NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Summary
Maintenance of glucose homeostasis requires ‘cross-talk’ between pancreatic insulin secretion and insulin signaling in the peripheral tissues. Both insulin secretion and glucose uptake are regulated exocytotic processes mediated by SNARE protein complexes. SNARE core complexes are heterotrimeric, composed of syntaxin, SNAP-25/23 and VAMP2 proteins in a 1:1:1 ratio. It has become clear that we must thoroughly define the precise mechanisms underlying insulin-stimulated GLUT4 vesicle translocation by skeletal muscle and adipose cells as well as glucose-stimulated insulin secretion by pancreatic beta cells in order to develop therapeutic strategies to better treat and eventually cure diabetic patients. In this chapter, I will examine the molecular interactions responsible for these SNARE dependent events. Similarities and differences in molecular machinery, exocytosis mechanisms as well as the impact of accessory proteins such as Munc18 and Rab GTPases will be discussed. New findings on alterations of particular SNARE proteins upon glucose homeostasis are described in terms of potential for pharmacological targets for intervention.
In 1957 Lacy and colleagues published immunofluorescent and later electron microscopy images of islet beta cells filled with darkened organelles, or insulin granules. These granules were later shown to fuse with the plasma membrane to release insulin.1-3 Analogously, in 1990 the localization of the insulin-responsive glucose transporter ‘GLUT4’ to intracellular vesicles of adipocytes was revealed, and these GLUT4-storage vesicles were later shown to fuse with the plasma membrane upon stimulation with insulin, to ultimately facilitate glucose disposal.4,5 However, it was not until 1993 that the molecular basis for vesicle fusion and secretion, termed ‘vesicle exocytosis’, was elucidated by the discovery of SNARE proteins (soluble N-ethylmaleimide sensitive factor attachment protein receptor).6,7
Vesicle Exocytosis
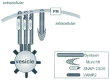
Vesicle exocytosis entails the pairing of a vesicle associated membrane protein (v)-SNARE (VAMP) with a binary cognate receptor complex at the target membrane composed of SNAP-25/ 23 and Syntaxin proteins (t-SNAREs)8-14 to form the SNARE core complex (Fig. 1). The v-SNARE VAMP2 (also called synaptobrevin) is an 18 kDa protein oriented with its N-terminus towards the cytoplasm and the carboxyl-terminus spanning the membrane facing the vesicle lumen.15-17 The t-SNARE Syntaxin is a 35 kDa protein oriented with its amino terminal domain towards the cytoplasm and carboxyl-terminus spanning the plasma membrane facing the extracellular matrix.8,18 The other t-SNARE, SNAP-23/25 (a 23 or 25 kDa synaptosome associated protein) is tethered to the target membrane via palmitoylated cysteine residues.19 A combination of electron microscopic, spectroscopic and X-ray crystallographic evidence shows that these SNAREs form a rod shaped complex that is a coiled-coil of four helices: one from VAMP, one from Syntaxin, and the remaining two from SNAP-25.20-23 Upon forming the core SNARE complex the syntaxin protein increases it helical content whereas VAMP and SNAP-25 convert from unstructured forms into a helices.20 These conformational changes result in the formation of the heterotrimeric complex that is extremely stable, melting only at temperatures greater than 95°C.6,10,12
Multiple isoforms of each SNARE protein were found to exist and it is hypothesized that by the particular pairing and compartmentalization of these proteins, specificity of vesicle targeting could be achieved.24-32 On the basis of these studies in neuroendocrine cells, investigators from diabetes-related fields discovered that SNARE protein core complexes were also responsible for regulated secretion of insulin from islet beta cells in response to increased blood glucose,33-40 as well as facilitating the downstream action of insulin on peripheral glucose disposal via the translocation to and integration of intracellular glucose transporter (GLUT4) vesicles into the plasma membrane of adipocytes and skeletal muscle transverse tubules/sarcolemma.41-50
Regulation of Exocytosis by Munc18 Proteins
Concurrent with the discovery of SNARE proteins came evidence from yeast genetics suggesting that other proteins also participate in exocytosis and play a role in the regulation of the SNARE complex,51 leading to the discovery of Sec1 secretory proteins. Yeast Sec1 was found to interact directly with the t-SNARE Syntaxin leading to the quick identification of Sec1 homologues in C. elegans (unc18), D. melanogaster (Rop)52-61 and mammals.53,54,59,62,63 Three homologues were identified in mammalian plasma membranes, and named Munc18 (for Mammalian unc18) proteins. Munc18 proteins are ˜66-68 kDa in size and are soluble factors with no transmembrane domain,54 although they are found localized to the plasma membrane through their high-affinity for binding to their cognate Syntaxin.64 Munc18-1 (also called Munc18a/n-Sec1/rbSec1) was demonstrated to interact with Syntaxin 1 in a manner mutually exclusive of the other SNARE core complex proteins,52,54,65 as depicted as a separate binary complex in Figure 1. By 1995 Munc18b and Munc18c were identified but found to be ubiquitously expressed, unlike Munc18a which was expressed only in neurons and islet cells.56,66-71 In addition, it was shown that Munc18a and Munc18b shared binding preferences for Syntaxin isoforms 1-3, while only Munc18c bound Syntaxin 4 (Syn4) with high affinity.67,68,70,71 Then it was in 1997 that Syntaxin 4 was demonstrated to be the functional isoform in insulin-stimulated GLUT4 vesicle translocation,46,72 and in 1998 we and others found that Munc18c over-expression inhibited this process64,73 (Table 1). Collectively, the Sec1 and Munc18 protein family are now referred to as ‘SM’ proteins (Sec1 and Munc18).
Table 1
Comparison of SNARE protein composition utilized for GLUT4 translocation and insulin exocytosis.
The evidence cited above strongly suggests that Munc18 proteins are essential regulators of SNARE mediated exocytosis. The most recent crystallographic and NMR structural analyses support and extend this concept, showing that Munc18-1 holds Syntaxin 1 in a “closed” conformational state in a 1:1 Munc18:Syntaxin molar ratio and unable to interact with VAMP2. Munc18-1 is proposed to orchestrate the conversion of Syntaxin 1 to the “open” conformational state to facilitate the interaction between Syntaxin 1, VAMP2 and SNAP-25.54,74-80 Structural studies have led to the general conclusion that Munc18 proteins share a similar overall structure in which a small folded N-terminal domain mediates the interaction with Syntaxins,81,82 whereas the remainder of the C-terminal domain carries out the poorly understood effector function that appears essential for fusion. Dulubova and colleagues have furthermore speculated that a particular loop2/3 domain of Munc18 proteins may be critical for this effector function,81,83 which is consistent with studies showing that an inhibitory peptide directed at this region or a single point mutation within it alters Syntaxin 4-Munc18c interaction in 3T3L1 adipocytes.84,85
One common theme in studying SNARE protein complexes and accessory proteins is that over-expression of one member of the complex can alter that stoichiometry which is optimal for the exocytotic event, resulting in loss/inhibition of function. Throughout the chapter this will become evident, as most initial studies of SNARE and SNARE accessory proteins were conducted using over-expression. Now as more data on SNARE knockout mice and siRNA-depletion evolves we will be able to disseminate the essentiality of these proteins in the various exocytotic processes.
Insulin Action: GLUT4 Vesicle Translocation
The insulin-responsive glucose transporter GLUT4 is predominantly expressed in striated muscle and adipose tissue and is responsible for the majority of insulin-stimulated glucose uptake.86 In the basal noninsulin stimulated state, GLUT4 localizes to tubulovesicular elements and small intracellular vesicles throughout the cell cytoplasm.87,88 Upon stimulation with insulin these GLUT4-containing compartments undergo a series of regulated steps leading to their eventual fusion with the plasma membrane.86,89-95 This ultimately results in a large increase in the number of functional glucose transporters on the cell surface (a process termed translocation), which accounts for nearly all of the insulin-stimulated glucose uptake.
