NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The notion proposed over a decade ago that ceramide is involved in cellular signaling events has stimulated a large number of studies that have attempted to define the precise function(s) of ceramide in signaling, and has revived interest in understanding the mechanisms of regulation of sphingolipid metabolism. Ceramide is now known to be involved in cellular events as diverse as proliferation, differentiation, senescence and apoptosis, and the ceramide that is involved in these processes can come from either sphingomyelin breakdown or from de novo synthesis. Studies from our laboratory have helped define the role of ceramide derived from sphingomyelin in the regulation of neuronal growth and development. Intriguingly, ceramide generated from sphingomyelin upon binding of nerve growth factor to neurotrophin receptors can either induce neuronal cell death, or induce survival and outgrowth. Which of these two processes occurs is dictated by the developmental stage of the neuron, which is itself reflected in the level of expression of the neurotrophin receptor, p75NTR. The biochemical status of downstream signaling molecules may be an additional determinant of how ceramide performs its disparate roles.
Ceramide and Neuronal Development and Death
The development of the nervous system is accompanied by a considerable amount of programmed cell death. Neurons are produced in excess and compete with each other for limiting amounts of neurotrophins secreted by target cells. Indeed, the assumption that neurons die simply by passive starvation in the absence of trophic factors has been challenged by the finding that neurotrophins themselves can induce apoptotic cell death under certain conditions.1
Apoptotic cell death is characterized by removal of the dying cell without an inflammatory response. Several characteristic morphological changes occur during apoptosis, including shrinking of the cytoplasm, plasma membrane blebbing, nuclear chromatin condensation, and fragmentation of genomic DNA. In the early stages of apoptosis, phosphatidylserine is exposed on the cell surface, triggering cell engulfment by neighboring cells or phagocytes. The fundamental biochemical elements of the apoptotic pathway are conserved throughout the animal kingdom. Among the factors that regulate mammalian apoptosis are the Bcl-2 family of proteins, the adaptor protein 'Apoptotic protease-activating factor 1' (Apaf-1), and the cysteine protease family of caspases. Neurons, as might be expected, share the same basic apoptotic program as other cell types, but different types of neurons, and neurons at different developmental stages, express different combinations of Bcl-2 and caspase family members, which might be one way of controling the specificity of regulation.2
Ceramide has been implicated as a key player in the apoptosis of various cell types, including neurons.3 Ceramide can be produced by sphingomyelin (SM) hydrolysis by sphingomyelinases (SMase)4 with either an acidic (A-SMase) or neutral (N-SMase) pH optimum, or by de novo synthesis.5 In our laboratory, we have used neurons cultured from embryonic mice or rat hippocampus to study the role of ceramide (and other sphingolipids) in both neuronal development and in neuronal death. These neurons are cultured in such a way that axons and dendrites can be distinguished both morphologically and biochemically.6 Briefly, post-mitotic hippocampal neurons are plated on cover slips coated with poly-L-lysine. Cover slips can either be transferred to culture dishes containing a monolayer of astroglia,6 or transferred into a culture dish that contains B27 medium,7 allowing neuronal survival in the absence of a glial monolayer.8 Importantly, in both systems, cultures are maintained in serumfree medium. The growth of cultured hippocampal neurons has been classified into five distinct developmental stages.9 Stage 1 is characterized by a neuronal cell body exhibiting many lamellipodia. In stage 2, the lamellipodia are lost and a number of short processes, known as 'minor processes', are formed from the cell body. Within hours, one of the minor processes starts to grow rapidly and develops axonal characteristics; such neurons are 'stage 3'. Branches are formed from the axon as collaterals, and as each new branch emerges, the growth cone of the original axon loses its lamellipodial appearance and elongation stops. Dendrites develop from minor processes in stage 4. Finally in stage 5, synaptic contacts between axons and dendrites are created.
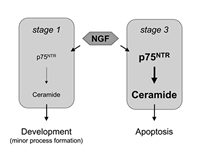
We originally demonstrated that ceramidegeneration could regulate neuronal development, since it enhanced the formation of minor processes from lamellipodia.3 Moreover, in stage 3 neurons, the glycosylated metabolite of ceramide, glucosylceramide (GlcCer), is required for normal10 and accelerated11 axonal growth. Intriguingly, at both stages high concentrations of ceramide induce apoptosis3. We have gone on to demonstrate that ceramide produced endogenously (rather than exogenously-applied short-acyl chain ceramides, or ceramide produced by exogenously added bacterial or human SMase) in response to the binding of neurotrophins to the p75NTR receptor8 can regulate the rate of minor process formation, or of apoptosis in hippocampal neurons. The decision whether to enter these diametrically opposed pathways depends on the expression status of neurotrophin receptors and, as a consequence, intracellular ceramide levels.12
Neurotrophins as Modulators of Neuronal Survival and Death
Neurotrophins were originally thought to be mainly involved in promoting neuronal differentiation and survival. Although this concept still remains true, recent work has demonstrated that neurotrophins can also directly induce neuronal apoptosis.2 In mammals, four neurotrophins are known, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). Neurotrophins are produced as precursor proteins, which are cleaved at dibasic amino acids to form mature forms of 118-120 amino acids. In their mature form they generally function as non-covalently associated homodimers, but at least some neurotrophin subunits are able to form heterodimers with other neurotrophins.13
All four neurotrophins interact with two receptor types, the shared p75NTR and three distinct receptor tyrosine kinases of the trk receptor family, trkA, trkB, and trkC. Neurotrophins directly bind and dimerize trk receptors, resulting in activation of the tyrosine kinase present in the cytoplasmic domain, which results in subsequent tyrosine phosphorylation of selective cellular substrates, such as phospholipase Cγ14 and phosphatidylinositol-3-kinase.15 Tyrosine kinase-mediated signaling by endogenous trk receptors promotes survival and/or differentiation in all neuronal populations examined to date.
