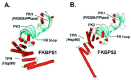NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Hsp90 functionally interacts with a broad array of client proteins, but in every case examined Hsp90 is accompanied by one or more cochaperones. One class of cochaperone contains a tetratricopeptide repeat domain that targets the cochaperone to the C-terminal region of Hsp90. Within this class are Hsp90-binding peptidylprolyl isomerases, most of which belong to the FK506-binding protein (FKBP) family. Despite the common association of FKBP cochaperones with Hsp90, it is now clear that the client protein influences, and is influenced by, the particular FKBP bound to Hsp90. Examples include Xap2 in aryl hydrocarbon receptor complexes and FKBP52 in steroid receptor complexes. In this chapter, we discuss the known functional roles played by FKBP cochaperones and, where possible, relate distinctive functions to structural differences between FKBP members.
Introduction
Immunophilins are a large, functionally diverse group of proteins that are defined by their ability to bind immunosuppressive ligands. The immunophilins minimally contain a peptidyl-prolyl cis-trans isomerase (PPIase; also termed rotamase) domain to which the immunosuppressive drugs bind. Early investigations into the PPIase enzymatic activity led to the belief that the immunosuppressive drugs elicited their effects by inhibiting the PPIase activity. However, some compounds binding the PPIase active site efficiently inhibit PPIase activity without inducing immunosuppression, so PPIase activity is not critical for immune responses. It is now known that effector domains on the immunosuppressive drugs project from the PPIase pocket. This allows the immunophilin-drug complex to bind tightly to and inhibit calcineurin or target of rapamycin, signal transduction proteins required for immune responses (see reference 1 for a detailed review on the mechanisms by which immunophilins and their ligands suppress immune responses).
Since the initial identification of the immunophilin proteins, multiple family members have been identified in all major branches of life. Some immunophilins are small proteins containing only a single PPIase domain while others are large multidomain proteins that contain one or more PPIase domains, as well as additional functional domains. The immunophilins are divided into two groups based on their ability to bind different immunosuppressive ligands: the FK506 binding proteins (FKBP), which also bind rapamycin, and the cyclosporin-A binding proteins or cyclophilins (CyP). The PPIase domains of FKBP and cyclophilins are structurally distinct and likely evolved independently. On the other hand, some members of either the FKBP or cyclophilin families contain a structurally similar tetratricopeptide repeat (TPR) domain that targets binding to heat shock protein 90 (Hsp90).
Hsp90 is an abundant molecular chaperone that interacts with a broad array of protein clients that regulate numerous important cellular pathways. Among the known Hsp90 clients are transcription factors (e.g., steroid hormone receptors, heat shock transcription factor 1, aryl hydrocarbon receptor), both serine/threonine and tyrosine kinases (e.g., Raf and Src-related kinases), and key regulatory enzymes (e.g., nitric oxide synthase and telomerase). A compilation of known Hsp90 clients maintained by Didier Picard at Univ. of Geneva can be accessed at: http://www.picard.ch/downloads/Hsp90interactors.pdf.
In concert with other chaperone proteins, Hsp90 facilitates client folding and proteolytic stability but can also promote client degradation. In the case of steroid receptors, Hsp90 and it's associated cochaperones also regulate receptor activity. Hsp90 binding to steroid receptors must be preceded by transient receptor interactions with Hsp40, Hsp70, and associated cochaperones. Hsp90, which is recruited as a dimer in the latter stages of complex assembly, binds directly to the receptor ligand binding domain and stabilizes a receptor conformation that is competent for hormone binding. Associated with Hsp90 in the functionally mature receptor complex are p23, a cochaperone that stabilizes Hsp90 binding to receptor, and any one of several TPR cochaperones, including the immunophilin/PPIases FKBP52 (also termed p59, Hsp56, p50, HBI, FKBP59, and FKBP4), FKBP51 (also termed p54, FKBP54, and FKBP5), and CyP40, or the protein phosphatase PP5. As discussed below, receptor activity can vary depending on the particular TPR cochaperone in mature receptor heterocomplexes.
The domain organization for several TPR cochaperones are compared in Figure 1. These cochaperones compete for a common binding site in the C-terminal region of Hsp90 that includes the highly conserved -MEEVD sequence that terminates Hsp90. Cocrystallographic structures have shown how an MEEVD pentapeptide associates with the TPR binding pocket.2,3 Although the TPR domains for each of these cochaperones are structurally similar and interact in a similar manner with Hsp90, the client protein bound by Hsp90 can influence the rank order of cochaperone recruitment to Hsp90-client complexes (reviewed in ref. 4). For instance, PP5 and FKBP51 are preferred components in glucocorticoid receptor (GR) complexes, FKBP51 is preferred in progesterone receptor (PR) complexes, and CyP40 is relatively enhanced in estrogen receptor (ER) complexes.5,6 On the other hand, another TPR-containing FKBP, the hepatitis B virus protein X associated protein 2 (Xap2; also termed AIP, ARA9, and FKBP37) shows little interaction with steroid receptors but is strongly associated with the aryl hydrocarbon receptor-Hsp90 complex.7,8 The distinctive patterns of preference for cochaperone association in client complexes is one line of evidence that the cochaperones bound to Hsp90 can also interact with the Hsp90-bound client.
In addition to FKBP52, FKBP51, and XAP2, several other FKBP family members contain TPR domains that are known or likely to bind Hsp90. FKBP6 (also termed FKBP36) is structurally similar to Xap2 but is required for male fertility and homologous chromosome pairing in meiosis.9 Drosophila melanogaster express a TPR-containing immunophilin (DmFKBP59) that has high similarity to FKBP52/51 in vertebrates.10,11 Plants have several FKBP genes that encode TPR domains; for example, in Arabidopsis thaliana there are 4 such genes: AtFKBP42, AtFKBP62, AtFKBP65 and AtFKBP72 (reviewed in refs. 12 and 13). Although prokaryotic and Archaeal genomes also contain FKBP family members,14 none of these genes encode a TPR domain.
