NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Introduction
The prostate is a male sex accessory organ whose development is regulated by androgens and mesenchymal/epithelial interactions. The organ comprises branched epithelial ducts within a stroma consisting of fibroblasts and smooth muscle as well as other components such as vasculature and nerves. The function of the prostate is to produce secretions that make up part of the seminal fluid, though it is not certain if these are essential for fertility or sperm function. There has been considerable interest in the identification of molecules and pathways that regulate prostatic growth, due to their relevance in prostatic disease. Few studies have focussed directly on prostatic branching though some have identified factors or pathways that play a role in prostatic growth and branching morphogenesis. Several pathways have been identified that appear to influence growth of the prostate and the process of branching morphogenesis simultaneously. However, genetic evidence suggests that prostatic growth and prostatic branching morphogenesis are processes that are independently regulated. The prostate is not one of the organs widely used for studies of branching morphogenesis, though this seems unfortunate as there are many factors which suggest that this organ would be an excellent model for the study of branching. These are: the ease with which these organs can be grown in vitro, the fact that the prostate is not required for viability, and the late genesis and growth of this organ relative to others during organogenesis.
Anatomical and Endocrine Aspects of Prostate Development
The prostate is an organ that is found only in male mammals and surrounds the urethra at the base of the bladder. The function of the prostate is to add various components to the seminal fluid, and these are emptied directly into the urethra. The contraction of smooth muscle within and surrounding the prostate leads to the expulsion of products made in the branched secretory acini through a branched ductal system and thence the urethra. The secretory epithelia show a tall columnar morphology while epithelia of the ducts show a more flattened morphology.
The formation of the prostate occurs relatively late in gestation when compared to the genesis of most organs. In humans the prostate starts to form between 10-12 weeks of gestation while in mice prostatic development begins at E16.5 and in rats at E17.5. At present, the onset of prostatic organogenesis is defined by the induction of epithelial buds from the urethra, however, it is likely that formation of a condensed mesenchyme involved in organ induction precedes this. During prostatic bud induction and subsequent branching morphogenesis, epithelial buds grow as solid cords which later differentiate and undergo canalisation.
Androgens are required for prostatic development, and it is important to note that the androgen receptor (AR) is not expressed in epithelia during organ induction but is expressed in the mesenchyme.1 This indicates that essential components of bud induction (in response to androgens) are found within the mesenchyme, though the identity of these signals is presently unknown. The importance of mesenchymal to epithelial signalling was established many years ago using tissue recombination studies, which clearly demonstrated that AR was required in the mesenchymal compartment for prostate development.2 AR is expressed in prostatic epithelia at later stages of prostatic growth and in adulthood, though epithelial AR is neither necessary nor sufficient for normal organ growth.3 This data also indicates that any involvement of the AR in branching is likely to be restricted to androgen signalling in the mesenchyme. Further details of genetic studies implicating androgens and the AR in prostatic development are described in the section on mutations that alter prostatic branching morphogenesis. The dependence of prostatic growth and branching upon androgens might make the prostate an excellent system in which to study branching, since the ability to inhibit or augment growth and branching allows for thorough examination of different pathways in these processes. Another important issue is that, while it is clear that androgens stimulate the growth and branching of the prostate, it is not known if androgens act upon growth or branching independently. Thus, the effects on branching may be mediated via general effects upon growth and it remains to be determined whether branching is directly affected by androgens.
In rodents, the prostate consists of anatomically distinct lobes (termed dorsal, lateral, ventral and anterior)4 while in humans it is more compact and composed of different zones that differ subtly in their histology.5 The different lobes of the rodent prostate exhibit functional differences in terms of the secretory proteins that they make and also differ in their branching patterns.6 This has allowed for some characterisation of how the separate branching patterns arise, using tissue recombination studies. This work has shown that aspects of epithelial identity (such as secretory protein profile) are specified by mesenchymal signals and suggests that the distinct branching patterns are also mesenchymally defined.7,8
Signals from the mesenchyme play a key role in defining branching pattern, however in some circumstances there are limitations of the epithelial response to inductive mesenchyme. For example, the mesenchyme of the seminal vesicle will induce prostatic branching in urethral or bladder epithelium. The seminal vesicle shows an epithelial architecture which is both highly folded as well as branched, yet the mesenchyme of the seminal vesicle will elicit a true branching pattern with a heterologous epithelium (such as bladder). It is worth noting that the seminal vesicle epithelium is derived from the mesoderm (pronephric/wolffian duct) while the urethral and bladder epithelium are endoderm derived. There are two important conclusions to be drawn from this work. Firstly, that some elements of branching pattern can be defined by the epithelium and secondly that instructive branching interactions can occur between tissues of different germ layer origin (mesoderm and endoderm).