The other neurotrophin receptor, p75NTR, is a distant member of the tumor necrosis factor (TNF) receptor family16 and was the first neurotrophin receptor identified. It comprises an extracellular region of four cysteine-rich repeats, all of which are required for ligand binding, a single transmembrane domain, and a cytoplasmic tail. No known catalytic motifs are present in the cytoplasmic tail but a region of approximately 80 amino acids near the C-terminus displays strong homology to the death domains of TNF receptors.17 Initially p75NTR was thought to be a lowaffinity receptor specific for NGF but later it has been shown to bind all neurotrophins with a very similar affinity. Recent studies have shown that unprocessed proneurotrophins represent a large amount of the secreted forms, and that proNGF binds to p75NTR with an affinity that is five-fold higher than the mature form; in contrast, trkA preferentially binds to the processed form of NGF.18
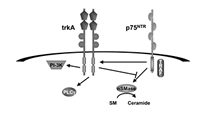
The first evidence (see Dobrowsky chapter) for the involvement of ceramide in the downstream signaling pathway elicited by NGF binding to p75NTR was obtained in T9 glioma cells, a cell line that does not express trkA. The latter is of particular importance since it has been suggested that the major function of p75NTR was to modify the activity of trk receptors; thus, ligand binding by p75NTR leads to an increase in high affinity trkA binding sites, enhanced trkA autophosphorylation in response to NGF, and increased selectivity of trkA for the neurotrophin.19 In T9 glioma cells, NGF binding led to an increase in intracellular ceramide levels,20 which resulted from binding of NGF to p75NTR since these cells do not express trkA receptors. In contrast, in PC12 cells that do express trkA and p75NTR, NGF did not induce ceramide generation,21 whereas BDNF, which binds p75NTR but not trkA, lead to increased ceramide levels, suggesting that trkA negates the signaling properties of p75NTR with respect to ceramide generation (Fig. 1). Although various p75NTR-interacting proteins have been identified, including members of the TRAF family,22 NRIF,23 NADE24 and NRAGE,25 their precise roles in the apoptotic signaling cascade has not yet been defined, and little is known about how these interact with the ceramide signaling pathway. Our data, discussed below, has addressed some of these issues by analyzing a number of the downstream interactors that are involved in NGFinduced ceramide generation via N-SMase, but not A-SMase, in hippocampal neurons that express p75NTR but not trkA.
Sphingomyelinases and their Regulation
The major form of SMase found in the brain is N-SMase, and we would like to speculate that N-SMase, rather than the A-SMase that is found at high levels in other tissues, is the SMase that is largely responsible for ceramide generation in the central nervous system during normal development, particularly that which is under control of trophic factors. This is based on our finding that A-SMase is not involved in signaling through the p75NTR in cultured hippocampal neurons,8 as neurons derived from wild type or A-SMase deficient26 mice are equally susceptible to NGF-induced cell death.8 In contrast, excitotoxic cell death is partially abolished in A-SMase deficient neurons,8,27 suggesting that different pathways of ceramide generation, via A-SMase or N-SMase, can exist in the same neuron, and are involved in ceramide generated from distinct ligandreceptor interactions. This would be consistent with many observations showing an important role for A-SMase in ceramide generation in response to apoptotic signals. For instance, in the case of the apoptotic pathway initiated by binding of TNF to the TNF receptor 1, A-SMase is activated by the TNF receptor death domainassociated proteins TRADD and FADD28, and in Kym-1 rhabdomyosarcoma cells, ceramide generated via A-SMase in response to TNF treatment occurs in the final stage of apoptosis.29
Unfortunately, progress in identifying and cloning N-SMase has lagged well behind that of A-SMase. Several putative N-SMase activities have been purified over the past few years, but a convincing case that any of them are the genuine N-SMase is still lacking. A neutral, membraneassociated, Mg2+-stimulated SMase sensitive to the inhibitor scyphostatin30 as well as to glutathione31 has been purified from bovine brain,32 but not cloned. Purification of N-SMase from rat liver plasma membrane has revealed a distinct enzyme not affected by glutathione,33 and six distinguishable activities were purified from heavy membrane fractions from bovine brain.34 The first cloned putative N-SMase35 was shown to reside in the endoplasmic reticulum and is most likely a phospholipase C specific for lyso-platelet activating factor.36,37 A second N-SMase was identified by expression cloning of a human kidney cDNA library38 and the product of a brain specific clone was shown to reside in the Golgi apparatus.39 Thus, to date identification of a genuine N-SMase remains elusive.