Structure/Function Relationships of Steroid Receptor-Associated FKBPs
Three-dimensional crystal structures have been solved for full-length FKBP51 and for overlapping fragments of FKBP52. Both have a similar TPR domain composed of three tandem repeats of the degenerate 34-amino acid motif that characterizes TPR proteins.15 Each repeat adopts a helix-turn-helix conformation and adjacent units stack in parallel to form a saddle-shaped domain with a concave binding pocket for Hsp90 (Figs. 2A and 2B). In addition to the TPR domain, both FKBP51 and FKBP52 have two N-terminal domains, each of which is structurally similar to FKBP12. FK506-binding and PPIase activities reside in the most N-terminal domain (FK1), which has a pocket and active site residues similar to FKBP12. Due to several amino acid differences, the second domain (FK2) lacks drug binding and PPIase activity;16 other functions for FK2 have not been identified.
FKBP52 and FKBP51 have distinct functional influences on steroid receptors (see below), but the underlying structure/function relationships are not well understood. FKBP51 and FKBP52 share greater than 60% amino acid sequence similarity, and individual domains do not differ markedly between FKBP51 and FKBP52. The most striking difference in crystal structures relates to apparent domain:domain orientations. The FKBP52 structure shown in (Fig. 2B) is a composite model derived from merging the separate FK1-FK2 and FK2-TPR structures. The composite model suggests that the FKBP52 TPR domain is aligned in a more linear fashion with the FK domains rather than in the kinked conformation seen with FKBP51 (Fig. 2A). In fact, the static orientations shown in crystal structures are likely more dynamic in solution, but the different crystal orientations are perhaps telling. Amino acid side chains unique to FKBP51 form a salt bridge between FK2 and TPR that would stabilize the domain:domain interaction in FKBP51 relative to FKBP52, which lacks this salt bridge. The apparently more flexible structure of FKBP52 might accommodate interactions within the receptor heterocomplex that are constrained in FKBP51.
FKBP51 and FKBP52 also differ in the hinge region connecting FK1 and FK2 domains (FK loop). The FK loop of FKBP52 contains a -TEEED- sequence that has been identified as an in vitro substrate for casein kinase II; the corresponding sequence in FKBP51, -FED-, lacks the threonine phosphorylation site. Phosphorylation of FKBP52 is potentially important since the phospho-protein is reported to lose Hsp90 binding.17 Finally, there are a few amino acid differences in and around the respective FK1 PPIase pockets of FKBP52 and FKBP51. Although these FKBPs have similar PPIase activity measured against a model small peptide substrate,18 FKBP specificity for prolines in the context of a full-length protein might be distinctive. So, for instance, FKBP52 might recognize a receptor proline site that is poorly accessed by FKBP51.
Cellular and Physiological Functions of Hsp90-Associated FKBPs
FKBP52
FKBP52 is expressed in most vertebrate tissues and cell lines, although its expression can be up-regulated by heat stress,19 by estrogen in MCF-7 breast cancer cells,20 and by the homeobox transcription factor HoxA-10 in the peri-implantation mouse uterus.21 FKBP52 associates with steroid receptor complexes in an Hsp90-dependent manner, but FKBP52 is not required in a defined cell-free assembly system for receptor to reach the mature conformation that is competent for hormone binding.22,23 Nonetheless, FKBP52 in cells potentiates hormone-dependent reporter gene activation by GR,24 AR,25 and PR.26 Potentiation of hormone signaling can be related to an increase in receptor affinity for hormone,24,27 but there may be additional mechanisms by which FKBP52 enhances receptor activity.
In concordance with hormone binding affinity changes, domain swapping experiments between GR and ER, which is not potentiated by FKBP52, demonstrated that FKBP52 potentiation is localized to the ligand binding domain of GR.24 FKBP52-dependent potentiation of receptor activity is abrogated in point mutants that are defective for Hsp90 binding or PPIase activity, and potentiation is blocked by the PPIase inhibitor FK506.24,25 One model to explain these findings is that Hsp90 recruits FKBP52 to the receptor heterocomplex such that the FK1 PPIase can effectively catalyze isomerization of one or more prolines in the receptor ligand binding domain. The resultant change in receptor conformation would translate as an alteration in receptor interactions with hormone or other cellular factors.
FKBP52 has been shown by in vitro studies to have a chaperone activity that is independent of Hsp90-binding or PPIase.28,29 Like Hsp90 and numerous other chaperone components, FKBP52 can hold misfolded model proteins in a nonaggregated state that is amenable to refolding. The possibility that chaperone holding activity displayed by FKBP52 plays some role in altering receptor activity cannot be dismissed, but this appears unlikely since holding activity is highly redundant among chaperone components. Furthermore, holding activity, unlike FKBP52-dependent potentiation of receptor activity, is neither PPIase- nor Hsp90-dependent. Unfortunately, no one has identified an FKBP52 mutation that disrupts holding activity in a discrete manner.
In an effort to extend biochemical and cellular data on FKBP functions to the physiological level our laboratory has generated FKBP52 gene knockout (52KO) mice. The mutant mice have striking reproductive phenotypes that can be attributed, at least in part, to loss of steroid receptor activity. Male 52KO mice are infertile and display abnormal virilization with persistent nipples, ambiguous external genitalia, and dysgenic seminal vesicles and prostate.25 These developmental defects are consistent with androgen insensitivity in these tissues. Testicular morphology, descent, histology, and spermatogenesis are normal, and androgen production and release from testes is unimpaired; these developmental features are not highly androgen-dependent. On the other hand, sperm isolated from the epididymis have abnormal tail morphology and reduced motility suggestive of a defect in sperm maturation within the epididymis, a process that is androgen-dependent. Cellular studies confirm that FKBP52 is required for full AR function, which provides a rational explanation for androgen insensitivity in tissues of 52KO males.