Epithelial Bud Induction
Prostatic bud induction from the epithelia of the urethra may be regarded as the initial branching event of prostatic growth and it is emerging that bud induction and subsequent growth and branching may have significant differences. For example, fibroblast growth factor 10 (FGF10) is required for the formation and growth of the prostate, but there is induction of a few prostatic buds in FGF10 null mice.9 This suggests that FGF10 may be required for prostatic growth but is not essential for bud induction, even though fewer prostatic buds are apparent in the FGF10 null mouse. Similarly, the sonic hedgehog pathway has recently been shown to be important for prostatic growth and branching,10 but is not required for prostatic bud induction.11 While bud induction versus subsequent branching morphogenesis have been clearly separated in other systems, these processes have only recently been separated in prostatic organogenesis.
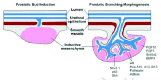
Signals from the mesenchyme are essential for the induction and growth of the prostate but there is little information regarding the identity of these molecules or how they are regulated. Recent work has shown that smooth muscle may play an important role in regulating prostatic induction.12 During prostatic induction it appears that a layer of smooth muscle forms between the mesenchyme and epithelium, and that the formation of this layer is sexually dimorphic. In females, the smooth muscle layer develops but in males testosterone inhibits the formation of this layer. The inhibition of the smooth muscle layer is proposed to allow continued signalling between the mesenchyme and epithelium, and instructive interactions leading to bud stabilisation and growth. A schematic diagram of prostatic bud induction and subsequent growth is shown in Figure 1. Additionally, it is possible that smooth muscle also plays a role in branching morphogenesis, as growing epithelial buds lack adjacent smooth muscle but mature ducts are surrounded by layers of well differentiated smooth muscle.13 Such a pattern of discontinuous smooth muscle distribution is also observed in the developing lung.14,15 At present it is not known precisely how smooth muscle might affect mesenchymal/ epithelial interactions though there are several possibilities. As mesenchyme differentiates into smooth muscle it is very likely that there is a change in the expression of growth or branching regulatory molecules and it is also possible that the smooth muscle acts as a barrier to the diffusion of regulatory molecules from the mesenchyme. Additionally, it is possible that smooth muscle is a source of molecules that are inhibitory to growth or branching. It is also possible that there is a combination of these mechanisms active which are involved in the mediating the effects of smooth muscle.
Growth Factor Signalling in Prostate Branching Morphogenesis
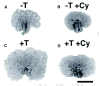
Various studies have examined the function of growth factor signalling in prostate branching morphogenesis, and this section will discuss those that have used organ culture studies of prostatic rudiments grown in vitro. This is a highly tractable system which allows for the addition of growth factors, blocking antibodies, or small molecules that activate or inhibit receptor signalling. Figure 2 shows an example of day-of-birth rat prostatic organs grown in vitro. Testosterone clearly stimulates growth and branching morphogenesis, and in this example the hedgehog (Hh) signalling pathway has been blocked using a small molecule (cyclopamine) in either the presence or absence of testosterone. The advantages of the organ culture system are the ability to perform a functional test of a given pathway, however a significant problem is that inhibition or stimulation are applied throughout the organ and are not restricted to sites of active branching morphogenesis within the organ. Most studies have not examined branching in detail but have looked at effects on the whole organ, though a few have studied branching directly. These limitations notwithstanding, growth factor pathways studied have included fibroblast growth factors (FGFs), Transforming Growth Factors betas (TGFβs), activin and inhibins, and sonic hedgehog (Shh).
The effects of FGF7 (also known as KGF) and FGF10 have been examined in ventral prostate rudiments grown in vitro.16,17 Both of these factors are made by the mesenchyme and act via a receptor which is restricted to the epithelium (FGFR2IIIb) and act as paracrine factors. Addition of recombinant FGF7 or 10 stimulated the growth of the prostate and did not appear to result in any distortion of branching pattern. In contrast, addition of TGFβ results in the inhibition of prostatic growth as well as changes in the branch number.18 Addition of TGFβ resulted in a decrease in ductal tip number, when the total number of branch tips were counted. However, it is quite difficult to define individual tips in the whole organ, and it is possible that TGFβ may have differential effects at the periphery of the organ versus the middle. It appears that, when TGFβ is added to organ cultures of ventral prostate, there is an increase in epithelial tip number at the periphery and an overall reduction in size (D. Tomlinson and AAT, unpublished observations). Another member of the TGFβ superfamily which inhibits prostatic growth is activin. Cancilla et al showed that addition of activin A inhibited the growth of the prostate, and led to little branching morphogenesis. Addition of follistatin (an antagonist of activin signalling) led to organ growth and branching in the absence of testosterone.19 These data suggest that activin signalling may be a modulator of growth and branching of the prostate.
