway. As mentioned later in the discussion of the role of TRAF2 in CD40 signaling, conditional knockout of TRAF2 in B-cells results in high levels of alternative NF-κB pathway activation, suggesting that TRAF2 can inhibit p100/p52 processing.25 On the other hand, mutation of the TRAF2/5 binding site on CD40 abolished p52/RelB translocation to the nucleus, suggesting that TRAF2 and TRAF5 may be required for noncanonical NF-κB pathway activation as well.28
Regulation of the alternative pathway by TRAF2 is complicated further by the involvement of TRAF3. In the context of CD40 signaling, TRAF3 overexpression has been recently found to inhibit of TRAF2/TRAF5 mediated activation of the alternative pathway, but not TRAF6 dependent activation of the canonical pathway.28 Interestingly, TRAF2-deficient B cells appear to have increased levels of TRAF3, indicating that TRAF2 helps target TRAF3 for ubiquitinantion and degradation(see Fig. 1).29
TRAF2 and JNK and MAPK Activation
TRAF2 and TRAF5 also signal through MAPK induction, primarily through activation of JNK. TRAF2 has been found to interact with a variety of upstream MAP3Ks, including MEKK1 and ASK-1, germinal center kinase and germinal center kinase kinase.30-33 TRAF5-deficient cells do not, however, demonstrate detectable impairment in JNK activation in response to TNF.7
K63 ubiquitination of TRAF2 appears to be critical for activation of JNK. TRAF2 is able to bind ASK1, GCK and GCKR through its RING domain, however, siRNA knockdown of Ubc13 has shown that activation of GCKR and the SAPK/JNK pathway also depends on the presence of Ubc13 E2 ligase complex. Activation of ASK1, in contrast, only marginally depends on Ubc13, and neither p38 MAPK nor IKKβ activation is affected by knockdown of Ubc13.15,34
Regulation of TRAF2
TRAF2 signaling also appears to be regulated by translocation. Recruitment of TNFR1, TRADD, RIP and TRAF2 to plasma membrane lipid rafts is important for signalling NF-κB activation.35 Furthermore, TRAF2 ubiquitination appears to coincide with the translocation of TRAF2 to the insoluble membrane/cytoskeletal fraction, and appears to have a role in regulating TRAF2 levels.
Studies have demonstrated that translocation to lipid rafts precedes ubiquitination, and have also suggested that compartments such as the endoplasmic reticulum may play roles in modulating TNFR signalling.35,36 Upon TNFR2 engagement, c-IAP1, an E3 ligase, can ubiquitinate TRAF2.37 Recent research has shown that c-IAP1 associates with the E2 ligase Ubc6, which is a an ER transmembrane protein. c-IAP1 and Ubc6 are responsible for synthesis of K48 type ubiquitin chains on TRAF2 that target it for degradation in a proteasome-dependent manner.36,37 In addition to c-IAP1, the E3 ligase Siah2 has also been found to regulate TRAF2 levels through ubiquitination. Using Siah2-deficient cells from knockout mice, it was found that Siah2 targeted TRAF2 for degradation under stress conditions, including TNF stimulation with cyclohexamide and UV irradiation.38
TRAF2 is also regulated by de-ubiquitinating enzymes. The tumour suppressor CYLD has be found to inhibit NF-κB activation. CYLD appears to regulate NF-κB by binding and removing ubiquitin chains on TRAF2, therefore preventing TRAF2 activation of the IKK complex.39-41 A20, a TNF-inducible gene, has also been found to interact with TRAF2 and inhibit NF-κB activation.18 A20 has been found to possess both ubiquitin ligase and de-ubiquitination activity, and is known to downregulate NF-κB activity by removing K63 ubiquitin chains from RIP1 and by adding K48 ubiquitin. It is probable that A20 is also involved in de-ubiquitinating and de-activating TRAF2.19
Finally, it is also important to note that TRAF2 can target itself for degradation through K48 ubiquitin chain synthesis. CD40 induced TRAF2 degradation, for example, requires an intact TRAF2 RING domain.42 The duality of the TRAF2 E3 ligase, in that it is able to generate ubiquitin chains that lead to both activation (such as RIP1) or inactivation (such as TRAF3), gives this molecule a unique role depending what it interacts with (see Fig. 1).
Receptors and Pathway Anchor Proteins that Utilize TRAF2 and TRAF5
TNFR1
The role of TRAF2 is perhaps best characterized for TNF signaling through TNF receptor 1 (TNFR1). TNF is a major proinflammatory mediator, and can induce apoptosis under certain circumstances. It is responsible for not only immune response, but also development and tissue regeneration, and has been found to have pathophysiological roles in septic shock, autoimmune disease, and cancer. TNFR1 appears to be the key mediator of TNF signalling in the majority of cells.
Upon TNF binding, TNFR1 recruits several signaling proteins to its cytoplasmic death domain. TNFR1-associated death domain protein (TRADD) is first recruited via its death domain to the death domain of TNFR1, and acts as an adaptor molecule. Fas-associated death domain protein (FADD) interacts with the carboxy-terminal death domain of TRADD, which exposes the death effector domain of FADD, allowing FADD to recruit caspase 8/FLICE, which leads to the activation of the apoptotic cysteine protease cascade.43-47 RIP1 is a serine-threonine kinase that binds the carboxy-terminal death domain of TRADD in a TNF-dependent manner. TRADD also directly interacts with TRAF2 via its amino-terminal half43,46-49 Recent studies have suggested that a complex involving TNFR1, TRADD, RIP1 and TRAF2 at the plasma membrane is formed first, and rapidly signals NF-κB activation and cell survival. A second complex consisting of TRADD, TRAF2, RIP1, FADD and caspase-8 is formed later in the cytoplasm to signal cell death under certain contexts.50 (see Fig. 2)
Half of mice deficient in TRAF2 die at E14.5 with a similar phenotype to RelA deficient mice, whereas the rest are born normal but are runted and die prematurely with atrophy of the thymus and spleen, and show elevated serum TNF levels. Thymocytes and other hematopoietic cells also show extreme sensitivity to TNF induced cell death. These phenotypes suggest that TRAF2 plays an important physiological role in regulating cell survival, particularly in response to TNF, since TRAF2 knockout mice can be rescued by crossing with TNF or TNFR1 knockout.6,51 On the other hand, targeted disruption of TRAF5 in mice does not lead to perinatal lethality, suggesting that it has a more minor role in TNF cytoprotection. Furthermore, hyperactivity of certain TNF responses, including increase NO and TNF production by macrophages, has also been observed in TRAF2 knockout mice, indicating that TRAF2 also has an important role in regulating TNF mediated immune responses.51
Activation of NF-κB and JNK/SAPK may be important pathways through which TRAF2 mediates cytoprotection against TNF. From knockout studies, it is known that RIP1 is essential for NF-κB activation induced by TNF, and that NF-κB activation is essential for cell survival in response to TNF.13,52 However, as mentioned previously, TRAF2-deficient cells are not significantly defective in NF-κB activation6,52,53 even though overexpression of TRAF2 or TRAF5 can activate NF-κB in cells.2,3,54 TRAF2 and TRAF5 double knockout cells, however, do demonstrate more impaired NF-κB activation than single knockouts, suggesting that TRAF5 may compensate for the lack of TRAF2 in this signalling pathway.8 The TNFR1-TRADD-RIP1-TRAF2 signaling complex primarily leads to induction of the classical NF-κB signalling pathway. As discussed in the previous section, this depends on the activation of IKKβ and IKKγ/NEMO, and results in the formation of primarily p65/RelA-p50 heterodimers.