52KO females have no gross morphological abnormalities, yet are completely infertile.26 Oocyte formation and release are not markedly impaired, and oocytes are competent by in vitro and in vivo fertilization. Infertility is due, at least in part, to a maternal failure of embryonic implantation and uterine decidualization. During the early stages of pregnancy, the 52KO uterus does not display the usual molecular or physiological markers for implantation. These events are largely dependent on progesterone actions, and both molecular and cellular studies confirm that FKBP52 is required for full PR activity.
Thus, FKBP52 is critical for reproductive development and success in both male and female mice, and its role can be traced to support of AR and PR function. FKBP52 does not alter ER function in cellular studies and 52KO mice show no signs of estrogen insensitivity. Conversely, results from cellular studies would predict that 52KO mice are resistant to glucocorticoids; consistent with this prediction, 52KO mice have elevated levels of serum corticosterone, although they clearly do not display features suggestive of major glucocorticoid deficits. Endocrine feedback mechanisms may be sufficient to compensate for partial reductions in GR activity, but more in-depth physiological studies are required to determine whether 52KO mice display less apparent immunological or behavioral alterations that could result from partial loss of GR activity.
Apart from the well-established roles of FKBP52 in steroid hormone receptor function, FKBP52, as with other Hsp90 cochaperones, has been identified in a variety of client-Hsp90 heterocomplexes such as those containing kinases, aryl hydrocarbon receptor, and heat shock transcription factor; however, many of these interactions might reflect passive, transient association of FKBP52 with Hsp90 and have no functional impact on client activity. FKBP52 also has been found to interact directly with the copper transport protein Atox1,30 which is part of the copper efflux machinery in neurons, interferon regulatory factor 4,31 which regulates gene expression in B and T lymphocytes, and FKBP associated protein 48,32 which influences proliferation of Jurkat T cells.33 A Drosophila homolog termed dFKBP59 was found to interact with the Ca2+ channel protein TRPL in photoreceptor cells and to influence Ca2+ influx.10 Subsequent studies revealed that FKBP52 similarly interacts with a subset of rat TRPC proteins that form Ca2+ channels in the mammalian brain.34 Each of these interactions were found to be disrupted by FK506 and to target the FKBP52 PPIase domain to specific proline sites in each partner protein. Phenotypes potentially related to these interactions have not yet been assessed in 52KO mice. Not only does FKBP52 interact with proteins, but also FKBP52 is capable of directly binding adeno-associated virus DNA and regulating replication of the viral genome.35,36 The relevant DNA binding site in FKBP52 has not been identified.
FKBP51
FKBP51/p54/FKBP54 was originally identified as a component of chicken PR complexes37-39 and is now known to assemble as an Hsp90 cochaperone with all steroid receptors and other Hsp90-client complexes. FKBP51 is functionally similar in some ways to FKBP52; both have similar PPIase activity in the presence of model peptide substrates, both hold misfolded proteins in a folding competent state, and they compete for binding a common site on Hsp90.18,40 As noted above, the overall structural similarity of these FKBPs is consistent with these shared functional properties, yet their distinct effects on steroid receptor activity belie these similarities. Another distinction is that the FKBP51 gene is highly inducible by glucocorticoids, androgens and progesterone.41-47
FKBP51 acts as an inhibitor of steroid receptor function. The first indication of its inhibitory role came from studies by Scammell and colleagues of glucocorticoid resistance in New World primates.48,49 In squirrel monkeys GR has a relatively low affinity for hormone yet the cloned monkey GR has an affinity similar to human GR in vitro. This observation led to a search for cellular factors in monkey cells that reduced GR binding affinity. A key factor identified was FKBP51, which is constitutively overexpressed in squirrel monkey cells as well as cells of other New World primates, all of which display some degree of glucocorticoid resistance. Human FKBP51 was also found to inhibit GR function but not to the degree of squirrel monkey FKBP51, which differs in amino acid sequence from its human counterpart at 15 of 457 amino acids. These differences are scattered fairly evenly along the sequence and mapping studies have shown that amino acid changes in several domains contribute to the more potent inhibitory actions of squirrel monkey FKBP51.50 Crystal structures for both human and squirrel monkey FKBP51 have been solved;16 although functionally relevant structural changes are not yet apparent, comparison of these structures should ultimately help to understand why inhibitory potencies differ.
In a yeast model for studying functional interactions between steroid receptors and human FKBPs, FKBP51 does not inhibit the activity of GR; however, FKBP51 can effectively reverse the potentiation of GR activity conferred by FKBP52.24 Therefore, FKBP51 acts as an antagonist of FKBP52, which is unexpected based on measured similarities of FKBP functional properties noted above. FKBP51 has also been shown to inhibit PR function,46 presumably through a similar inhibition of FKBP52-mediated potentiation. The mechanism by which FKBP51 antagonizes FKBP52's ability to enhance steroid receptor function is not understood. Other Hsp90-binding TPR proteins do not block FKBP52 actions, so it does not appear that competitive displacement of FKBP52 from receptor complexes by FKBP51 can fully account for antagonism. On the other hand, FKBP51 is known to preferentially associate with PR and GR complexes.6,40 Domain swapping studies indicate that the FK1 PPIase domain partially contributes to antagonism but sequences in the FK2 and TPR domain also play a role.24,50 Currently, we are genetically mapping sequence differences in FKBP52 and FKBP51 to distinguish how FKBP52 potentiates steroid receptor function and FKBP51 blocks potentiation.