Shh signalling has been studied in relation to prostatic growth and recent studies indicate that this pathway is involved in branching and epithelial differentiation. Initial studies suggested that Shh was required for the formation of the prostate,10 but more recent data indicate that formation of the prostate is not dependent upon Shh signalling.11 In addition, inhibition of Shh signalling causes an increase in epithelial tips at the periphery of the organ, suggesting that Shh may be an inhibitor of the branching process (Fig. 2).
Genetic Analysis of Prostatic Branching
The phenotypes created by spontaneous and engineered mutations in mice and humans have provided direct evidence for the roles of several genes in prostatic branching morphogenesis. In humans, mutations affecting the prostate were identified because they result in androgen insensitivity syndrome (AIS) and pseudovaginal perineoscrotal hypospadias (PPSH). In mice, spontaneous mutations affecting the prostate were identified because of external phenotypes at other anatomical locations. These mutations include Testicular feminization (Tfm) and Hypodactyly (Hd). The remaining mutations affecting prostatic branching morphogenesis were engineered in mice using homologous recombination and transgenic approaches. A clear advantage of using a genetic approach to define the roles of particular genes in prostatic branching morphogenesis is that genetics can prove that a gene is required for branching morphogenesis in the context of the intact mouse or human. A potential disadvantage of using a genetic approach is that many mutations cause defects in other organ systems that can complicate the interpretation of prostatic phenotypes. The prostatic phenotypes created by spontaneous and engineered mutations in mice and humans are summarized in Table 1 and discussed in greater detail in the sections that follow.
Table 1
Mutations affecting prostatic branching morphogenesis.
Mutations That Affect Endocrine Signalling
Several circulating hormones regulate prostatic development. The most important of these are the androgens.20 Cellular response to systemic androgens is mediated by nuclear androgen receptors that are activated by testosterone or dihydrotestosterone. The requirement for the androgen receptor (AR) gene in prostatic epithelial budding and branching morphogenesis is shown by the phenotypes caused by AIS mutations in humans and the Tfm mutation in mice.21,22 These mutations are recessive, loss of function mutations in the AR. The prostatic phenotypes caused by the AIS and Tfm mutations include the absence of epithelial budding and branching morphogenesis, and the absence of organ-specific cellular differentiation.
The phenotypes caused by inactivating mutations in the steroid 5-alpha reductase 2 (Srd5a2) gene further clarify the roles of specific androgens. Mutations in this gene include the pseudovaginal perineoscrotal hypospadias (PPSH) mutations in humans23 and a mouse knockout allele created by homologous recombination in mouse embryonic stem (ES) cells.24 Srd5a2 encodes the enzyme Δ4-3-ketosteroid-5α-reductase (5α-reductase) type 2 that converts testosterone to the more potent androgen receptor agonist dihydrotestosterone.25 During prostatic development, testosterone is the major androgen in circulation. Srd5a2 is expressed in the mesenchyme of the developing prostate. Thus, testosterone could activate androgen receptors in the prostate through direct binding to androgen receptors, or through local conversion of testosterone to dihydrotestosterone that could subsequently bind androgen receptors. When conversion of testosterone to dihydrotestosterone is blocked in the prostate by mutations in Srd5a2, the urogenital sinus is specified as prostate but prostatic growth and branching morphogenesis are greatly reduced.23,24 These phenotypes demonstrate that dihydrotestosterone is not required for prostatic branching morphogenesis to occur, but dihydrotestosterone is required to achieve the normal extent of growth and morphogenesis. Further studies have confirmed that nonreducible analogues of testosterone will stimulate prostatic growth and branching.6
Genetic experiments in mice have implicated two additional circulating hormones in prostatic development. Expression of an antagonist for the growth hormone receptor (GHR) in transgenic mice resulted in a small prostate with reduced branching.27 In mice transgenic for the growth hormone receptor antagonist, reduced prostatic morphogenesis occurred in the context of an overall dwarfism syndrome caused by the transgene. GHR antagonist transgenic mice were about 50% the size of wild type control mice. These phenotypes demonstrated that growth hormone (GH) and the GHR act as positive factors that stimulate prostatic branching morphogenesis. However, the effects of GH and GHR to stimulate growth and morphogenesis are not specific to the prostate. Inactivation of prolactin in a mouse knockout model resulted in a smaller ventral prostate.28 Steger et al did not measure the dorsolateral prostate or quantify prostatic branching morphogenesis so the potential effects on these aspects of prostatic development are not clear.
