NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The formation of the enteric nervous system (ENS) is a particularly interesting example of the migratory ability of the neural crest, and of the complexity of structures to which neural crest cells contribute. The distance that neural crest cells migrate to colonize the entire length of the gastrointestinal tract exceeds that of any other neural crest cell population. Furthermore, this migration takes a long time—over 25% of the gestation period for mice and around 3 weeks in humans. After colonizing the gut, neural crest-derived cells within the gut wall then differentiate into glial cells plus many different types of neurons, and generate the most complex part of the peripheral nervous system.
The Enteric Nervous System (ENS)
The ENS contains more neurons than the spinal cord.1,2 There are many different classes of enteric neurons that differ in their neurotransmitters, electrophysiological properties, targets (circular muscle, longitudinal muscle, other neurons, blood vessels, epithelium etc), inputs and the direction along the gut in which their axons project.3,4 The neurons are grouped into ganglia, and each ganglion contains many different types of neurons. Myenteric ganglia are located between the circular and longitudinal muscle layers, and submucosal ganglia are located internal to the circular muscle layer. The ENS plays a critical role in mediating motility reflexes, as well as regulating blood flow within the gut wall and water and electrolyte transport across the mucosal epithelium. The fact that some regions of the gastrointestinal tract can function autonomously (without CNS input),5 and because of its high degree of complexity, the ENS has been termed “the second brain”.2
Origins of the ENS
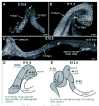
Although neural crest cells arise along the entire length of the body axis, studies by Yntema and Hammond (1954) and later by Le Douarin and Teillet (1973) showed that the ENS is derived from crest cells that originate from two specific regions of the neuraxis – the vagal (defined as post-otic hindbrain adjacent to somites 1-7) and sacral (caudal to somite 28 in chick embryos and caudal to somite 24 in embryonic mice and humans) levels6 (Fig. 1). The vagal region includes the transition zone between the head and neck.7

Figure 1
Diagram of an E10 embryo showing the origins of neural crest cells that colonize the developing gastrointestinal tract. nc - neural crest; OV - otic vesicle; pelvic gang. - pelvic ganglion primordia (note that pelvic ganglia are bilateral).
By ablating neural crest cells from the chick post-otic hindbrain, Yntema and Hammond (1954) showed that enteric ganglia were absent from the esophagus, stomach and small and large intestine. In a series of elegant chick-quail transplantation studies, Le Douarin and Teillet (1973) later showed that vagal neural crest cells adjacent to somites1-7 are the major source of enteric neuron precursors, and that sacral level neural crest cells also contribute to the ENS, mostly in the hindgut. A sub-population of vagal neural crest cells also gives rise to major elements in the cardiac outflow tracts, as well as to neurons and support cells in intrinsic cardiac ganglia.8-10
More recent studies have further fine tuned the axial origins of the ENS. In chick embryos, vagal neural crest cells adjacent to somites 1-2 contribute predominantly to the esophagus and stomach; those adjacent to somites 3-6 contribute to the entire length of the gut; while neural crest cells adjacent to somites 6-7 are located mainly in the hindgut.11-15 In mice, neural crest cell adjacent to somites 1-5 contribute enteric neurons along the entire length of the gut, while those adjacent to somites 6-7 give rise to cells only in the esophagus.16 There therefore appears to be some species differences in the origin of esophageal neurons.
Vagal Neural Crest
The emigration of vagal level neural crest cells from the dorsal neural tube commences about the 7-10 somite stage. In avians, most vagal level neural crest cells migrate in a ventral route through the rostral half of somites 3, 4 and 5,12 with some passing through branchial arch 6.17,18 Chick vagal neural crest cells then migrate further ventrally to form the circumpharyngeal crest,19,20 which is a complex pattern of crest cells that gives rise to a range of different derivatives including the ENS.21 The first vagal neural crest cells reach the vicinity of the chick foregut within 9 hours, at the 13 somite stage.22 In mice, vagal neural crest cells migrate underneath the epithelium, through the somites, between the somites, and between the somites and the neural tube.23 Neural crest cell that emigrate adjacent to somites 1-4 migrate around the pharynx along a pathway that is later followed by the vagus nerve, while neural crest cells adjacent to somites 4-7 form a continuous stream of cells that migrate ventrally beyond the dorsal aortae.16,24 Near the foregut, neural crest cells form left and right strings along the route that is later followed by vagal axons from the hindbrain.17,25 Vagal neural crest cells enter the nearby foregut mesenchyme at the 20-25 somite stage.24
Once in the foregut, vagal neural crest cells migrate caudally along the entire length of the gut (Figs. 1, 2). Neural crest cells have been reported to migrate at speeds of between 35-40 μm/h in both birds and in explants from embryonic mice.26,27 In chick, vagal neural crest cells enter the foregut around E2.5-3, reach the level of the umbilicus at E5, the cecal region at E6 and the colorectum at E7.5. The entire length of the chick gut is completely colonized by E8.5.13 In mice (Fig. 2A-C), the colonization of the gut by vagal neural crest cells takes around 5 days; crest cells enter the foregut around E9.5; reach the cecal region at E11.5 (Fig. 2B); and completely colonize the gut by E14.5.28 In humans, vagal neural crest cells enter the foregut at week 4 and reach the terminal hindgut by week 7.29 Some of the crest cells that colonize the foregut emigrate out into the lung buds and give rise to ganglia within the airways,30 and some of the crest-derived cells in the small intestine emigrate into the pancreas and give rise to pancreatic ganglia.31,32
In humans, birds and mice, vagal neural crest cells initially migrate in the outer half of the fore and midgut mesenchyme, which comprises an initially uniform population of cells that lie between the endoderm tube and the squamous epithelial serosal layer that surrounds the gut externally.21,29 After each fore and midgut region is colonized, vagal neural crest cells then form a narrow layer in close proximity to the serosa, where myenteric ganglia will later form.26,33 By the time vagal neural crest cells have reached the proximal hindgut in birds, the circular muscle layer has begun to develop, and the cells migrate internal to the circular muscle in the region where the submucosal plexus will form; vagal crest cells then undergo a secondary migration that gives rise to the myenteric plexus.13 However in the hindgut of mice and humans, vagal crest cells migrate in the outer part of the mesenchyme and aggregrate in the region where myenteric ganglia will form (as they do in the fore and midgut); the submucosal plexus arises several days later from a secondary migration of neural crest cells from the myenteric plexus.33,34 In mice, the netrin family of guidance molecules and their receptors has been shown to be required for the secondary migration of vagal crest cells to form the submucosal plexus.32
Sacral Neural Crest
Sacral neural crest cells migrate ventrally through the rostral halves of the adjacent somatic sclerotomes and congregate near the dorsal wall of the hindgut where they formed extramural ganglia (i.e., paired pelvic ganglia and, additionally in birds, the nerve of Remak)13,24,35 (Fig. 2C). The avian-specific nerve of Remak is a large ganglionated chain running along the mesentery adjacent to the hindgut and distal midgut. Sacral neural crest cells form the nerve of Remak at E3.5 and remain in this structure until E7, when axons from neurons in the nerve of Remak project into the hindgut. Sacral neural crest cells then migrate into the hindgut along these extrinsic axons and colonize the hindgut in larger number from E10.13 In mice, sacral neural crest cells form the pelvic plexus (Fig. 2C) adjacent to the distal hindgut from around E10.5 and do not enter the hindgut until E14.5.24,35 In both chick and mice, sacral neural crest cells directly adjacent to the gut undergo a “waiting period” and only colonize the hindgut after it has been fully colonized by vagal neural crest cells. It was initially suggested that sacral neural crest cells might require the presence of vagal neural crest cell in the hindgut, in order for them to enter. However, this has subsequently been shown not to be the case. Following ablation of the vagal level neural tube in chick, the migration of sacral neural crest cells into the hindgut was found to be unaffected,14 and combination grafts of aneural hindgut with sacral cells, with and without vagal neural crest cells, have reported the same effect.36 Similar finding have also been reported in mice, using Wnt1-lacZ transgene expression as an early marker of neural crest cells.35
The sacral “waiting period” could be due to an absence of attractive molecules (or their receptors on the sacral neural crest cells) and/or the transient expression of repulsive molecules in the distal hindgut. The chemorepulsive molecule Sema3A (formerly known as Collapsin-1) is expressed transiently in the distal hindgut of the embryonic chick, and is responsible for the delay in the ingrowth of axons from neurons in the nearby nerve of Remak.37 Since sacral neural crest cells migrate into the hindgut along the axons of neurons in the nerve of Remak,13 it is possible that the expression of Sema3A, either directly or indirectly, regulates the time of entry of the sacral neural crest cells into the hindgut.