Functional Interactions between FKBP52 and FKBP51
Nuclear Transport
There is some evidence to suggest that FKBP51and FKBP52 have a role in hormone-dependent translocation of GR from the cytosol to nucleus.51-54 The Pratt laboratory has demonstrated that FKBP52, CyP40 and PP5 bind dynein and therefore link the receptor/ Hsp90 complex to the cytoskeleton.55 Unlike FKBP52, FKBP51 lacks the ability to bind dynein, and hormone-induced switching from FKBP51 to FKBP52 in GR-Hsp90 heterocomplexes has been reported.52 Thus, one can imagine a model in which FKBP51 holds the receptor in the cytosol until hormone is present, then upon hormone binding FKBP52 replaces FKBP51 in the complex and FKBP52 mediates translocation of the receptor to the nucleus. This is an attractive model, but several observations raise concerns about the general relevance of FKBP52-dynein interactions to steroid receptor function. First, there is a dynamic exchange of Hsp90 cochaperones within mature Hsp90/steroid receptor complexes before binding hormone, 6 which would seemingly confound receptor localization. Next, although rapid nuclear translocation of GR can be slowed by disrupting cytoskeletal interactions or altering FKBP levels, hormone-bound GR still concentrates in the nucleus within 30 minutes. While one might expect that hormone-dependent activation of a reporter gene might lag in parallel with delayed GR transport, inhibition of reporter expression persists for as long as 16 hours in cells lacking FKBP52. Moreover, FKBP52-dependent potentiation of GR activity is unaltered in yeast that express or lack dynein.24 Another concern with the transport model is the lack of explanation for why PP5, which binds dynein similar to FKBP52 and assembles preferentially with GR heterocomplexes,5,55 fails to compensate for loss of FKBP52-dependent GR activity. Clearly, FKBP52 is functioning as more than a linker between GR and dynein complexes. A final consideration relates to differences in subcellular localization of GR, AR, and PR, each of which is similarly potentiated by FKBP52. Although GR is largely localized to the cytosol in the absence of hormone, and thus requires nuclear translocation in response to hormone, AR and PR are more typically localized to the nuclear compartment even in the absence of hormone. It is unlikely that FKBP52 actions relate to nuclear translocation of AR and PR.
Mutual Antagonism
Given that FKBP51 gene expression is inducible by some steroid hormones and FKBP51 can inhibit receptor function, one can reasonably speculate that FKBP51 serves as a cellular modulator of hormone responsiveness. In cells unexposed to hormone, FKBP52 actions would predominate and promote a robust response to hormone; as a consequence, however, FKBP51 levels would rise and partially desensitize cells to a secondary hormone exposure. These effects can be demonstrated in cellular models, but the physiological importance of this mechanism must be established with animal models. Toward this goal, our laboratory has recently generated FKBP51 gene knockout mice. Homozygous mutant animals are grossly normal and reproductively viable (unpublished observations), so FKBP51 does not appear to be critical in the same physiological processes as FKBP52. Nonetheless, modulatory actions of FKBP51 might be relevant but subject to compensatory physiological mechanisms. Interestingly, double knockout of both FKBP51 and FKBP52 genes is embryonic lethal in mice (unpublished observation), suggesting either that FKBP51 and FKBP52 have a critical, mutually redundant function or that FKBP51 and FKBP52 function in a common developmental pathway that requires the distinct actions of both immunophilins.
Aside from its role in steroid receptor complexes, FKBP51 has been shown to regulate NFκB pathways. FKBP51 was identified56 by a proteomic approach in complex with IKKα, one of the serine/threonine kinases that stimulates phosphorylation and degradation of the NFκB inhibitor IκB. Knockdown of FKBP51 expression was shown to inhibit IKKα activation and thereby block TNFα-induced activation of NFκB, which confirmed the functional significance of FKBP51 in IKKα complexes. Perhaps related to FKBP51-dependent regulation of NFκB pathways, overexpression of FKBP51 has been correlated57 with idiopathic myelofibrosis, a rare clonal stem cell disorder. Experimental overexpression of FKBP51 was subsequently shown to stimulate NFκB activity and, as a consequence, to increase secretion of pro-fibrotic TGF-β1.58 IKKα had previously been shown to be an Hsp90 client,59 so it is possible that, analogous to steroid receptor complexes, FKBP51 assembles with IKKα as a heterocomplex with Hsp90. Whether FKBP51 Hsp90 binding or PPIase is required for regulation of IKKα has not been determined.
Xap2 and FKBP6
Apart from the highly characterized steroid hormone receptor-associated FKBPs, several other TPR-containing FKBPs are present in higher vertebrates. As mentioned in earlier sections of this chapter, Xap2 is a TPR-containing immunophilin that is found almost exclusively in AhR complexes. As the name implies, Xap2 also functionally interacts with the hepatitis B virus protein X.60 In addition Xap2 is known to have functional interactions with peroxisome proliferator activated receptor α (PPARα),61 however these interactions have not been extensively characterized. AhR is a ligand-dependent transcription factor that mediates the physiological response to specific environmental contaminants termed polycyclic aromatic hydrocarbons, the most notorious of which is 2,3,7,8-tetrachlorodibenzo-p-dioxin. Similar to steroid receptors, AhR requires assembly with Hsp90 and p23 to achieve a mature ligand-binding conformation (reviewed in 62), although the AhR ligand binding domain is unrelated to steroid receptor ligand binding domains. AhR complexes also contain an FKBP component, but in this case it is Xap2 rather than FKBP52 or FKBP51.