However, despite the lack of a genuine cloned N-SMase, there is little doubt that this enzyme is involved in ceramide generation in signaling pathways. Support for this has been obtained, amongst others, by studies showing that activation of N-SMase by TNF occurs via a distinct proteinprotein interaction, involving the cytoplasmic domain of the TNF receptor 1 called the NSD (neutral SMase activation domain), which binds to the adaptor protein FAN40 (factor associated with neutral SMase activation) (see also Chapter X/Levade). FAN, a member of the family of WD-repeat proteins, activates N-SMase by an unknown mechanism. Other potential players involved in N-SMase activation have been identified. Activation of phospholipase A2 and arachidonic acid accumulation precedes SM hydrolysis in response to TNF in HL60 cells;41 moreover, cells lacking phospholipase A2 are resistant to TNF-induced apoptosis and susceptibility to TNF can be restored by ectopic expression of phospholipase A2.42 A further level of regulation of N-SMase occurs by intracellular oxidation through the inhibition of N-SMase by reduced glutathione.31 Levels of oxidized glutathione are elevated in response to TNF in rat primary astrocytes, oligodendrocytes, microglia, and C6 glial cells.43 Thus, some up- and down-stream players in the N-SMase pathway are beginning to emerge.
Downstream Players in the Ceramide Response
Recent studies in our laboratory have identified two downstream players in the pathway initiated by binding of NGF to p75NTR and subsequent activation of N-SMase, namely deathassociated protein (DAP)-kinase44 and jun kinase,8 although it should be stressed that neither of these kinases are the initial proteins with which N-SMase interacts.
DAP-kinase is a Ca2+/calmodulin-regulated serine/threonine kinase mediating the apoptotic responses of several intra- and extracellular stress signals. It is a multi-domain protein containing a conserved death domain.17 The proapoptotic function of DAP-kinase requires the joint action of several domains and depends on autophosphorylation.45 In the developing and adult rat central nervous system, DAP-kinase mRNA is widely expressed in proliferative and post mitotic regions within the cerebral cortex, hippocampus and cerebellar Purkinje cells, from embryonic day 13 onwards.46 Expression in the brain is markedly decreased post-natally, but remains high in a number of areas, particularly the hippocampus.
In culture, hippocampal neurons derived from DAP-kinase-deficient mice are almost completely resistant to treatment with short-chain ceramide and to NGF.44 Interestingly, phosphorylation of serine 308, which is required for auto-inhibition of DAP-kinase, is rapidly reduced in response to ceramide.45 However, a C-terminal peptide of DAP-kinase distinct from the autophosphorylation site efficiently blocks apoptosis by either short-chain ceramide or treatment with bacterial Smase.44 Moreover, over-expression of a kinase-dead mutant of DAP-kinase in PC12 cells abolishes ceramide-induced apoptosis.47 In addition, protein levels of DAP-kinase increase early in response to ceramide in hippocampal neurons, suggesting a further level of regulation. Together, these data suggest that although DAP-kinase is a central player in ceramide-induced cell death, the pathway in which DAP-kinase is involved is not the only one via which ceramide can induce apoptosis in neurons, since ceramide can kill neurons after long times even in DAP kinase deficient mice,44 again indicating the variety of levels at which the response to ceramide can be regulated.
Ceramide generation is also up-stream to jun kinase in the signaling pathway elicited by NGF binding to p75NTR in hippocampal neurons. Previous studies48 had implicated jun kinase in neuronal death induced by neurotrophins, and we recently demonstrated that incubation of neurons with scyphostatin prior to incubation with NGF, blocked both neuronal cell death, and importantly, jun kinase activation.8 Dual roles have been suggested for jun kinase, in development and stress responses, with different jun kinase pools serving different functions.49 Our data show that ceramide generation is necessary for both NGF-induced neuronal cell death and jun kinase activation, suggesting that ceramide somehow regulates or modulates one of the interactors or kinases up-stream of jun kinase. The current state of knowledge regarding activation pathways to jun kinase50 does not allow the delineation of a precise mechanism by which ceramide might activate jun kinase. An intriguing option might involve modulation of the accessibility of p75NTR and interactor proteins to jun kinase via scaffolding proteins such as the juninteracting proteins51. Mediators that could provide an initiating link from p75NTR to jun kinase have not yet been determined, although a growing list of p75NTR interactors has emerged in recent years, as discussed above. It will obviously be of interest to examine the effects of manipulating ceramide levels on the interaction of these diverse proteins with p75NTR, and on the downstream cascades thus activated.