TRAF2 and TRAF5 have also been implicated in MAPK activation and regulation of the AP-1 transcription factor, as cells lacking TRAF2 also demonstrate severe impairment in JNK/SAPK activation upon TNFR1 stimulation.6 Cells deficient in both TRAF2 and TRAF5 have been found in some cases to demonstrate a delayed but prolonged MAPK activation in response to TNF, which has been linked to increased TNF-induced reactive oxygen species signalling and induction of cell death.6,55
TRAF2 also promotes survival in response to TNF by recruiting c-IAP1 and c-IAP2 to the TNFR1 complex. c-IAP1 and c-IAP2, both typical members of the BIR domain containing inhibitors of apoptosis family, are able to prevent caspase-3 activation and apoptosis.48,56,57
TNFR2
Unlike TNFR1, TNFR2 does not possess a Carboxy-terminal death domain, and TRAF2 directly binds to the cytoplasmic tail of TNFR2. While TRAF1 cannot bind directly to TNFR2, TRAF1 can be recruited to complex indirectly via interaction with TRAF2, and may act as a negative regulator of TNFR2 signaling through TRAF2.1,58 TRAF5 has not been found to bind to the cytoplasmic tail of TNFR2.2 Signaling downstream of TNFR2 and TRAF2 is relatively similar to TNFR1. As mentioned previously, RIP1 can bind to TRAF2, and also associates with TNFR2. TNFR2 recruitment of TRAF2 is also involved in both NF-κB and MAPK activation, indicating that TRAF2 is important in the crosstalk between TNFR2 and TNFR1.59,60
As mentioned previously, TRAF2 interacts with both c-IAP1 and c-IAP2. This interaction was initially identified as part of TNFR2 complex.61 More recent studies looking at the TNFR2-TRAF2 complex have demonstrated that the carboxy-terminal of c-IAP1 acts as an E3 ubiquitin ligase that is able to ubiquitinate TRAF2 and target it for proteasomal degradation.37 As TRAF2 typically signals cell survival through NF-κB and JNK activation, this suggests a mechanism through which proteins recruited by TRAF2 can enhance TNF induced apoptosis, and that TNFR2 activation can help regulate TNFR1 signals.
CD40
CD40 is a TNFR family member that is expressed constitutively by antigen presenting cells, such as B-lymphocytes, macrophages and dendritic cells. Activation by its ligand, CD40L/CD154, induces a variety of effector functions, including upregulation of molecules involved in antigen presentation and B and T cell interactions, antibody production, isotype switching, cytokine secretion, and protection from apoptosis.62,63
Both TRAF2 and TRAF5 have been implicated in CD40 signaling. Although TRAF5 was originally identified as a protein binding to the cytoplasmic domain of CD40, subsequent studies have shown that TRAF5 recruitment to CD40 is indirect through hetero-oligomerization with TRAF3. TRAF2, on the other hand, is able to directly associate with CD40.5 TRAF1, TRAF2, and TRAF3 associate with CD40 via a PVQET motif, wherease TRAF6 associates in a different region. Competition and different combinations of TRAF recruitment to CD40 may therefore contribute to modulating receptor signals across different cell types.64
While CD40 can induce p65RelA-p50 NF-κB activation, CD40 is also known to induce NFκB2/p100 processing and the alternative NF-κB pathway.27 Dominant negative TRAF2, which lacks the amino-terminal RING finger domain, inhibits CD40 mediated NF-κB activation.54 Studies using TRAF2-deficient B cell lines expressing mutant CD40 defective in TRAF6 binding have also shown that NF-κB pathway activation, as demonstrated through IκB phosphorylation and degradation, is impaired when both TRAF2 and TRAF6 binding are absent. However, neither TRAF2 nor TRAF6 binding alone are indispensable for CD40-induced NF-κB activation.29
More recent studies looking at conditional knockout of TRAF2 in B-cells have shown that while TRAF2 is necessary for canonical activation of NF-κB in response to CD40, deficiency in TRAF2 actually results in hyperactivity of the alternative NF-κB pathway. TRAF2-deficient B-cells demonstrated a survival advantage and upregulation of CD21/35. TRAF2 can therefore act as a negative regulator of p100/p52 processing.25 In contrast to TRAF2, deficiency in TRAF5 does not affect NF-κB or JNK signalling in response to CD40.7 (see Fig. 3)
TRAF2 has also been found to be important in B-cell receptor (BCR) and CD40 synergy. Antigen stimulation of BCR leads to activation of a variety of downstream signalling molecules and second messengers, including members of the protein kinase C family (PKC) and protein kinase D (PKD). Pharmacological inhibition of PKD in B-lymphocytes was found to prevent CD40 and BCR synergy. B cells expressing a mutant CD40 defective in TRAF2 binding also demonstrate a BCR-CD40 synergy defect, however, overexpression of constitutively active PKD in these cells is unable to overcome the defect observed, indicating that TRAF2 is required for PKD-mediated enhancement of BCR-CD40 signals.29,65,66