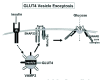
The insulin-stimulated translocation of GLUT4-containing vesicles is a complex multi-step process (Fig. 2). Several specific molecular events and signaling molecules have been identified. Initially, insulin binding to the insulin receptor activates the intrinsic protein kinase of the receptor β subunit resulting in its autophosphorylation and tyrosine phosphorylation of several proteins, most notably the IRS family of insulin receptor substrate proteins.96 The tyrosine phosphorylation of IRS results in the association, activation and targeting of the phosphatidylinositol kinase (PI 3-kinase).97-100 The active PI 3-kinase can then generate phosphatidylinositol-3,4,5-trisphosphate, which is necessary for the stimulation of both Protein Kinase B (PKB/Akt) and atypical Protein Kinase C isoforms through activation of the phosphoinositide-dependent protein kinases (PDK1 and PDK2) and/or through engagement of the PKB pleckstrin homology (PH) domain.101-103 In 2002, the Akt substrate AS160 was identified.104 AS160, a Rab GTPase-activating protein, becomes phosphorylated in response to insulin and is required for insulin-stimulated GLUT4 translocation.105-107 Unlike neuronal vesicles, the GLUT4 vesicles are mostly intracellularly localized and physically separated from the plasma membrane, and following insulin receptor signaling, these intracellular GLUT4 storage vesicles traffic towards the plasma membrane, afterwards becoming docked just below the plasma membrane. Docked vesicles undergo priming mediated by binding between syntaxin 4, SNAP-23 and VAMP2 and ultimately the primed vesicles are functionally incorporated into the plasma membrane through the physical mixing of the two lipid bilayers by membrane fusion events, leaving the GLUT4 protein positioned to facilitate the uptake of glucose.92,94
Substantial advances have been made in our understanding of the priming and fusion steps of GLUT4 translocation. GLUT4 vesicles copurify with the VAMP2 v-SNARE, and specific proteolytic cleavage of VAMP2, expression of a dominant-negative VAMP2 mutant or inhibitory peptides all impair insulin-stimulated GLUT4 translocation.42,43,45,46,48,49 Although VAMP2 (-/-) knockout mice have been generated, no reports on their glucose homeostasis are available at this time as they fail to thrive upon birth.108 Using dominant-interfering mutants and inhibitory peptides, Syntaxin 4 and SNAP-23 have been identified as the t-SNARE proteins required for insulin-stimulated GLUT4 translocation.41,44,46,47,72 Syntaxin 4 (-/+) mice show deficiencies in skeletal muscle GLUT4 translocation and glucose uptake, although adipocyte glucose uptake is normal.109 While VAMP3 was initially found to be part of this process in 3T3L1 adipocytes, it was later determined to be nonessential since VAMP3 (-/-) knockout mice showed no defects in skeletal muscle or adipocyte insulin-stimulated GLUT4 translocation or alterations in whole-body glucose homeostasis.110 In all, these data provide compelling evidence that VAMP2 functions by directing the association of the GLUT4 containing vesicles with Syntaxin 4 and SNAP-23 at the plasma membrane.
Impact of Syntaxin4 Binding Proteins Upon GLUT4 Vesicle Translocation
Munc18c
We and others have demonstrated that the Munc18c-Syntaxin 4 complex is functional in insulin-stimulated GLUT4 translocation in 3T3L1 adipocytes. Over-expression of Munc18c resulted in the inhibition of insulin-stimulated glucose uptake and GLUT4 translocation, while having no effect upon GLUT1 translocation or the trafficking of mannose-6-phosphate receptor or transferrin receptor.64,73,111 Furthermore, competition for the association of endogenous Munc18c-Syntaxin 4 complexes using an interfering peptide of Munc18c demonstrated a specific requirement of this complex for insulin-stimulated GLUT4 vesicle fusion,84 but was not necessary for proximal trafficking steps.85 This functional profile is consistent with the role of the homologous Sec1 protein in yeast.112,113
Post-translational modification of Munc18c or alteration of Munc18c abundance has been related to insulin resistance. For example, glucosamine-induced insulin resistance in adipocytes has recently been correlated with increased O-linked glycosylation of Munc18c and a reduced association of VAMP2 with Syntaxin 4.114,115 In vivo, localized over-expression of Munc18c in skeletal muscle via adenoviral particle injection markedly impaired insulin-stimulated GLUT4 translocation to sarcolemmal and transverse tubule membranes.116 Moreover, over-expression of a tetracycline-repressible (tet-off) CMV-driven Munc18c transgene in skeletal muscle, adipose tissue and pancreas of mice significantly impaired whole body glucose tolerance, skeletal muscle glucose uptake and GLUT4 translocation.117 Most recently we have shown that Munc18c (-/+) knockout mice are insulin resistant and have severely impaired insulin-stimulated GLUT4 translocation in skeletal muscle.118 This contrasts however with the response of adipocytes differentiated from mouse embryonic fibroblasts (MEF) derived from an independent line of Munc18c (-/-) knockout mice: the MEF-derived adipocytes showed enhanced appearance of GLUT4 at the cell surface in response to insulin.119 These seemingly conflicting data are actually quite consistent with the emerging data from other genetically-altered SNARE mouse models of insulin resistance, showing defects in GLUT4 translocation and glucose uptake in skeletal muscle but not in adipocytes (Table 2).109,117,118,120 An alternate explanation for these differences might be that different genes encoding Munc18c were targeted, a concept which is supported by differences in exon-intron structure depicted in the two reports.117,118
Table 2
Transgenic and knockout strains of mice with genetic alterations in SNARE proteins.
Rabs
In yeast, the SM protein Sly1p interacts with the small GTPase protein Ypt1p.83 However, an analogous Munc18c interacting protein has remained elusive. Members of the small GTPase Rab protein family play crucial regulatory roles in vesicular trafficking. In neurotransmitter release, Rab3A is involved in regulating the efficiency of vesicle priming and membrane fusion.121 Also, in the yeast trafficking pathway from Golgi to vacuole, the Rab effector protein Vac1 has been shown to interact with the Sec1 family member Vps45.122 Vps45 binds to the yeast syntaxin Pep12,123 connecting the functions of a Rab, a soluble Rab effector and the target-membrane-localized Sec1/syntaxin family. To date, there are more than 30 different Rab GTPases, most with distinct cellular localizations and individual roles in secretory and exocytic pathways. Rabs cycle between an active GTP-bound form and the inactive GDP-bound form such that vesicle-associated Rabs are found in the GTP-bound state and following membrane fusion are hydrolyzed to the inactive form.124 Rab4 has been shown to be associated with GLUT4-containing vesicles and implicated in the insulin action on glucose transport in rat adipocytes.125 Upon insulin stimulation Rab4 is depleted from the GLUT4 containing microsomal fraction of adipocytes and is redistributed to the cytosolic fraction, where it specifically interacts with the GDP-dissociation inhibitor GDI-1.126-128 In addition, introduction of a carboxyl-terminal peptide, expression of a carboxyl-terminal Rab4 deletion mutant, or increased expression of wild type Rab4 all inhibit insulin-stimulated GLUT4 translocation,129-131 consistent with a vital functional role for Rab4 in GLUT4 trafficking.
Tomosyn and Synip
In 1998 the Syntaxin 1 binding protein Tomosyn was identified in neuronal cells and found to dissociate Munc18a from Syntaxin 1.132 Tomosyn has a VAMP2-like region that is necessary for its interaction with Syntaxin 1 and for complex formation with Syntaxin 1 and SNAP-25.133,134 Tomosyn has further been shown to exist as multiple isoforms sharing a conserved structure.134,135 Since tomosyn over-expression results in down-regulated exocytosis,132,133 and it dissociates the Munc18a-Syntaxin 1 complex known to be important for exocytosis, tomosyn has been characterized as a negative-regulator of neuronal exocytosis. More specifically, it is proposed to inhibit the priming step.136 In an analogous manner, the b-tomosyn isoform is found to bind to Syntaxin 4 through a VAMP2-like domain, form a ternary complex with Syntaxin 4 and SNAP-23, and inhibit the priming of GLUT4 vesicles in 3T3L1 adipocytes when over-expressed.137 Thus tomosyn and Munc18c appear to function with Syntaxin 4 at the priming step of GLUT4 vesicle fusion, although the details of their roles remain unclear.
The Syntaxin 4 binding protein Synip was isolated and characterized in 1999 in 3T3L1 adipocytes, and the syntaxin 4-synip complex was found to be dissociated upon stimulation with insulin.138 Moreover, deletion analyses suggested that the amino terminal domain may provide a regulatory role in modulating the interaction of the carboxyl terminal domain of synip with syntaxin 4.138 More recently, synip was shown to contain an unusual dual Akt/PKB consensus phosphorylation motif, where serine 99 is a substrate for Akt2 but not Akt1 or Akt3.139 The expression of a synip-ser99 mutant inhibited insulin-stimulated GLUT4 vesicle docking/fusion, concurrent with its inability to undergo insulin-stimulated dissociation from syntaxin 4. It is thus postulated that insulin activation of Akt2 in adipocytes regulates the docking/fusion step of GLUT4 vesicle translocation through regulation of synip phosphorylation.
Exocyst Complex
In the yeast S. cerevisiae a complex of seven proteins were found to function together at sites on the plasma membrane to promote exocytosis, and were so named collectively the Exocyst.140 Mammalian homologues were isolated shortly thereafter and one Exocyst protein, rSec6, was specifically immunolocalized to sites of granule exocytosis in neurosecretory PC12 cell processes.141 Another exocyst protein, Exo70, has been identified in 3T3L1 adipocytes and found to associate with other exocyst proteins Sec6 and Sec8.142 Exo70 translocates to the plasma membrane in response to insulin through the activation of TC10, which is suggested to function in the insulin signaling cascade as part of a PI-3-kinase independent pathway convergent at stimulation of GLUT4 trafficking.94,143 Most recently, Sec6 and Sec8 have also been shown to redistribute to the plasma membrane in response to insulin in 3T3L1 adipocytes,144 altogether supporting a model whereby the exocyst complex functions in insulin-stimulated GLUT4 vesicle targeting to the docking/fusion sites at the plasma membrane.