Once sacral neural crest cells have entered the hindgut, they give rise to both neurons and glial cells.13 The contribution of chick sacral neural crest cells was found to be confined predominantly to the distal hindgut, where they comprised up to 17% of myenteric neurons, and far fewer submucosal neurons.13 Thus, even in the rectum, the vast majority of enteric neurons arise from precursors that originate in the hindbrain (vagal crest cells). In mice, the quantitative contribution of sacral neural crest cells to the ENS is still unclear. Mice in which the genes encoding members of the glial cell line-derived neurotrophic factor (GDNF) signaling pathway (GDNF, Ret or GFRα1) have been knocked out, lack enteric neurons in the gut caudal to the stomach16,38 (Fig. 3). However, a small number of enteric neurons have been reported in the distal hindgut of these mice, which are almost certainly derived from sacral neural crest cells.16,24,38 The small number of enteric neurons present in the hindgut of these mice is likely to be due to the fact that many of the sacral crest cells that normally enter the hindgut are dependent on the GDNF signaling pathway, but it is also possible that in mice, sacral neural crest cells contribute only a small number of enteric neurons.
Migratory Behavior of Neural Crest Cells
Images of fixed samples, and time-lapse imaging of living enteric crest-derived cells, have both shown that enteric crest cells migrate in chains, indicating the presence of cell-cell adhesive interactions between migrating neural crest cells.28,39,40 The cell adhesion molecule, L1, is expressed by migratory and post-migratory crest cells in the embryonic mouse gut,41,42 and in cultured gut explants, perturbing L1 activity with function blocking antibodies retards the rate of crest cell migration and increases the number of solitary cells (cells not in chains) near the migratory wavefront.42 Other cell adhesion molecules, in addition to L1, are almost certainly involved in adhesive interactions between enteric crest-derived cells, as perturbing L1 function does not noticeably disrupt cell-cell contacts between post-migratory cells.42 The gap junction protein, connexin 43, is also expressed by crest-derived cells in the embryonic mouse gut.43 Direct cell-cell communication via gap junctions has been shown to be important for the migration and survival of trunk neural crest cells in culture,44,45 but the role of gap junctions in enteric crest cells has yet to be determined.
Cell number can also influence the migratory ability of vagal crest cells. The partial ablation of vagal crest cells in chick embryos, results in aganglionosis of the distal gut.11,14,46 Furthermore, when a small number of crest cells at the migratory wavefront in explants of embryonic mouse gut is isolated from the crest cells behind them, the isolated cells migrate more slowly.40 These studies have lead to the idea of “population pressure” as a major driving force behind the colonization of the gut by crest-derived cells, and that high cell density and/or cell-cell contact stimulates migration and colonization.47
The migratory behaviour of crest-derived cells in the gut is also influenced by molecules expressed by the gut mesenchyme that could either promote or inhibit the motility of enteric crest-derived cells nondirectionally, or act as chemoattractive or chemorepulsive cues. GDNF, which is expressed by the gut mesenchyme, is chemoattractive to enteric crest-derived cells and appears to induce vagal cells to enter the gut and may also promote their rostrocaudal migration along the gut (see below).
Hirschsprung's Disease
Hirschsprung's disease (HSCR) is a congenital birth defect, with an incidence of around 1 in 5000 live births, in which enteric neurons are absent from variable lengths of the distal-most gut. Studies of mouse models have shown that HSCR is almost certainly caused by a failure of neural crest cells to colonize the distal regions of the gut, rather than a failure of crest cells to survive in the affected regions.21,48 As enteric neurons are essential for propulsive activity in the gut, HSCR patients suffer from intestinal obstruction or severe constipation. The region lacking enteric neurons, termed the “aganglionic” zone, is usually contracted and devoid of contents. Although the region proximal to the aganglionic zone contains enteric neurons, it is distended (forming a “mega-colon”) due to an accumulation of faecal contents. HSCR is treated by surgical resection of the aganglionic gut, although functional problems often persist. The genetics of HSCR is complex.48-51 Eleven “HSCR susceptibility genes” have been identified in humans, but mutations in known genes account for only about 50% of HSCR cases.48,52,53 Many of the known susceptibility genes encode members of the RET- or EDNRB-signaling pathways or transcription factors that regulate RET or EDNRB expression. The importance of many of the known HSCR genes for normal ENS development is discussed below.