Mutations That Affect Homeobox-Containing Transcription Factors
Several homeobox-containing transcription factors have been implicated in prostatic branching morphogenesis by genetic data. These include three members of the Hox gene family: Hoxa10, Hoxa13, and Hoxd13. Mice homozygous for a knockout allele of Hoxa10 have reduced branching in the anterior prostate and a partial anterior prostate to dorsolateral prostate transformation.29 Mice heterozygous for a deletion in Hoxa13 [the mouse Hypodactyly (Hd) mutation] have reduced size and reduced branching in the anterior prostate and dorsolateral prostate.30 In the case of the Hd mutation, phenotypes in other organ systems result in lethality in late gestation for homozygous mutants. Prostatic development primarily occurs after birth. Consequently, the phenotypic consequences of removing both copies of Hoxa13 for prostatic development cannot be determined using the Hd mutation. Mice homozygous for a knockout allele of Hoxd13 have smaller anterior prostates and dorsolateral prostate and reduced dorsolateral prostate branching.31 These phenotypes demonstrate that each of these Hox genes acts to promote branching morphogenesis in one or more prostatic lobes.
In mice and humans, the Hox genes are organized into 4 paralogous clusters (Hoxa, Hoxb, Hoxd, and Hoxd clusters). In many tissues, a comparison of paralogous Hox genes between clusters has identified similar gene expression patterns and partially redundant gene functions. This is true for paralogous genes Hoxa13 and Hoxd13 that act in a partially redundant fashion in the prostate. This is shown by a more severe failure of prostatic development in compound Hoxa13 Hd/+: Hoxd13 -/- mutant mice than the individual mutants.32 The most dramatic example of this is for the AP. Mice homozygous for the Hoxd13 mutation have an AP of wild type size and wild type branching.31 Mice heterozygous for the Hd mutation have only a modest decrease in the extent of AP morphogenesis that was not statistically significant in the study by Podlasek et al.30 In contrast, compound Hoxa13 Hd/+: Hoxd13 -/- mutant mice lack the AP entirely.32
The three Hox gene mutations that alter prostatic morphogenesis also cause developmental defects in multiple unrelated organ systems. Mutations in a fourth homeobox-containing transcription factor, Nkx3.1, have developmental phenotypes that are limited to a small number of glandular organs including the prostate.33-35 Mice homozygous for a knockout allele of Nkx3.1 have reduced branching in all prostatic lobes without defects in prostatic size. These phenotypes demonstrate that Nkx3.1 acts to promote prostatic branching morphogenesis independent of prostatic growth. The anatomical sites of other developmental defects in Nkx3.1 mutant mice are the bulbourethral glands and salivary glands (see Chapter 9).
Mutations That Affect Secreted Signalling Molecules
Branching morphogenesis during prostatic development is dependent upon mesenchymalepithelial interactions such that morphogenesis and differentiation of both the epithelium and mesenchyme are abortive if the epithelium and mesenchyme are grown separately. Not surprisingly, the genes implicated in prostatic branching morphogenesis by genetic data include three genes encoding secreted signalling molecules, Bmp4, Igf1, and Fgf10, that can mediate intercellular communication. Mice heterozygous for a knockout allele of Bmp4 have increased branching in the anterior prostate and ventral prostate.36 Mice homozygous for a knockout allele of Bmp4 die during embryogenesis prior to prostatic development so the affects of the complete absence of Bmp4 on prostatic morphogenesis cannot be determined using the allele studied by Lamm et al. These data are consistent with a role for BMP4 as a factor that limits prostatic branching morphogenesis.
Mice homozygous for a knockout allele of Igf1 have reduced size and reduced branching for all prostatic lobes.27,37 These phenotypes are observed in the context of a severe dwarfism syndrome. In the study by Ruan et al only 10% of homozygous mutants survived to the adult stage when prostatic phenotypes were assessed. At that age, mutant males were approximately 20% the size of wild type controls. These phenotypes demonstrated that Igf1 acts to stimulate prostatic branching morphogenesis. However, the effects of Igf1 to stimulate growth and morphogenesis are not specific to the prostate. In addition, Igf1 mutant mice have decreased circulating androgen levels.37 Consequently, the prostatic phenotypes in Igf1 mutant mice may be indirect.