Insulin Exocytosis in Pancreatic Beta Cells
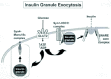
Greater than 99% of insulin secreted from the pancreatic beta cell proceeds via a regulated secretory pathway.145 Insulin is contained within dense core granules located inside the pancreatic beta cell. These granules form in the trans-golgi network (TGN), at which time they are loaded with proinsulin. In these immature beta cell granules the proinsulin is cleaved to insulin and C-peptide. Glucose stimulates these granules to move from their intracellular location to the cell surface, and also from more interior pools to the readily releasable pools located just beneath the cell surface. The amount of insulin contained in beta cells is very constant, as the balance between insulin secretion, proinsulin biosynthesis and insulin granule degradation is carefully maintained.146,147 Based upon the amount of stored insulin in normal beta cells, it is generally accepted that at least for the initial release of insulin, insulin content is not rate-limiting.148
As depicted in Figure 3, when extracellular glucose rises, the glucose transporters (GLUT2) at the beta cell surface facilitate the uptake of glucose into the cell, resulting in an increase in ATP through metabolism of the glucose. This ATP upsets the basal ATP/ADP ratio causing the KATP-channels to close and cell depolarization,149,150 leading to the opening of Ca2+-channels, overall increasing cytoplasmic Ca2+ concentration.151 In response to the rise in intracellular Ca2+, some docked and primed insulin storage granules fuse and release insulin, while storage granules traffic to join the readily releasable pool at the cell surface (see review, ref. 152). Thus, insulin secretion in islets is biphasic,153,154 with the first phase resulting from the release of insulin granules from an immediate releasable pool preprimed at the plasma membrane.155 First phase secretion peaks within 5-10 min, and without returning to baseline insulin secretion gradually rises over time after this if glucose is present (representing a second phase of secretion).153 The second phase only occurs with nutrient stimulation156 and is currently thought to represent secretion from those granules which are mobilized to refill the readily and immediate releasable pools of granules.157
SNARE Proteins in Insulin Secretion
To date, insulin secretion is known to involve the same SNARE isoforms as those utilized in synaptic vesicle exocytosis and neurotransmitter release, namely Syntaxin 1, VAMP2, and SNAP-25.33-40 Similar to neurosecretory cell over-expression of Syntaxin 1, over-expression of Syntaxin 1 in insulin-secreting beta cells as well as in transgenic mice results in decreased insulin release.158,159 Cleavage of VAMP2 by botulinum toxin severely reduces insulin release from islets.37,38 Cleavage of SNAP-25 only reduces insulin secretion by 50%, but can be substituted for by the abundant SNAP-23 isoform in Syntaxin 1 based SNARE complexes.38,160 Cleavage of Syntaxin 1 inhibited 95% of K+-induced insulin secretion, but only 25% of glucose induced secretion was inhibited.40,161 Taken together, these data suggest that although the Syntaxin 1-based SNARE complexes are responsible for K+ induced secretion, glucose-stimulated secretion likely involves a second VAMP2-dependent SNARE complex.
In actuality, pancreatic beta cells contain numerous other Syntaxin isoforms (Table 1), suggesting that additional SNARE complexes participate in the regulation of insulin secretion.34,39,160 Recently we and others have determined that Syntaxin 4 plays a positive functional role in glucose-stimulated insulin secretion. This was initially shown indirectly in over-expression studies whereby expression of a dominant-negative form of Syntaxin 4 resulted in inhibition of insulin secretion in βHC9 cultured beta cells.162 However this has now been substantiated by detection of a 50% reduction of insulin secretion in islets isolated from Syntaxin 4 (-/+) knockout mice.163 Furthermore, we have shown that Syntaxin 4 is specifically required by the beta cells of the islet by using siRNA-mediated depletion and immunodepletion of Syntaxin 4 from MIN6 beta cells in culture.163 While over-expression studies have provided some evidence for a role for Syntaxin 3 but not Syntaxin 2 in beta cells,164 further studies using depletion of these Syntaxin isoforms will be required to determine their essential nature to insulin secretion.
In addition to isoform specificity, the abundance of SNARE proteins in pancreatic beta cells has been shown to impinge upon insulin secretion in two different rodent models of diabetes. For example, in islets isolated from diabetic GK rats, a nonobese rodent model of Type II diabetes, reduced levels of SNAP-25, Syntaxin 1, Syntaxin 2 and VAMP2 SNARE isoform proteins were correlated with impaired insulin secretion, and exogenous replacement of these particular SNARE proteins improved insulin secretion.165 The Type II diabetic Zucker fa/fa rat also has reduced levels of these SNARE proteins in beta cells.166 These findings illustrate the importance of SNARE protein stoichiometry to overall islet function.
SNARE Binding Proteins in Insulin Secretion
Calcium and Potassium Channels
The insulin granules found primed at the plasma membrane release insulin rapidly in response to stimuli, and thus this group of granules is referred to as the ‘readily releasable pool’. It was found that granules in this pool are tightly associated with the voltage-dependent α1C Ca2+ channels (VDCC)155 (Fig. 3). This occurs through an interaction between a region in α1C subunit of the L-type channel, corresponding to the ‘synprint’ site which binds to Syntaxin 1 and SNAP-25.167 Expression of this peptide inhibits exocytosis evoked by voltage clamp depolarization, suggesting that this interaction facilitated first phase insulin secretion. Moreover, over-expression of Syntaxin 1A in beta cell lines inhibits L-type channel activity.164 In addition, Syntaxin 1A also binds to the Kv2.1 channel, the dominant membrane-repolarizing voltage gated K+ channel in pancreatic beta cells,168 and the open form of Syntaxin 1A was found to inhibit the channel, which was proposed to limit K+ efflux during exocytosis to optimize insulin release.169 SNAP-25 also associates with the voltage-gated a1C Ca2+ channels and Kv2.1 channels.170,171 Thus, Syntaxin 1A and SNAP-25 mediate secretion by regulating calcium entry and membrane potential though their associations with calcium and potassium channels in pancreatic beta cells.
Munc18a and Mint1
The Syntaxin 1 binding protein Munc18a (n-Sec1/Munc18-1/rbSec1) was initially immunolocalized in rat pancreatic islet cells and shown to interact with Syntaxin 1 in extracts of HIT-T15 cultured beta cells.172 Functionally Munc18a is described as a negative regulator of exocytosis in the beta cell, as evidenced by an increase in insulin secretion in streptolysin-O treated HIT-T15 cells administered antibody to Munc18 or a Munc18 peptide.172 Munc18b is also expressed in islet cells, although its function is unknown. Mint1, a Munc18a interacting protein, is expressed in rat pancreatic islets, localized primarily to the cell periphery.173 In addition to Mint1, RT-PCR analysis of rat islets and two culture beta cells lines (HIT-T15 and RINm5F) showed the presence of mRNA for Mint2 and Mint3. Mint2 but not Mint3 can bind to Munc18a or Munc18b (Munc18-2),174 thus leaving the importance of Mint3 expression in beta cells unknown.
Munc18c
Munc18c has been immunolocalized in rat islets and multiple cultured insulin secreting cell lines.39 Munc18c transgenic mice, which have over-expression of Munc18c in pancreas, showed inhibited insulin secretion in isolated islets,117 indicating for the first time, that Munc18c played a role in the islet beta cell (Table 2). However, just as in GLUT4 translocation, while over-expression of Munc18c resulted in inhibition, so did depletion of Munc18c in the skeletal muscle of the Munc18c (-/+) knockout mouse.118 Consistent with this, we have now found that islets isolated from Munc18c (-/+) knockout mice have a 50% reduction in glucose-stimulated insulin secretion.118 This requirement for Munc18c is further supported by siRNA-mediated depletion and antibody immunodepletion of endogenous Munc18c in MIN6 beta cells (D.C. Thurmond, unpublished results). Thus, since the islet beta cells were thought previously to use the same t-SNAREs as neurons, namely Syntaxin 1 and Munc18a, our data now suggests that Syntaxin 4 and its binding partner Munc18c regulates insulin secretion as well.