Deficiencies in either TRAF2 or TRAF5, however, demonstrated CD40 signaling defects in vivo. Crossing with TNF or TNFR1-knockout mice aids the survival of TRAF2-knockout mice and has allowed the investigation of TRAF2-deficiency on CD40 responses in lymphocytes. TRAF2-deficiency results in impaired isotype switching and failure to mount IgG responses induced by vesicular stomatitis viral infection. TRAF2-deficiency also leads to defective CD40 mediated proliferation and NF-κB activation in splenocytes.51 TRAF5-deficient mice reveal impairment of CD40 stimulated B-cell proliferation and upregulation of surface markers, and also show mild defects in affinity maturation of IgG antibodies.7
TACI, BCMA
BCMA (B-cell maturation antigen) and TACI (transmembrane activator and CAML interactor) are TNF receptor superfamily members that share the ligands BAFF (B-cell activating factor) and APRIL. Both receptors are expressed primarily on B-lymphocytes. TRAF2, TRAF5 and TRAF6 have been shown to associate with TACI. TRAF2 and TRAF5 share a binding motif, but the majority of positive clones from yeast-two hybrid were TRAF2-TACI interactions, suggesting that TRAF2 may play a more prominent role. Like other TNF receptor superfamily members interacting with TRAFs, activation of TACI also results in NF-κB and JNK activation.67
TRAF1, TRAF2 and TRAF3 interact with the cytoplasmic region of BCMA. Analyses of deletion mutants of the TRAF binding domain in BCMA demonstrated that TRAF association is required for NF-κB, Elk-1 and JNK activation in response to BCMA.67,68 However, although BAFF stimulation is important for B cell survival and proliferation, the phenotypes of mice deficient in TACI and BCMA indicated that these receptors are not responsible for the survival signal. There is no obvious phenotype for the BCMA knockout,69,70 and TACI-deficient mice actually show increased numbers of B-cells.71 The survival signal was actually found to be mediated primarily through TRAF3 by BAFF activation of BAFF-R. Furthermore, while activation of BAFF-R leads to alternative NF-κB pathway activation, which has been implicated in B-cell survival, activation of BCMA or TACI does not.71,72 Therefore, signalling through BCMA or TACI through TRAF2 appears to mediate signals other than BAFF-R signals.
CD30
CD30 is a cell surface receptor characteristic of activated T-lymphocytes. CD30 stimulation can lead to cell proliferation, survival, differention, or cell death, depending on cell type and costimulation. CD30 is also an important marker for Hodgkin's and other lymphomas, and is upregulated in several virally transformed cell lines.73
Like many other TNFR family members, signalling through CD30 is transduced through TRAFs and can lead to activation of NF-κB and MAPKs.74-76 Two different regions in the c-terminal tail of CD30 are capable of binding TRAFs. The more N-terminal domain in the tail can bind TRAF3, TRAF2, and TRAF5, whereas the more C-terminal domain can bind TRAF1, TRAF2 and TRAF5. Expression of a dominant negative TRAF2 or TRAF5 resulted in impaired CD30 mediated NF-κB activation. While TRAF2 and TRAF5 are both implicated in NF-κB activation in response to CD30 stimulation, mutation of a more membrane proximal domain that is not known to bind TRAFs can also abrogate CD30 induced NF-κB activation.74,75,77,78
CD27
CD27 is a receptor expressed on T, B, and NK cells. CD27 plays an important role in T cell interactions and T and B cell interactions, and provides an important costimulatory signal for proliferation.73 Both TRAF2 and TRAF5 interact with the cytoplasmic tail of CD27. Deletion analysis of the cytoplasmic domain identified a critical motif that is necessary for CD27 mediated NF-κB and JNK activation, and that this motif coincides with the binding site for TRAF2 and TRAF5. Overexpression of dominant negative TRAF2 or TRAF5 was also found to block NF-κB activation.79,80 In vivo, thymocytes from TRAF5-deficient mice demonstrate defects in CD27 costimulation of CD3-induced T cell proliferation. However, NF-κB and JNK activation are not noticeably altered in these cells, which may be due to either compensation from other TRAFs or a role for TRAF5 in CD27 signaling that does not require NF-κB or JNK.7 Furthermore, since CD27 has been implicated in regulation of humoral responses, the effect of TRAF deficiency on both CD40 and CD27 responses may contribute to observed lymphocyte phenotypes.