Proliferation, Cell Death and Differentiation in the Developing ENS
Proliferation
It is thought that somewhere in the order of 1000-2000 vagal neural crest cells enter the foregut of embryonic chicks and mice.54 In small intestine and colon of adult mice, there are over one million neurons plus an equal number or higher number of glial cells.55 Thus, crest-derived cells must undergo extensive proliferation in the gut during development. In fact, 2 hours following the administration of the thymidine analogue, BrdU, to pregnant female mice, over 40% of crest-derived cells in the gut of their E11.5 or E12.5 progeny are BrdU-labelled (Fig. 2C, D).56 The expression of molecules that can influence the rate of crest cell proliferation (such as GDNF and endothelin-3) are higher in the caecum than in other regions of the developing gut. This has lead to the suggestion that the caecum may be a proliferative zone in which sufficient precursors to form the ENS of the colon are generated. However, a recent study has shown that the rate of proliferation of crest-derived cells does not vary in different regions of the embryonic mouse small and large intestine including the caecum, or at different distances from the migratory wavefront56 (Fig. 2C, D). On the other hand, there does appear to be a lower rate of proliferation of crest-derived cells in the stomach of E12.5 mice compared to the midgut.57
Apoptosis
In most parts of the developing nervous system, excess neurons are generated, and then surplus neurons, usually those that have not projected to a target and have insufficient access to survival factors, are removed by programmed cell death (apoptosis). Surprisingly, studies of embryonic mouse and rat gut have failed to detect any evidence for apoptosis in the developing ENS.55,58 Thus enteric neuron number appears to be regulated largely by the rate of proliferation.
Neuronal and Glial Differentiation
When crest-derived cells enter the mouse foregut at E10, a sub-population express pan-neuronal proteins (such as neurofilament, PGP9.5 and Hu).25,59 By E14.5, around 50% of crest-derived cells in the small intestine express neuronal proteins, and the percentage of crest-derived cells that can be classified as neurons does not change much at later developmental stages.60 Early differentiating neurons project their axons in the same direction (caudally) and along the same pathway through the mesenchyme as the migrating vagal cells.61 Neural cells commonly migrate in close association with axons, for example in the development of peripheral nerves.62 In the lateral line of the zebrafish, axons are the source of instructive cue(s) that guide migrating neural crest cells.63 However, in the developing gut, it is still unknown whether axons are a source of guidance cue for migrating crest cells or vice versa, and hence whether crest cell migration can occur without axon growth.
The expression of molecules characteristic of particular neuron types (such as neurotransmitter synthesizing enzymes, etc) commences after the expression of pan neuronal proteins, and different neuron classes develop at very different developmental stages.60 For example, in mice nitric oxide neurons develop at E12.5,64 whereas cholinergic neurons do not develop in significant numbers until after birth.65 In embryonic mice, cells expressing glial cell precursor markers are not detected until a day or so after crest cells have colonized a particular region of the gut, whereas in embryonic chicks, cells expressing glial markers are present close to the migratory wavefront.39,60 Little is known about the molecular control of the differentiation of glial cells and different types of neurons from undifferentiated crest cells, but the transcription factor, Sox10, appears to be required for the differentiation of enteric glial cells,66 and another transcription factor, Mash1, is required for the differentiation of serotonin neurons.67
Molecules and Signaling Pathways Involved in ENS Development
A variety of signaling pathways and molecules have been shown to be required for normal enteric neural crest cell survival, proliferation, migration and differentiation. These genes and molecules have been identified from both basic research and from molecular genetic studies of patients with HSCR. Recent genetic and cell biological studies have shown that there are complex interactions between the different signaling pathways.68-72
GDNF Family
GDNF/Ret-GFRα1
GDNF is a secreted protein that is a distant member of the TGF-β superfamily. GDNF signals predominantly through the Ret receptor tyrosine kinase, but Ret is only activated if GDNF is bound to another protein, GFRα1, which is attached to the cell membrane by a glycosyl phosphatidylinositol (GPI) anchor.73 GDNF is expressed by the gut mesenchyme prior to the entry of neural crest cells; Ret is expressed exclusively by neural crest-derived cells; and GFRα1 is expressed by both crest-derived cells and the gut mesenchyme.74-77 GDNF-, GFRα1- or Ret-null mice die within 24 hours of birth, and lack neurons from most of the gastrointestinal tract, although they do have some neurons (but in greatly reduced numbers) in the esophagus, stomach and distal hindgut78 (Fig. 3). Gdnf+/- mice have reduced numbers of enteric neurons throughout the gastrointestinal tract and motility defects.79 Although Ret+/- mice have normal numbers of enteric neurons,55 humans that are heterozygous for RET mutations can exhibit aganglionosis of the distal gut.80
There are two main isoforms of Ret, Ret9 and Ret51. Mice lacking Ret51 have neurons throughout the gut, while mice lacking Ret9 have no neurons in the colon (Fig. 3); this indicates that Ret9 is the most important isoform for the development of the ENS.81 Recently, mice were generated that express a dominant negative mutation in Ret (RetDN) by inserting human cDNA encoding a mutant Ret9 (with two point mutations) into the first coding exon of the Ret gene. In contrast to Ret+/- mice, RetDN/+ mice have an aganglionic colon82 (Fig. 3). Therefore, reduced Ret signaling in mice can be tolerated to some extent (as in Ret+/- mice), however a reduction in Ret signaling beyond a certain level results in defects in the ENS. The extent of aganglionosis is in all likelihood correlated with the degree to which Ret signaling is reduced. The developing ENS in humans appears to be more sensitive to reduced Ret signaling than the ENS of mice. In mice, it appears that excessive GDNF/Ret-GFRα1 signaling can also cause ENS defects. Mice lacking Sprouty2, an inhibitor of receptor tyrosine kinases, have ENS hyperplasia and intestinal pseudo-obstruction (delayed transit of intestinal contents) which can be corrected by inhibiting GDNF/Ret signaling.83 Thus, the negative regulation of Ret by Sprouty2 appears to be important for maintaining appropriate levels of GDNF/Ret signaling during ENS development.
GDNF/Ret-GFRα1 signaling plays multiple and varied roles during ENS development. In mice lacking Ret, vagal neural crest cells die around the time at which they reach the foregut, suggesting that GDNF/Ret-GFRα1 signaling is required for survival.16 Studies of enteric neural crest-derived cells grown in vitro have shown that GDNF also promotes their differentiation and proliferation.76,84-87 Furthermore, in vitro assays have shown that GDNF is chemoattractive to migrating neural crest cells.75,88 Therefore, GDNF expressed by the gut mesenchyme probably plays a role in inducing vagal neural crest cells to enter the gut, retaining them within the gut, and may also play a role in their caudally directed migration along the gut. Although GDNF/Ret-GFRα1 signaling plays multiple roles in ENS development, the circumstances under which Ret activation induces different processes (survival, proliferation, differentiation or migration) are unknown.