Rab Proteins and Effectors
Rab3 has been suggested to participate in the dissociation of the Munc18a-Syntaxin 1 complex.175 In fact, the number of synaptic vesicles that fuse with the plasma membrane in response to stimuli is increased in Rab3A (-/-) knockout mice.176 Rab3 was originally identified in homogenates of rat pancreatic islets and in HIT-T15 and RINm5F insulin secreting cell lines,177,178 and later was immunolocalized to the insulin secretory granules.179 Munc18a is also associated with Rab3 in pancreatic beta cells,180 and over-expression of dominant active mutants of Rab3 decreases exocytosis.179,181 Numerous Rab3 effectors have since been identified in pancreatic beta cells, such as RIM, granuphilin and Noc2. RIM was localized on the plasma membrane of rat islet beta cells, INS-1E and HIT-T15 beta cell lines.182 Competitive over-expression of the Rab3 binding domain of RIM resulted in enhanced glucose-stimulated insulin secretion, implicating RIM in the regulation of insulin exocytosis. Granuphilin interacts with Rab3 in its GTP-bound form and Munc18a, and over-expression of granuphilin inhibits stimulated insulin secretion.180,183 Granuphilin also binds to Rab27 on the insulin granule,184 which forms a heterotrimeric complex with Syntaxin 1 at the plasma membrane, effectively tethering granules to the fusion sites at the plasma membrane.185 Noc2 was cloned and characterized as a Rab3 effector protein expressed in the MIN6 insulin-secreting cell line.186 RNA interference was used to silence Noc2 in INS-1E beta cells, which strongly impaired secretagogue-induced insulin secretion.187 Furthermore, the defect was particularly detrimental during the sustained release phase, suggesting that Noc2 may be involved in the recruitment of secretory granules. Noc2 also binds to Munc13-1,187 which has been implicated in insulin granule priming188 and is also a RIM binding protein189 and is functional in synaptic vesicle priming. Munc13-1 (-/-) knockout mice have been generated but have yet to be examined for defects in insulin secretion.190 This is consistent with data showing Noc2 interaction with the cytoskeletal-associated protein zyxin, and thus Noc2 is postulated to regulate exocytosis by interacting with the actin cytoskeleton.186
Insulin Granule Trafficking and Actin Remodeling
Filamentous actin (F-actin) is known to be important to the process of insulin secretion.191-193 Our recent studies in MIN6 beta cells and isolated rat islets demonstrate that glucose transiently modulates cortical actin organization and disrupts the interaction of F-actin with the t-SNARE complex at the plasma membrane to facilitate glucose-stimulated insulin secretion.194 Further evidence indicates that the cortical actin reorganization induced by glucose occurs at a proximal step in the stimulus-secretion pathway, perhaps at a step concurrent with the KATP channel closure.195 However, our understanding of the coupling of SNARE mediated exocytosis to F-actin reorganization remains incomplete.
Multiple lines of evidence suggest that the F-actin effector protein Cdc42, a Rho family small GTPase, is a downstream target of glucose signaling in beta cells. For example, we have recently shown that stimulation with glucose results in alterations in the cycling of Cdc42 between the GTP-bound activated and GDP-bound inactivated states, which correlate with a transient depolymerization of cortical F-actin.195 Cdc42 has also been demonstrated to colocalize with VAMP2-containing insulin secretory granules in pancreatic beta cells.196,197 Moreover, Cdc42 has been shown to interact indirectly with the t-SNARE Syntaxin 1, linking Cdc42 and the actin cytoskeleton to the plasma membrane exocytotic machinery.198 We have recently demonstrated that VAMP2 bridged the interaction between Cdc42 and Syntaxin 1 and that the interactions were functionally important for SNARE dependent insulin exocytosis.199
Perspectives
Diabetes now strikes 6.2% of the U.S. population, having risen to afflict approximately 1 in every 17 Americans, and is expected to continue to rise. In addition, although it is well-recognized that frank diabetes develops over years of cellular dysfunction in insulin secretion and insulin action, clear markers of this predisposition have remained elusive. Significant progress has been made in defining signaling cascades leading to the secretion of insulin from the islets and uptake of glucose into skeletal muscle and adipose tissues, however each signaling cascade is rate-limited by the distal steps of vesicle exocytosis.85,148,200 The functional regulation of Syntaxins by the Munc18 proteins is thought to be crucial in these distal steps, although the details remain unclear. There are also numerous animal models of insulin resistance and diabetes which exhibit alterations in SNARE protein abundance with which therapeutic agents are tested.165,166,201,202 However, given the evidence showing that Syntaxin 4-based SNARE core complexes and Syntaxin 4-Munc18c complexes participate in both insulin secretion and insulin action, therapies which target or even affect secondarily the SNARE protein abundances will have consequences in both insulin secretion and insulin action. This could be beneficial if the regulation occurred in parallel, but hazardous if the complex regulation were opposing (i.e., upregulation of Syntaxin 4 resulted in decreased glucose uptake but increased insulin secretion). Lack of such knowledge represents an important gap in our understanding of the etiology of insulin resistance and diabetes. Progress in therapeutic treatment of diabetes will need to take into account the mechanism(s) underlying the regulation of insulin secretion in islets cells and glucose uptake in adipocytes by these SNARE complexes and SNARE accessory proteins, and relate these tissue-specific effects to the overall control of glucose homeostasis.
Acknowledgements
I would like to thank Dr. Herbert Gaisano for personal communications regarding his Syntaxin 1 transgenic mice, and Angela Nevins for her critical review of the manuscript. This work was supported by research grants from the National Institute of Health (DK067912) and the American Diabetes Association (1-03-CD-10).
References
- 1.
- Lacy PE. Electron microscopy of the beta cell of the pancreas. Am J Med. 1961;31:851. [PubMed: 14461448]
- 2.
- Lacy PE, Davies J. Preliminary studies on the demonstration of insulin in the islets by the fluorescent antibody technic. Diabetes. 1957;6:354. [PubMed: 13447765]
- 3.
- Orci L, Amherdt M, Malaisse-Lagae F. et al. Insulin release by emiocytosis: Demonstration with freeze-etching technique. Science. 1973;179(68):82–84. [PubMed: 4565325]
- 4.
- Clancy BM, Czech MP. Hexose transport stimulation and membrane redistribution of glucose transporter isoforms in response to cholera toxin, dibutyryl cyclic AMP, and insulin in 3T3-L1 adipocytes. J Biol Chem. 1990;265(21):12434–12443. [PubMed: 2165064]
- 5.
- Holman GD, Kozka IJ, Clark AE. et al. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990;265:18172–18179. [PubMed: 2211693]
- 6.
- Sollner T, Bennett MK, Whiteheart SW. et al. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75(3):409–418. [PubMed: 8221884]
- 7.
- Sollner T, Whiteheart SW, Brunner M. et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362(6418):318–324. [PubMed: 8455717]
- 8.
- Calakos N, Bennett MK, Peterson KE. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. [PubMed: 8108733]
- 9.
- Chapman E, An S, Barton N. et al. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269(44):27427–27432. [PubMed: 7961655]
- 10.
- Fasshauer D, Otto H, Eliason WK. et al. Structural changes are associated with soluble N-ethylmaleimide- Sensitive fusion protein attachment protein receptor complex formation. J Biol Chem. 1997;272(44):28036–28041. [PubMed: 9346956]
- 11.
- Hayashi T, McMahon H, Yamasaki S. et al. Synaptic vesicle membrane fusion complex: Action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. [PMC free article: PMC395451] [PubMed: 7957071]
- 12.
- Hayashi T, Yamasaki S, Nauenburg S. et al. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 1995;14(10):2317–2325. [PMC free article: PMC398339] [PubMed: 7774590]
- 13.
- Kee Y, Lin RC, Hsu SC. et al. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995;14(5):991–998. [PubMed: 7748566]
- 14.
- Poirier MA, Hao JC, Malkus PN. et al. Protease resistance of syntaxin SNAP-25 VAMP complexes. Implications for assembly and structure. J Biol Chem. 1998;273(18):11370–11377. [PubMed: 9556632]
- 15.
- Baumert M, Mollard GFV, Jahn R. et al. Synaptobrevin: An integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989;8:379–384. [PMC free article: PMC400817] [PubMed: 2498078]
- 16.
- Sudhof TC, Baumert M, Perin MS. et al. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989;2:1475–1481. [PubMed: 2560644]
- 17.
- Trimble WS, Cowan DM, Scheller RH. Vamp-1: A synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci USA. 1988;85:4538–4542. [PMC free article: PMC280466] [PubMed: 3380805]
- 18.
- Pevsner J, Hsu S-C, Braun JEA. et al. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. [PubMed: 8060616]
- 19.
- Hess DT, Slater TM, Wilson MC. et al. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12(12):4634–4641. [PMC free article: PMC6575770] [PubMed: 1281490]
- 20.
- Canaves JM, Montal M. Assembly of a ternary complex by the predicted minimal coiled-coilforming domains of syntaxin, SNAP-25, and synaptobrevin. A circular dichroism study. J Biol Chem. 1998;273(51):34214–34221. [PubMed: 9852083]
- 21.
- Lin RC, Scheller RH. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19(5):1087–1094. [PubMed: 9390521]
- 22.