A number of other putative down-stream targets of ceramide have been identified in non-neuronal cells. By affinity chromatography, cathepsin D was identified as a ceramide-binding protein,52 and a number of protein phosphatases (see also Chapter X/Hannun) and protein kinases have also been identified. However, the mode of their activation or inhibition by ceramide has not been fully characterized, and none of them has been shown to unambiguously directly bind to ceramide.
A relatively well-studied example is protein phosphatase 2A, which is activated upon ceramide treatment.53 Protein phosphatase 2A inactivates protein kinase C (PKC)54 and protein kinase B/Akt55, as well as downstream targets of these kinases involved in apoptosis, such as bcl-2.56 The dephosphorylation of bcl-2 results in loss of its anti-apoptotic potency. Protein phosphatase 1 is also involved in the ceramide dependent dephosphorylation of the transcriptional regulator retinoblastoma gene product (Rb)57. In addition, classical and novel PKC isoforms are inhibited by ceramide, most probably via protein phosphatase 2A, whereas the activity of the atypical isoform PKCζ is stimulated;58 interestingly, PKCζ is involved in activation of jun kinases.59 In contrast, kinase suppressor of Ras (KSR) has been shown to be required for ceramideinduced activation of the mitogen activated protein kinase (MAPK) p42/p4460 (see also Chapter X/Kolesnick).
It is not possible to conclude a section on downstream targets of ceramide without a brief discussion of the role of mitochondria in this process, particularly as there is emerging evidence suggesting a direct role for ceramide in the biochemical events that occur in mitochondria during apoptosis. By way of example, the stimulation of the CD95 receptor upon binding of a CD95 ligand induces formation of a signaling complex,61 in which caspase-8 is activated, which subsequently cleaves procaspase-3 resulting in activation of the main executor caspase, caspase-3. In the majority of cell types, caspase-3, and thus apoptosis, is not directly activated but requires the activation of caspase-9 which involves the mitochondria;62 this is also the case in neurons.63 Jun kinase may provide a link between ceramide and mitochondria since active junkinase can induce cytochrome c release from mitochondria in a cell free assay.64 All mitochondrial activities in apoptosis can be blocked by overexpression of bcl-2 or bcl-xL.65 Thus, ceramide-induced cytochrome c release from isolated mitochondria can be prevented by preincubation with Bcl-2.66 Importantly, Bcl-2 expression is widespread in the developing central nervous system, remains high in the adult peripheral nervous system,67 and is up-regulated in response to ischemia. Moreover, bcl-2 overexpression can block apoptosis induced by neurotrophin withdrawal,68 suggesting that the mitochondria, as it does in other cells, will also play a key role in ceramide-induced apoptosis in the nervous system.
A Role for de Novo Ceramide Synthesis
To date, we have mainly discussed ceramide generated via the action of SMases. However, there is good evidence that ceramide synthesized de novo also plays an important role in apoptosis. Ceramide synthesis occurs on the cytosol leaflet of the endoplasmic reticulum69 and therefore has a distinct topology from ceramide generated via SMases, which is probably produced at the inner leaflet of the plasma membrane although this issue has still not been completely clarified.70 Nevertheless, inhibition of ceramide synthesis by fumonisin B1 often blocks apoptosis,71 suggesting that de novo ceramide synthesis also participates in apoptotic and developmental processes. Ceramide production by this route is induced by DNA-damaging agents5 and also by TNF,72 and shows the same apoptotic features as ceramide generated from SM. Likewise, in hippocampal neurons, NGF-induced cell death can be abolished by longterm (24 h) treatment with fumonisin B1 (unpublished data), although it is not clear if this effect is due to altering cellular levels of SM, or directly due to de novo ceramide synthesis. In contrast, the development of hippocampal neurons is not altered by treatment with fumonisin B1 within the first 24 to 48 h in culture,10,73 suggesting that sphingolipid synthesis is not required for minor process formation at stage 2, or the initiation of axonal outgrowth in early stage 3. Later in stage 3, fumonisin B1 abolishes axonal elongation and this effect is due to a reduction in the rate of synthesis of GlcCer,3,74 which is required for axonal growth, rather than accumulation of ceramide. Thus, an integrated view of the role of ceramide in regulating neuronal growth or death must take into account the possibility, at least over longtime periods, that ceramide is rapidly metabolized, and the effect of the metabolites needs to be carefully distinguished from that of ceramide per se.