Other GDNF Family Members
In addition to GDNF, there are three other known members of the GDNF family – neurturin, artemin and persephin.73 All GDNF family members signal through Ret, but bind to specific GFRα coreceptors. Persephin is not expressed outside of the CNS. Although artemin is expressed by the esophagus and stomach of embryonic rats,89 its preferred coreceptor, GFRα3, is not expressed by enteric neurons,90 and the sizes and numbers of enteric neurons in the small and large intestine of mice lacking artemin are no different from wildtype mice.91 Neurturin is expressed by the mesenchyme of the developing gut, but at later developmental stages than GDNF, and its preferred coreceptor, GFRα2 is expressed by neural crest-derived cells within the gut.90,92,93 In vitro studies have shown that neurturin promotes the survival, proliferation, differentiation, neurite outgrowth and migration of neural crest cells in the developing gut.85,86,90 Mice lacking neurturin or GFRα2 are viable and fertile and have only minor differences in enteric neuron numbers from wild-type mice.55,94,95 Thus, neurturin does not appear to be essential for the survival, proliferation or differentiation of enteric neural crest cells. However mice lacking neurturin or GFRα2 have smaller enteric neurons, reduced numbers of nerve fibres in the gut wall and some motility defects,93 suggesting that neurturin may provide trophic support to enteric neurons.
Endothelin-3 (Et-3)/Endothelin Receptor B (Ednrb)
Et-3 is a secreted peptide that signals through a G-protein-coupled 7 trans-membrane spanning receptor, Ednrb. Lethal spotted (ls) and piebald lethal (sl) are naturally occurring mutants for Et-3 and Ednrb respectively, and have been shown to lack enteric neurons in the distal bowel96-98 (Fig. 3). Mutations in ET-3 and EDNRB in humans can also result in Hirschsprung's disease.80 Et-3 is expressed by the mesenchyme of the developing gut, with highest expression in the caecum.69,99 Ednrb is expressed by both migrating neural crest cells and some mesenchymal cells.69,100 Although enteric neurons are absent only from the distal colon of Et-3 and Ednrb-null mice and rats, the migration of neural crest cells through the small intestine is also delayed.58,69,100-102
It is still unclear why perturbations in Et-3/Ednrb signaling result in delayed neural crest cell migration, and their failure to colonize the distal regions of the gastrointestinal tract. In vivo and in vitro studies have shown that Et-3 signaling does not effect the survival of enteric neural crest cells.58,100,103 Different studies have reported variable effects of Et-3 on enteric crest cell proliferation,58,69,84,87,103 and it appears that if Et-3 does influence proliferation, the effect is slight. Some in vitro studies have found that Et-3 inhibits neuronal differentiation induced by GDNF; it was therefore proposed that Ednrb signaling normally prevents the premature differentiation of crest cells into neurons, and thereby maintains sufficient numbers of undifferentiated migratory cells to colonize the entire gastrointestinal tract.84,87 However, a recent study of Ednrb null embryonic rats has shown that the percentage of crest cells expressing neuronal markers was lower in the mutant rats than in wild-type animals.58
Other Secreted Factors
Neurotrophin 3 (NT-3) acting via TrkC receptors appears to be the only neurotrophin involved in the development of the ENS.104 In vitro, NT-3 promotes the neuronal differentiation of crest-derived cells, and mice with null mutations in Nt-3 or Trk3 have reduced numbers of enteric neurons.105 Interestingly, crest-derived cells respond to NT-3 at later developmental stages than they respond to GDNF.76
A number of studies have shown that BMP-2 and BMP-4 influence the differentiation of crest-derived cells in vitro, and mice that overexpress noggin, a BMP antagonist, have more enteric neurons.58,106-108 Thus, BMPs seem to contribute to the regulation of enteric neuron number and differentiation. BMPs could also play a role in the concentric patterning of enteric ganglia within the gut wall and to ganglion size.109,110
Both Indian hedgehog (Ihh) and sonic hedgehog (Shh) have been shown to play a role in the development of the ENS.109,111,112 Embryonic Ihh-/- mice are missing enteric neurons from some regions of the small intestine (Fig. 3), but the cellular and molecular mechanisms underlying the phenotype have not been elucidated.111 In embryonic Shh-/- mice, some neurons are ectopically located under the epithelium instead of being restricted to myenteric and submucosal ganglia.111 In vitro, Shh promotes the proliferation of enteric crest-derived cells directly, and inhibits GDNF-induced neuronal differentiation and migration.112 Thus, the ectopic location of enteric neurons observed in the null mutants could be due to direct effects of Shh on the migratory behaviour of crest cells, or to influences of Shh on the mesenchyme and the concentric patterning of the gut wall.109,111,112
Transcription Factors
Gene knockout studies have revealed some of the transcription factors necessary for the development of the ENS. Mice lacking Phox2b or Sox10 lack enteric neurons throughout the entire gastrointestinal tract113-115 (Fig. 3). Mice lacking Pax3 lack enteric neurons from the small and large intestine,43 while mice lacking Mash1 lack enteric neurons in the esophagus (Fig. 3).116 These null mutant mice all die during late embryogenesis or shortly after birth.
Phox2b is required for the expression of Ret.115 Sox10 regulates the expression of a number of different genes; it can activate Ret directly by interacting with Pax3,43,117 or indirectly by inducing Phox2b.118 Sox10 also binds to the Ednrb gene and regulates the temporal and spatial expression of Ednrb in the gut.72 Mash1 can also indirectly activate Ret by regulating Phox2a, a closely related protein to Phox2b.119,120 Thus, Phox2b, Sox10, Pax3 and Mash1 seem to be required for normal ENS development because they regulate the expression of Ret and/or Ednrb.
In Sox10 null mutants, crest-derived cells die early.113 Most Sox10 heterozygous mutant mice are viable, although some exhibit aganglionosis of the terminal colon113,121 (Fig. 3). In Sox10+/- mice, the migration of crest cells through the gut is delayed and there is a reduction in the numbers of progenitor cells and an increase in the proportion of cells expressing neuronal markers.66,121 These data show that Sox10 is required for the survival and correct cell fate of neural crest cells, in particular for the development of glial cells.66 Sox10 is probably required for the early survival of enteric crest cells because it is necessary for Ret expression, and its role in glial development is probably due to interactions with other genes.122 Sox8 is a transcription factor that is closely related to Sox10. Sox8 is expressed by enteric neural crest cells, but Sox8-/- mice are viable and fertile and show no ENS phenotype.57 However, double heterozygous mice (Sox8+/-, Sox10+/- mice) have a more severe ENS phenotype than Sox10+/- mice alone, suggesting that Sox8 functions as a modifier gene.57
SIP1 (smad-interacting protein-1), encoded by the ZFHX1B gene, is a transcription factor that appears to repress expression of target genes. Mutations in ZFHX1B have been implicated in HSCR-mental retardation syndrome, which is HSCR associated with multiple congenital anomalies.80,123 In mice lacking Zfhx1b, the neural tube does not close and vagal neural crest cells fail to form.124 Hence Sip1 appears to be required for the formation of the ENS because it is necessary for the formation of vagal neural crest cells in the neural tube.
