NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Slit was identified in Drosophila embryo as a gene involved in the patterning of larval cuticle.1 It was later shown that Slit is synthesized in the fly central nervous system by midline glia cells.2-5 Slit homologues have since been found in C. elegans6 and many vertebrate species, from amphibians,7 fishes,8 birds9-11 to mammals.7,12-14 A single slit was isolated in invertebrates, whereas there are three slit genes (slit1-slit3) in mammals, that have around 60% homology.12 All encodes large ECM glycoproteins of about 200 kDa15,16 (Fig. 1A), comprising, from their N terminus to their C terminus, a long stretch of four leucine rich repeats (LRR) connected by disulphide bonds, seven to nine EGF repeats, a domain, named ALPS (Agrin, Perlecan, Laminin, Slit) or laminin G-like module (see ref. 17), and a cystein knot (Fig. 1A). Alternative spliced transcripts have been reported for Drosophila Slit2, human Slit2 and Slit3,14 and Slit1.18,19 Moreover, two Slit1 isoforms exist in zebrafish as a consequence of gene duplication.20 Last, in mammals, two Slit2 isoforms can be purified from brain extracts, a long 200 kDa one15,16 and a shorter 150 kDa form (Slit2-N) that was shown to result from the proteolytic processing of full-length Slit2.21 Human Slit1 and Slit3 and Drosophila Slit are also cleaved by an unknown protease in a large N-terminal fragment and a shorter C-terminal fragment, suggesting conserved mechanisms for Slit cleavage across species.12,21-23 Moreover, Slit fragments have different cell association characteristics in cell culture suggesting that they may also have different extents of diffusion, different binding properties, and, hence, different functional activities in vivo. This conclusion is supported by in vitro data showing that full-length Slit2 functions as an antagonist of Slit2-N in the DRG branching assay, and that Slit2-N, not full-length Slit2, causes collapse of OB growth cones.24 In addition, Slit1-N and full-length Slit1 can induce branching of cortical neurons (see below), but only full-length Slit1 repels cortical axons.23
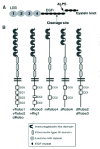
Figure 1
A) Structure of the slit proteins. B) Diagrammatic comparison of the structure of Robo receptors in invertebrate and vertebrate species. v: vertebrate; d: Drosophila; z: zebrafish. ALPS: domain found in Agrin, Laminin, Perlecan and Slit.
Structure-function analysis in vertebrates and Drosophila demonstrated that the LRRs of Slits are required and sufficient to mediate their repulsive activities in neurons.24-26 More recent detailed structure function analysis of the LRR domains of Drosophila Slit,27 revealed that the active site of Slit (at least regarding its pro-angiogenic activity) is located on the second of the fourth LRR (LRR2), which is highly conserved between Slits. Slit can also dimerize through the LRR4 domain and the cystein knot.18 However, a Slit1 spliced-variant that lacks the cystein knot and does not dimerize is still able to repel OB axons.18
Introduction
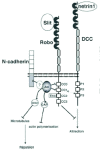
The first roundabout gene, robo, was identified in Drosophila during a comprehensive screen for genes regulating midline crossing in the CNS.28 If SAX-3 is the unique robo ortholog in C.elegans,6 three robo genes have been found in Drosophila,5,29,30 zebrafish,31,32 chick9 (Chedotal unpublished data) and mammals.7,12,33 Robo proteins belong to the immunoglobulin (Ig) superfamily and have five Ig-like domains followed by three fibronectin type III (FNIII) repeats, a transmembrane portion and a long intracellular tail containing up to four conserved cytoplasmic motifs, CC0-CC3, with no obvious catalytic domains (Fig. 1B). The first two Ig domains are the most highly conserved portion and are also found in another protein called Robo4 or magic roundabout that is only expressed by endothelial cells and plays a role in angiogenesis.34,35 However, Robo4 lacks the last three Ig domains, some FNIII domains and the CC1 and CC3 motifs found in other Robo proteins. Moreover, its capacity to bind Slits is still debated.36,37
CC0 has no known function but is a site of tyrosine phosphorylation.38 CC1 also contains tyrosine residues that can be phosphorylated and was shown to bind to the P3 domain of the netrin-1 receptor, deleted in colorectal cancer (DCC; see below).
CC2 is a proline-rich sequence that matches the consensus binding site for Drosophila Enabled (Ena; see below), CC3 is also a polyproline stretch.29 Drosophila Robo2 and Robo3 lack the CC2 and CC3 domains5,30 and the second half of CC2 is also not conserved in mouse and zebrafish Robo3. Furthermore, mouse Robo3/Rig-1 (Rig-1 for retinoblastoma inhibiting gene 139) lacks the CC1 motif33,39 but zebrafish Robo3 has it.32 In addition, some spliced variants of mouse Robo3/Rig-1, including a secreted form, may exist.39 Last, Robo 1 can be cleaved in transfected cells.26
In Drosophila, genetic and biochemical evidence demonstrated that Slit is a ligand of the Robo1-Robo3 receptors.4,5,29,30,40 Likewise, mammalian Slits can bind to all Robo receptors with comparable affinity.7,12,41 Slit cleavage fragments appear to have different cell association characteristics, with the smaller C-terminal fragment being more diffusible and the larger N-terminal and full length fragments being more tightly cell-associated.21 In addition, the C-fragment does not bind to Robo.24 More recent studies have shown that in Drosophila all three Robo receptors compete for a single active binding site in the second LRR of Slit27 and that neither the FNIII domains nor Robo dimerization are required for Slit binding. The major Robo1-3 binding site of Slit is in the second of the four LRRs, is evolutionary conserved and has a similar affinity for all Robos. However, Slit affinity is higher when all LRRs are present, probably due to its dimerization. On the receptor side, several results suggest that the first two Ig domains of Robos are required for Slit binding. First, the genetic deletion of Ig1 and Ig2 results in abnormal lung development.42 Second, antibodies against Robo Ig1 inhibit tumor growth in mice43 and neurite outgrowth in vitro.44 Third, Robo1 Ig1-2 are important for Slit binding and function in vitro.45
Several studies suggest that Slit can bind to other proteins than Robo, in particular heparan sulfate glycosaminoglycans that are negatively charged carbohydrates found on the cell surface. Slit1 and Slit2 were shown to bind to heparin column21,46 and to Glypican-1,46 a glycosyl phosphatidyl inositol (GPI)-anchored heparan sulfate proteoglycan known to interact with positively charged molecules. Biochemical data suggest that Slit binds to glypican-1 through its C-terminus.47 Moreover, heparinase III treatment reduces Slit2 activity and binding to Robo1.48 In Drosophila, expression of the transmembrane heparan sulfate proteoglycan syndecan in target cells appears to be required for Slit signaling.49 There is also genetic evidence in mouse supporting interaction between Slit and heparan sulfates in vivo.50 Heparan sulfates could help stabilizing the Robo/Slit complex or function as coreceptors presenting Slits to Robos or to alternative receptors.
Robo Partners
The analysis of Frazzled-Robo chimeric proteins in Drosophila, first revealed that the cytoplasmic domain of Robo is required to control the lateral positioning of post-crossing axons.51 Genetic and biochemical studies have then led to the identification of a number of transmembrane and cytoplasmic proteins that may participate or modulate Slit signaling through Robo receptors (Fig. 2). However, only a few of these proteins have been shown to directly participate to Robo signaling upon binding Robo CC domains.