Signaling Through Ceramide Metabolites
Notable amongst ceramide metabolites is sphingosine-1-phosphate, which is produced by the hydrolysis of ceramide to sphingosine and its subsequent phosphorylation by sphingosine kinase. Several ceramidases potentially responsible for the first step in this pathway have been identified, residing in lysosomes,75 plasma membrane76 and mitochondria77 (see chapter by Ito and chapter by Obeid). Sphingosine is known to cause apoptosis in several cell types, including hippocampal neurons.78 In contrast, sphingosine1phosphate, which is a paracrine as well as an intracellular survival factor, often works in direct contrast to ceramide.79 However, it induces cell death in hippocampal neurons80 and can cause their developmental arrest in stage 1 (unpublished results).
GlcCer can also directly affect neuronal development, and the development of other cell types, and its synthesis from ceramide can protect against the pro-apoptotic effects of ceramide. In cancer cells, ceramide glycosylation to GlcCer rescues them from ceramide-induced apoptosis and is associated with multidrug resistance81 (see also Chapter X/Cabot). In hippocampal neurons, GlcCer is required for normal axon growth during stage 33 and its synthesis is stimulated by laminin and basic fibroblast growth factor (bFGF).11 Treatment of neurons at this stage with either fumonisin B1 or D-erythro-1-phenyl-2-deaconoylamino-3-morpholino-1-propanol (D-PDMP) blocks axonal outgrowth.10 In contrast, the glucocerebrosidase inhibitor, conduritol-B-epoxide, stimulates axonal growth by enhancing either the rate of formation or stabilization of axonal branches. Axonal outgrowth is accompanied by a tremendous increase of membrane surface, and in this respect it is of interest that the activity of the ratelimiting enzyme of phosphatidylcholine biosynthesis, CTP:phosphocholine cytidylyltransferase (CCT), in brain and hippocampal neurons is directly activated by GlcCer.82 Thus, GlcCer may 'signal' via direct activation of CCT, whereas ceramide essentially has no effect on this enzyme.82
Conclusions
Ceramide is an important signaling molecule involved in the regulation of the development and the death of neurons. It can be generated via binding of NGF to p75NTR leading to activation of N-SMase. The level of ceramide generated can dictate whether development is stimulated or whether apoptosis is induced. The mechanism by which ceramide induces these disparate effects is not known (Fig. 2), but may involve activation of different downstream signaling pathways, such as DAP kinase and jun kinase. Alternatively, the possibility that a specific concentration of ceramide must be reached to allow formation of membrane rafts,83,84 and thus sequestering and activation of receptors in this membrane domain, is an attractive new hypothesis that deserves wide-spread attention. Although this aspect of ceramide signaling has not been discussed in this review, renewed interest in the biophysics of ceramide (see chapter by Holopanien), and the effect of its generation on properties of the lipid bilayer,70 are sure to add an additional layer of excitement to the study of ceramide signaling in neurons and in other cells types.
Acknowledgments
Christian Riebeling is supported by a Research Training Network fellowship from the European Union (RTN1-1999-00382). Work in the Futerman laboratory is currently supported by the Israel Science Foundation, the European Union (RTN1-1999-00382, and QLG3-CT-1999-573), the Buddy Taub Foundation, the National NiemannPick Foundation, and the Minerva Foundation, Munich, Germany.
References
- 1.
- Frade JM, RodriguezTebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. [PubMed: 8774880]
- 2.
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. [PubMed: 11048732]
- 3.
- Schwarz A, Futerman AH. Distinct roles for ceramide and glucosylceramide at different stages of neuronal growth. J Neurosci. 1997;17:2929–2938. [PMC free article: PMC6573634] [PubMed: 9096129]
- 4.
- Jarvis WD, Kolesnick RN, Fornari et al. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994;91:73–77. [PMC free article: PMC42888] [PubMed: 8278410]
- 5.
- Bose R, Verheij M, Haimovitz et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. [PubMed: 7634330]
- 6.
- Goslin K, Asmussen H, Banker G. Rat Hippocampal Neurons in Low Density In Culturing Nerve Cells (Banker G, Goslin K, eds.)1998Vol, pp.339–370., MIT press, Cambridge, Massachusetts.
- 7.
- Brewer GJ, Torricelli JR, Evege EK. et al. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. [PubMed: 8377226]
- 8.
- Brann AB, Tcherpakov M, Williams IM. et al. NGF-induced p75-mediated death of cultured hippocampal neurons is age dependent and transduced through ceramide generated by neutral sphingomyelinase. J Biol Chem. 2002;277:9812–9818. [PubMed: 11777929]
- 9.
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. [PMC free article: PMC6569279] [PubMed: 3282038]
- 10.
- Schwarz A, Rapaport E, Hirschberg K. et al. A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J Biol Chem. 1995;270:10990–10998. [PubMed: 7738041]
- 11.
- Boldin S, Futerman AH. Glucosylceramide synthesis is required for basic fibroblast growth factor and laminin to stimulate axonal growth. J Neurochem. 1997;68:882–885. [PubMed: 9003082]
- 12.