Hox11L1 is expressed by enteric smooth muscle cells and by developing enteric neurons.125 Mice with a null mutation in the Hox11L1 gene exhibit pseudo-obstruction (mega-colon),125 and have been reported to have ENS hyperplasia in the colon.126,127 However, a more recent study reported no detectable differences in the numbers of enteric neurons in the colon of mutant mice compared to wild-type mice.125 Thus, it seems likely that the pseudo-obstruction phenotype observed in the Hox11L1 null mutant mice is due to myogenic, rather than ENS, defects.128
Other Molecules
Retinoic acids (RA) act at nuclear retinoic acid receptors to regulate the transcriptional activity of target genes. The main synthetic enzyme for retinoic acids during development is retinaldehyde dehydrogenase 2 (RALD2). Raldh2-/- mice die around E10 because of severe cardiovascular defects, but maternal retinoic acid supplementation can prolong the survival of Raldh2-/- embryos until late gestational stages.129 RA-rescued Raldh2-/-- embryos lack an ENS, probably due to defects in the posterior pharyngeal arches and vagal level hindbrain, and consequent defects in vagal level crest cell migration.129
Conclusions
The development of the ENS from the neural crest encapsulates nearly all the major events of development, such as induction, cell migration, proliferation, stem cell replication and lineage restriction, cell associations, differentiation, neurite elaboration and connectivity. ENS development from the neural crest shows many desirable attributes both intrinsically and as a model of general developmental processes. This system appears to be highly conserved in vertebrates,130-133 and many of these processes are of greater scale or duration, better defined and timetabled, and more accessible to experimental manipulation, compared to other early developmental systems. This is allied to a large number of genes implicated in ENS development, several serious clinical problems stemming from ENS dysgenesis (many already related to gene defects), and an array of informative animal mutant models (mostly mouse) (see above). In addition mathematical simulations of early ENS development are also being formulated.134 Combined, these have placed the ENS at the forefront of research into clinically relevant, genetically complex developmental events.
ENS research is sufficiently detailed now to give an idea of what is not known; it drives home how complex development really is, and highlights the common difficulty in bridging the knowledge gap between genotype and phenotype. For the future, the discovery and isolation of ENS stem cells107,135-138 and the recent use of neural stem cells in replenishing ENS neural deficits in animal models,58,139 and even in providing some functional recovery140 provides hope for clinical applications. But to reap these rewards requires even more exact basic research on ENS genes, molecules and cell biology.
References
- 1.
- Karaosmanoglu T, Aygun B, Wade PR. et al. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: An evaluation of markers used to count neurons. Anat Rec. 1996;244(4):470–480. [PubMed: 8694282]
- 2.
- Gershon MD. The Second Brain. New York: Harper Collins. 1998
- 3.
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262(1):58–70. [PubMed: 11146429]
- 4.
- Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81(1-3):87–96. [PubMed: 10869706]
- 5.
- Furness JB, Johnson PJ, Pompolo S. et al. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995;7(2):89–96. [PubMed: 7621324]
- 6.
- Le Douarin N. The Neural Crest. Vol 12. 1st ed. Cambridge: Cambridge University Press. 1982
- 7.
- Ferguson CA, Graham A. Redefining the head-trunk interface for the neural crest. Dev Biol. 2004;269(1):70–80. [PubMed: 15081358]
- 8.
- Kirby ML, Stewart DE. Neural crest origin of cardiac ganglion cells in the chick embryo: Identification and extirpation. Dev Biol. 1983;97(2):433–443. [PubMed: 6852374]
- 9.
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220(4601):1059–1061. [PubMed: 6844926]
- 10.
- Verberne ME, Gittenberger-de Groot AC, van Iperen L. et al. Distribution of different regions of cardiac neural crest in the extrinsic and the intrinsic cardiac nervous system. Dev Dyn. 2000;217(2):191–204. [PubMed: 10706143]
- 11.
- Peters-van der Sanden MJ, Kirby ML, Gittenberger-de Groot A. et al. Ablation of various regions within the avian vagal neural crest has differential effects on ganglion formation in the fore-, midand hindgut. Dev Dyn. 1993;196(3):183–194. [PubMed: 8400404]
- 12.
- Epstein ML, Mikawa T, Brown AM. et al. Mapping the origin of the avian enteric nervous system with a retroviral marker. Dev Dyn. 1994;201(3):236–244. [PubMed: 7881127]
- 13.
- Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the postumbilical gut: Spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125(21):4335–4347. [PubMed: 9753687]
- 14.
- Burns AJ, Champeval D, Le Douarin NM. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000;219:30–43. [PubMed: 10677253]
- 15.
- Burns AJ, Le Douarin NM. Enteric nervous system development: Analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;262(1):16–28. [PubMed: 11146425]
- 16.
- Durbec PL, Larsson-Blomberg LB, Schuchardt A. et al. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122(1):349–358. [PubMed: 8565847]
- 17.
- Tucker GC, Ciment G, Thiery JP. Pathways of avian neural crest cell migration in the developing gut. Dev Biol. 1986;116:439–450. [PubMed: 3732616]
- 18.
- Phillips MT, Kirby ML, Forbes G. Analysis of cranial neural crest distribution in the developing heart using quail-chick chimeras. Circ Res. 1987;60(1):27–30. [PubMed: 3568286]
- 19.
- Kuratani SC, Kirby ML. Initial migration and distribution of the cardiac neural crest in the avian embryo: An introduction to the concept of the circumpharyngeal crest. Am J Anat. 1991;191(3):215–227. [PubMed: 1927968]
- 20.
- Kuratani SC, Kirby ML. Migration and distribution of circumpharyngeal crest cells in the chick embryo. Formation of the circumpharyngeal ridge and E/C8+ crest cells in the vertebrate head region. Anat Rec. 1992;234(2):263–280. [PubMed: 1384396]
- 21.
- Newgreen D, Young HM. Enteric nervous system: Development and developmental disturbances-part 1. Pediatr Dev Pathol. 2002;5(3):224–247. [PubMed: 12007016]
- 22.
- Andrew A. The origin of intramural ganglia. I. The early arrival of precursor cells in the presumptive gut of chick embryos. J Anat. 1964;98:421–428. [PMC free article: PMC1261367] [PubMed: 14193199]
- 23.
- Chan WY, Cheung CS, Yung KM. et al. Cardiac neural crest of the mouse embryo: Axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 2004;131(14):3367–3379. [PubMed: 15226254]
- 24.
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2005:1–15. [PubMed: 16133146]
- 25.
- Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: Relationship to the development of enteric neurons. Dev Biol. 1989;132(1):189–211. [PubMed: 2563710]
- 26.
- Allan IJ, Newgreen DF. The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat. 1980;157(2):137–154. [PubMed: 7405865]
- 27.
- Young HM, Bergner AJ, Anderson RB. et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270(2):455–473. [PubMed: 15183726]
- 28.
- Young HM, Hearn CJ, Ciampoli D. et al. A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret, and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol. 1998;202(1):67–84. [PubMed: 9758704]
- 29.
- Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319(3):367–382. [PubMed: 15672264]
- 30.