Abelson Tyrosine Kinase
In Drosophila, Robo was found to be a substrate for the cytoplasmic tyrosine kinase Abelson (Abl). Abl is able to phosphorylate Robo's CC0 and CC1 leading to Robo inactivation.38 In the Drosophila visual system, Abl was also found to interact with Robo2 and Robo3. An Abl substrate, the actin binding protein Enabled (Ena), is also involved in Robo repulsion in Drosophila38 and C. elegans.52 Ena was shown to bind Robo's CC2 motif, therefore participating to Robo signaling. However, other studies showed that Abl rather than inactivating Robo, could promote repulsion downstream of Robo.53 The adenylyl cyclase associated proteins (CAP) regulate actin polymerization and bind to the SH3 domain of Abl. Interestingly, axon guidance defects at the midline were observed in the Drosophila CAP homolog capulet (capt), or in capt-slit or capt-robo-robo2 transheterozygotes. In this system, Abl and Capt are recruited by Robo activation and inhibit actin polymerization, therefore acting positively in the Slit pathway. Thus, Abl may play both positive and negative roles in Slit signaling.54 Slit binding to Robo was also shown to inactivate the cell adhesion molecule N-cadherin that mediates homophilic binding. Interestingly, Abl is required for Robo binding to N-cadherin. Robo is thought to inhibit N-cadherin function by interfering with its cytoplasmic domain and by inducing a decrease in N-cadherin-mediated adhesion. Slit binding to Robo increases the phosphorylation of β-catenin and thus its ability to bind to N-cadherin. This induces the binding of Robo to N-cadherin resulting in its inhibition.55
Abl function downstream of Robo may involve microtubule associated proteins (MAP) in addition to actin binding proteins.56 The MAP Orbit/MAST, ortholog of the vertebrate cytoplasmic linker protein (CLIP)-associated proteins (CLASP) are microtubule-associated plus end tracking proteins that seem to reduce microtubule stability. Genetic evidence supports a role for Orbit/MAST downstream of Abl in the Slit repellent pathway.
Although all these studies suggest that Abl plays a pivotal role in mediating Robo signaling in Drosophila, it remains to determine if Abl function is conserved in vertebrates.
Rho Family of Small GTPases
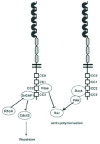
Rho family of small GTP-binding proteins (Rho GTPases) are major modulators of the actin cytoskeleton and play a central role in axonal growth and cell migration.57 Rho GTPases are activated upon GTP binding and inactive when bound to GDP. The switch from their active to their inactive state is controlled by two families of proteins: the guanine nucleotide exchange factors (GEFs) and the GTPase activating proteins (GAPs). GEFs activate Rho GTPAses while GAPs inactivate them by inducing GTP hydrolysis.57
Many studies have shown that Rho GTPases play an important role in the modulation of Slit function (Fig. 3). First, activation of RhoA in Drosophila causes axons to cross the midline.58 Second, In this system, Rac1 inactivation or Cdc42 activation can overcome the effect of constitutively active Robo suggesting that Cdc42 and Rac1 function downstream of Robo.59 Likewise, in vertebrate neurons, a constitutively active Cdc42 blocks the repulsive effect of Slit.60 Biochemical studies have shown that the SH3-SH2 adaptator protein Dock, can bind directly to Robo and that this interaction requires the SH3 domain of Dock and the CC2 and CC3 motifs of Robo.61,62 Dock is known to interact with key regulators of the actin cytoskeleton such as p21-activated protein kinase (Pak), a serine-threonine kinase which in turn can interact with RhoGTPases such as Rac1 and Cdc42. Slit binding to Robo increases the level of Dock-Robo association, recruits Pak and stimulates Rac (in particular Rac1). Thus Slit regulates the assembly of a multiproteic complex composed of Dock, Pak and Rac that couples Robo receptor activation to the regulation of the actin cytoskeleton.
Robo also controls the activity of Rho GTPases through a family of Slit/Robo specific GAPs, SrGAP1-3, that were identified using yeast two hybrid and Robo1 CC3 domain as a bait. SrGAPs consist of a RhoGAP domain, a SH3 domain and a Fes/CIP4 (FCH) homology domain. SrGAP1 and srGAP3 bind to the CC3 domain of Robo1 through their SH3 domain. Slit2 increases Robo1 binding to srGAP1 and its activity and this regulation requires CC3. In turn, SrGAP1 inactivates Cdc42 and activates RhoA but not Rac1.60 Contrary to srGAP1, srGAP3 is mainly a repressor of Rac1.63 Recently, it was also shown that in Drosophila, Slit/ Robo signaling could also control Rac activity upon binding the GAP Vilse/CrossGAP, that is conserved in vertebrates.64,65 Genetic evidence showed that CrossGAP may be involved in Robo-dependent axonal repulsion and tracheal cell migration and that it specifically inactivates Rac. Moreover, CrossGAP WW domains can directly bind to the CC2 domain of Robo and thus may not function downstream of other Robo receptors that lack the CC2 domain.64 Moreover, the two proteins can be coimmunoprecipitated from brain extracts.65 Although it inactivates Rac, Vilse seems to have a positive role in Robo repulsion.64
The Netrin Receptor DCC
Deleted in colorectal cancer (DCC) is a transmembrane receptor for the secreted protein netrin-1 (see Kennedy and colleagues, this issue). Robo1 can bind DCC and this results in the inhibition of netrin-1 attraction.66 This silencing activity requires the binding of the CC1 domain of Robo 1 and to the P3 domain of DCC. The C. elegans homolog of DCC, UNC-40, can also bind SAX-3.52 Interestingly, Slit also binds netrin-1,12 but the functional consequence of this interaction is unknown.
Other Modulators of Slit/Robo Function
ECM molecules, specially laminin-1 have been shown to influence the response of retinal axons to netrin-1.67 Thus, exposure of Xenopus retinal axons to laminin converts their response to netrin-1 from attraction to repulsion, apparently by lowering cAMP levels in the growth cones.67 The level of cyclic nucleotides in the growth cone in part determines the action of many guidance cues.68 It was found that Slit2-N growth-promoting action could be converted into an inhibition by lowering cGMP levels. Along this line, the activation of CXCR4 by the chemokine SDF-1 reduces the repulsive activity of Slit2 on retinal axons indirectly, by stimulating PKA69 and increasing cAMP levels. Other second messengers such as calcium may also participate to Slit signaling.70 Interestingly, as for netrin-1, a laminin-1 peptide was able to convert Slit2-N activity and this may also involve integrins.71 Accordingly, there is genetic evidence in flies for a regulation of Slit action by integrins.62
Last, there is also genetic evidence suggesting that receptor-linked tyrosine phosphatases,72 Calmodulin and the Ras/Rho GEF Son of Sevenless (SOS) critical for Ras activation73 may participate to Slit/Robo signaling. Both Sos and CaM signaling pathways are required to prevent certain axons from crossing the midline. However, the link with the transduction of Robo signaling is unclear and these proteins may just function in parallel pathways.