- Nicholson KL, Munson M, Miller RB. et al. Regulation of SNARE complex assembly by an N-terminal domain of the t- SNARE Sso1p. Nat Struct Biol. 1998;5(9):793–802. [PubMed: 9731774]
- 23.
- Sutton RB, Fasshauer D, Jahn R. et al. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395(6700):347–353. [PubMed: 9759724]
- 24.
- Advani RJ, Bae H-R, Bock JB. et al. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273(17):10317–10324. [PubMed: 9553086]
- 25.
- Bock JB, Matern HT, Peden AA. et al. A genomic perspective on membrane compartment organization. Nature. 2001;409(6822):839–841. [PubMed: 11237004]
- 26.
- Chen YA, Scales SJ, Patel SM. et al. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97(2):165–174. [PubMed: 10219238]
- 27.
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2(2):98–106. [PubMed: 11252968]
- 28.
- McNew JA, Parlati F, Fukuda R. et al. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407(6801):153–159. [PubMed: 11001046]
- 29.
- McNew JA, Weber T, Parlati F. et al. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J Cell Biol. 2000;150(1):105–118. [PMC free article: PMC2185554] [PubMed: 10893260]
- 30.
- Pombo I, Rivera J, Blank U. Munc18-2/syntaxin3 complexes are spatially separated from syntaxin3-containing SNARE complexes. FEBS Letters. 2003;550(1-3):144–148. [PubMed: 12935901]
- 31.
- Scales SJ, Chen YA, Yoo BY. et al. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26(2):457–464. [PubMed: 10839363]
- 32.
- Watson RT, Pessin JE. Transmembrane domain length determines intracellular membrane compartment localization of syntaxins 3, 4, and 5. Am J Physiol Cell Physiol. 2001;281(1):C215–223. [PubMed: 11401844]
- 33.
- Daniel S, Noda M, Straub SG. et al. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48(9):1686–1690. [PubMed: 10480595]
- 34.
- Jacobsson G, Bean AJ, Scheller RH. et al. Identification of synaptic proteins and their isoform mRNAs in compartments of pancreatic endocrine cells. Proc Natl Acad Sci USA. 1994;91(26):12487–12491. [PMC free article: PMC45463] [PubMed: 7809063]
- 35.
- Kiraly-Borri CE, Morgan A, Burgoyne RD. et al. Soluble N-ethylmaleimide-sensitive-factor attachment protein and N-ethylmaleimide-insensitive factors are required for Ca2+-stimulated exocytosis of insulin. Biochem J. 1996;314(Pt 1):199–203. [PMC free article: PMC1217025] [PubMed: 8660283]
- 36.
- Nakamichi Y, Nagamatsu S. Alpha-SNAP functions in insulin exocytosis from mature, but not immature secretory granules in pancreatic beta cells. Biochem Biophys Res Commun. 1999;260(1):127–132. [PubMed: 10381355]
- 37.
- Regazzi R, Wollheim CB, Lang J. et al. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995;14(12):2723–2730. [PMC free article: PMC398391] [PubMed: 7796801]
- 38.
- Sadoul K, Lang J, Montecucco C. et al. SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J Cell Biol. 1995;128(6):1019–1028. [PMC free article: PMC2120411] [PubMed: 7896868]
- 39.
- Wheeler MB, Sheu L, Ghai M. et al. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996;137(4):1340–1348. [PubMed: 8625909]
- 40.
- Yang SN, Larsson O, Branstrom R. et al. Syntaxin 1 interacts with the L(D) subtype of voltage-gated Ca(2+) channels in pancreatic beta cells. Proc Natl Acad Sci USA. 1999;96(18):10164–10169. [PMC free article: PMC17860] [PubMed: 10468580]
- 41.
- Araki S, Tamori Y, Kawanishi M. et al. Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c. Biochem Biophys Res Commun. 1997;234:257–262. [PubMed: 9168999]
- 42.
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. [PubMed: 1601842]
- 43.
- Cheatham B, Volchuk A, Kahn CR. et al. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA. 1996;93:15169–15173. [PMC free article: PMC26375] [PubMed: 8986782]
- 44.
- Kawanishi M, Tamori Y, Okazawa H. et al. Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2. J Biol Chem. 2000;275(11):8240–8247. [PubMed: 10713150]
- 45.
- Martin LB, Shewan A, Millar CA. et al. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J Biol Chem. 1998;273:1444–1452. [PubMed: 9430681]
- 46.
- Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. [PMC free article: PMC232091] [PubMed: 9111311]
- 47.
- Rea S, Martin LB, McIntosh S. et al. Syndet, an adipocyte target SNARE involved in the insulin-induced translocation of GLUT4 to the cell surface. J Biol Chem. 1998;273:18784–18792. [PubMed: 9668052]
- 48.
- Tamori Y, Hashiramoto M, Araki S. et al. Cleavage of vesicle-associated membrane protein (VAMP)-2 and cellubrevin on GLUT4-containing vesicles inhibits the translocation of GLUT4 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1996;220:740–745. [PubMed: 8607835]
- 49.
- Volchuk A, Mitsumoto Y, He L. et al. Expression of vesicle-associated membrane protein 2 (VAMP-2)/synaptobrevin II and cellubrevin in rat skeletal muscle and in a muscle cell line. Biochem J. 1994;304(Pt 1):139–145. [PMC free article: PMC1137463] [PubMed: 7998925]
- 50.
- Timmers KI, Clark AE, Omatsu-Kanbe M. et al. Identification of SNAP receptors in rat adipose cell membrane fractions and in SNARE complexes coimmunoprecipitated with epitope-tagged N-ethylmaleimide-sensitive fusion protein. Biochem J. 1996;320:429–436. [PMC free article: PMC1217948] [PubMed: 8973549]
- 51.
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370(6486):191–193. [PubMed: 8028665]
- 52.
- Garcia EP, Gatti E, Butler M. et al. A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc Natl Acad Sci USA. 1994;91:2003–2007. [PMC free article: PMC43297] [PubMed: 8134339]
- 53.
- Harrison SD, Broadie K, van de Goor J. et al. Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron. 1994;13:555–566. [PubMed: 7917291]
- 54.
- Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. [PubMed: 8247129]
- 55.
- Hosono R, Hekimi S, Kamuya Y. et al. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J Neurochem. 1992;58:1517–1525. [PubMed: 1347782]
- 56.
- Katagiri H, Terasaki J, Murata T. et al. A novel isoform of syntaxin-binding protein homologous to yeast Sec1 expressed ubiquitously in mammalian cells. J Biol Chem. 1995;270:4963–4966. [PubMed: 7890599]
- 57.
- Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. [PMC free article: PMC383491] [PubMed: 377286]
- 58.
- Ogawa H, Harada S, Sassa T. et al. Functional properties of the unc-64 gene encoding a Caenorhabditis elegans syntaxin. J Biol Chem. 1998;273(4):2192–2198. [PubMed: 9442061]
- 59.
- Pevsner J, Hsu SC, Scheller RH. n-Sec1: A neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA. 1994;91(4):1445–1449. [PMC free article: PMC43176] [PubMed: 8108429]
- 60.
- Salzberg A, Cohen N, Halachmi N. et al. The Drosophila Ras2 and Rop gene pair: A dual homology with a yeast Ras-like gene and a suppressor of its loss-of-function phenotype. Development. 1993;117(4):1309–1319. [PubMed: 8404533]
- 61.
- Schulze KL, Littleton JT, Salzberg A. et al. Rop, a Drosophila homolog of yeast Sec1 and vertebrate n-Sec1/Munc-18 proteins, is a negative regulator of neurotransmitter release in vivo. Neuron. 1994;13:1099–1108. [PubMed: 7946348]
- 62.
- Gengyo-Ando K, Kamiya Y, Yamakawa A. et al. The C. elegans unc-18 gene encodes a protein expressed in motor neurons. Neuron. 1993;11(4):703–711. [PubMed: 8398155]
- 63.
- Verhage M, Maia AS, Plomp JJ. et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion: Dynamics of munc18-1 phosphorylation/dephosphorylation in rat brain nerve terminals. Science. 2000;287(5454):864–869. [PubMed: 10657302]
- 64.
- Thurmond DC, Ceresa BP, Okada S. et al. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J Biol Chem. 1998;273:33876–33883. [PubMed: 9837979]
- 65.
- Wu MN, Fergestad T, Lloyd TE. et al. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23(3):593–605. [PubMed: 10433270]
- 66.
- Fujita Y, Sasaki T, Fukui K. et al. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: Its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J Biol Chem. 1996;271(13):7265–7268. [PubMed: 8631738]
- 67.
- Garcia EP, McPherson PS, Chilcote TJ. et al. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995;129:105–120. [PMC free article: PMC2120371] [PubMed: 7698978]
- 68.