- Burns AJ, Delalande JM. Neural crest cell origin for intrinsic ganglia of the developing chicken lung. Dev Biol. 2005;277(1):63–79. [PubMed: 15572140]
- 31.
- Kirchgessner AL, Adlersberg MA, Gershon MD. Colonization of the developing pancreas by neural precursors from the bowel. Dev Dyn. 1992;194(2):142–154. [PubMed: 1421524]
- 32.
- Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258(2):364–384. [PubMed: 12798294]
- 33.
- McKeown SJ, Chow CW, Young HM. Development of the submucous plexus in the large intestine of the mouse. Cell Tissue Res. 2001;303(2):301–305. [PubMed: 11291776]
- 34.
- Payette RF, Bennett GS, Gershon MD. Neurofilament expression in vagal neural crest-derived precursors of enteric neurons. Dev Biol. 1984;105(2):273–287. [PubMed: 6383899]
- 35.
- Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: Experimental support for an evolutionarily conserved model. Dev Biol. 2000;227(1):146–155. [PubMed: 11076683]
- 36.
- Hearn C, Newgreen D. Lumbo-sacral neural crest contributes to the avian enteric nervous system independantly of vagal neural crest. Dev Dyn. 2000;218:525–530. [PubMed: 10878617]
- 37.
- Shepherd IT, Raper JA. Collapsin-1/semaphorin D is a repellent for chick ganglion of Remak axons. Dev Biol. 1999;212(1):42–53. [PubMed: 10419684]
- 38.
- Cacalano G, Farinas I, Wang LC. et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21(1):53–62. [PMC free article: PMC2710137] [PubMed: 9697851]
- 39.
- Conner PJ, Focke PJ, Noden DM. et al. Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Dev Dyn. 2003;226(1):91–98. [PubMed: 12508228]
- 40.
- Young HM, AJB, Anderson RB. et al. Dynamics of neural crest cell migration in the embryonic mouse gut. Proc Australian Neurosci Soc. 2004;15 [PubMed: 15183726]
- 41.
- Probstmeier R, Martini R, Tacke R. et al. Expression of the adhesion molecules L1, N-CAM and J1/tenascin during development of the murine small intestine. Differentiation. 1990;44(1):42–55. [PubMed: 1701406]
- 42.
- Anderson RB, Young HM. The cell adhesion molecule, L1, is required for the migration of neural crest cells in the developing gut. Paper presented at: Mech Dev. 2005
- 43.
- Lang D, Chen F, Milewski R. et al. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J Clin Invest. 2000;106(8):963–971. [PMC free article: PMC314346] [PubMed: 11032856]
- 44.
- Xu X, Li WE, Huang GY. et al. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol. 2001;154(1):217–230. [PMC free article: PMC2196865] [PubMed: 11449002]
- 45.
- Bannerman P, Nichols W, Puhalla S. et al. Early migratory rat neural crest cells express functional gap junctions: Evidence that neural crest cell survival requires gap junction formation. J Neuro Res. 2000;61:605–615. [PubMed: 10972957]
- 46.
- Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–541. [PubMed: 13221667]
- 47.
- Newgreen DF. Control of the directional migration of mesenchyme cells and neurites. Sem Dev Biol. 1990;1:301–311.
- 48.
- Kapur RP. Multiple endocrine neoplasia type 2B and Hirschsprung's disease. Clin Gastroenterol Hepatol. 2005;3(5):423–431. [PubMed: 15880310]
- 49.
- Belknap WM. The pathogenesis of Hirschsprung disease. Current opininion in gastroenterology. 2002;18:74–81. [PubMed: 17031234]
- 50.
- Kashuk CS, Stone EA, Grice EA. et al. Phenotype-genotype correlation in Hirschsprung disease is illuminated by comparative analysis of the RET protein sequence. Proc Natl Acad Sci USA. 2005;102(25):8949–8954. [PMC free article: PMC1157046] [PubMed: 15956201]
- 51.
- Emison ES, McCallion AS, Kashuk CS. et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature. 2005;434(7035):857–863. [PubMed: 15829955]
- 52.
- Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12(6):610–617. [PubMed: 11106284]
- 53.
- Gariepy CE. Developmental disorders of the enteric nervous system: Genetic and molecular bases. J Pediatr Gastroenterol Nutr. 2004;39(1):5–11. [PubMed: 15187773]
- 54.
- Young HM, Newgreen D. Enteric neural crest-derived cells: Origin, identification, migration, and differentiation. Anat Rec. 2001;262(1):1–15. [PubMed: 11146424]
- 55.
- Gianino S, Grider JR, Cresswell J. et al. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130(10):12187–2198. [PubMed: 12668632]
- 56.
- Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell Tissue Res. 2005;320(1):1–9. [PubMed: 15714282]
- 57.
- Maka M, Stolt CC, Wegner M. Identification of Sox8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev Biol. 2005;277(1):155–169. [PubMed: 15572147]
- 58.
- Kruger GM, Mosher JT, Tsai YH. et al. Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron. 2003;40(5):917–929. [PubMed: 14659091]
- 59.
- Young HM, Ciampoli D, Hsuan J. et al. Expression of ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn. 1999;216(2):137–152. [PubMed: 10536054]
- 60.
- Young HM, Bergner AJ, Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol. 2003;456(1):1–11. [PubMed: 12508309]
- 61.
- Young HM, Jones BR, McKeown SJ. The projections of early enteric neurons are influenced by the direction of neural crest cell migration. J Neurosci. 2002;22(14):6005–6018. [PMC free article: PMC6757928] [PubMed: 12122062]
- 62.
- Noakes PG, Hornbruch A, Wolpert L. The relationship between migrating neural crest cells and growing limb nerves in the developing chick forelimb. (In Limb Development and Regeneration. Part A). In: Fallon JF GPF, Kelly RO, Stocum DL, eds. Progress in Clinical and Biological Research. Vol 383A. New York: Wiley-Liss. 1993:381–390. [PubMed: 7508129]
- 63.
- Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34(4):577–588. [PubMed: 12062041]
- 64.
- Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285(2):262–273. [PubMed: 2788179]
- 65.
- Vannucchi MG, Faussone-Pellegrini MS. Differentiation of cholinergic cells in the rat gut during pre and postnatal life. Neurosci Lett. 1996;206(2-3):105–108. [PubMed: 8710162]
- 66.
- Paratore C, Eichenberger C, Suter U. et al. Sox10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum Mol Genet. 2002;11(24):3075–3085. [PubMed: 12417529]
- 67.
- Blaugrund E, Pham TD, Tennyson VM. et al. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development. 1996;122(1):309–320. [PubMed: 8565843]
- 68.
- Carrasquillo MM, McCallion AS, Puffenberger EG. et al. Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat Genet. 2002;32(2):237–244. [PubMed: 12355085]
- 69.
- Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40(5):905–916. [PubMed: 14659090]
- 70.