Molecular Control of Slit and Robo Expression
Transcriptional Regulation
In Drosophila, Slit function is controlled by the BTB transcription factor Lola.74 In addition, several transcription factors were shown to control Slit expression in fly embryo75 such as the PAS bHLH single minded.76 Slit promoter region also contains binding sites for the SOX HMG domain protein Fish-Hook and the POU domain protein Drifter.75 Likewise, in the chick retina optic layer, the Irx homeobox gene family member, Irx4, negatively controls Slit1 expression.77 Last, Islet-2, a LIM/homeodomain-type transcription factor of the Islet-1 family was also proposed to control in zebrafish sensory neurons the expression of some factors important for Slit signaling.78 One of these factors may be the semaphorin receptor plexin-A4.79
Similarly, Robos could be subject to transcriptional regulation.74,80 For instance, in fly embryo, Robo2 expression in the mesoderm is likely to be controlled by homeotic genes such as homothorax81 and there is a hox binding site in the robo2 gene.
Post Transcriptional and Post-Translational Regulation Commissureless
In Drosophila and rodents, Robo expression is regionally restricted29,41,82 to longitudinal axons and absent from commissures. In fly, this localization of Robo to the post-crossing segment of commissural axons is controlled by the transmembrane protein commissureless (Comm).82,83
Comm was initially proposed to be expressed and required at the midline for appropriate midline crossing and to triggers Robo internalization.84 However, more recent studies have shown that Comm is expressed by commissural axons and acts autonomously in commissural neurons.85 Moreover, there is no need for Comm at the midline for restoring midline crossing in comm mutants.86 Comm protein appears to be prevented from reaching the contralateral portion of commissural axons and accumulates at the midline. In turn, Comm prevents thedelivery of Robo at the growth cone, by recruiting it to late endosomes.86 It is still unclear why Robo is present on the post-crossing segment.
Comm is a predicted transmembrane protein of 370 amino acids with no known domains. Structure-function analysis revealed that the N-terminal and transmembrane domains of Comm are required to downregulate Robo.87 The intracellular portion of Comm is also essential for its function and contains an endosomal sorting domain that is required, together with the membrane proximal region of comm (108-131) to relocalize Robo in transfected cells.86 It also includes a binding site for the ubiquitine ligase dNedd4 and interaction with Nedd4 is required for Comm to localize within vesicles in transfected S2 cells.88 In yeast two hybrid, Nedd4 was shown to bind Robo.88 However, more recent studies have shown that Comm ubiquitination is not required for its function and that Nedd4 does not influence midline guidance in vivo.86 Robo2 and Robo3 expression can also be negatively regulated by Comm when the protein is overexpressed but this probably does not occur in vivo.4,5,30 In normal condition, the restricted expression of Robo2 and Robo3 expression may be controlled by other Comm proteins, but may also be transcriptionally regulated.5
Despite its major role in Drosophila, so far no commissureless homolog has been found in vertebrates suggesting the existence of additional regulatory mechanisms and modulators one of which could be Robo3/Rig1.
In the mouse spinal cord, commissural axons become responsive to midline repellents, including Slit2, after crossing.89 Moreover, in mouse spinal cord, Robo1 and Robo2 expression is upregulated after crossing.90 This suggests that the expression and function of vertebrate Robo is also precisely controlled at the midline. Surprisingly, this regulation seems to involve the receptor Robo3/Rig1. Rig1 expression overlaps with Robo1 in dorsal spinal cord41 and is downregulated in post-crossing axons and neurons.41,91 In addition, axons from Rig1 knockout exhibit a premature response to Slit. In the spinal cord and hindbrain,91 Rig1 seems to function as an inhibitor of Slit signaling in precrossing axons. Accordingly, there is a significant rescue of midline crossing by commissural axons in rig1/slit2 and rig1/robo1 double mutants and rig1/slit1/slit2 triple mutants.41,90 Rig1 exact function is unknown. It may sequester Slit, or interfere with Robo1 signaling, but there is still no evidence for direct Robo/Rig1 interaction.41
Other Regulators
As mentioned above, all Slits, and possibly some Robo receptors can be proteolytically processed into shorter fragments. The enzymes regulating the cleavage of these proteins are unknown, although there is some evidence92 for a role of the metalloprotease of the ADAM family kuzbanian in Drosophila. There is also some data supporting a postranscriptional modulation of Slit function by the Arf6-GEF, Schizo, through a regulation of endocytosis or membrane dynamics.93
Multiple Functions for Slit/Robo in the Nervous System
Slits play a major role in axon guidance in many systems and animal species. In most cases Slits act as repellents but there is some evidence that they may act positively on some axons.71,77
Midline Crossing
Slit and Robo are primarily known for their function in regulating midline crossing in the nervous system. In Drosophila robo mutants, many axons abnormally cross the CNS midline and some multiple times.29 In Drosophila, Robo also controls midline crossing in the olfactory system.94 In the CNS of slit mutant, axons converge to the midline and remains there. Slit was later shown to be a repellent for noncrossing axons and for commissural axons once they have crossed the midline. Biochemical and genetic studies showed that Slit is produced by midline glia cells and that its binding to Robo triggers axonal repulsion. The different midline phenotype between robo and slit mutants suggested that additional Slit receptors may be present on commissural axons. Accordingly, Robo2 was shown to act redundantly with Robo to control midline crossing in Drosophila.4,5 However, each receptor has a unique role and their function in controlling midline crossing is only partially redundant. In contrast, Robo3 does not seem to play a role in midline crossing in fly.5
Interestingly, this essential function of Slit/Robo at the CNS midline is evolutionary conserved from C. elegans to humans.33 In all these species, Slits are expressed at or near the midline, such as the floor plate and septum in vertebrates, or are expressed around decussating axons, canalizing them as they approach the midline. Thus, in vertebrates, Slit/Robo were shown to govern midline crossing by retinal axons95,96 commissural axons in the spinal cord,41,90 olfactory bulb axons,97 cortical axons,98,99 precerebellar axons.91 They were also shown to control midline crossing by migrating neurons in the hindbrain.91
In the vertebrate visual system both ipsilaterally and controlaterally projecting axons respond to Slits and in their absence, pathfinding errors are observed prior to crossing. In this system, Slit expressing cells surround retinal axons, channeling the axons before and after the chiasm up to the diencephalon.96,100 The same occurs in the neocortex where Slit2 expression in the glial wedge and induseum griseum prevent callosal axons from entering the septum.98,99 In vertebrates, the function of the three slit genes, that are often totally or partially coexpressed101 appears largely redundant. Thus, axonal tracts are only slightly perturbed in mice deficient for a single slit gene and sometimes for two slit genes.90,95,97,98 This redundancy may explain why some major commissures such as the anterior commissure and the hippocampal commissure are normal in mice deficient for both Slit1 and Slit2. Accordingly, it is only in the spinal cord of triple Slit1/2/3 knockouts90 that many commissural axons stay at the midline and recross it, a phenotype reminiscent of the Drosophila slit mutant. The organization of the brain of slit1/slit2/slit3 triple knockouts will have to be fully studied to determine if Slit/Robo controls the development of all commissural tracts in vertebrates.