- Brann AB, Scott R, Neuberger Y. et al. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. [PMC free article: PMC6783007] [PubMed: 10493721]
- 13.
- Arakawa T, Haniu M, Narhi LO. et al. Formation of heterodimers from three neurotrophins, nerve growth factor, neurotrophin3, and brainderived neurotrophic factor. J Biol Chem. 1994;269:27833–27839. [PubMed: 7961712]
- 14.
- Blanquet PR. Identification of two persistently activated neurotrophinregulated pathways in rat hippocampus. Neuroscience. 2000;95:705–719. [PubMed: 10670437]
- 15.
- Qiu YH, Zhao X, Hayes RL, PerezPolo JR. et al. Activation of phosphatidylinositol 3kinase by brainderived neurotrophic factor gene transfection in septohippocampal cultures. J Neurosci Res. 1998;52:192–200. [PubMed: 9579409]
- 16.
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. [PubMed: 11239407]
- 17.
- Feinstein E, Kimchi A, Wallach D. et al. The death domain: a module shared by proteins with diverse cellular functions. Trends Biochem Sci. 1995;20:342–344. [PubMed: 7482697]
- 18.
- Lee R, Kermani P, Teng KK. et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. [PubMed: 11729324]
- 19.
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci U S A. 1993;90:7859–7863. [PMC free article: PMC47242] [PubMed: 8356095]
- 20.
- Dobrowsky RT, Werner MH, Castellino AM. et al. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. [PubMed: 8079174]
- 21.
- Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis. Modulation by co expression of p75NTR with Trk receptors. J Biol Chem. 1995;270:22135–22142. [PubMed: 7673191]
- 22.
- Ye X, Mehlen P, Rabizadeh S. et al. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem. 1999;274:30202–30208. [PubMed: 10514511]
- 23.
- Casademunt E, Carter BD, Benzel I. et al. The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. Embo J. 1999;18:6050–6061. [PMC free article: PMC1171670] [PubMed: 10545116]
- 24.
- Mukai J, Hachiya T, ShojiHoshino S. et al. NADE, a p75NTR-associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR. J Biol Chem. 2000;275:17566–17570. [PubMed: 10764727]
- 25.
- Salehi AH, Roux PP, Kubu CJ. et al. NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor-dependent apoptosis. Neuron. 2000;27:279–288. [PubMed: 10985348]
- 26.
- Horinouchi K, Erlich S, Perl DP. et al. Acid sphingomyelinase-deficient mice: a model of types A and B Niemann Pick disease. Nat Genet. 1995;10:288–293. [PubMed: 7670466]
- 27.
- Yu ZF, NikolovaKarakashian M, Zhou D. et al. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000; 15:85–97. [PubMed: 11220788]
- 28.
- Schwandner R, Wiegmann K, Bernardo K. et al. TNF receptor death domain-associated proteins TRADD and FADD signal activation of acid sphingomyelinase. J Biol Chem. 1998;273:5916–5922. [PubMed: 9488730]
- 29.
- Bourteele S, Hausser A, Döppler H. et al. Tumor necrosis factor induces ceramide oscillations and negatively controls sphingolipid synthases by caspases in apoptotic Kym1 cells. J Biol Chem. 1998;273:31245–31251. [PubMed: 9813032]
- 30.
- Tanaka M, Nara F, SuzukiKonagi K. et al. Structural Elucidation of Scyphostatin, an Inhibitor of Membrane Bound Neutral Sphingomyelinase. J Am Chem Soc. 1997;119:7871–7872.
- 31.
- Liu B, AndrieuAbadie N, Levade T. et al. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–11320. [PubMed: 9556624]
- 32.
- Bernardo K, Krut O, Wiegmann K. et al. Purification and characterization of a magnesium-dependent neutral sphingomyelinase from bovine brain. J Biol Chem. 2000;275:7641–7. [PubMed: 10713073]
- 33.
- Martin SF, Navarro F, Forthoffer N. et al. Neutral magnesium-dependent sphingomyelinase from liver plasma membrane: purification and inhibition by ubiquinol. J Bioenerg Biomembr. 2001;33:143–153. [PubMed: 11456220]
- 34.
- Jung SY, Suh JH, Park HJ. et al. Identification of multiple forms of membrane-associated neutral sphingomyelinase in bovine brain. J Neurochem. 2000;75:1004–1014. [PubMed: 10936181]
- 35.
- Tomiuk S, Hofmann K, Nix M. et al. Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc Natl Acad Sci U S A. 1998;95:3638–3643. [PMC free article: PMC19888] [PubMed: 9520418]
- 36.
- Sawai H, Domae N, Nagan N. et al. Function of the cloned putative neutral sphingomyelinase as lyso platelet activating factor-phospholipase C. J Biol Chem. 1999;274:38131–38139. [PubMed: 10608884]
- 37.