- Halachmi N, Lev Z. The Sec1 family: A novel family of proteins involved in synaptic transmission and general secretion. J Neurochem. 1996;66(3):889–897. [PubMed: 8769846]
- 69.
- Hata Y, Sudhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. J Biol Chem. 1995;270:13022–13028. [PubMed: 7768895]
- 70.
- Tellam JT, Macaulay SL, McIntosh S. et al. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J Biol Chem. 1997;272:6179–6186. [PubMed: 9045631]
- 71.
- Tellam JT, McIntosh S, James DE. Molecular identification of two novel Munc-18 isoforms expressed in nonneuronal tissues. J Biol Chem. 1995;270:5857–5863. [PubMed: 7890715]
- 72.
- Volchuk A, Wang Q, Ewart HS. et al. Syntaxin 4 in 3T3-L1 adipocytes: Regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell. 1996;7:1075–1082. [PMC free article: PMC275959] [PubMed: 8862521]
- 73.
- Tamori Y, Kawanishi M, Niki T. et al. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J Biol Chem. 1998;273:19740–19746. [PubMed: 9677404]
- 74.
- Bracher A, Perrakis A, Dresbach T. et al. The X-ray crystal structure of neuronal Sec1 from squid sheds new light on the role of this protein in exocytosis. Structure Fold Des. 2000;8(7):685–694. [PubMed: 10903948]
- 75.
- Dulubova I, Sugita S, Hill S. et al. A conformational switch in syntaxin during exocytosis: Role of munc18. EMBO J. 1999;18(16):4372–4382. [PMC free article: PMC1171512] [PubMed: 10449403]
- 76.
- Fernandez I, Ubach J, Dulubova I. et al. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94(6):841–849. [PubMed: 9753330]
- 77.
- Lerman JC, Robblee J, Fairman R. et al. Structural analysis of the neuronal SNARE protein syntaxin-1A. Biochemistry. 2000;39(29):8470–8479. [PubMed: 10913252]
- 78.
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404(6776):355–362. [PubMed: 10746715]
- 79.
- Rowe J, Corradi N, Malosio ML. et al. Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells: Prevention by rbSEC1. J Cell Sci. 1999;112(Pt 12):1865–1877. [PubMed: 10341206]
- 80.
- Yang B, Steegmaier M, Gonzalez Jr LC. et al. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148(2):247–252. [PMC free article: PMC2174276] [PubMed: 10648557]
- 81.
- Dulubova I, Yamaguchi T, Arac D. et al. Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins. PNAS. 2003;100(1):32–37. [PMC free article: PMC140874] [PubMed: 12506202]
- 82.
- Grusovin J, Stoichevska V, Gough KH. et al. Definition of a minimal munc18c domain that interacts with syntaxin 4. Biochem J. 2000;350(Pt 3):741–746. [PMC free article: PMC1221305] [PubMed: 10970787]
- 83.
- Dascher C, Ossig R, Gallwitz D. et al. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11(2):872–885. [PMC free article: PMC359739] [PubMed: 1990290]
- 84.
- Thurmond DC, Kanzaki M, Khan AH. et al. Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol Cell Biol. 2000;20(1):379–388. [PMC free article: PMC85093] [PubMed: 10594040]
- 85.
- Thurmond DC, Pessin JE. Discrimination of GLUT4 vesicle trafficking from fusion using a temperature-sensitive Munc18c mutant. EMBO J. 2000;19(14):3565–3575. [PMC free article: PMC313977] [PubMed: 10899111]
- 86.
- Olson AL, Pessin JE. Structure, function and regulation of the mammalian facilitative glucose transporter gene family. Ann Rev Nutr. 1996;16:235–256. [PubMed: 8839927]
- 87.
- Slot JW, Geuze HJ, Gigengack S. et al. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci USA. 1991;88:7815–7819. [PMC free article: PMC52394] [PubMed: 1881917]
- 88.
- Slot JW, Geuze HJ, Gigengack S. et al. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. [PMC free article: PMC2288909] [PubMed: 2007617]
- 89.
- Czech MP. Molecular actions of insulin on glucose transport. Annu Rev Nutr. 1995;15:441–471. [PubMed: 8527229]
- 90.
- Kandror KV, Pilch PF. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol. 1996;271:E1–E14. [PubMed: 8760075]
- 91.
- Klip A, Tsakiridis T, Marette A. et al. Regulation of expression of glucose transporters by glucose: A review of studies in vivo and in cell cultures. FASEB J. 1994;8:43–53. [PubMed: 8299889]
- 92.
- Pessin JE, Thurmond DC, Elmendorf JS. et al. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! location! location! J Biol Chem. 1999;274:2593–2596. [PubMed: 9915783]
- 93.
- Rea S, James DE. Moving GLUT4: The biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. [PubMed: 9356011]
- 94.
- Thurmond DC, Pessin JE. Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane. Mol Membr Biol. 2001;18(4):237–245. [PubMed: 11780752]
- 95.
- Kahn BB. Facilitative glucose transporters: Regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992;89:1367–1374. [PMC free article: PMC443004] [PubMed: 1569179]
- 96.
- White MF. The IRS-signalling system: A network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed: 9609109]
- 97.
- Chen KS, Friel JC, Ruderman NB. Regulation of phosphatidylinositol 3-kinase by insulin in rat skeletal muscle. Am J Physiol. 1993;265:E736–E742. [PubMed: 8238500]
- 98.
- Folli F, Saad MJ, Backer JM. et al. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993;92(4):1787–1794. [PMC free article: PMC288341] [PubMed: 7691886]
- 99.
- Kelly KL, Ruderman NB, Chen KS. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992;267(5):3423–3428. [PubMed: 1310686]
- 100.
- Ruderman NB, Kapeller R, White MF. et al. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci USA. 1990;87:1411–1415. [PMC free article: PMC53485] [PubMed: 2154747]
- 101.
- Bandyopadhyay G, Standaert ML, Zhao L. et al. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. [PubMed: 8999972]
- 102.
- Kotani K, Ogawa W, Matsumoto M. et al. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. [PMC free article: PMC109280] [PubMed: 9819385]
- 103.
- Vollenweider P, Clodi M, Martin SS. et al. An SH2 domain-containing 5' inositolphosphatase inhibits insulin-induced GLUT4 translocation and growth factor-induced actin filament rearrangement. Mol Cell Biol. 1999;19:1081–1091. [PMC free article: PMC116038] [PubMed: 9891043]
- 104.
- Kane S, Sano H, Liu SC. et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277(25):22115–22118. [PubMed: 11994271]
- 105.
- Bruss MD, Arias EB, Lienhard GE. et al. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54(1):41–50. [PubMed: 15616009]
- 106.
- Sano H, Kane S, Sano E. et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278(17):14599–14602. [PubMed: 12637568]
- 107.
- Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell. 2004;15(10):4406–4415. [PMC free article: PMC519136] [PubMed: 15254270]
- 108.
- Schoch S, Deak F, Konigstorfer A. et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294(5544):1117–1122. [PubMed: 11691998]
- 109.
- Yang C, Coker KJ, Kim JK. et al. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest. 2001;107(10):1311–1318. [PMC free article: PMC209300] [PubMed: 11375421]
- 110.
- Yang C, Mora S, Ryder JW. et al. VAMP3 null mice display normal constitutive, insulin- and exercise-regulated vesicle trafficking. Mol Cell Biol. 2001;21(5):1573–1580. [PMC free article: PMC86703] [PubMed: 11238894]
- 111.
- Macaulay SL, Grusovin J, Stoichevska V. et al. Cellular munc18c levels can modulate glucose transport rate and GLUT4 translocation in 3T3L1 cells. FEBS Letters. 2002;528(1-3):154–160. [PubMed: 12297296]
- 112.
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. [PMC free article: PMC1170560] [PubMed: 9545229]
- 113.
- Van Rheenen SM, Cao X, Sapperstein SK. et al. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J Cell Biol. 1999;147(4):729–742. [PMC free article: PMC2156162] [PubMed: 10562277]
- 114.
- Chen G, Liu P, Thurmond DC. et al. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett. 2003;534(1-3):54–60. [PubMed: 12527361]
- 115.
- Nelson BA, Robinson KA, Buse MG. Insulin acutely regulates Munc18-c subcellular trafficking: Altered response in insulin-resistant 3T3-L1 adipocytes. J Biol Chem. 2002;277(6):3809–3812. [PubMed: 11751846]
- 116.
- Khan AH, Thurmond DC, Yang C. et al. Munc18c regulates insulin-stimulated GLUT4 translocation to the transverse tubules in skeletal muscle. J Biol Chem. 2001;276(6):4063–4069. [PMC free article: PMC5540311] [PubMed: 11054418]
- 117.
- Spurlin BA, Thomas RM, Nevins AK. et al. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes. 2003;52(8):1910–1917. [PubMed: 12882905]
- 118.