Projection Map Formation
In many systems, in particular those conveying sensory informations, axonal projections are topographically ordered in the target territory. Slit and Robo seem to play an important role in regulating axonal targeting in vertebrates and invertebrates. Thus, in the Drosophila visual system, Slit and Robo control the segregation of lamina cells (that express Slit) and lobula cells (that express all Robo receptors) by preventing cell mixing.102 Likewise, in the visual system of zebrafish, Robo2 (Astray) in addition to control axon guidance at the chiasm regulates pathfinding within the tectum.96,100
In the Drosophila olfactory system, distinct subtypes of olfactory axons express various combinations of Robo receptors and Robo controls axonal positioning in the olfactory lobes.94 In rodents, the projection from the vomeronasal organ (VNO) to the accessory olfactory bulb (AOB) is topographically organized. Neurons in the apical part of the VNO send axons to glomeruli in the anterior half of the AOB and VNO neurons in the basal part project to the posterior AOB. All VNO axons were shown to express robo1 mRNA during development, while robo2 is present only in basal ones.101,103 Slit1 and Slit3 are also expressed in the VNO (preferentially in the apical part ) and the anterior AOB and VNO axons are repelled by Slit in collagen gel.101,103,104 The important role of Slit1 in VNO axon targeting was recently confirmed in vivo using slit1-deficient mice.104 In zebrafish, Robo2 controls the development of olfactory projections from to the olfactory bulb, in particular the establishment of the glomerular map.105
Branching
In vertebrate, Slit2 was originally purified as a factor able to stimulate the formation of axon collateral branches by NGF-responsive neurons of the dorsal root ganglia (DRG).21 It was also shown that only the N-terminal fragment of Slit2, but not the full length protein is capable of stimulating DRG elongation and branching.21,24 Moreover, full-length Slit2 can antagonize the effect of Slit2-N.24 Slit2 also controls the branching/arborization of central trigeminal sensory axons in the brainstem of rodents106 and in zebrafish.78 In this later case, the branching activity of Slit2 is modulated by the semaphorin receptor plexin-A4.79 Last, although DRG express Robo2,21 and trigeminal axons express both Robo1 and Robo2, the axonal receptor mediating Slit branching activity is unknown.
Interestingly, Slit/Robo not only influence axonal branching but also dendritic branching. First, in Drosophila, the directionality of dendritic outgrowth at the midline is controlled cell-autonomously by Robo.107 In robo mutant, dendrites of motor neurons grow abnormally toward the midline while no phenotype was observed in robo2 and robo3 mutants. Likewise, Slit1 has a dual activity on rat cortical neurons,108 as it repels their axons but induces dendritic growth and branching. This effect appears to involve Robo signaling.
Longitudinal Tract Formation
In Drosophila, Robo, Robo2 and Robo3 are expressed in overlapping domains within longitudinal tracts of the CNS, and this combination of Robo receptors is thought to control the lateral position of longitudinal axons. Thus, genetic alterations of the Robo code displace longitudinal axons along the mediolateral axis. However, it is not known if these changes involves Slit signaling. As Robos are immunoglobulins and able to mediate homophilic and heterophilic binding, it is possible that the control of lateral positioning by Robo involves Robo-Robo interactions and selective axonal fasciculation.
In mouse, there is also some data supporting a differential expression of Robo receptors by longitudinal axons that project at distinct ventro-dorsal position in the spinal cord.41
Control of Cell Differentiation
In fly, serotoninergic neurons are bilaterally organized and must cross the midline to achieve their differentiation. Robo2 and Robo3 were shown to regulate the expression of the serotonin transporter (SerT) as many serotoninergic neurons fail to express SerT in robo2 and robo3 mutants. Moreover, Robo2 and Robo3 are required for eagle expression, a transcription factor controlling serotoninergic differentiation.109 Interestingly, SerT activity is normal in slit mutants suggesting that Robo2/3 function in serotoninergic differentiation is Slit independent. In Drosophila, Slit also promotes the terminal asymmetric division of ganglion mother cells by regulating the asymmetric distribution of Inscuteable and by downregulating the expression of POU genes.110 In vertebrates,111 Robo1 may also control cell differentiation as its overexpression in Xenopus leads to ectopic neuronal differentiation. Last, during kidney development in mouse, slit2 and robo2 inactivation leads to supernumerary ureteric buds, possibly through postranscriptional effect on other developmental genes.112
Cell Migration
Another important function for Slits and Robos is the control of cell migration in the nervous system (both neurons and glia) and in several other tissues. As for axons, Slits were found to be important regulators of the behavior of migrating cells at the midline. But in contrast with axons, migrating cells can either be attracted or repelled by Slits.
In Drosophila, longitudinal glia is generated from glioblasts that migrate ventrally to contact pioneer neurons at a distance from the midline. These cells express Robo1 and in robo mutant, glial cells migrate over the midline113 suggesting that Slit is repulsive. Likewise, Muscle precursors in Drosophila embryos114 fail to migrate away from the midline in slit mutant. In this system also, Slit produced by midline glia acts as a repellent. However, at later stages Slit expressed at muscle attachment sites attracts muscle precursors that express both Robo and Robo2. Interestingly, Comm also cooperates with Robo and Robo2 to control muscle precursors migration.30 The mechanism responsible for the switch from repulsion to attraction is still unknown but may involve signaling through different Robo receptors as suggested in other systems. Hence, Robo2 was proposed to mediate the long-range attraction of tracheal cell into the CNS115 while Robo may mediate a repulsive action of Slit on tracheal cells. This different activity of the two receptors may rely on differences in their cytoplasmic domains. In C. elegans, Slit was also shown to be positive regulator of neuronal migration C.elegans along the anterior posterior axis.6
In vertebrates, Slits and Robo participate to the migration of many neurons in the CNS and PNS but so far there is only evidence for a repulsive activity. Moreover, whereas Slit and Robo were shown to guide tangentially migrating neurons, they do not seem to participate to radial migration. During development and throughout adulthood, several types of olfactory bulb (OB) interneurons (the granule cells and the tufted cells) are generated from progenitors located in the so-called subventricular zone (SVZ) that surrounds the lateral ventricles116 and migrate to the OB via the rostral migratory stream (RMS). The rostral migration of SVZ-derived neuroblasts was shown to involve chemorepellents secreted by the septum.117-119 Biochemical and in vitro studies have since demonstrated that Slit1 and Slit2 are mediating this repulsive activity.15 Accordingly, some SVZ-derived neuroblasts showed abnormal migration pattern in slit1 deficient-mice.119 However, those cells were also shown to express Slit1119 that may act cell autonomously. Migrating OB neuroblasts express robo2 and robo3 mRNAs101 and srGAP1.60 Although dominant-negative srGAP1 blocks Slit repulsion of SVZ cells,60 the contribution of Robo signaling in this system is largely unknown. Elegant in vitro assay also showed that Slit repels migrating svz cells without blocking their migration. Thus, Slit may just control the directionality of the migration without affecting cell motility,120 although this issue is still controversial.121 In chick embryo, Slit2 repels the migration of trunkal but not vagal neural crest cells122 that take different migratory pathways during development. As observed in the SVZ system, Slit2 appears to enhance cell motility: neural crest cells migrate further in the presence of soluble Slit2.
In the telencephalon, Slit1 repels in vitro the migration of GABAergic interneurons from the ganglionic eminence.123 However, the phenotypic analysis of slit1/slit2 deficient mice revealed that they are not necessary for tangential migration of GABAergic interneurons to the cortex in vivo.124 On the other hand, this study revealed that Slits influence the migration of cholinergic neurons of the basal magnocellular complex.