Mice homozygous for a knockout allele of Fgf10 lack nearly all prostatic buds at birth.9 In wild type mice, many prostatic buds and some early ductal branches have formed by birth. Homozygous Fgf10 mutant mice die at birth due to defects in other organ systems. Donjacour et al performed rescue and grafting experiments of prostatic rudiments into immuno-deficient male host mice to determine the affects of Fgf10 deficiency on postnatal prostatic branching morphogenesis and cellular differentiation. In these experiments, Fgf10 mutant prostatic rudiments exhibited little growth and failed to undergo branching morphogenesis, but they did express mature differentiation markers for prostatic epithelial cells. Under the same experimental conditions wild type rudiments exhibited extensive growth and branching morphogenesis. These phenotypes demonstrate that Fgf10 is required for prostatic growth and branching morphogenesis.
Other Mutations
The p63 gene encodes a nuclear protein that is expressed in many epithelial tissues. Mice homozygous for a knockout allele of p63 lack any sign of prostatic budding or branching morphogenesis at birth.38 In wild type mice, many prostatic buds and some early ductal branches have formed by birth. p63 homozygous mutant mice die at birth due to defects in multiple other epithelia throughout the body. These data are consistent with a requirement for p63 in prostatic morphogenesis. However, since p63 mutant mice die shortly after prostatic morphogenesis begins in wild type mice, it is not possible to exclude the possibility that prostatic morphogenesis is merely delayed in the absence of p63 based on the study by Signoretti et al.
Prostatic Growth and Branching Morphogenesis Are Under Separate Genetic Control
Several pathways have been identified that simultaneously affect prostatic growth and prostatic branching morphogenesis. However, the phenotypes caused by some mutations demonstrate that these are two distinct processes under separate genetic control. For example, the ventral prostate has dramatically reduced branching in Nkx3.1 deficient mice, but the ventral prostate grows to its normal size in these mice.3,28,35 Similarly, the ventral prostate has increased branching morphogenesis in heterozygous Bmp4 mutant mice without an increase in prostatic size.16 These types of phenotypes demonstrate that genetic mechanisms are present within the prostate to regulate prostatic branching morphogenesis independently of prostatic growth.
Conclusions
Branching morphogenesis in the prostate is regulated by several factors though we are far from a comprehensive knowledge of their identity. Since the prostate is a target of steroid hormone action there is additional complexity regarding the role of androgens in growth versus branching, and much needs to be done in separating the pathways involved in these two processes. At present, it appears that many mechanisms identified in other organ systems are involved in regulating prostatic branching morphogenesis and it seems unlikely that there are prostate-specific mechanisms of branching. However, since the organ is dependent upon androgens for its development there may be specific pathways involved in the response to androgens and it will be interesting to determine how these endocrine pathways interact with those involved in growth and branching.
References
- 1.
- Takeda H, Mizuno T, Lasnitzki I. Autoradiographic studies of androgen-binding sites in the rat urogenital sinus and postnatal prostate. J Endocrinol. 1985;104:87–92. [PubMed: 3968508]
- 2.
- Cunha GR, Chung LW. Stromal-epithelial interactions—I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. [PubMed: 6460136]
- 3.
- Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen- stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Research. 2001;61:5038–5044. [PubMed: 11431338]
- 4.
- Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–1432. [PubMed: 8158800]
- 5.
- McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12:619–633. [PubMed: 2456702]
- 6.
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. [PubMed: 3730488]
- 7.
- Hayashi N, Cunha GR, Parker M. Permissive and instructive induction of adult rodent prostatic epithelium by heterotypic urogenital sinus mesenchyme. Epithelial Cell Biol. 1993;2:66–78. [PubMed: 8353595]
- 8.
- Timms BG. et al. Instructive induction of prostate growth and differentiation by a defined urogenital sinus mesenchyme. Microsc Res Tech. 1995;30:319–332. [PubMed: 7606051]
- 9.
- Donjacour AA, Thomson AA, Cunha GR. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261:39–54. [PubMed: 12941620]
- 10.
- Podlasek CA. et al. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209:28–39. [PubMed: 10208740]
- 11.
- Freestone SH. et al. Sonic hedgehog regulates prostatic growth and epithelial differentiation Dev Biol 2003 . In press. [PubMed: 14651923]
- 12.