- McCallion AS, Stames E, Conlon RA. et al. Phenotype variation in two-locus mouse models of Hirschsprung disease: Tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci USA. 2003;100(4):1826–1831. [PMC free article: PMC149918] [PubMed: 12574515]
- 71.
- Cantrell VA, Owens SE, Chandler RL. et al. Interactions between Sox10 and EdnrB modulate penetrance and severity of aganglionosis in the Sox10Dom mouse model of Hirschsprung disease. Hum Mol Genet. 2004;13(19):2289–2301. [PubMed: 15294878]
- 72.
- Zhu L, Lee HO, Jordan CS. et al. Spatiotemporal regulation of endothelin receptor-B by SOX10 in neural crest-derived enteric neuron precursors. Nat Genet. 2004;36(7):732–737. [PubMed: 15170213]
- 73.
- Airaksinen MS, Saarma M. The gdnf family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. [PubMed: 11988777]
- 74.
- Worley DS, Pisano JM, Choi ED. et al. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development. 2000;127(20):4383–4393. [PubMed: 11003838]
- 75.
- Natarajan D, Marcos-Gutierrez C, Pachnis V. et al. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129(22):5151–5160. [PubMed: 12399307]
- 76.
- Chalazonitis A, Rothman TP, Chen J. et al. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: Expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998;204(2):385–406. [PubMed: 9882478]
- 77.
- Enomoto H, Hughes I, Golden J. et al. GFRalpha1 Expression in cells lacking RET is dispensable for organogenesis and nerve regeneration. Neuron. 2004;44(4):623–636. [PubMed: 15541311]
- 78.
- Taraviras S, Pachnis V. Development of the mammalian enteric nervous system. Curr Opin Genet Dev. 1999;9(3):321–327. [PubMed: 10377293]
- 79.
- Shen L, Pichel JG, Mayeli T. et al. Gdnf haploinsufficiency causes Hirschsprung-like intestinal obstruction and early-onset lethality in mice. Am J Hum Genet. 2002;70(2):435–447. [PMC free article: PMC384918] [PubMed: 11774071]
- 80.
- Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: A review. J Med Genet. 2001;38(11):729–739. [PMC free article: PMC1734759] [PubMed: 11694544]
- 81.
- de Graaff E, Srinivas S, Kilkenny C. et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15(18):2433–2444. [PMC free article: PMC312785] [PubMed: 11562352]
- 82.
- Jain S, Naughton CK, Yang M. et al. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development. 2004;131(21):5503–5513. [PubMed: 15469971]
- 83.
- Taketomi T, Yoshiga D, Taniguchi K. et al. Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci. 2005;8(7):855–857. [PubMed: 15937482]
- 84.
- Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197(1):93–105. [PubMed: 9578621]
- 85.
- Heuckeroth RO, Lampe PA, Johnson EM. et al. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200(1):116–129. [PubMed: 9698461]
- 86.
- Taraviras S, Marcos-Gutierrez CV, Durbec P. et al. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126(12):2785–2797. [PubMed: 10331988]
- 87.
- Wu JJ, Chen JX, Rothman TP. et al. Inhibition of in vitro enteric neuronal development by endothelin-3: Mediation by endothelin B receptors. Development. 1999;126(6):1161–1173. [PubMed: 10021336]
- 88.
- Young HM, Hearn CJ, Farlie PG. et al. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229(2):503–516. [PubMed: 11150245]
- 89.
- Enomoto H, Crawford PA, Gorodinsky A. et al. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128(20):3963–3974. [PubMed: 11641220]
- 90.
- Yan H, Bergner AJ, Enomoto H. et al. Neural cells in the esophagus respond to glial cell line-derived neurotrophic factor and neurturin, and are RET-dependent. Dev Biol. 2004;272(1):118–133. [PubMed: 15242795]
- 91.
- Honma Y, Araki T, Gianino S. et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35(2):267–282. [PubMed: 12160745]
- 92.
- Golden JP, DeMaro JA, Osborne PA. et al. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158(2):504–528. [PubMed: 10415156]
- 93.
- Rossi J, Herzig KH, Voikar V. et al. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor alpha2. J Clin Invest. 2003;112(5):707–716. [PMC free article: PMC182204] [PubMed: 12952919]
- 94.
- Heuckeroth RO, Kotzbauer P, Copeland NG. et al. Neurturin, a novel neurotrophic factor, is localized to mouse chromosome 17 and human chromosome 19p13.3. Genomics. 1997;44(1):137–140. [PubMed: 9286710]
- 95.
- Rossi J, Luukko K, Poteryaev D. et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron. 1999;22(2):243–252. [PubMed: 10069331]
- 96.
- Baynash AG, Hosoda K, Giaid A. et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79(7):1277–1285. [PubMed: 8001160]
- 97.
- Hosoda K, Hammer RE, Richardson JA. et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. [PubMed: 8001159]
- 98.
- Puffenberger EG, Hosoda K, Washington SS. et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994;79(7):1257–1266. [PubMed: 8001158]
- 99.
- Leibl MA, Ota T, Woodward MN. et al. Expression of endothelin 3 by mesenchymal cells of embryonic mouse caecum. Gut. 1999;44(2):246–252. [PMC free article: PMC1727386] [PubMed: 9895385]
- 100.
- Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol. 2003;259(1):162–175. [PubMed: 12812796]
- 101.
- Cass DT, Zhang AL, Morthorpe J. Aganglionosis in rodents J Pediatr Surg 199227(3):351–355. (discussion 355-356) [PubMed: 1501010]
- 102.
- Newgreen DF, Hartley L. Extracellular matrix and adhesive molecules in the early development of the gut and its innervation in normal and spotting lethal rat embryos. Acta Anat (Basel). 1995;154(4):243–260. [PubMed: 8773711]
- 103.
- Woodward MN, Sidebotham EL, Connell MG. et al. Analysis of the effects of endothelin-3 on the development of neural crest cells in the embryonic mouse gut. J Pediatr Surg. 2003;38(9):1322–1328. [PubMed: 14523813]
- 104.
- Chalazonitis A. Neurotrophin-3 in the development of the enteric nervous system. Prog Brain Res. 2004;146:243–263. [PubMed: 14699968]
- 105.
- Chalazonitis A, Pham TD, Rothman TP. et al. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21(15):5620–5636. [PMC free article: PMC6762643] [PubMed: 11466433]
- 106.
- Pisano JM, Colon-Hastings F, Birren SJ. Postmigratory enteric and sympathetic neural precursors share common, developmentally regulated, responses to BMP2. Dev Biol. 2000;227(1):1–11. [PubMed: 11076672]
- 107.
- Bixby S, Kruger G, Mosher J. et al. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35(4):643. [PubMed: 12194865]
- 108.
- Chalazonitis A, D'Autreaux F, Guha U. et al. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24(17):4266–4282. [PMC free article: PMC6729284] [PubMed: 15115823]
- 109.