As with FKBP51 and FKBP52, Xap2 has a C-terminal TPπ™domain that is known to facilitate binding to the MEEVD motif on Hsp9063 (Fig. 1). In addition Xap2 contains one N-terminal FK domain that lacks drug binding and also likely lacks PPIase activity. Although the FK domain is not required for Hsp90 binding, it is required for an interaction with the AhR-Hsp90 complex that functionally influences receptor activity.63,64 In a cell-free assembly system that lacks Xap2, AhR is capable of assembling with Hsp90 and binding ligand, and upon ligand binding AhR is capable of binding AhR response elements on DNA.8 Again, similar to FKBP52 or FKBP51 in steroid receptor complexes, Xap2 is not required for basal maturation of AhR activity, but in both yeast and mammalian systems, Xap2 can modulate AhR-mediated reporter gene expression.7,8,63,65 By titrating the relative level of Xap2 protein in cells, AhR activity can be enhanced or decreased. For example, when Xap2 is expressed at a level 2- to 3-fold higher than normal, binding of p23 in the AhR-Hsp90 complex is reduced.66 Displacement of p23 by high levels of Xap2 would destabilize binding of Hsp90 to AhR and reduce the proportion of AhR in functionally mature complexes. Conversely, there is also evidence that at elevated Xap2 levels AhR is protected from ubiquitination and proteosomal degradation which would increase total AhR levels.67-71 Finally, several studies suggest that Xap2 facilitates nucleocytoplasmic shuttling of AhR following ligand binding.71-75
The physiological relevance of Xap2 interactions with AhR complexes has not been examined in a whole animal model, but Xap2 could potentially influence any of several physiological and pathological pathways mediated by AhR. Mice that are homozygous for a disrupted AhR gene have many physiological and developmental defects; among these are immune system impairment, hepatic fibrosis, cardiac hypertrophy, impaired insulin regulation, and defects in ovarian and vascular development.76-79 In addition, many of the toxic and teratogenic effects produced by AhR ligands require an intact AhR signaling pathway.80,81 For example, dioxin induced defects in prostate development are absent in AhR knockout mice.82 Development of a mouse strain lacking Xap2 could be very helpful in determining the role Xap2 plays in these processes and might validate Xap2 as a potential target for therapeutic intervention.
In addition to Xap2, vertebrates contain FKBP36 (gene name FKBP6 in humans), another TPR-containing FKBP that is structurally similar to Xap2 yet functionally distinct. FKBP36 has a single N-terminal FK domain and a C-terminal TPR domain. In vitro studies show that FKBP36 binds Hsp90 and can assemble with steroid receptor complexes (unpublished observation), but there is currently no evidence that FKBP36 alters receptor activity. FKBP36 mRNA is broadly expressed in vertebrate tissues with an exceptionally high level observed in the testis; male FKBP6 knockout mice lack sperm and FKBP36 was shown to be a critical component in meiotic synaptonemal complexes.9 Patients with Williams syndrome, which is characterized by congenital cardiovascular defects, dysmorphic facial features, mental retardation, growth defects, azoospermia, and hypercalcemia, are typically haploinsufficient for FKBP6;83 however, the contribution of FKBP6 deletion in this syndrome is not clear since several contiguous genes on chromosome 11, including genes for elastin and LIM-Kinase 1, are also deleted in these patients and clearly contribute to some phenotypic aspects.
Plant FKBPs
Hsp90-binding TPR immunophilins have been identified in all eukaryotes examined. A few examples of plant TPR-containing FKBPs are shown in Figure 1. The TPR domain of each FKBP is very similar in amino acid sequence to that of vertebrate proteins; these are presumed to bind Hsp90, but that has not been determined in all cases. The plant and insect FKBPs contain one or more PPIase-related domain and can contain other functional domains. For example, AtFKBP42 contains a C-terminal transmembrane domain that localizes the protein to the inner plasma membrane and the vacuolar membrane.84-86
There is ample evidence to suggest that the plant and insect FKBPs are physiologically important. Mutations in AtFKBP42 cause the severe developmental phenotypes termed twisted dwarf 1 (TWD)85 and ultracurvata (UCU2).87 The mechanism by which these phenotypes occur likely involves impairment of membrane transport as AtFKBP42 is known to interact with several ATP-binding cassette transporters on the plasma and vacuolar membranes.85,86,88 Mutations in AtFKBP72 result in a class of mutants termed pasticcino or pas mutants, which are characterized by a wide variety of developmental defects.89 Two Hsp90-binding TPR FKBPs in wheat, wFKBP72 and the heat shock-inducible wFKBP77, have been shown in transgenic plants to distinctively influence developmental patterns.90
Summary
In addressing the physiological importance of PPIases, Heitman and colleagues91 generated an S. cerevisiae strain that lacked all 12 PPIase genes in the FKBP and cyclophilin families; the pluri-mutant strain displayed some growth abnormalities but was viable, thus demonstrating that these genes collectively are nonessential in yeast. Nonetheless, it has become increasingly clear that the Hsp90-binding PPIases, through interactions with steroid receptors, kinases, and other cellular factors, play important physiological and potentially pathological roles in mammals. Elucidation of these roles, definition of underlying molecular mechanisms, and identification of specific inhibitors will likely quicken in the coming few years and lead to therapeutic targeting of individual PPIases.
Acknowledgements
Studies in the authors' laboratory were supported by NIH R01-DK48218 and the Mayo Foundation.
References
- 1.
- Hamilton GS, Steiner JP. Immunophilins: Beyond immunosuppression. J Med Chem. 1998;41(26):5119–5143. [PubMed: 9857082]
- 2.
- Scheufler C, Brinker A, Bourenkov G. et al. Structure of TPR domain-peptide complexes: Critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101(2):199–210. [PubMed: 10786835]
- 3.
- Wu B, Li P, Liu Y. et al. 3D structure of human FK506-binding protein 52: Implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Natl Acad Sci USA. 2004;101(22):8348–8353. [PMC free article: PMC420397] [PubMed: 15159550]
- 4.
- Riggs DL, Cox MB, Cheung-Flynn J. et al. Functional specificity of cochaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. [PubMed: 15763706]
- 5.
- Silverstein AM, Galigniana MD, Chen MS. et al. Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272(26):16224–16230. [PubMed: 9195923]
- 6.
- Barent RL, Nair SC, Carr DC. et al. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12(3):342–354. [PubMed: 9514152]
- 7.