- Oh E, Spurlin BA, Pessin JE. et al. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes. 2005;54(3):638–647. [PubMed: 15734838]
- 119.
- Kanda H, Tamori Y, Shinoda H. et al. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest. 2005;115(2):291–301. [PMC free article: PMC546422] [PubMed: 15690082]
- 120.
- Spurlin BA, Park SY, Nevins AK. et al. Syntaxin 4 transgenic mice exhibit enhanced insulin-mediated glucose uptake in skeletal muscle. Diabetes. 2004;53(9):2223–2231. [PubMed: 15331531]
- 121.
- Geppert M, Bolshakov VY, Siegelbaum SA. et al. The role of Rab3A in neurotransmitter release. Nature. 1994;369(6480):493–497. [PubMed: 7911226]
- 122.
- Tall GG, Hama H, De Wald DB. et al. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10(6):1873–1889. [PMC free article: PMC25384] [PubMed: 10359603]
- 123.
- Webb GC, Hoedt M, Poole LJ. et al. Genetic interactions between a pep7 mutation and the PEP12 and VPS45 genes: Evidence for a novel SNARE component in transport between the Saccharomyces cerevisiae Golgi complex and endosome. Genetics. 1997;147(2):467–478. [PMC free article: PMC1208171] [PubMed: 9335586]
- 124.
- Schimmoller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem. 1998;273(35):22161–22164. [PubMed: 9712825]
- 125.
- Cormont M, Tanti JF, Zahraoui A. et al. Insulin and okadaic acid induce Rab4 redistribution in adipocytes. J Biol Chem. 1993;268(26):19491–19497. [PubMed: 8366094]
- 126.
- Li L, Omata W, Kojima I. et al. Direct interaction of Rab4 with syntaxin 4. J Biol Chem. 2000;276:5265–5273. [PubMed: 11063739]
- 127.
- Shibata H, Omata W, Kojima I. Insulin stimulates guanine nucleotide exchange on Rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J Biol Chem. 1997;272(23):14542–14546. [PubMed: 9169411]
- 128.
- Shisheva A, Czech MP. Association of cytosolic Rab4 with GDI isoforms in insulin-sensitive 3T3-L1 adipocytes. Biochemistry. 1997;36(22):6564–6570. [PubMed: 9184135]
- 129.
- Cormont M, Bortoluzzi MN, Gautier N. et al. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol Cell Biol. 1996;16:6879–6886. [PMC free article: PMC231691] [PubMed: 8943343]
- 130.
- Mora S, Monden I, Zorzano A. et al. Heterologous expression of rab4 reduces glucose transport and GLUT4 abundance at the cell surface in oocytes. Biochem J. 1997;324:455–459. [PMC free article: PMC1218451] [PubMed: 9182703]
- 131.
- Shibata H, Omata W, Suzuki Y. et al. A synthetic peptide corresponding to the Rab4 hypervariable carboxyl-terminal domain inhibits insulin action on glucose transport in rat adipocytes. J Biol Chem. 1996;271:9704–9709. [PubMed: 8621647]
- 132.
- Fujita Y, Shirataki H, Sakisaka T. et al. Tomosyn: A syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20(5):905–915. [PubMed: 9620695]
- 133.
- Hatsuzawa K, Lang T, Fasshauer D. et al. The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. J Biol Chem. 2003;278(33):31159–31166. [PubMed: 12782620]
- 134.
- Yokoyama S, Shirataki H, Sakisaka T. et al. Three splicing variants of tomosyn and identification of their syntaxin-binding region. Biochem Biophys Res Commun. 1999;256(1):218–222. [PubMed: 10066450]
- 135.
- Groffen AJ, Jacobsen L, Schut D. et al. Two distinct genes drive expression of seven tomosyn isoforms in the mammalian brain, sharing a conserved structure with a unique variable domain. J Neurochem. 2005;92(3):554–568. [PubMed: 15659226]
- 136.
- Yizhar O, Matti U, Melamed R. et al. Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proc Natl Acad Sci USA. 2004;101(8):2578–2583. [PMC free article: PMC356992] [PubMed: 14983051]
- 137.
- Widberg CH, Bryant NJ, Girotti M. et al. Tomosyn interacts with the t-SNAREs syntaxin4 and SNAP23 and plays a role in insulin-stimulated GLUT4 translocation. J Biol Chem. 2003;278(37):35093–35101. [PubMed: 12832401]
- 138.
- Min J, Okada S, Kanzaki M. et al. Synip: A novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol Cell. 1999;3(6):751–760. [PubMed: 10394363]
- 139.
- Yamada E, Okada S, Saito T. et al. Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4-containing vesicles. J Cell Biol. 2005;168(6):921–928. [PMC free article: PMC2171785] [PubMed: 15753124]
- 140.
- TerBush DR, Maurice T, Roth D. et al. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15(23):6483–6494. [PMC free article: PMC452473] [PubMed: 8978675]
- 141.
- Kee Y, Yoo JS, Hazuka CD. et al. Subunit structure of the mammalian exocyst complex. Proc Natl Acad Sci USA. 1997;94(26):14438–14443. [PMC free article: PMC25013] [PubMed: 9405631]
- 142.
- Inoue M, Chang L, Hwang J. et al. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422(6932):629–633. [PubMed: 12687004]
- 143.
- Thurmond DC, Pessin JE. Molecular basis for insulin-stimulated GLUT4 translocation. Current Opinion in Endocrinology and Diabetes. 2001;8(2):67–73.
- 144.
- Ewart MA, Clarke M, Kane S. et al. Evidence for a role of the exocyst in insulin-stimulated Glut4 trafficking in 3T3-L1 adipocytes. J Biol Chem. 2005;280(5):3812–3816. [PubMed: 15550383]
- 145.
- Rhodes CJ. Processing of the insulin molecule. In: LeRoith T, Olefsky, eds. Diabetes Mellitus: A fundamental and clinical text. Philadelphia, PA: Lippincott Williams and Wilkins. 2000:20–38.
- 146.
- Halban PA, Renold AE. Influence of glucose on insulin handling by rat islets in culture. A reflection of integrated changes in insulin biosynthesis, release, and intracellular degradation. Diabetes. 1983;32(3):254–261. [PubMed: 6337904]
- 147.
- Halban PA, Wollheim CB. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980;255(13):6003–6006. [PubMed: 6993463]
- 148.
- Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259(1-2):3–17. [PubMed: 9914469]
- 149.
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311(5983):271–273. [PubMed: 6090930]
- 150.
- Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2:163–214. [PubMed: 2943567]
- 151.
- Satin LS, Cook DL. Voltage-gated Ca2+ current in pancreatic B-cells. Pflugers Arch. 1985;404(4):385–387. [PubMed: 2414720]
- 152.
- Rorsman P, Eliasson L, Renstrom E. et al. The cell physiology of biphasic insulin secretion. News Physiol Sci. 2000;15:72–77. [PubMed: 11390882]
- 153.
- Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83(3):572–584. [PubMed: 4877098]
- 154.
- Heinemann C, von Ruden L, Chow RH. et al. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 1993;424(2):105–112. [PubMed: 8414901]
- 155.
- Barg S, Eliasson L, Renstrom E. et al. A subset of 50 secretory granules in close contact with L-type Ca(2+) channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51(Suppl 1):S74–82. [PubMed: 11815462]
- 156.
- Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89(4):1288–1295. [PMC free article: PMC442990] [PubMed: 1556189]
- 157.
- Renstrom E, Eliasson L, Bokvist K. et al. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol. 1996;494(Pt 1):41–52. [PMC free article: PMC1160613] [PubMed: 8814605]
- 158.
- Lam PL, Leung YM, Sheu L. et al. Transgenic mouse over-expressing Syntaxin-1A as a diabetes model. Diabetes. 2005;54:2744–54. [PubMed: 16123365]
- 159.
- Watanabe T, Fujiwara T, Komazaki S. et al. HPC-1/syntaxin 1A suppresses exocytosis of PC12 cells. J Biochem (Tokyo). 1999;125(4):685–689. [PubMed: 10101280]
- 160.
- Sadoul K, Berger A, Niemann H. et al. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J Biol Chem. 1997;272(52):33023–33027. [PubMed: 9407084]
- 161.
- Land J, Zhang H, Vaidyanathan VV. et al. Transient expression of botulinum neurotoxin C1 light chain differentially inhibits calcium and glucose induced insulin secretion in clonal beta-cells. FEBS Lett. 1997;419(1):13–17. [PubMed: 9426210]
- 162.
- Saito T, Okada S, Yamada E. et al. Syntaxin 4 and Synip (Syntaxin 4 Interacting Protein) regulate insulin secretion in the pancreatic {beta} HC-9 cell. J Biol Chem. 2003;278(38):36718–36725. [PubMed: 12855681]
- 163.
- Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic insulin secretion from pancreatic beta cells Mol Endocrinol 2005 . in press. [PubMed: 16099818]
- 164.