Ox40
Ox40 is another TNFR superfamily member involved in costimulation, and is expressed on activated T cells. Studies from Ox40-deficient mice demonstrate important roles for this receptor in regulating the number of effector T cells during primary immune response, and the number of memory T cells that develop and remain.73
TRAF1, TRAF2, TRAF3 and TRAF5 have all been found to associate with the cytoplasmic domain of Ox40. NF-κB activation in response to Ox40 stimulation appears to depend on TRAF2 and TRAF5 as deletion of the TRAF binding site in Ox40 or overexpression of dominant negative TRAF2 or TRAF5 can block NF-κB activation. In contrast, TRAF3 appears to act as a negative modulator.81,82 In vivo, TRAF2 has been implicated in Ox40-mediated memory T cell expansion. T cells from OVA-specific TCR transgenic mice crossed with dominant negative TRAF2 mice were adoptively transferred to naï BALB/c recipients, and stimulated with antibody to Ox40. The increase in antigen-specific T cells after Ox40 engagement was reduced with TRAF2 deficiency, and Ox40 engagement only enhanced the survival of antigen specific cells in wildtype but not mutant cells expressing dominant negative TRAF2.83 TRAF5 has also recently been implicated in regulating T cell differentiation to Th1 and Th2 lineages by modulating Ox40 stimulation. Immunization of TRAF5-deficient mice with protein in adjuvant plus anti-Ox40 antibody leads to increased Th2 development.84
4-1BB
4-1BB, like CD27 and OX40, is another T cell costimulatory molecule, and is thought be be involved in antigen presentation, generation and long term survival of cytotoxic T lymphocytes, and induction of helper T cell anergy.73
TRAF1, TRAF2 and TRAF3 are known to bind to the cytoplasmic domain of 4-1BB. Like many other TNF receptor superfamily members, activation of 4-1BB leads to NF-κB activation. However, expression of dominant negative TRAF2 can inhibit 4-1BB induction of NF-κB.81,85 Furthermore, while 4-1BB engagement results in activation of NF-κB and IL-2 production in wild-type T-lymphocytes, TRAF2-deficient lymphocytes are defective in this response.86 4-1BB induced TRAF2 dependent IL-2 production, however, appears to be mediated primarily through JNK activation through ASK-1.87 TRAF2 has also been shown to be required for p38 MAPK activation in response to 4-1BB, which is thought to be critical for the development of Th1 and Th2 reponses.88
LTβR
Lymphotoxin (LT) α and β can heterotrimerize to form three distinct ligands for lymphotoxin β receptor (LTβR). LIGHT is another ligand for LTβR, but also interacts with HVEM. The signalling pathways controlled by these receptors and ligands are involved in lymphoid tissue development and organization, adaptive and innate immune responses, and central tolerance.89 LTs can also bind TNFR1 and TNFR2. While LTβR and TNFR1/2 activation elicit distinct downstream signals, they also have complementary and overlapping functions, and employ shared mechanisms of signal propagation, including TRAF2 and TRAF5. LTβR is also known to bind TRAF3.3,90,91
TRAF2 is able to interact directly with the cytoplasmic domain of LTβR.92 Recent studies have shown that LTβR stimulation is able to activate the alternative NF-κB pathway.93,94 NF-κB2/p100 knockout and LTβR knockout have similar phenotypes - both showing aberrant development of peripheral lymphoid organs - indicating that alternative pathway activation significantly contributes to the physiological role of LTβR signalling.95-97 TRAF2 appears to participate directly in LTβR mediated induction of both classical and alternative NF-κB pathways. However, TRAF2-deficient animals do not show defects in lympho-organogenesis. JNK activation induced by LIGHT stimulation of LTβR is also absent in TRAF2-deficient cells. Interestingly, unlike TNF signalling, LIGHT induced NF-κB and JNK activation are normal in both TRAF5-deficient and RIP1-deficient cells.90
HVEM/ATAR
The other receptor bound by LIGHT and LTα is the herpes virus entry mediator HVEM, which is expressed on lymphocytes. HVEM activation generally confers anti-apoptotic and proliferative signals to cells, and is thought to be important in T-cell costimulation, activation and modulation.73,89
Yeast two hybrid analyses have shown that HVEM interacts directly with TRAF2 and TRAF5 but not TRAF3.98 Like other TNF receptor super family members discussed so far, recruitment of TRAF2 and TRAF5 to HVEM leads to NF-κB, JNK and AP-1 activation.98,99 Remarkably, coexpression of HVEM with TRAF5, but not TRAF2, leads to synergistic NF-κB activation,98 suggesting that TRAF2 and TRAF5 may play different roles downstream of HVEM.
RANK/TRANCER
TRANCE/RANKL/OPGL, a survival factor for activated dendritic cells, binds TRANCE-R/ RANK. More importantly, RANK signalling is crucial for osteoclast activation and differentiation and therefore critical for maintaining bone homeostasis.100,101
TRAF2 and TRAF5, in addition to TRAF 1 and TRAF3, can interact with the cytoplasmic tail of RANK via two different motifs.102 TRAF6 also binds RANK, but in a distinct region more proximal to the membrane.103 RANK signalling leads to NF-κB activation and JNK activation that is mediated through TRAFs. Dominant negative forms of TRAF2, TRAF5 and TRAF6 are all able to inhibit NF-κB activation induced through RANK.104 TRAF6, however, is likely the key adapter for TRANCE-R, as TRAF6-deficient mice are phenotypically similar to TRANCE-R-deficient mice. Unlike TRAF6-deficient mice, however, neither TRAF2 nor TRAF5-deficient mice exhibit osteopetrosis, suggesting a more minor role for these TRAFs in osteoclastogenesis induced by RANK signalling.105,106
EDAR
Mutation of the ectodysplasin-A (Eda) receptor (EDAR) or the X-linked Eda receptor (XEDAR) leads to hypohidrotic ectodermal dysplasia (HED), a disease characterized by loss of hair, sweat glands and teeth.107
Unlike XEDAR, which can asscociate directly with TRAF3 and TRAF6, EDAR is similar to TNFR1 and unable to bind to TRAFs directly. EDAR utilizes the adaptor EDARADD, which associates via its death domain to the cytoplasmic death domain of EDAR. EDARADD is then able to recruit TRAF1, TRAF2 and TRAF3, and possibly with TRAF5 and TRAF6 as well.108,109 There is considerable evidence suggesting that NF-κB is important for EDAR signalling. Hypomorphic mutations that inhibit IKKγ/NEMO activity result in defects similar to those seen in HED.110,111 Although it is likely that TRAF2 and TRAF5 are involved in EDAR induced NF-κB activation, it is currently unknown whether these TRAFs play a role in ectodermal organ development.
p75NTR
The common neurotrophin receptor p75NTR is unusual as it binds dimeric neurotrophins, unlike the majority of TNF receptor superfamily members which bind trimeric ligands. Signaling through this receptor controls apoptosis in neurons under conditions such as neurotrophin withdrawal or exposure to inappropriate neurotrophins.112
All six TRAF proteins have been shown to bind p75NTR in vitro. Curiously, TRAF2 appears to bind preferentially to the monomeric form of the receptor, unlike TRAF4 or TRAF6. Interactions with different TRAFs also have different effects - whereas coexpression of p75NTR with TRAF2 appears to enhance cell death, coexpression with TRAF6 is cytoprotective. Both TRAF2 and TRAF6 are able to induce NF-κB activation, albeit to a lesser extent by TRAF2.113
TAJ/TROY
TAJ/TROY is a TNF superfamily orphan receptor, recently identified to have a role in regulation of axonal regeneration, via association with Nogo-66 receptor 1.114 Earlier coimmunoprecipitation experiments have shown that TAJ/TROY is capable of binding TRAFs 1, 2, 3 and 5 in vitro. TAJ was also shown to activate the JNK pathway, however, dominant negative TRAF2 or TRAF5 is unable to block TAJ mediated JNK activation,115 so these TRAFs may be involved in other TAJ signalling pathways.