- Sukegawa A, Narita T, Kameda T. et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127(9):1971–1980. [PubMed: 10751185]
- 110.
- De Santa Barbara P, Williams J, Goldstein AM. et al. Bone morphogenetic protein signaling pathway plays multiple roles during gastrointestinal tract development. Dev Dyn. 2005 [PubMed: 16110505]
- 111.
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127(12):2763–2772. [PubMed: 10821773]
- 112.
- Fu M, Lui VC, Sham MH. et al. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J Cell Biol. 2004;166(5):673–684. [PMC free article: PMC2172437] [PubMed: 15337776]
- 113.
- Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998;18(1):60–64. [PubMed: 9425902]
- 114.
- Kapur RP. Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr Dev Pathol. 1999;2(6):559–569. [PubMed: 10508880]
- 115.
- Pattyn A, Morin X, Cremer H. et al. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399(6734):366–370. [PubMed: 10360575]
- 116.
- Guillemot F, Lo LC, Johnson JE. et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75(3):463–476. [PubMed: 8221886]
- 117.
- Lang D, Epstein JA. Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Hum Mol Genet. 2003;12(8):937–945. [PubMed: 12668617]
- 118.
- Kim J, Lo L, Dormand E. et al. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38(1):17–31. [PubMed: 12691661]
- 119.
- Hirsch MR, Tiveron MC, Guillemot F. et al. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125(4):599–608. [PubMed: 9435281]
- 120.
- Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125(4):609–620. [PubMed: 9435282]
- 121.
- Kapur RP, Livingston R, Doggett B. et al. Abnormal microenvironmental signals underlie intestinal aganglionosis in Dominant megacolon mutant mice. Dev Biol. 1996;174(2):360–369. [PubMed: 8631507]
- 122.
- Wegner M, Stolt CC. From stem cells to neurons and glia: A Soxist's view of neural development. Trends Neurosci. 2005 [PubMed: 16139372]
- 123.
- Wakamatsu N, Yamada Y, Yamada K. et al. Mutations in SIP1, encoding Smad interacting protein- 1, cause a form of Hirschsprung disease. Nat Genet. 2001;27(4):369–370. [PubMed: 11279515]
- 124.
- Van De Putte T, Maruhashi M, Francis A. et al. Mice lacking Zfhx1b, the gene that codes for Smad-interacting protein- 1, reveal a role for multiple neural crest cell defects in the etiology of hirschsprung disease-mental retardation syndrome. Am J Hum Genet. 2003;72(2):2. [PMC free article: PMC379238] [PubMed: 12522767]
- 125.
- Parisi MA, Baldessari AE, Iida MH. et al. Genetic background modifies intestinal pseudo-obstruction and the expression of a reporter gene in Hox11L1-/- mice. Gastroenterology. 2003;125(5):1428–1440. [PubMed: 14598259]
- 126.
- Hatano M, Aoki T, Dezawa M. et al. A novel pathogenesis of megacolon in Ncx/Hox11L.1 deficient mice. J Clin Invest. 1997;100(4):795–801. [PMC free article: PMC508250] [PubMed: 9259577]
- 127.
- Shirasawa S, Yunker AM, Roth KA. et al. Enx (Hox11L1)-deficient mice develop myenteric neuronal hyperplasia and megacolon. Nat Med. 1997;3(6):646–650. [PubMed: 9176491]
- 128.
- Kapur RP, Clarke CM, Doggett B. et al. Hox11L1 expression by precursors of enteric smooth muscle: An alternative explanation for megacecum in HOX11L1-/- mice. Pediatr Dev Pathol. 2005;8(2):148–161. [PubMed: 15803212]
- 129.
- Niederreither K, Vermot J, Le Roux I. et al. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130(11):2525–2534. [PubMed: 12702665]
- 130.
- Epperlein HH, Krotoski D, Halfter W. et al. Origin and distribution of enteric neurones in Xenopus. Anat Embryol (Berl). 1990;182(1):53–67. [PubMed: 1700645]
- 131.
- Shepherd IT, Beattie CE, Raible DW. Functional analysis of zebrafish GDNF. Dev Biol. 2001;231(2):420–435. [PubMed: 11237470]
- 132.
- Shepherd IT, Pietsch J, Elworthy S. et al. Roles for GFR{alpha}1 receptors in zebrafish enteric nervous system development. Development. 2004;131(1):241–249. [PubMed: 14660438]
- 133.
- Elworthy S, Pinto JP, Pettifer A. et al. Phox2b function in the enteric nervous system is conserved in zebrafish and is sox10-dependent. Mech Dev. 2005;122(5):659–669. [PubMed: 15817223]
- 134.
- Landman KA, Pettet GJ, Newgreen DF. Mathematical models of cell colonization of uniformly growing domains. Bull Math Biol. 2003;65(2):235–262. [PubMed: 12675331]
- 135.
- Bondurand N, Natarajan D, Thapar N. et al. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130(25):6387–6400. [PubMed: 14623827]
- 136.
- Kruger G, Mosher J, Bixby S. et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657. [PMC free article: PMC2728576] [PubMed: 12194866]
- 137.
- Iwashita T, Kruger GM, Pardal R. et al. Hirschsprung disease is linked to defects in neural crest stem cell function. Science. 2003;301(5635):972–976. [PMC free article: PMC2614078] [PubMed: 12920301]
- 138.
- Schafer KH, Hagl CI, Rauch U. Differentiation of neurospheres from the enteric nervous system. Pediatr Surg Int. 2003;19(5):340–344. [PubMed: 12845455]
- 139.
- Natarajan D, Grigoriou M, Marcos-Gutierrez CV. et al. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126(1):157–168. [PubMed: 9834195]
- 140.
- Micci MA, Kahrig KM, Simmons RS. et al. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice Gastroenterology 2005. (in press) [PubMed: 16344050]
- 141.
- Kapur RP, Yost C, Palmiter RD. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992;116(1):167–175. [PubMed: 1483385]
- Neural Crest and the Development of the Enteric Nervous System - Madame Curie Bi...Neural Crest and the Development of the Enteric Nervous System - Madame Curie Bioscience Database
- Calcium Signaling During Phagocytosis - Madame Curie Bioscience DatabaseCalcium Signaling During Phagocytosis - Madame Curie Bioscience Database
- Regulation of Cell Adhesion Responses by Abl Family Kinases - Madame Curie Biosc...Regulation of Cell Adhesion Responses by Abl Family Kinases - Madame Curie Bioscience Database
- X Chromosome Inactivation and Embryonic Stem Cells - Madame Curie Bioscience Dat...X Chromosome Inactivation and Embryonic Stem Cells - Madame Curie Bioscience Database
- NOTCH SIGNALING AND THE DEVELOPING INNER EAR - Madame Curie Bioscience DatabaseNOTCH SIGNALING AND THE DEVELOPING INNER EAR - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...