- Neuberger Y, Shogomori H, Levy Z. et al. A lysoplatelet activating factor phospholipase C, originally suggested to be a neutral sphingomyelinase, is located in the endoplasmic reticulum. FEBS Lett. 2000;469:44–46. [PubMed: 10708753]
- 38.
- Chatterjee S, Han H, Rollins S. et al. Molecular cloning, characterization, and expression of a novel human neutral sphingomyelinase. J Biol Chem. 1999;274:37407–37412. [PubMed: 10601312]
- 39.
- Hofmann K, Tomiuk S, Wolff G. et al. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97:5895–5900. [PMC free article: PMC18530] [PubMed: 10823942]
- 40.
- AdamKlages S, Adam D, Wiegmann K. et al. FAN, a novel WD-repeat protein, couples the p55 TNFreceptor to neutral sphingomyelinase. Cell. 1996;86:937–947. [PubMed: 8808629]
- 41.
- Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994;269:5757–5763. [PubMed: 8119915]
- 42.
- Jayadev S, Hayter HL, Andrieu N. et al. Phospholipase A2 is necessary for tumor necrosis factor alpha-induced ceramide generation in L929 cells. J Biol Chem. 1997;272:17196–17203. [PubMed: 9202042]
- 43.
- Singh I, Pahan K, Khan M. et al. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J Biol Chem. 1998;273:20354–20362. [PubMed: 9685387]
- 44.
- Pelled D, Raveh T, Riebeling C. et al. Death-associated protein (DAP) kinase plays a central role in ceramide induced apoptosis in cultured hippocampal neurons. J Biol Chem. 2002;277:1957–1961. [PubMed: 11709549]
- 45.
- Shohat G, SpivakKroizman T, Cohen O. et al. The proapoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem. 2001;276:47460–47467. [PubMed: 11579085]
- 46.
- Yamamoto M, Takahashi H, Nakamura T. et al. Developmental changes in distribution of death-associated protein kinase mRNAs. J Neurosci Res. 1999;58:674–683. [PubMed: 10561695]
- 47.
- Yamamoto M, Hioki T, Ishii T. et al. DAP kinase activity is critical for C(2)ceramide-induced apoptosis in PC12 cells. Eur J Biochem. 2002;269:139–147. [PubMed: 11784307]
- 48.
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. [PMC free article: PMC6772976] [PubMed: 10964939]
- 49.
- Bilderback TR, Grigsby RJ, Dobrowsky RT. Association of p75(NTR) with caveolin and localization of neurotrophin induced sphingomyelin hydrolysis to caveolae. J Biol Chem. 1997;272:10922–10927. [PubMed: 9099750]
- 50.
- Coffey ET, Hongisto V, Dickens M. et al. Dual roles for cJun Nterminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci. 2000;20:7602–13. [PMC free article: PMC6772887] [PubMed: 11027220]
- 51.
- Basu S, Kolesnick R. Stress signals for apoptosis: ceramide and cJun kinase. Oncogene. 1998;17:3277–85. [PubMed: 9916990]
- 52.
- Heinrich M, Wickel M, SchneiderBrachert W. et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. Embo J. 1999;18:5252–5263. [PMC free article: PMC1171596] [PubMed: 10508159]
- 53.
- Dobrowsky RT, Kamibayashi C, Mumby MC. et al. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed: 8393446]
- 54.
- Lee JY, Hannun YA, Obeid LM. Ceramide inactivates cellular protein kinase Calpha. J Biol Chem. 1996;271:13169–13174. [PubMed: 8662781]
- 55.
- Salinas M, LopezValdaliso R, Martin D. et al. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15:156–169. [PubMed: 10673324]
- 56.
- Ruvolo PP, Deng X, Ito T. et al. Ceramide induces Bcl-2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274:20296–20300. [PubMed: 10400650]
- 57.
- Dbaibo GS, Pushkareva MY, Jayadev S. et al. Retinoblastoma gene product as a downstream target for a ceramide dependent pathway of growth arrest. Proc Natl Acad Sci U S A. 1995;92:1347–1351. [PMC free article: PMC42516] [PubMed: 7877980]
- 58.
- Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. [PubMed: 10962008]
- 59.
- Wang YM, Seibenhener ML, Vandenplas ML. et al. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NFkappaB/JNK kinase and cell survival. J Neurosci Res. 1999;55:293–302. [PubMed: 10348660]
- 60.
- Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–969. [PubMed: 11221891]
- 61.
- Kischkel FC, Hellbardt S, Behrmann I. et al. Cytotoxicity-dependent APO1 (Fas/CD95)associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. [PMC free article: PMC394672] [PubMed: 8521815]
- 62.
- Scaffidi C, Fulda S, Srinivasan A. et al. Two CD95 (APO1/Fas) signaling pathways. Embo J. 1998;17:1675–1687. [PMC free article: PMC1170515] [PubMed: 9501089]
- 63.