GITR
Glucocorticoid-induced TNFR-related receptor (GITR) is a TNFR superfamily member expressed on T lymphocytes, and is activated by GITRL, which is expressed mainly on endothelial and antigen presenting cells. GITR is thought to have a role in augmenting T cell responses and a pathophysiological role in autoimmune disease.73,116
TRAF1, TRAF2, TRAF3, and TRAF4 have been found to interact with the cytoplasmic domain of GITR in a ligand-dependent manner. GITR stimulation also leads to activation of NF-κB, and this was found to require TRAF recruitment. However, recent studies have also shown that TRAF2 can have an inhibitory effect on NF-κB activation in response to GITR signalling.117,118
LMP-1
Epstein Barr Virus is an etiological factor in many lymphomas, including Burkitt's lymphoma and Hodgkin's disease. The latent membrane protein 1 (LMP-1) of Epstein Barr Virus is crucial for B-lymphocyte transformation, and is known to have transforming effects on nonlymphoid cells as well.119 It was found that LMP-1 essentially a constitutively active TNF receptor family member, and able to associate with TRAF1, TRAF2 and TRAF3. LMP-1 induction of NF-κB appears to partially depend on TRAF1 and TRAF2, since dominant negative TRAF2 is able to block NF-κB activation.120,121
ER stress and IRE1
TRAF2 also has a unique role in endoplasmic reticulum (ER) stress pathways. Misfolded proteins in the ER, induced by stress conditions such as starvation or hypoxia, can induce cellular stress responses. These responses are mediated by IRE1s, which are ER membrane receptors that sense stress through their lumenal domains and transduce the signal across the ER via their cytoplasmic domains, leading to JNK activation. IRE1 was originally identified in yeast as the inositol auxotrophy gene, and mammalian homologs have been recently identified.122-124
Induction of IRE1 leads to JNK activation that is dependent on TRAF2. TRAF2 was found to bind the cytoplasmic region of IRE1, a dominant negative TRAF2 is able to inhibit IRE1 induction of JNK.125 Additional studies have shown that JNK inhibitory kinase (JIK) also associates with IRE1 and TRAF2 in a complex to modulate IRE1-TRAF2 activation of the JNK pathway. Furthermore, in this pathway, TRAF2 is capable of binding and inducing oligomerization of caspase-12 and therefore its cleavage and activation. Activation of caspase-12 then promotes an apoptosis in response to ER stress.126
Conclusion
TRAF2, and to a lesser extent TRAF5, play critical roles in the signalling of many TNF receptor superfamily members. As these pathways share TRAF2 and TRAF5, these proteins are likely critical for signal integration and crosstalk. TRAF2 has a particularly diverse set of functions, as it is able to act as an activator or as an inhibitor under different contexts, in addition to its role as an adaptor protein.
The diverse set of receptors that rely on TRAF2 and TRAF5 for signal transduction also highlights their importance in the regulation of a wide range of physiological processes, including adaptive and innate immunity, inflammation, development, and cell survival. Dysregulation of these signalling pathways can result in pathophysiological states such as autoimmune disease and cancer. Since effective strategies for therapy may be derived from targeting molecules in these pathways, an understanding of the key roles played by TRAF2 and TRAF5 is critical.
References
- 1.
- Rothe M, Wong SC, Henzel WJ. et al. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78(4):681–692. [PubMed: 8069916]
- 2.
- Ishida TK, Tojo T, Aoki T. et al. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93(18):9437–9442. [PMC free article: PMC38446] [PubMed: 8790348]
- 3.
- Nakano H, Oshima H, Chung W. et al. TRAF5, an activator of NF-kappaB and putative signal transducer for the lymphotoxin-beta receptor. J Biol Chem. 1996;271(25):14661–14664. [PubMed: 8663299]
- 4.
- Cao Z, Xiong J, Takeuchi M. et al. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383(6599):443–446. [PubMed: 8837778]
- 5.
- Pullen SS, Miller HG, Everdeen DS. et al. CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: Regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry. 1998;37(34):11836–11845. [PubMed: 9718306]
- 6.
- Yeh WC, Shahinian A, Speiser D. et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7(5):715–725. [PubMed: 9390694]
- 7.
- Nakano H, Sakon S, Koseki H. et al. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc Natl Acad Sci USA. 1999;96(17):9803–9808. [PMC free article: PMC22291] [PubMed: 10449775]
- 8.
- Tada K, Okazaki T, Sakon S. et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276(39):36530–36534. [PubMed: 11479302]
- 9.
- Takaesu G, Surabhi RM, Park KJ. et al. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326(1):105–115. [PubMed: 12547194]
- 10.
- Rudolph D, Yeh WC, Wakeham A. et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14(7):854–862. [PMC free article: PMC316493] [PubMed: 10766741]
- 11.
- Devin A, Lin Y, Yamaoka S. et al. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol. 2001;21(12):3986–3994. [PMC free article: PMC87061] [PubMed: 11359906]
- 12.
- Li Q, Van Antwerp D, Mercurio F. et al. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284(5412):321–325. [PubMed: 10195897]
- 13.
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274(5288):782–784. [PubMed: 8864118]
- 14.
- Van Antwerp DJ, Martin SJ, Kafri T. et al. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274(5288):787–789. [PubMed: 8864120]
- 15.
- Habelhah H, Takahashi S, Cho SG. et al. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. Embo J. 2004;23(2):322–332. [PMC free article: PMC1271753] [PubMed: 14713952]
- 16.
- Takeuchi M, Rothe M, Goeddel DV. Anatomy of TRAF2. Distinct domains for nuclear factor-kappaB activation and association with tumor necrosis factor signaling proteins. J Biol Chem. 1996;271(33):19935–19942. [PubMed: 8702708]
- 17.
- Lee TH, Shank J, Cusson N. et al. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279(32):33185–33191. [PubMed: 15175328]
- 18.
- Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci USA. 1996;93(13):6721–6725. [PMC free article: PMC39093] [PubMed: 8692885]
- 19.
- Wertz IE, O'Rourke KM, Zhou H. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. [PubMed: 15258597]
- 20.