In the hindbrain, Slit and Robo participate to the migration of rhombic lip derivatives both in chick and rodents.11,70,91 Although Slits primarily act as repellents for rhombic lip-derived cells, they were also proposed to antagonize the attractive activity of floor plate-derived netrin-1.125
Last, Slits and Robo also influence the migration of other vertebrate cells; either negatively as shown for leukocytes,126 but sometimes positively as shown for endothelial cells27,127 In this later case, Slit attractive activity involves Robo1 signaling.
A Role for Slit and Robo in Neurological Disorders?
There is relatively little direct evidence so far indicating that Slit and Robo may be involved in pathological processes except in cancers.128 However, several patients suffering from a rare congenital syndrome named Horizontal gaze palsy with progressive scoliosis and hindbrain dysplasia (HGPPS) were recently shown to bear mutations in the ROBO3 gene. In these patients, the pyramidal tract and the dorsal column-medial lemniscus are uncrossed.33 Moreover, there is a reduced pontine nucleus and abducens nuclei. As shown in robo3 knockout mice the pontine nucleus defect is probably caused by an abnormal migration during development.91 It is still unknown if the other human brain defects are also present in mice lacking Robo3 and as those die at birth, behavioral analysis are not possible.
One of the Slit/Robo GAP, SrGAP3 has a putative role in idiopathic mental retardation63 as it is mutated in patients with X chromosome-linked MR. However, the cellular basis for these defects and the normal function of SrGAP3 in the CNS are unknown.
The incapacity of adult axons to regenerate in the CNS of mammals is known to rely for a large extent on the existence of inhibitors of axonal growth expressed either in the glial scar or in myelin. Several studies have shown that repulsive axon guidance molecules may be responsible for some of the inhibition.129 Slit an Robo expression after injury has not been extensively studied so far. However, in a model of cryoinjury, Slit2 was found to be expressed in reactive astrocytes together with glypican-1.130 In addition, adult DRG neurons also express mRNAs for Robo2 and Slit1 but their expression does not change after sciatic nerve transection or dorsal column lesion in the spinal cord.131 In contrast, Glypican-1 and SrGAP2 expression are upregulated after such lesions.131,132 Thus, it is still unclear if Slit and Robo play any role in preventing axonal regeneration.
Perspectives
There is mounting evidence that Slits regulate a large range of biological functions, from axon guidance, neuronal migration, immune response133 to cell differentiation most likely through Robo signaling. However there are still many open questions.
First, could Robo functions independently of Slit in particular through Robo/Robo interaction and what are the signaling pathways involved. Thus, in the Drosophila PNS, Robo2 expressed on visceral mesoderm binds Slit and present it to Robo expressing chordotonal sensory neurons. This may also involve Robo-Robo2 direct interaction.81,134 In vitro experiments also showed that the growth of retinal and olfactory Robo expressing axons is stimulated on Robo expressing cells, suggesting that Robo might work as cell-adhesion molecule to regulate outgrowth.44 In Drosophila, Robo and Robo2 can dimerize in vitro40 and ectopic expression of low level of Robo2 causes a Robo-like phenotype suggesting that Robo2 could interfere with Robo function.5
The function of Slits and Robos to in the normal adult brain and in pathological condition also remains to be clarified. Many data support a role for these molecules in tumorigenesis, in particular in gliomas128 but this needs to be further demonstrated. As all Slits and Robos are expressed in adult neurons it is likely that they modulate synaptic transmission as shown recently for other secreted axon guidance molecules of the semaphorin family.135 Many answers to these questions should come from the analysis of mouse deficient for one or several of these proteins.
References
- 1.
- Nusslein-Volhard C, Wiechaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Roux Arch Dev Biol. 1984;193:267–283. [PubMed: 28305337]
- 2.
- Rothberg JM, Artavanis-Tsakonas S. Modularity of the Slit protein characterization of a conserved carboxy-terminal sequence in secreted proteins and a motif implicated in extracellular protein interactions. J Mol Biol. 1992;227:367–370. [PubMed: 1404356]
- 3.
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in drosophila. Cell. 1999;96:785–794. [PubMed: 10102267]
- 4.
- Rajagopalan S, Nicolas E, Vivancos V. et al. Crossing the midline: Roles and regulation of robo receptors. Neuron. 2000;28:767–777. [PubMed: 11163265]
- 5.
- Simpson JH, Kidd T, Bland KS. et al. Short-range and long-range guidance by slit and its robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. [PubMed: 11163264]
- 6.
- Hao JC, Yu TW, Fujisawa K. et al. C. elegans Slit Acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32(1):25–38. [PubMed: 11604136]
- 7.
- Li HS, Chen JH, Wu W. et al. Vertebrate Slit, a secreted ligand for the transmembrane protein roudabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. [PubMed: 10102269]
- 8.
- Yee KT, Simon HH, Tessier-Lavigne M. et al. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24(3):607–622. [PubMed: 10595513]
- 9.
- Vargesson N, Luria V, Messina I. et al. Expression patterns of Slit and Robo family members during vertebrate limb development. Mech Dev. 2001;106(1-2):175–180. [PubMed: 11472852]
- 10.
- Holmes G, Niswander L. Expression of slit-2 and slit-3 during chick development. Dev Dyn. 2001;222(2):301–307. [PubMed: 11668607]
- 11.
- Gilthorpe JD, Papantoniou EK, Chedotal A. et al. The migration of cerebellar rhombic lip derivatives. Development. 2002;129(20):4719–4728. [PubMed: 12361964]
- 12.
- Brose K, Bland KS, Wang KH. et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. [PubMed: 10102268]
- 13.
- Holmes GP, Negus K, Burridge L. et al. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech Dev. 1998;79:57–72. [PubMed: 10349621]
- 14.
- Itoh A, Miyabayashi T, Ohno M. et al. Cloning and expressions of three mammalian homologues of Drosophila slit suggest possible roles for slit in the formation and maintenance of the nervous system. Mol Brain Res. 1998;62:175–186. [PubMed: 9813312]
- 15.
- Hu H. Chemorepulsion of neuronal migration by slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. [PubMed: 10482237]
- 16.
- Niclou SP, Jia L, Raper JA. Slit2 is a repellent for retinal ganglion cell axons. J Neurosci. 2000;20(13):4962–4974. [PMC free article: PMC6772294] [PubMed: 10864954]
- 17.
- Nguyen-Ba-Charvet KT, Chédotal A. Role of Slit proteins in the vertebrate brain. J Physiol Paris. 2002;96:91–98. [PubMed: 11755787]
- 18.
- Tanno T, Takenaka S, Tsuyama S. Expression and function of Slit1{alpha}, a novel alternative splicing product for Slit1. J Biochem. 2004;136(5):575–581. [PubMed: 15632296]
- 19.
- Little M, Rumballe B, Georgas K. et al. Conserved modularity and potential for alternate splicing in mouse and human Slit genes. Int J Dev Biol. 2002;46(4):385–391. [PubMed: 12141424]
- 20.
- Hutson LD, Jurynec MJ, Yeo SY. et al. Two divergent slit1 genes in zebrafish. Dev Dyn. 2003;228(3):358–369. [PubMed: 14579375]
- 21.
- Wang KH, Brose K, Arnott D. et al. Biochemical purification of a mammalian Slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. [PubMed: 10102266]
- 22.
- Patel K, Nash JAB, Itoh A. et al. Slit proteins are dominant chemorepellents for olfactory tract and spinal motor axons. Development. 2001;128:5031–5037. [PubMed: 11748139]
- 23.