- Kang Y, Huang X, Pasyk EA. et al. Syntaxin-3 and syntaxin-1A inhibit L-type calcium channel activity, insulin biosynthesis and exocytosis in beta-cell lines. Diabetologia. 2002;45(2):231–241. [PMC free article: PMC2970522] [PubMed: 11935155]
- 165.
- Nagamatsu S, Nakamichi Y, Yamamura C. et al. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: Restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999;48(12):2367–2373. [PubMed: 10580425]
- 166.
- Chan CB, MacPhail RM, Sheu L. et al. Beta-cell hypertrophy in fa/fa rats is associated with basal glucose hypersensitivity and reduced SNARE protein expression. Diabetes. 1999;48(5):997–1005. [PubMed: 10331403]
- 167.
- Wiser O, Bennett MK, Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J. 1996;15(16):4100–4110. [PMC free article: PMC452132] [PubMed: 8861939]
- 168.
- Leung YM, Kang Y, Gao X. et al. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2.1 to regulate channel gating and trafficking. J Biol Chem. 2003;278(19):17532–17538. [PubMed: 12621036]
- 169.
- Leung YM, Kang Y, Xia F. et al. Open form syntaxin-1A is a more potent inhibitor than wild type syntaxin-1A of Kv2.1 channels. Biochem J. 2005;387(Pt 1):195–202. [PMC free article: PMC1134947] [PubMed: 15518587]
- 170.
- Ji J, Yang SN, Huang X. et al. Modulation of L-type Ca(2+) channels by distinct domains within SNAP-25. Diabetes. 2002;51(5):1425–1436. [PubMed: 11978639]
- 171.
- MacDonald PE, Wang G, Tsuk S. et al. Synaptosome-associated protein of 25 kilodaltons modulates Kv2.1 voltage-dependent K(+) channels in neuroendocrine islet beta-cells through an interaction with the channel N terminus. Mol Endocrinol. 2002;16(11):2452–2461. [PubMed: 12403834]
- 172.
- Zhang W, Efanov A, Yang SN. et al. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta -cell. J Biol Chem. 2000;275(52):41521–41527. [PubMed: 11024017]
- 173.
- Zhang W, Lilja L, Bark C. et al. Mint1, a Munc-18-interacting protein, is expressed in insulin-secreting beta-cells. Biochem Biophys Res Commun. 2004;320(3):717–721. [PubMed: 15240107]
- 174.
- Okamoto M, Sudhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem. 1997;272:31459–31464. [PubMed: 9395480]
- 175.
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 1997;276(5316):1255–1258. [PubMed: 9157884]
- 176.
- Geppert M, Goda Y, Stevens CF. et al. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387(6635):810–814. [PubMed: 9194562]
- 177.
- Regazzi R, Kikuchi A, Takai Y. et al. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267(25):17512–17519. [PubMed: 1517204]
- 178.
- Regazzi R, Vallar L, Ullrich S. et al. Characterization of small-molecular-mass guanine-nucleotide-binding regulatory proteins in insulin-secreting cells and PC12 cells. Eur J Biochem. 1992;208(3):729–737. [PubMed: 1327767]
- 179.
- Regazzi R, Ravazzola M, Iezzi M. et al. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci. 1996;109(Pt 9):2265–2273. [PubMed: 8886977]
- 180.
- Coppola T, Frantz C, Perret-Menoud V. et al. Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell. 2002;13(6):1906–1915. [PMC free article: PMC117613] [PubMed: 12058058]
- 181.
- Iezzi M, Escher G, Meda P. et al. Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol Endocrinol. 1999;13(2):202–212. [PubMed: 9973251]
- 182.
- Iezzi M, Regazzi R, Wollheim CB. The Rab3-interacting molecule RIM is expressed in pancreatic beta-cells and is implicated in insulin exocytosis. FEBS Lett. 2000;474(1):66–70. [PubMed: 10828453]
- 183.
- Torii S, Zhao S, Yi Z. et al. Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1A. Mol Cell Biol. 2002;22(15):5518–5526. [PMC free article: PMC133943] [PubMed: 12101244]
- 184.
- Yi Z, Yokota H, Torii S. et al. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22(6):1858–1867. [PMC free article: PMC135591] [PubMed: 11865063]
- 185.
- Torii S, Takeuchi T, Nagamatsu S. et al. Rab27 effector granuphilin promotes the plasma membrane targeting of insulin granules via interaction with syntaxin 1A. J Biol Chem. 2004;279(21):22532–22538. [PubMed: 15028737]
- 186.
- Kotake K, Ozaki N, Mizuta M. et al. Noc2, a putative zinc finger protein involved in exocytosis in endocrine cells. J Biol Chem. 1997;272(47):29407–29410. [PubMed: 9367993]
- 187.
- Cheviet S, Coppola T, Haynes LP. et al. The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis. Mol Endocrinol. 2004;18(1):117–126. [PubMed: 14593078]
- 188.
- Sheu L, Pasyk EA, Ji J. et al. Regulation of Insulin Exocytosis by Munc13-1. J Biol Chem. 2003;278(30):27556–27563. [PubMed: 12871971]
- 189.
- Betz A, Thakur P, Junge HJ. et al. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30(1):183–196. [PubMed: 11343654]
- 190.
- Augustin I, Rosenmund C, Sudhof TC. et al. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–461. [PubMed: 10440375]
- 191.
- Li G, Rungger-Brandle E, Just I. et al. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol Biol Cell. 1994;5(11):1199–1213. [PMC free article: PMC301146] [PubMed: 7865885]
- 192.
- Somers G, Blondel B, Orci L. et al. Motile events in pancreatic endocrine cells. Endocrinology. 1979;104(1):255–264. [PubMed: 376285]
- 193.
- Wilson JR, Ludowyke RI, Biden TJ. A redistribution of actin and myosin IIA accompanies Ca(2+)-dependent insulin secretion. FEBS Lett. 2001;492(1-2):101–106. [PubMed: 11248245]
- 194.
- Thurmond DC, Gonelle-Gispert C, Furukawa M. et al. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (Target Membrane Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor Protein) complex. Mol Endocrinol. 2003;17(4):732–742. [PubMed: 12554769]
- 195.
- Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol. 2003;285(3):C698–710. [PubMed: 12760905]
- 196.
- Kowluru A, Metz SA. Regulation of guanine-nucleotide binding proteins in islet subcellular fractions by phospholipase-derived lipid mediators of insulin secretion. Biochim Biophys Acta. 1994;1222(3):360–368. [PubMed: 8038204]
- 197.
- Kowluru A, Seavey SE, Li G. et al. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98(2):540–555. [PMC free article: PMC507460] [PubMed: 8755667]
- 198.
- Daniel S, Noda M, Cerione RA. et al. A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry. 2002;41(30):9663–9671. [PubMed: 12135388]
- 199.
- Nevins AK, Thurmond DC. A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J Biol Chem. 2005;280(3):1944–1952. [PubMed: 15537656]
- 200.
- Parsons TD, Coorssen JR, Horstmann H. et al. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15(5):1085–1096. [PubMed: 7576652]
- 201.
- Maier V, Melvin D, Lister C. et al. v- and t-SNARE protein expression in models of insulin resistance: Normalization of glycemia by rosiglitazone treatment corrects overexpression of cellubrevin, vesicle-associated membrane protein-2, and syntaxin 4 in skeletal muscle of Zucker diabetic fatty rats. Diabetes. 2000;49(4):618–625. [PubMed: 10871200]
- 202.
- Miura T, Suzuki W, Ishihara E. et al. Impairment of insulin-stimulated GLUT4 translocation in skeletal muscle and adipose tissue in the Tsumura Suzuki obese diabetic mouse: A new genetic animal model of type 2 diabetes. Eur J Endocrinol. 2001;145(6):785–790. [PubMed: 11720905]
- Regulation of Insulin Action and Insulin Secretion by SNARE-Mediated Vesicle Exo...Regulation of Insulin Action and Insulin Secretion by SNARE-Mediated Vesicle Exocytosis - Madame Curie Bioscience Database
- Structure, Function and Assembly of Flagellar Axial Proteins - Madame Curie Bios...Structure, Function and Assembly of Flagellar Axial Proteins - Madame Curie Bioscience Database
- The ø29 DNA Packaging Motor: Seeking the Mechanism - Madame Curie Bioscience Dat...The ø29 DNA Packaging Motor: Seeking the Mechanism - Madame Curie Bioscience Database
- Molecular Phylogeny and Evolution of the Coronin Gene Family - Madame Curie Bios...Molecular Phylogeny and Evolution of the Coronin Gene Family - Madame Curie Bioscience Database
- Cell-Cell Fusion: Transient Channels Leading to Plasma Membrane Merger - Madame ...Cell-Cell Fusion: Transient Channels Leading to Plasma Membrane Merger - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...