- Devin A, Cook A, Lin Y. et al. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12(4):419–429. [PubMed: 10795740]
- 21.
- Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. Embo J. 1999;18(23):6694–6704. [PMC free article: PMC1171732] [PubMed: 10581243]
- 22.
- Bonnard M, Mirtsos C, Suzuki S. et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. Embo J. 2000;19(18):4976–4985. [PMC free article: PMC314216] [PubMed: 10990461]
- 23.
- Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity. 2004;21(4):477–489. [PubMed: 15485626]
- 24.
- Lin X, Mu Y, Cunningham Jr ET. et al. Molecular determinants of NF-kappaB-inducing kinase action. Mol Cell Biol. 1998;18(10):5899–5907. [PMC free article: PMC109176] [PubMed: 9742107]
- 25.
- Grech AP, Amesbury M, Chan T. et al. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21(5):629–642. [PubMed: 15539150]
- 26.
- Dobrzanski P, Ryseck RP, Bravo R. Differential interactions of Rel-NF-kappa B complexes with I kappa B alpha determine pools of constitutive and inducible NF-kappa B activity. Embo J. 1994;13(19):4608–4616. [PMC free article: PMC395393] [PubMed: 7925301]
- 27.
- Coope HJ, Atkinson PG, Huhse B. et al. CD40 regulates the processing of NF-kappaB2 p100 to p52. Embo J. 2002;21(20):5375–5385. [PMC free article: PMC129074] [PubMed: 12374738]
- 28.
- Hauer J, Puschner S, Ramakrishnan P. et al. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci USA. 2005;102(8):2874–2879. [PMC free article: PMC549490] [PubMed: 15708970]
- 29.
- Hostager BS, Haxhinasto SA, Rowland SL. et al. Tumor necrosis factor receptor-associated factor 2 (TRAF2)-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J Biol Chem. 2003;278(46):45382–45390. [PubMed: 12958312]
- 30.
- Baud V, Liu ZG, Bennett B. et al. Signaling by proinflammatory cytokines: Oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13(10):1297–1308. [PMC free article: PMC316725] [PubMed: 10346818]
- 31.
- Hoeflich KP, Yeh WC, Yao Z. et al. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1). Oncogene. 1999;18(42):5814–5820. [PubMed: 10523862]
- 32.
- Yuasa T, Ohno S, Kehrl JH. et al. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem. 1998;273(35):22681–22692. [PubMed: 9712898]
- 33.
- Shi CS, Leonardi A, Kyriakis J. et al. TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related. J Immunol. 1999;163(6):3279–3285. [PubMed: 10477597]
- 34.
- Shi CS, Kehrl JH. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J Biol Chem. 2003;278(17):15429–15434. [PubMed: 12591926]
- 35.
- Legler DF, Micheau O, Doucey MA. et al. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18(5):655–664. [PubMed: 12753742]
- 36.
- Wu CJ, Conze DB, Li X. et al. TNF-alpha induced c-IAP1/TRAF2 complex translocation to a Ubc6-containing compartment and TRAF2 ubiquitination. Embo J. 2005;24(10):1886–1898. [PMC free article: PMC1142588] [PubMed: 15861135]
- 37.
- Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416(6878):345–347. [PubMed: 11907583]
- 38.
- Habelhah H, Frew IJ, Laine A. et al. Stress-induced decrease in TRAF2 stability is mediated by Siah2. Embo J. 2002;21(21):5756–5765. [PMC free article: PMC131073] [PubMed: 12411493]
- 39.
- Kovalenko A, Chable-Bessia C, Cantarella G. et al. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424(6950):801–805. [PubMed: 12917691]
- 40.
- Brummelkamp TR, Nijman SM, Dirac AM. et al. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424(6950):797–801. [PubMed: 12917690]
- 41.
- Trompouki E, Hatzivassiliou E, Tsichritzis T. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424(6950):793–796. [PubMed: 12917689]
- 42.
- Brown KD, Hostager BS, Bishop GA. Regulation of TRAF2 signaling by self-induced degradation. J Biol Chem. 2002;277(22):19433–19438. [PubMed: 11909853]
- 43.
- Hsu H, Shu HB, Pan MG. et al. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84(2):299–308. [PubMed: 8565075]
- 44.
- Muzio M, Salvesen GS, Dixit VM. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem. 1997;272(5):2952–2956. [PubMed: 9006941]
- 45.
- Varfolomeev EE, Schuchmann M, Luria V. et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–276. [PubMed: 9729047]
- 46.
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81(4):495–504. [PubMed: 7758105]
- 47.
- Yeh WC, Pompa JL, McCurrach ME. et al. FADD: Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–1958. [PubMed: 9506948]
- 48.
- Park YC, Ye H, Hsia C. et al. A novel mechanism of TRAF signaling revealed by structural and functional analyses of the TRADD-TRAF2 interaction. Cell. 2000;101(7):777–787. [PubMed: 10892748]
- 49.
- Hsu H, Huang J, Shu HB. et al. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4(4):387–396. [PubMed: 8612133]
- 50.
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. [PubMed: 12887920]
- 51.
- Nguyen LT, Duncan GS, Mirtsos C. et al. TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity. 1999;11(3):379–389. [PubMed: 10514016]
- 52.
- Kelliher MA, Grimm S, Ishida Y. et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. [PubMed: 9529147]
- 53.
- Lee SY, Reichlin A, Santana A. et al. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7(5):703–713. [PubMed: 9390693]
- 54.
- Rothe M, Sarma V, Dixit VM. et al. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269(5229):1424–1427. [PubMed: 7544915]
- 55.
- Sakon S, Xue X, Takekawa M. et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. Embo J. 2003;22(15):3898–3909. [PMC free article: PMC169052] [PubMed: 12881424]
- 56.
- Uren AG, Pakusch M, Hawkins CJ. et al. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci USA. 1996;93(10):4974–4978. [PMC free article: PMC39390] [PubMed: 8643514]
- 57.
- Wang CY, Mayo MW, Korneluk RG. et al. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281(5383):1680–1683. [PubMed: 9733516]
- 58.
- Tsitsikov EN, Laouini D, Dunn IF. et al. TRAF1 is a negative regulator of TNF signaling Enhanced TNF signaling in TRAF1-deficient mice. Immunity 200115(4):647–657. [PubMed: 11672546]
- 59.