- Whitford KL, Dijkhuizen P, Polleux F. et al. Molecular control of cortical dendrite development. Annu Rev Neurosci. 2002;25:127–149. [PubMed: 12052906]
- 24.
- Nguyen Ba-Charvet KT, Brose K, Ma L. et al. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21:4281–4289. [PMC free article: PMC6762758] [PubMed: 11404413]
- 25.
- Battye R, Stevens A, Jacobs JR. Axon repulsion from the midline of the Drosophila CNS requires slit function. Development. 1999;126:2475–2481. [PubMed: 10226006]
- 26.
- Chen JH, Wen L, Dupuis S. et al. The N-terminal leucine-rich regions in slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001;21:1548–1556. [PMC free article: PMC6762944] [PubMed: 11222645]
- 27.
- Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 2004;23(22):4406–4412. [PMC free article: PMC526463] [PubMed: 15496984]
- 28.
- Seeger M, Tear G, Ferres-Marco D. et al. Mutations affecting growth cone guidance in Drosophila: Genes necessary for guidance toward or away from the midline. Neuron. 1993;10(3):409–426. [PubMed: 8461134]
- 29.
- Kidd T, Brose K, Mitchell KJ. et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. [PubMed: 9458045]
- 30.
- Rajagopalan S, Vivancos V, Nicolas E. et al. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. [PubMed: 11163180]
- 31.
- Yuan W, Zhou L, Chen JH. et al. The mouse SLIT family: Secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. [PubMed: 10433822]
- 32.
- Lee JS, Ray R, Chien CB. Cloning and expression of three zebrafish roundabout homologs suggest roles in axon guidance and cell migration. Dev Dyn. 2001;221(2):216–230. [PubMed: 11376489]
- 33.
- Jen JC, Chan WM, Bosley TM. et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304(5676):1509–1513. [PMC free article: PMC1618874] [PubMed: 15105459]
- 34.
- Huminiecki L, Gorn M, Suchting S. et al. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79(4):547–552. [PubMed: 11944987]
- 35.
- Bedell VM, Yeo SY, Park KW. et al. Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102(18):6373–6378. [PMC free article: PMC1088354] [PubMed: 15849270]
- 36.
- Park KW, Morrison CM, Sorensen LK. et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. [PubMed: 12941633]
- 37.
- Suchting S, Heal P, Tahtis K. et al. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. Faseb J. 2005;19(1):121–123. [PubMed: 15486058]
- 38.
- Bashaw GJ, Kidd T, Murray D. et al. Repulsive axon guidance: Abelson and enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. [PubMed: 10892742]
- 39.
- Yuan S-SF, Cox LA, Dasika GK. et al. Cloning and functional studies of a novel gene aberrantly expressed in RB-deficient embryos. Dev Biol. 1999;207:62–75. [PubMed: 10049565]
- 40.
- Simpson JH, Bland KS, Fetter RD. et al. Short-range and long-range guidance by slit and its robo receptors: A combinatorial code of Robo receptors controls lateral position. Cell. 2000;103:1019–1032. [PubMed: 11163179]
- 41.
- Sabatier C, Plump AS, Le M. et al. The divergent robo family protein rig-1/robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117(2):157–169. [PubMed: 15084255]
- 42.
- Xian J, Clark KJ, Fordham R. et al. Inadequate lung development and bronchial hyperplasia in mice with a targeted deletion in the Dutt1/Robo1 gene. Proc Natl Acad Sci USA. 2001;4:4. [PMC free article: PMC64983] [PubMed: 11734623]
- 43.
- Xian J, Aitchison A, Bobrow L. et al. Targeted disruption of the 3p12 gene, Dutt1/Robo1, predisposes mice to lung adenocarcinomas and lymphomas with methylation of the gene promoter. Cancer Res. 2004;64(18):6432–6437. [PubMed: 15374951]
- 44.
- Hivert B, Liu Z, Chuang JY. et al. Robo1 and Robo2 are hompohilic binding molecules that promote axonal growth. Mol Cell Neurosci. 2002;21:534–545. [PubMed: 12504588]
- 45.
- Liu Z, Patel K, Schmidt H. et al. Extracellular Ig domains 1 and 2 of Robo are important for ligand (Slit) binding. Mol Cell Neurosci. 2004;26(2):232–240. [PubMed: 15207848]
- 46.
- Liang Y, Annan RS, Carr SA. et al. Mammalian homologues of the Drosophila slit protein are ligands of the heparan sulfate proteoglycan glypican-1 in brain. J Biol Chem. 1999;274(25):17885–17892. [PubMed: 10364234]
- 47.
- Ronca F, Andersen JS, Paech V. et al. Characterization of Slit protein interactions with glypican-1. J Biol Chem. 2001;276:29141–29147. [PubMed: 11375980]
- 48.
- Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nature Neurosci. 2001;4:695–701. [PubMed: 11426225]
- 49.
- Steigemann P, Molitor A, Fellert S. et al. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by Slit/Robo signaling. Curr Biol. 2004;14:225–230. [PubMed: 14761655]
- 50.
- Inatani M, Irie F, Plump AS. et al. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302(5647):1044–1046. [PubMed: 14605369]
- 51.
- Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: The cytoplasmic domains of slit and netrin receptors specificity attraction versus repulsion. Neuron. 1999;97:917–926. [PubMed: 10399919]
- 52.
- Yu TW, Hao JC, Lim W. et al. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/ Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5(11):1147–1154. [PubMed: 12379860]
- 53.
- Wills Z, Emerson M, Rusch J. et al. A Drosophila homolog of cyclase-associated proteins collaborates with the Abl tyrosine kinase to control midline axon pathfinding. Neuron. 2002;36(4):611–622. [PubMed: 12441051]
- 54.
- Hsouna A, Kim YS, VanBerkum MF. Abelson tyrosine kinase is required to transduce midline repulsive cues. J Neurobiol. 2003;57(1):15–30. [PubMed: 12973825]
- 55.
- Rhee J, Mahfooz NS, Arregui C. et al. Activation of the repulsive receptor Roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol. 2002;4(10):798–805. [PubMed: 12360290]
- 56.
- Lee H, Engel U, Rusch J. et al. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 2004;42(6):913–926. [PubMed: 15207236]
- 57.
- Huber AB, Kolodkin AL, Ginty DD. et al. Signaling at the growth cone: Ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. [PubMed: 12677003]
- 58.
- Bashaw GJ, Hu H, Nobes CD. et al. A novel Dbl family RhoGEF promotes Rho-dependent axon attractin to the central nervous system midline in Drosophila and overcomes Robo repulsion. JCB. 2001;155:1117–1122. [PMC free article: PMC2199320] [PubMed: 11756465]
- 59.
- Matsuura R, Tanaka H, Go MJ. Distinct functions of Rac1 and Cdc42 during axon guidance and growth cone morphogenesis in Drosophila. Eur J Neurosci. 2004;19(1):21–31. [PubMed: 14750960]
- 60.
- Wong K, Ren XR, Huang YZ. et al. Signal transduction in neuronal migration. roles of gtpase activating proteins and the small gtpase cdc42 in the slit-robo pathway. Cell. 2001;107(2):209–221. [PubMed: 11672528]
- 61.
- Fan X, Labrador JP, Hing H. et al. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40(1):113–127. [PubMed: 14527437]
- 62.