- Ma Q, Whitlock Jr JP. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272(14):8878–8884. [PubMed: 9083006]
- 8.
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP. et al. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18(2):978–988. [PMC free article: PMC108810] [PubMed: 9447995]
- 9.
- Crackower MA, Kolas NK, Noguchi J. et al. Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science. 2003;300(5623):1291–1295. [PMC free article: PMC2882960] [PubMed: 12764197]
- 10.
- Goel M, Garcia R, Estacion M. et al. Regulation of drosophila trpl channels by immunophilin fkbp59. J Biol Chem. 2001;276(42):38762–38773. [PubMed: 11514552]
- 11.
- Zaffran S. Molecular cloning and embryonic expression of dFKBP59, a novel Drosophila FK506-binding protein. Gene. 2000;246(1-2):103–109. [PubMed: 10767531]
- 12.
- Romano P, Gray J, Horton P. et al. Plant immunophilins: Functional versatility beyond protein maturation. New Phytol. 2005;166(3):753–769. [PubMed: 15869639]
- 13.
- He Z, Li L, Luan S. Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol. 2004;134(4):1248–1267. [PMC free article: PMC419802] [PubMed: 15047905]
- 14.
- Maruyama T, Suzuki R, Furutani M. Archaeal peptidyl prolyl cis-trans isomerases (PPIases) update 2004. Front Biosci. 2004;9:1680–1720. [PubMed: 14977579]
- 15.
- Blatch GL, Lassle M. The tetratricopeptide repeat: A structural motif mediating protein-protein interactions. Bioessays. 1999;21(11):932–939. [PubMed: 10517866]
- 16.
- Sinars CR, Cheung-Flynn J, Rimerman RA. et al. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci USA. 2003;100(3):868–873. [PMC free article: PMC298693] [PubMed: 12538866]
- 17.
- Miyata Y, Chambraud B, Radanyi C. et al. Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: Regulation of HSP90-binding activity of FKBP52. Proc Natl Acad Sci USA. 1997;94(26):14500–14505. [PMC free article: PMC25035] [PubMed: 9405642]
- 18.
- Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol. 2001;308(4):795–806. [PubMed: 11350175]
- 19.
- Sanchez ER. Hsp56: A novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990;265(36):22067–22070. [PubMed: 2266108]
- 20.
- Kumar P, Mark PJ, Ward BK. et al. Estradiol-regulated expression of the immunophilins cyclophilin 40 and FKBP52 in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2001;284(1):219–225. [PubMed: 11374893]
- 21.
- Daikoku T, Tranguch S, Friedman DB. et al. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19(3):683–697. [PubMed: 15528267]
- 22.
- Dittmar KD, Hutchison KA, Owens-Grillo JK. et al. Reconstitution of the steroid receptor.hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271(22):12833–12839. [PubMed: 8662785]
- 23.
- Kosano H, Stensgard B, Charlesworth MC. et al. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem. 1998;273(49):32973–32979. [PubMed: 9830049]
- 24.
- Riggs DL, Roberts PJ, Chirillo SC. et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22(5):1158–1167. [PMC free article: PMC150341] [PubMed: 12606580]
- 25.
- Cheung-Flynn J, Prapapanich V, Cox MB. et al. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19(6):1654–1666. [PubMed: 15831525]
- 26.
- Tranguch S, Cheung-Flynn J, Daikoku T. et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102(40):14326–14331. [PMC free article: PMC1242310] [PubMed: 16176985]
- 27.
- Davies TH, Ning YM, Sanchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44(6):2030–2038. [PubMed: 15697228]
- 28.
- Bose S, Weikl T, Bugl H. et al. Chaperone function of Hsp90-associated proteins. Science. 1996;274(5293):1715–1717. [PubMed: 8939863]
- 29.
- Pirkl F, Fischer E, Modrow S. et al. Localization of the chaperone domain of FKBP52. J Biol Chem. 2001;276(40):37034–37041. [PubMed: 11473108]
- 30.
- Sanokawa-Akakura R, Dai H, Akakura S. et al. A novel role for the immunophilin FKBP52 in copper transport. J Biol Chem. 2004;279(27):27845–27848. [PubMed: 15133031]
- 31.
- Mamane Y, Sharma S, Petropoulos L. et al. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity. 2000;12(2):129–140. [PubMed: 10714679]
- 32.
- Chambraud B, Radanyi C, Camonis JH. et al. FAP48, a new protein that forms specific complexes with both immunophilins FKBP59 and FKBP12. Prevention by the immunosuppressant drugs FK506 and rapamycin. J Biol Chem. 1996;271(51):32923–32929. [PubMed: 8955134]
- 33.
- Krummrei U, Baulieu EE, Chambraud B. The FKBP-associated protein FAP48 is an antiproliferative molecule and a player in T cell activation that increases IL2 synthesis. Proc Natl Acad Sci USA. 2003;100(5):2444–2449. [PMC free article: PMC151360] [PubMed: 12604780]
- 34.
- Sinkins WG, Goel M, Estacion M. et al. Association of immunophilins with mammalian TRPC channels. J Biol Chem. 2004;279(33):34521–34529. [PubMed: 15199065]
- 35.
- Qing K, Hansen J, Weigel-Kelley KA. et al. Adeno-associated virus type 2-mediated gene transfer: Role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75(19):8968–8976. [PMC free article: PMC114465] [PubMed: 11533160]
- 36.
- Zhong L, Li W, Yang Z. et al. Improved transduction of primary murine hepatocytes by recombinant adeno-associated virus 2 vectors in vivo. Gene Ther. 2004;11(14):1165–1169. [PubMed: 15164097]
- 37.
- Smith DF, Faber LE, Toft DO. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990;265(7):3996–4003. [PubMed: 2303491]
- 38.
- Smith DF, Baggenstoss BA, Marion TN. et al. Two FKBP-related proteins are associated with progesterone receptor complexes. J Biol Chem. 1993;268(24):18365–18371. [PubMed: 7688746]
- 39.