- Reinhard C, Shamoon B, Shyamala V. et al. Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997;16(5):1080–1092. [PMC free article: PMC1169707] [PubMed: 9118946]
- 60.
- Jupp OJ, McFarlane SM, Anderson HM. et al. Type II tumour necrosis factor-alpha receptor (TNFR2) activates c-Jun N-terminal kinase (JNK) but not mitogen-activated protein kinase (MAPK) or p38 MAPK pathways. Biochem J. 2001;359(Pt 3):525–535. [PMC free article: PMC1222173] [PubMed: 11672426]
- 61.
- Rothe M, Pan MG, Henzel WJ. et al. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83(7):1243–1252. [PubMed: 8548810]
- 62.
- Noelle RJ, Roy M, Shepherd DM. et al. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89(14):6550–6554. [PMC free article: PMC49539] [PubMed: 1378631]
- 63.
- Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L). Crit Rev Immunol. 1996;16(1):59–108. [PubMed: 8809473]
- 64.
- Pullen SS, Labadia ME, Ingraham RH. et al. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry. 1999;38(31):10168–10177. [PubMed: 10433725]
- 65.
- Haxhinasto SA, Hostager BS, Bishop GA. Cutting edge: Molecular mechanisms of synergy between CD40 and the B cell antigen receptor: Role for TNF receptor-associated factor 2 in receptor interaction. J Immunol. 2002;169(3):1145–1149. [PubMed: 12133933]
- 66.
- Haxhinasto SA, Bishop GA. A novel interaction between protein kinase D and TNF receptor-associated factor molecules regulates B cell receptor-CD40 synergy. J Immunol. 2003;171(9):4655–4662. [PubMed: 14568940]
- 67.
- Xia XZ, Treanor J, Senaldi G. et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192(1):137–143. [PMC free article: PMC1887716] [PubMed: 10880535]
- 68.
- Hatzoglou A, Roussel J, Bourgeade MF. et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000;165(3):1322–1330. [PubMed: 10903733]
- 69.
- Schiemann B, Gommerman JL, Vora K. et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–2114. [PubMed: 11509691]
- 70.
- Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol Cell Biol. 2001;21(12):4067–4074. [PMC free article: PMC87068] [PubMed: 11359913]
- 71.
- Yan M, Wang H, Chan B. et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2(7):638–643. [PubMed: 11429549]
- 72.
- Thompson JS, Bixler SA, Qian F. et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293(5537):2108–2111. [PubMed: 11509692]
- 73.
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. [PubMed: 15771565]
- 74.
- Aizawa S, Nakano H, Ishida T. et al. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFkappaB activation. J Biol Chem. 1997;272(4):2042–2045. [PubMed: 8999898]
- 75.
- Duckett CS, Gedrich RW, Gilfillan MC. et al. Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17(3):1535–1542. [PMC free article: PMC231879] [PubMed: 9032281]
- 76.
- Wendtner CM, Schmitt B, Gruss HJ. et al. CD30 ligand signal transduction involves activation of a tyrosine kinase and of mitogen-activated protein kinase in a Hodgkin's lymphoma cell line. Cancer Res. 1995;55(18):4157–4161. [PubMed: 7545087]
- 77.
- Boucher LM, Marengere LE, Lu Y. et al. Binding sites of cytoplasmic effectors TRAF1, 2, and 3 on CD30 and other members of the TNF receptor superfamily. Biochem Biophys Res Commun. 1997;233(3):592–600. [PubMed: 9168896]
- 78.
- Lee SY, Kandala G, Liou ML. et al. CD30/TNF receptor-associated factor interaction: NF-kappa B activation and binding specificity. Proc Natl Acad Sci USA. 1996;93(18):9699–9703. [PMC free article: PMC38492] [PubMed: 8790394]
- 79.
- Akiba H, Nakano H, Nishinaka S. et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273(21):13353–13358. [PubMed: 9582383]
- 80.
- Gravestein LA, Amsen D, Boes M. et al. The TNF receptor family member CD27 signals to Jun N-terminal kinase via Traf-2. Eur J Immunol. 1998;28(7):2208–2216. [PubMed: 9692890]
- 81.
- Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18(1):558–565. [PMC free article: PMC121523] [PubMed: 9418902]
- 82.
- Kawamata S, Hori T, Imura A. et al. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem. 1998;273(10):5808–5814. [PubMed: 9488716]
- 83.
- Prell RA, Evans DE, Thalhofer C. et al. OX40-mediated memory T cell generation is TNF receptor-associated factor 2 dependent. J Immunol. 2003;171(11):5997–6005. [PubMed: 14634111]
- 84.
- So T, Salek-Ardakani S, Nakano H. et al. TNF receptor-associated factor 5 limits the induction of Th2 immune responses. J Immunol. 2004;172(7):4292–4297. [PubMed: 15034043]
- 85.
- Jang IK, Lee ZH, Kim YJ. et al. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochem Biophys Res Commun. 1998;242(3):613–620. [PubMed: 9464265]
- 86.
- Saoulli K, Lee SY, Cannons JL. et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187(11):1849–1862. [PMC free article: PMC2212301] [PubMed: 9607925]
- 87.
- Cannons JL, Hoeflich KP, Woodgett JR. et al. Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. J Immunol. 1999;163(6):2990–2998. [PubMed: 10477561]
- 88.
- Cannons JL, Choi Y, Watts TH. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J Immunol. 2000;165(11):6193–6204. [PubMed: 11086053]
- 89.
- Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. [PubMed: 15546385]
- 90.
- Kim YS, Nedospasov SA, Liu ZG. TRAF2 plays a key, nonredundant role in LIGHT-lymphotoxin beta receptor signaling. Mol Cell Biol. 2005;25(6):2130–2137. [PMC free article: PMC1061604] [PubMed: 15743811]
- 91.
- Force WR, Cheung TC, Ware CF. Dominant negative mutants of TRAF3 reveal an important role for the coiled coil domains in cell death signaling by the lymphotoxin-beta receptor. J Biol Chem. 1997;272(49):30835–30840. [PubMed: 9388227]
- 92.