- Stevens A, Jacobs JR. Integrins regulate responsiveness to slit repellent signals. J Neurosci. 2002;22(11):4448–4455. [PMC free article: PMC6758817] [PubMed: 12040052]
- 63.
- Endris V, Wogatzky B, Leimer U. et al. The novel Rho-GTPase activating gene MEGAP/ srGAP3 has a putative role in severe mental retardation. Proc Natl Acad Sci USA. 2002;99(18):11754–11759. [PMC free article: PMC129341] [PubMed: 12195014]
- 64.
- Lundstrom A, Gallio M, Englund C. et al. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes Dev. 2004;18(17):2161–2171. [PMC free article: PMC515293] [PubMed: 15342493]
- 65.
- Hu H, Li M, Labrador JP. et al. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc Natl Acad Sci USA. 2005;102(12):4613–4618. [PMC free article: PMC555501] [PubMed: 15755809]
- 66.
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: Silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291(5510):1928–1938. [PubMed: 11239147]
- 67.
- Höpker VH, Shewan D, Tessier-Lavigne M. et al. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. [PubMed: 10485706]
- 68.
- Song H, Ming G, He Z. et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281(5382):1515–1518. [PubMed: 9727979]
- 69.
- Chalasani SH, Sabelko KA, Sunshine MJ. et al. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23(4):1360–1371. [PMC free article: PMC6742262] [PubMed: 12598624]
- 70.
- Xu HT, Yuan XB, Guan CB. et al. Calcium signaling in chemorepellant Slit2-dependent regulation of neuronal migration. Proc Natl Acad Sci USA. 2004;101(12):4296–4301. [PMC free article: PMC384735] [PubMed: 15020772]
- 71.
- Nguyen-Ba-Charvet KT, Brose K, Marillat V. et al. Sensory axons response to substrate-bound Slit2 is modulated by laminin and cyclicGMP. Mol Cell Neurosci. 2001;17:1048–1058. [PubMed: 11414793]
- 72.
- Sun Q, Bahri S, Schmid A. et al. Receptor tyrosine phosphatases regulate axon guidance across the midline of the drosophila embryo. Development. 2000;127:801–812. [PubMed: 10648238]
- 73.
- Fritz JL, VanBerkum MFA. Calmodulin and son of sevenless dependent signaling pathways regulate midline crossing of axons in the Drosophila CNS. Development. 2000;127:1991–2000. [PubMed: 10751187]
- 74.
- Crowner D, Madden K, Goeke S. et al. Lola regulates midline crossing of CNS axons in Drosophila. Development. 2002;129(6):1317–1325. [PubMed: 11880341]
- 75.
- Ma Y, Certel K, Gao Y. et al. Functional interactions between Drosophila bHLH/PAS, Sox, and POU transcription factors regulate CNS midline expression of the slit gene. J Neurosci. 2000;20(12):4596–4605. [PMC free article: PMC6772444] [PubMed: 10844029]
- 76.
- Wharton Jr K, Franks RG, Kasai Y. et al. Control of CNS midline transcription by asymetric E-box-like elements: Similarity to xenobiotic responsive regulation. Development. 1994;120(12):3563–3569. [PubMed: 7821222]
- 77.
- Jin Z, Zhang J, Klar A. et al. Irx4-mediated regulation of Slit1 expression contributes to the definition of early axonal paths inside the retina. Development. 2003;130:1037–1048. [PubMed: 12571096]
- 78.
- Yeo SY, Miyashita T, Fricke C. et al. Involvement of Islet-2 in the Slit signaling for axonal branching and defasciculation of the sensory neurons in embryonic zebrafish. Mech Dev. 2004;121(4):315–324. [PubMed: 15110042]
- 79.
- Miyashita T, Yeo SY, Hirate Y. et al. PlexinA4 is necessary as a downstream target of Islet2 to mediate Slit signaling for promotion of sensory axon branching. Development. 2004;131(15):3705–3715. [PubMed: 15229183]
- 80.
- Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors. Atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37(1):41–51. [PubMed: 12526771]
- 81.
- Kraut R, Zinn K. Roundabout 2 regulates migration of sensory neurons by signaling in trans. Curr Biol. 2004;14(15):1319–1329. [PMC free article: PMC3566263] [PubMed: 15296748]
- 82.
- Kidd T, Russell C, Goodman CS. et al. Dosage-sensitive and complementary functions of roudabout and commissureless control axon crossing of the CNS midline. Neuron. 1998;20:25–33. [PubMed: 9459439]
- 83.
- Tear G, Harris R, Sutaria S. et al. Commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron. 1996;16(3):501–514. [PubMed: 8785048]
- 84.
- Georgiou M, Tear G. Commissureless is required both in commissural neurones and midline cells for axon guidance across the midline. Development. 2002;129(12):2947–2956. [PubMed: 12050141]
- 85.
- Keleman K, Rajagopalan S, Cleppien D. et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110(4):415. [PubMed: 12202032]
- 86.
- Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: Cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8(2):156–163. [PubMed: 15657595]
- 87.
- Georgiou M, Tear G. The N-terminal and transmembrane domains of Commissureless are necessary for its function and trafficking within neurons. Mech Dev. 2003;120(9):1009–1019. [PubMed: 14550530]
- 88.
- Myat A, Henry P, McCabe V. et al. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. 2002;35(3):447–459. [PubMed: 12165468]
- 89.
- Zou Y, Stoeckli E, Chen H. Squeezing axons out of the gray matter: A role for slit and semaphorin proteins from the midline and ventral spinal cord. 2000. pp. 363–375. [PubMed: 10975526]
- 90.
- Long H, Sabatier C, Le M. et al. Conserved roles for slit and robo proteins in midline commissural axon guidance. Neuron. 2004;42(2):213–223. [PubMed: 15091338]
- 91.
- Marillat V, Sabatier C, Failli V. et al. The Slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43:1–20. [PubMed: 15233918]
- 92.
- Schimmelpfeng K, Gogel S, Klambt C. The function of leak and kuzbanian during growth cone and cell migration. Mech Dev. 2001;106(1-2):25–36. [PubMed: 11472832]
- 93.
- Onel S, Bolke L, Klambt C. The Drosophila ARF6-GEF Schizo controls commissure formation by regulating Slit. Development. 2004;131(11):2587–2594. [PubMed: 15148300]
- 94.
- Jhaveri D, Saharan S, Sen A. et al. Positioning sensory terminals in the olfactory lobe of Drosophila by Robo signaling. Development. 2004;131(9):1903–1912. [PubMed: 15056612]
- 95.
- Plump AS, Erskine L, Sabatier C. et al. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. [PubMed: 11804570]
- 96.
- Hutson LD, Chien CB. Pathfinding and error correction by retinal axons: The role of astray/ robo2. Neuron. 2002;33(2):205–217. [PubMed: 11804569]
- 97.
- Nguyen-Ba-Charvet KT, Plump AS, Tessier-Lavigne M. et al. Slit1 and Slit2 proteins control the development of the lateral olfactory tract. J Neurosci. 2002;22:5473–5480. [PMC free article: PMC6758232] [PubMed: 12097499]
- 98.
- Bagri A, Marin O, Plump AS. et al. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. [PubMed: 11804571]
- 99.
- Shu T, Sundaresan V, McCarthy MM. et al. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J Neurosci. 2003;23(22):8176–8184. [PMC free article: PMC6740498] [PubMed: 12954881]
- 100.