- Smith DF, Albers MW, Schreiber SL. et al. FKBP54, a novel FK506-binding protein in avian progesterone receptor complexes and HeLa extracts. J Biol Chem. 1993;268(32):24270–24273. [PubMed: 7693698]
- 40.
- Nair SC, Rimerman RA, Toran EJ. et al. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol. 1997;17(2):594–603. [PMC free article: PMC231784] [PubMed: 9001212]
- 41.
- Baughman G, Wiederrecht GJ, Campbell NF. et al. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15(8):4395–4402. [PMC free article: PMC230679] [PubMed: 7542743]
- 42.
- Kester HA, van der Leede BM, van der Saag PT. et al. Novel progesterone target genes identified by an improved differential display technique suggest that progestin-induced growth inhibition of breast cancer cells coincides with enhancement of differentiation. J Biol Chem. 1997;272(26):16637–16643. [PubMed: 9195978]
- 43.
- Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22(9):1399–1403. [PubMed: 11532861]
- 44.
- Yoshida NL, Miyashita T, UM. et al. Analysis of gene expression patterns during glucocorticoid-induced apoptosis using oligonucleotide arrays. Biochem Biophys Res Commun. 2002;293(4):1254–1261. [PubMed: 12054511]
- 45.
- Vermeer H, Hendriks-Stegeman BI, van der Burg B. et al. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: A potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab. 2003;88(1):277–284. [PubMed: 12519866]
- 46.
- Hubler TR, Denny WB, Valentine DL. et al. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144(6):2380–2387. [PubMed: 12746298]
- 47.
- Febbo PG, Lowenberg M, Thorner AR. et al. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol. 2005;173(5):1772–1777. [PubMed: 15821585]
- 48.
- Reynolds PD, Ruan Y, Smith DF. et al. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84(2):663–669. [PubMed: 10022435]
- 49.
- Denny WB, Valentine DL, Reynolds PD. et al. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141(11):4107–4113. [PubMed: 11089542]
- 50.
- Denny WB, Prapapanich V, Smith DF. et al. Structurefunction analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005;146(7):3194–3201. [PubMed: 15802496]
- 51.
- Czar MJ, Lyons RH, Welsh MJ. et al. Evidence that the FK506-binding immunophilin heat shock protein 56 is required for trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus. Mol Endocrinol. 1995;9(11):1549–1560. [PubMed: 8584032]
- 52.
- Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277(7):4597–4600. [PubMed: 11751894]
- 53.
- Wochnik GM, Ruegg J, Abel GA. et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280(6):4609–4616. [PubMed: 15591061]
- 54.
- Pratt WB, Galigniana MD, Harrell JM. et al. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16(8):857–872. [PubMed: 15157665]
- 55.
- Galigniana MD, Harrell JM, Murphy PJ. et al. Binding of hsp90-associated immunophilins to cytoplasmic dynein: Direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41(46):13602–13610. [PubMed: 12427021]
- 56.
- Bouwmeester T, Bauch A, Ruffner H. et al. A physical and functional map of the human TNF-alpha/ NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6(2):97–105. [PubMed: 14743216]
- 57.
- Giraudier S, Chagraoui H, Komura E. et al. Overexpression of FKBP51 in idiopathic myelofibrosis regulates the growth factor independence of megakaryocyte progenitors. Blood. 2002;100(8):2932–2940. [PubMed: 12351405]
- 58.
- Komura E, Tonetti C, Penard-Lacronique V. et al. Role for the nuclear factor kappaB pathway in transforming growth factor-beta1 production in idiopathic myelofibrosis: Possible relationship with FK506 binding protein 51 overexpression. Cancer Res. 2005;65(8):3281–3289. [PubMed: 15833861]
- 59.
- Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23(31):5378–5386. [PubMed: 15077173]
- 60.
- Kuzhandaivelu N, Cong YS, Inouye C. et al. XAP2, a novel hepatitis B virus X-associated protein that inhibits X transactivation. Nucleic Acids Res. 1996;24(23):4741–4750. [PMC free article: PMC146319] [PubMed: 8972861]
- 61.
- Sumanasekera WK, Tien ES, Turpey R. et al. Evidence that peroxisome proliferator-activated receptor alpha is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem. 2003;278(7):4467–4473. [PubMed: 12482853]
- 62.
- Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 2002;141(1-2):25–40. [PubMed: 12213383]
- 63.
- Carver LA, LaPres JJ, Jain S. et al. Characterization of the Ah receptor-associated protein, ARA9. J Biol Chem. 1998;273(50):33580–33587. [PubMed: 9837941]
- 64.
- Kazlauskas A, Poellinger L, Pongratz I. Two distinct regions of the immunophilin-like protein XAP2 regulate dioxin receptor function and interaction with hsp90. J Biol Chem. 2002;277(14):11795–11801. [PubMed: 11805120]
- 65.
- Miller CA. Two tetratricopeptide repeat proteins facilitate human aryl hydrocarbon receptor signalling in yeast. Cell Signal. 2002;14(7):615–623. [PubMed: 11955954]
- 66.
- Hollingshead BD, Petrulis JR, Perdew GH. The aryl hydrocarbon (Ah) receptor transcriptional regulator hepatitis B virus X-associated protein 2 antagonizes p23 binding to Ah receptor-Hsp90 complexes and is dispensable for receptor function. J Biol Chem. 2004;279(44):45652–45661. [PubMed: 15322122]
- 67.
- Lees MJ, Peet DJ, Whitelaw ML. Defining the role for XAP2 in stabilization of the dioxin receptor. J Biol Chem. 2003;278(38):35878–35888. [PubMed: 12837759]
- 68.