- Krajewski S, Krajewska M, Ellerby LM. et al. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:5752–5757. [PMC free article: PMC21932] [PubMed: 10318956]
- 64.
- Aoki H, Kang PM, Hampe J. et al. Direct activation of mitochondrial apoptosis machinery by cJun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. [PubMed: 11786558]
- 65.
- Yang J, Liu X, Bhalla K. et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. [PubMed: 9027314]
- 66.
- Ghafourifar P, Klein SD, Schucht O. et al. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274:6080–6084. [PubMed: 10037689]
- 67.
- Merry DE, Veis DJ, Hickey WF. et al. Bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994;120:301–311. [PubMed: 8149910]
- 68.
- Garcia I, Martinou I, Tsujimoto Y. et al. Prevention of programmed cell death of sympathetic neurons by the Bcl-2 protooncogene. Science. 1992;258:302–304. [PubMed: 1411528]
- 69.
- Hirschberg K, Rodger J, Futerman AH. The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J. 1993;290:751–757. [PMC free article: PMC1132344] [PubMed: 8457204]
- 70.
- Venkataraman K, Futerman AH. Ceramide as a second messenger: sticky solutions to sticky problems. Trends Cell Biol. 2000;10:408–412. [PubMed: 10998592]
- 71.
- Herget T, Esdar C, Oehrlein SA. et al. Production of ceramides causes apoptosis during early neural differentiation in vitro. J Biol Chem. 2000;275:30344–30354. [PubMed: 10862608]
- 72.
- Xu J, Yeh CH, Chen S. et al. Involvement of de novo ceramide biosynthesis in tumor necrosis factor alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998;273:16521–16526. [PubMed: 9632721]
- 73.
- Harel R, Futerman AH. Inhibition of sphingolipid synthesis affects axonal outgrowth in cultured hippocampal neurons. J Biol Chem. 1993;268:14476–14481. [PubMed: 8314804]
- 74.
- Boldin SA, Futerman AH. Upregulation of glucosylceramide synthesis upon stimulation of axonal growth by basic fibroblast growth factor. Evidence for post translational modification of glucosylceramide synthase. J Biol Chem. 2000;275:9905–9909. [PubMed: 10744663]
- 75.
- Bernardo K, Hurwitz R, Zenk T. et al. Purification, characterization, and biosynthesis of human acid ceramidase. J Biol Chem. 1995;270:11098–11102. [PubMed: 7744740]
- 76.
- Mitsutake S, Tani M, Okino N. et al. Purification, characterization, molecular cloning, and subcellular distribution of neutral ceramidase of rat kidney. J Biol Chem. 2001;276:26249–26259. [PubMed: 11328816]
- 77.
- El Bawab S, Roddy P, Qian T. et al. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275:21508–21513. [PubMed: 10781606]
- 78.
- Mitoma J, Ito M, Furuya S. et al. Bipotential roles of ceramide in the growth of hippocampal neurons: promotion of cell survival and dendritic outgrowth in dose and developmental stage-dependent manners. J Neurosci Res. 1998;51:712–722. [PubMed: 9545085]
- 79.
- Cuvillier O, Pirianov G, Kleuser B. et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. [PubMed: 8657285]
- 80.
- Moore AN, Kampfl AW, Zhao X. et al. Sphingosine-1-phosphate induces apoptosis of cultured hippocampal neurons that requires protein phosphatases and activator protein-1 complexes. Neuroscience. 1999;94:405–415. [PubMed: 10579204]
- 81.
- Lavie Y, Cao H, Bursten SL. et al. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem. 1996;271:19530–19536. [PubMed: 8702646]
- 82.
- Bodennec J, Pelled D, Riebeling C. et al. Phosphatidylcholine synthesis is elevated in neuronal models of Gaucher disease due to direct activation of CTP phosphocholine cytidylyltransferase by glucosylceramide Faseb J 2002; submitted. [PubMed: 12223447]
- 83.
- Cremesti A, Paris F, Grassme H. et al. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–61. [PubMed: 11287428]
- 84.
- Grassme H, Jekle A, Riehle A. et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–96. [PubMed: 11279185]
- Ceramide in the Regulation of Neuronal Development: Two Faces of a Lipid - Madam...Ceramide in the Regulation of Neuronal Development: Two Faces of a Lipid - Madame Curie Bioscience Database
- Learning from Deficiency: Gene Targeting of Caspases - Madame Curie Bioscience D...Learning from Deficiency: Gene Targeting of Caspases - Madame Curie Bioscience Database
- Neuronal Survival and Cell Death Signaling Pathways - Madame Curie Bioscience Da...Neuronal Survival and Cell Death Signaling Pathways - Madame Curie Bioscience Database
- Gossypium hirsutum strain:TM-1Gossypium hirsutum strain:TM-1Gossypium hirsutum strain:TM-1 Transcriptome or Gene expressionBioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...