- Fricke C, Lee J-S, Geiger-Rudolph S. et al. Astray, a zebrafish roundabout homolog required for retinal axon guidance. Science. 2001;292:507–510. [PubMed: 11313496]
- 101.
- Marillat V, Cases O, Nguyen-Ba-Charvet KT. et al. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442:130–155. [PubMed: 11754167]
- 102.
- Tayler TD, Robichaux MB, Garrity PA. Compartmentalization of visual centers in the Drosophila brain requires Slit and Robo proteins. Development. 2004;131(23):5935–5945. [PMC free article: PMC1201521] [PubMed: 15525663]
- 103.
- Knoll B, Schmidt H, Andrews W. et al. On the topographic targeting of basal vomeronasal axons through Slit-mediated chemorepulsion. Development. 2003;130:5073–5082. [PubMed: 12954717]
- 104.
- Cloutier JF, Sahay A, Chang EC. et al. Differential requirements for semaphorin 3F and Slit-1 in axonal targeting, fasciculation, and segregation of olfactory sensory neuron projections. J Neurosci. 2004;24(41):9087–9096. [PMC free article: PMC6730055] [PubMed: 15483127]
- 105.
- Miyasaka N, Sato Y, Yeo SY. et al. Robo2 is required for establishment of a precise glomerular map in the zebrafish olfactory system. Development. 2005;132:1283–1293. [PubMed: 15716341]
- 106.
- Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the Mammalian CNS. J Neurosci. 2002;22(11):4540–4549. [PMC free article: PMC4260804] [PubMed: 12040061]
- 107.
- Furrer MP, Kim S, Wolf B. et al. Robo and Frazzled/DCC mediate dendritic guidance at the CNS midline. Nat Neurosci. 2003;6(3):223–230. [PubMed: 12592406]
- 108.
- Whitford KL, Marillat V, Stein E. et al. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. [PubMed: 11779479]
- 109.
- Couch JA, Chen J, Rieff HI. et al. Robo2 and robo3 interact with eagle to regulate serotonergic neuron differentiation. Development. 2004;131(5):997–1006. [PubMed: 14973268]
- 110.
- Mehta B, Bhat KM. Slit signaling promotes the terminal asymmetric division of neural precursor cells in the Drosophila CNS. Development. 2001;128(16):3161–3168. [PubMed: 11688564]
- 111.
- Connor RM, Key B. Expression and role of Roundabout-1 in embryonic Xenopus forebrain. Dev Dyn. 2002;225(1):22–34. [PubMed: 12203717]
- 112.
- Grieshammer U, Ma L, Plump AS. et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6(5):709–717. [PubMed: 15130495]
- 113.
- Kinrade EFV, Bartes T, Tear G. et al. Roundabout signalling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development. 2001;128:207–216. [PubMed: 11124116]
- 114.
- Kramer SG, Kidd T, Simpson JH. et al. Switching repulsion to attraction: Changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. [PubMed: 11326102]
- 115.
- Englund C, Steneberg P, Falileeva L. et al. Attractive and repulsive functions of Slit are mediated by different receptors in the Drosophila trachea. Development. 2002;129(21):4941–4951. [PubMed: 12397103]
- 116.
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. [PubMed: 14583753]
- 117.
- Hu H, Rutishauser U. A septum-derived chemorepulsive factor for migrating olfactory interneuron precursors. Neuron. 1996;16:933–940. [PubMed: 8630251]
- 118.
- Wu W, Wong K, Chen JH. et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. [PMC free article: PMC2041931] [PubMed: 10432110]
- 119.
- Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M. et al. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24(6):1497–1506. [PMC free article: PMC6730320] [PubMed: 14960623]
- 120.
- Ward M, McCann C, DeWulf M. et al. Distinguishing between directional guidance and motility regulation in neuronal migration. J Neurosci. 2003;23(12):5170–5177. [PMC free article: PMC2041933] [PubMed: 12832541]
- 121.
- Mason HA, Ito S, Corfas G. Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: Inducers, inhibitors, and repellents. J Neurosci. 2001;21:7654–7663. [PMC free article: PMC6762882] [PubMed: 11567055]
- 122.
- De Bellard ME, Rao Y, Bronner-Fraser M. Dual function of Slit2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells. J Cell Biol. 2003;162(2):269–279. [PMC free article: PMC2172792] [PubMed: 12876276]
- 123.
- Zhu Y, Li HS, Zhou L. et al. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. [PubMed: 10433260]
- 124.
- Marin O, Plump AS, Flames N. et al. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130:1889–1901. [PubMed: 12642493]
- 125.
- Causeret F, Danne F, Ezan F. et al. Slit antagonizes netrin-1 attractive effects during the migration of inferior olivary neurons. Dev Biol. 2002;246(2):429–440. [PubMed: 12051827]
- 126.
- Wu JY, Feng L, Park H-T. et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. [PMC free article: PMC2072862] [PubMed: 11309622]
- 127.
- Wang B, Xiao Y, Ding BB. et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4(1):19–29. [PubMed: 12892710]
- 128.
- Chédotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: New role for axon guidance molecules in cancer. Cell Death Differ. 2005;12(8):1044–1056. [PubMed: 16015381]
- 129.
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4(9):703–713. [PubMed: 12951563]
- 130.
- Hagino S, Iseki K, Mori T. et al. Slit and glypican-1 mRNAs are coexpressed in the reactive astrocytes of the injured adult brain. Glia. 2003;42(2):130–138. [PubMed: 12655597]
- 131.
- Bloechlinger S, Karchewski LA, Woolf CJ. Dynamic changes in glypican-1 expression in dorsal root ganglion neurons after peripheral and central axonal injury. Eur J Neurosci. 2004;19(5):1119–1132. [PubMed: 15016071]
- 132.
- Madura T, Yamashita T, Kubo T. et al. Changes in mRNA of Slit-Robo GTPase-activating protein 2 following facial nerve transection. Brain Res Mol Brain Res. 2004;123(1-2):76–80. [PubMed: 15046868]
- 133.
- Guan H, Zu G, Xie Y. et al. Neuronal repellent Slit2 inhibits dendritic cell migration and the development of immune responses. J Immunol. 2003;171(12):6519–6526. [PubMed: 14662852]
- 134.
- Parsons L, Harris KL, Turner K. et al. Roundabout gene family functions during sensory axon guidance in the drosophila embryo are mediated by both Slit-dependent and Slit-independent mechanisms. Dev Biol. 2003;264(2):363–375. [PubMed: 14651924]
- 135.
- Sahay A, Kim CH, Sepkuty JP. et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25(14):3613–3620. [PMC free article: PMC6725392] [PubMed: 15814792]
- Slits and Their Receptors - Madame Curie Bioscience DatabaseSlits and Their Receptors - Madame Curie Bioscience Database
- FOXP Genes, Neural Development, Speech and Language Disorders - Madame Curie Bio...FOXP Genes, Neural Development, Speech and Language Disorders - Madame Curie Bioscience Database
- Regulation of Paracellular Transport across Tight Junctions by the Actin Cytoske...Regulation of Paracellular Transport across Tight Junctions by the Actin Cytoskeleton - Madame Curie Bioscience Database
- Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity - Ma...Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity - Madame Curie Bioscience Database
- The Role of Maspin in Tumor Progression and Normal Development - Madame Curie Bi...The Role of Maspin in Tumor Progression and Normal Development - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...