- Thomson AA. et al. The role of smooth muscle in regulating prostatic induction. Development. 2002;129:1905–1912. [PubMed: 11934856]
- 13.
- Hayward SW. et al. Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat. 1996;155:94–103. [PubMed: 8828707]
- 14.
- Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn. 2001;221:48–60. [PubMed: 11357193]
- 15.
- Tollet J, Everett AW, Sparrow MP. Development of neural tissue and airway smooth muscle in fetal mouse lung explants: a role for glial-derived neurotrophic factor in lung innervation. Am J Respir Cell Mol Biol. 2002;26:420–429. [PubMed: 11919078]
- 16.
- Sugimura Y. et al. Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. International Journal of Developmental Biology. 1996;40:941–951. [PubMed: 8946242]
- 17.
- Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. [PubMed: 10409514]
- 18.
- Itoh N. et al. Developmental and hormonal regulation of transforming growth factor-beta1 (TGFbeta1), -2, and -3 gene expression in isolated prostatic epithelial and stromal cells: Epidermal growth factor and TGF-beta interactions. Endocrinology. 1998;139:1378–1388. [PubMed: 9492075]
- 19.
- Cancilla B. et al. Regulation of prostate branching morphogenesis by activin A and follistatin. Dev Biol. 2001;237:145–158. [PubMed: 11518512]
- 20.
- Marker PC. et al. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–174. [PubMed: 12645922]
- 21.
- Brown TR. et al. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci USA. 1988;85:8151–8155. [PMC free article: PMC282385] [PubMed: 3186717]
- 22.
- He WW, Kumar MV, Tindall DJ. A frameshift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19:2373–2378. [PMC free article: PMC329445] [PubMed: 2041777]
- 23.
- Andersson S. et al. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161. [PMC free article: PMC4451825] [PubMed: 1944596]
- 24.
- Mahendroo MS. et al. Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology. 2001;142:4652–4662. [PMC free article: PMC4446976] [PubMed: 11606430]
- 25.
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. [PubMed: 7979239]
- 26.
- Foster BA, Cunha GR. Efficacy of various natural and synthetic androgens to induce ductal branching morphogenesis in the developing anterior rat prostate. Endocrinology. 1999;140:318–328. [PubMed: 9886841]
- 27.
- Ruan W. et al. Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology. 1999;140:1984–1989. [PubMed: 10218945]
- 28.
- Steger RW. et al. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology. 1998;139:3691–3695. [PubMed: 9724019]
- 29.
- Podlasek CA. et al. Hoxa-10 deficient male mice exhibit abnormal development of the accessory sex organs. Dev Dyn. 1999;214:1–12. [PubMed: 9915571]
- 30.
- Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999;161:1655–1661. [PubMed: 10210434]
- 31.
- Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997;208:454–465. [PubMed: 9097018]
- 32.
- Warot X. et al. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. [PubMed: 9428414]
- 33.
- Bhatia-Gaur R. et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–977. [PMC free article: PMC316645] [PubMed: 10215624]
- 34.
- Schneider A. et al. Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: Parallels to glandular duct morphogenesis in prostate. Mech Dev. 2000;95:163–174. [PubMed: 10906459]
- 35.
- Tanaka M. et al. Nkx3.1, a murine homolog of Drosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219:248–260. [PubMed: 11002344]
- 36.
- Lamm ML. et al. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232:301–314. [PubMed: 11401393]
- 37.
- Baker J. et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. [PubMed: 8813730]
- 38.
- Signoretti S. et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. [PMC free article: PMC1885786] [PubMed: 11106548]
- Branching Morphogenesis of the Prostate - Madame Curie Bioscience DatabaseBranching Morphogenesis of the Prostate - Madame Curie Bioscience Database
- From Artificial Antibodies to Nanosprings:The Biophysical Properties of Repeat P...From Artificial Antibodies to Nanosprings:The Biophysical Properties of Repeat Proteins - Madame Curie Bioscience Database
- Cell Cycle and Chromosome Segregation Defects in Alzheimer's Disease - Madame Cu...Cell Cycle and Chromosome Segregation Defects in Alzheimer's Disease - Madame Curie Bioscience Database
- Genetic Susceptibility to Infectious Diseases Linked to NRAMP1 Gene in Farm Anim...Genetic Susceptibility to Infectious Diseases Linked to NRAMP1 Gene in Farm Animals - Madame Curie Bioscience Database
- Conformational Dynamics within the Ribosome - Madame Curie Bioscience DatabaseConformational Dynamics within the Ribosome - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...