- LaPres JJ, Glover E, Dunham EE. et al. ARA9 modifies agonist signaling through an increase in cytosolic aryl hydrocarbon receptor. J Biol Chem. 2000;275(9):6153–6159. [PubMed: 10692406]
- 69.
- Meyer BK, Petrulis JR, Perdew GH. Aryl hydrocarbon (Ah) receptor levels are selectively modulated by hsp90-associated immunophilin homolog XAP2. Cell Stress Chaperones. 2000;5(3):243–254. [PMC free article: PMC312890] [PubMed: 11005382]
- 70.
- Meyer BK, Perdew GH. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry. 1999;38(28):8907–8917. [PubMed: 10413464]
- 71.
- Kazlauskas A, Poellinger L, Pongratz I. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem. 2000;275(52):41317–41324. [PubMed: 11013261]
- 72.
- Berg P, Pongratz I. Two parallel pathways mediate cytoplasmic localization of the dioxin (aryl hydrocarbon) receptor. J Biol Chem. 2002;277(35):32310–32319. [PubMed: 12065584]
- 73.
- Petrulis JR, Hord NG, Perdew GH. Subcellular localization of the aryl hydrocarbon receptor is modulated by the immunophilin homolog hepatitis B virus X-associated protein 2. J Biol Chem. 2000;275(48):37448–37453. [PubMed: 10986286]
- 74.
- Kazlauskas A, Sundstrom S, Poellinger L. et al. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol Cell Biol. 2001;21(7):2594–2607. [PMC free article: PMC86890] [PubMed: 11259606]
- 75.
- Petrulis JR, Kusnadi A, Ramadoss P. et al. The hsp90 Cochaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J Biol Chem. 2003;278(4):2677–2685. [PubMed: 12431985]
- 76.
- Fernandez-Salguero P, Pineau T, Hilbert DM. et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268(5211):722–726. [PubMed: 7732381]
- 77.
- Lahvis GP, Pyzalski RW, Glover E. et al. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol. 2005;67(3):714–720. [PubMed: 15590894]
- 78.
- Thackaberry EA, Bedrick EJ, Goens MB. et al. Insulin regulation in AhR-null mice: Embryonic cardiac enlargement, neonatal macrosomia, and altered insulin regulation and response in pregnant and aging AhR-null females. Toxicol Sci. 2003;76(2):407–417. [PubMed: 12970579]
- 79.
- Benedict JC, Lin TM, Loeffler IK. et al. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56(2):382–388. [PubMed: 10910997]
- 80.
- Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619(3):263–268. [PubMed: 12573486]
- 81.
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S. et al. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173–179. [PubMed: 8806883]
- 82.
- Lin TM, Ko K, Moore RW. et al. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68(2):479–487. [PubMed: 12151645]
- 83.
- Meng X, Lu X, Morris CA. et al. A novel human gene FKBP6 is deleted in Williams syndrome. Genomics. 1998;52(2):130–137. [PubMed: 9782077]
- 84.
- Kamphausen T, Fanghanel J, Neumann D. et al. Characterization of Arabidopsis thaliana AtFKBP42 that is membrane-bound and interacts with Hsp90. Plant J. 2002;32(3):263–276. [PubMed: 12410806]
- 85.
- Geisler M, Kolukisaoglu HU, Bouchard R. et al. TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol Biol Cell. 2003;14(10):4238–4249. [PMC free article: PMC207015] [PubMed: 14517332]
- 86.
- Geisler M, Girin M, Brandt S. et al. Arabidopsis immunophilin-like TWD1 functionally interacts with vacuolar ABC transporters. Mol Biol Cell. 2004;15(7):3393–3405. [PMC free article: PMC452592] [PubMed: 15133126]
- 87.
- Perez-Perez JM, Ponce MR, Micol JL. The ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol. 2004;134(1):101–117. [PMC free article: PMC316291] [PubMed: 14730066]
- 88.
- Liu G, Sanchez-Fernandez R, Li ZS. et al. Enhanced multispecificity of Arabidopsis vacuolar multidrug resistance-associated protein-type ATP-binding cassette transporter, AtMRP2. J Biol Chem. 2001;276(12):8648–8656. [PubMed: 11115509]
- 89.
- Vittorioso P, Cowling R, Faure JD. et al. Mutation in the Arabidopsis PASTICCINO1 gene, which encodes a new FK506- binding protein-like protein, has a dramatic effect on plant development. Mol Cell Biol. 1998;18(5):3034–3043. [PMC free article: PMC110682] [PubMed: 9566922]
- 90.
- Kurek I, Stoger E, Dulberger R. et al. Overexpression of the wheat FK506-binding protein 73 (FKBP73) and the heat-induced wheat FKBP77 in transgenic wheat reveals different functions of the two isoforms. Transgenic Res. 2002;11(4):373–379. [PubMed: 12212840]
- 91.
- Dolinski K, Muir S, Cardenas M. et al. All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94(24):13093–13098. [PMC free article: PMC24268] [PubMed: 9371805]
- Functions of the Hsp90-Binding FKBP Immunophilins - Madame Curie Bioscience Data...Functions of the Hsp90-Binding FKBP Immunophilins - Madame Curie Bioscience Database
- Important Mammalian Respiratory Allergens Are Lipocalins - Madame Curie Bioscien...Important Mammalian Respiratory Allergens Are Lipocalins - Madame Curie Bioscience Database
- Biochemical Studies of Voltage-Gated Ca2+ Channels - Madame Curie Bioscience Dat...Biochemical Studies of Voltage-Gated Ca2+ Channels - Madame Curie Bioscience Database
- Tyrosyl-tRNA Synthetases - Madame Curie Bioscience DatabaseTyrosyl-tRNA Synthetases - Madame Curie Bioscience Database
- CATHEPSIN PROTEASES IN TOXOPLASMA GONDII - Madame Curie Bioscience DatabaseCATHEPSIN PROTEASES IN TOXOPLASMA GONDII - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...