- Kuai J, Nickbarg E, Wooters J. et al. Endogenous association of TRAF2, TRAF3, cIAP1, and Smac with lymphotoxin beta receptor reveals a novel mechanism of apoptosis. J Biol Chem. 2003;278(16):14363–14369. [PubMed: 12571250]
- 93.
- Dejardin E, Droin NM, Delhase M. et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17(4):525–535. [PubMed: 12387745]
- 94.
- Derudder E, Dejardin E, Pritchard LL. et al. RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: Critical roles for p100. J Biol Chem. 2003;278(26):23278–23284. [PubMed: 12709443]
- 95.
- Koni PA, Sacca R, Lawton P. et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6(4):491–500. [PubMed: 9133428]
- 96.
- Futterer A, Mink K, Luz A. et al. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9(1):59–70. [PubMed: 9697836]
- 97.
- Caamano JH, Rizzo CA, Durham SK. et al. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187(2):185–196. [PMC free article: PMC2212102] [PubMed: 9432976]
- 98.
- Hsu H, Solovyev I, Colombero A. et al. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272(21):13471–13474. [PubMed: 9153189]
- 99.
- Marsters SA, Ayres TM, Skubatch M. et al. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272(22):14029–14032. [PubMed: 9162022]
- 100.
- Wong BR, Josien R, Lee SY. et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186(12):2075–2080. [PMC free article: PMC2199171] [PubMed: 9396779]
- 101.
- Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003;14(3-4):251–263. [PubMed: 12787563]
- 102.
- Darnay BG, Haridas V, Ni J. et al. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998;273(32):20551–20555. [PubMed: 9685412]
- 103.
- Darnay BG, Ni J, Moore PA. et al. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274(12):7724–7731. [PubMed: 10075662]
- 104.
- Wong BR, Josien R, Lee SY. et al. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 1998;273(43):28355–28359. [PubMed: 9774460]
- 105.
- Lomaga MA, Yeh WC, Sarosi I. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015–1024. [PMC free article: PMC316636] [PubMed: 10215628]
- 106.
- Dougall WC, Glaccum M, Charrier K. et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–2424. [PMC free article: PMC317030] [PubMed: 10500098]
- 107.
- Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 2003;14(3-4):211–224. [PubMed: 12787560]
- 108.
- Headon DJ, Emmal SA, Ferguson BM. et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414(6866):913–916. [PubMed: 11780064]
- 109.
- Yan M, Zhang Z, Brady JR. et al. Identification of a novel death domain-containing adaptor molecule for ectodysplasin-A receptor that is mutated in crinkled mice. Curr Biol. 2002;12(5):409–413. [PubMed: 11882293]
- 110.
- Zonana J, Elder ME, Schneider LC. et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am J Hum Genet. 2000;67(6):1555–1562. [PMC free article: PMC1287930] [PubMed: 11047757]
- 111.
- Doffinger R, Smahi A, Bessia C. et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–285. [PubMed: 11242109]
- 112.
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67(3):203–233. [PubMed: 12169297]
- 113.
- Ye X, Mehlen P, Rabizadeh S. et al. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem. 1999;274(42):30202–30208. [PubMed: 10514511]
- 114.
- Shao Z, Browning JL, Lee X. et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45(3):353–359. [PubMed: 15694322]
- 115.
- Eby MT, Jasmin A, Kumar A. et al. TAJ, a novel member of the tumor necrosis factor receptor family, activates the c-Jun N-terminal kinase pathway and mediates caspase-independent cell death. J Biol Chem. 2000;275(20):15336–15342. [PubMed: 10809768]
- 116.
- Ronchetti S, Zollo O, Bruscoli S. et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34(3):613–622. [PubMed: 14991590]
- 117.
- Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor, a costimulatory receptor on naive and activated T cells, uses TNF receptor-associated factor 2 in a novel fashion as an inhibitor of NF-kappaB activation. J Immunol. 2005;174(12):7875–7882. [PubMed: 15944293]
- 118.
- Esparza EM, Arch RH. TRAF4 functions as an intermediate of GITR-induced NF-kappaB activation. Cell Mol Life Sci. 2004;61(24):3087–3092. [PubMed: 15583869]
- 119.
- Knecht H, Berger C, Rothenberger S. et al. The role of Epstein-Barr virus in neoplastic transformation. Oncology. 2001;60(4):289–302. [PubMed: 11408795]
- 120.
- Devergne O, Hatzivassiliou E, Izumi KM. et al. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: Role in NF-kappaB activation. Mol Cell Biol. 1996;16(12):7098–7108. [PMC free article: PMC231713] [PubMed: 8943365]
- 121.
- Kaye KM, Devergne O, Harada JN. et al. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93(20):11085–11090. [PMC free article: PMC38288] [PubMed: 8855313]
- 122.
- Nikawa J, Yamashita S. IRE1 encodes a putative protein kinase containing a membrane-spanning domain and is required for inositol phototrophy in Saccharomyces cerevisiae. Mol Microbiol. 1992;6(11):1441–1446. [PubMed: 1625574]
- 123.
- Wang XZ, Harding HP, Zhang Y. et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17(19):5708–5717. [PMC free article: PMC1170899] [PubMed: 9755171]
- 124.
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: Coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–1233. [PubMed: 10346810]
- 125.
- Urano F, Wang X, Bertolotti A. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. [PubMed: 10650002]
- 126.
- Yoneda T, Imaizumi K, Oono K. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276(17):13935–13940. [PubMed: 11278723]
- Physiological Roles and Mechanisms of Signaling by TRAF2 and TRAF5 - Madame Curi...Physiological Roles and Mechanisms of Signaling by TRAF2 and TRAF5 - Madame Curie Bioscience Database
- Sympathetic Nervous System Regulation of Metastasis - Madame Curie Bioscience Da...Sympathetic Nervous System Regulation of Metastasis - Madame Curie Bioscience Database
- VEGF and Endothelial Guidance in Angiogenic Sprouting - Madame Curie Bioscience ...VEGF and Endothelial Guidance in Angiogenic Sprouting - Madame Curie Bioscience Database
- Physical Background and Technical Realizations of Hyperthermia - Madame Curie Bi...Physical Background and Technical Realizations of Hyperthermia - Madame Curie Bioscience Database
- The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway - Madame C...The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...