NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
All living organisms possess the ability to translocate proteins across biological membranes. This is a fundamental necessity since proteins function in different locations yet are synthesized in one compartment only, the cytosol. Even though different transport systems exist, the pathway that is dominantly used to translocate secretory and membrane proteins is known as the cotranslational transport pathway. It evolved only once and is in its core conserved throughout all kingdoms of life. The process is characterized by a well understood sequence of events: first, an N-terminal signal sequence of a nascent polypeptide is recognized on the ribosome by the signal recognition particle (SRP), then the SRP-ribosome complex is targeted to the membrane via the SRP receptor. Next, the nascent chain is transferred from SRP to the protein conducting channel, through which it is cotranslationally threaded. All the essential components of the system have been identified. Recent structural and biochemical studies have unveiled some of the intricate regulatory circuitry of the process. These studies also shed light on the accessory components unique to eukaryotes, pointing to early events in eukaryotic evolution.
Introduction
A system to integrate or translocate proteins into or across lipid bilayers is a fundamental requirement for all autonomous cellular life forms. Proteins destined for membrane translocation are synthesized with an N-terminal signal sequence composed of about 10 hydrophobic residues.1 The signal sequence of a nascent protein is recognized as it emerges from the exit tunnel of the ribosome by the signal recognition particle (SRP), a large RNA-protein complex. Next, the SRP-bound ribosome-nascent-chain complex (SRP-RNC) is targeted to the membrane through the SRP receptor (SR). Once localized to the membrane, the nascent chain is transferred from the SRP to the protein conducting channel (PCC). Secretory proteins are threaded through the PCC and adopt their native structure on the other side of the membrane, whereas the PCC can open laterally to insert membrane proteins into the lipid bilayer. In cotranslational transport protein synthesis and translocation are temporally closely coupled. Such synchronization needs regulation, which is provided by guanine nucleotide binding proteins (G proteins) acting as molecular switches.
Cotranslational transport has been studied extensively over the past 30 years.2 After identifying the essential molecules governing the process we have learned a great deal in recent years from x-ray crystallographic and cryo-electron microscopic studies. These structural characterizations have provided snapshots of components of the system in various functional states and gradually unveil the mechanistic underpinning. The reader is referred to excellent recent reviews on the subject.3-6 This review focuses on differences between the mediators of cotranslational transport in the three kingdoms of life. Even though universally conserved, cotranslational transport has become increasingly more sophisticated over time. Some features are only found in eukaryotes and it is likely that these features point to early events in eukaryogenesis.
Mediators of Cotranslational Transport
The Signal Recognition Particle
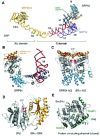
Signal recognition particles are conserved throughout all kingdoms of life7 and were initially discovered in mammalian cells.8 In mammals and other metazoans, SRP consists of a ˜300nt (90kD) elongated and largely double-stranded RNA scaffold structure to which six proteins bind. These proteins are named according to their apparent molecular mass (in kD) SRP9, 14, 19, 54, 68 and 72. SRP is organized in two functionally distinct regions, the Aluand the S-domain, that form two ends of the SRP RNA (Fig. 1). The S-domain, in addition to the architectural proteins SRP19, 68 and 72, also harbors the regulatory G protein SRP54. SRP54 recognizes the signal sequence of an emerging nascent chain at the exit site of the ribosome and also targets SRP-RNC to the membrane via interaction with SR. SRP54 is made of two functional domains and belongs to the small GTPase superfamily.9 The N-terminus of SRP54 contains a composite domain made of a four-helix bundle (N domain) tightly connected to a G domain. The NG domain is universally conserved in SRP54, repeated in the SRs, but is not found in any other cellular context. The second functional domain of SRP54 is C-terminal, flexibly linked to the NG domain, and called the M domain for its methionine-rich composition. The M domain directly binds SRP RNA (Fig. 1A,B) as well as the signal sequence. The SRP RNA Alu domain binds heterodimeric SRP9/14 and is responsible for attenuating elongation by blocking the elongation-factor binding site upon SRP binding of RNC.10
The composition of SRP is largely conserved among all eukaryotes, however some irregularities occur in fungi and protozoans. In Saccharomyces cerevisiae SRP-RNA contains additional insertions in the Alu domain of yet unknown function.11 In several fully sequenced eukaryotic parasites some of the architectural SRP proteins have not been identified. For example, in Leishmania major, Trypanosoma cruzi, and Giardia lamblia no SRP9 or SRP14 is found. Encephalitozoon cuniculi appears to lack SRP68 and SRP72.
The SRP repertoire of prokaryotes is much simpler then in eukaryotes. In archaea, only SRP19 and SRP54 are found.12 Gram-negative bacteria, such as Escherichia coli, possess the simplest version of SRP. Here the particle contains a ˜110 nt RNA and only the SRP54 ortholog Ffh. E. coli SRP can functionally replace its mammalian counterpart in vitro indicating the essential role of the G protein SRP54 for cotranslational transport.13
The SRP Receptor
SRs are an essential component of cotranslational transport pathways14 and are thus phylogenetically well conserved. In bacteria and archaea, neither of which contain intracellular organelles, SR is a single-subunit protein termed FtsY. FtsY homologs share a C-terminal NG domain, which directly interacts with the NG domain of Ffh (the SRP54 ortholog) in the targeting reaction (Fig. 1C). The N-terminal region of FtsY is not strongly conserved. E. coli has a glutamate-rich region called A domain, which is able to reversibly attach to the plasma membrane.15 Instead of an A domain homolog, some Gram-positive bacteria carry a transmembrane (TM) helix at the N-terminus of FtsY, thus permanently anchoring the receptor to the cytoplasmic membrane.16 Replacing the A region of E.coli FtsY with a TM helix of an unrelated protein resulted in a fully functional receptor.17
In contrast to the single-subunit prokaryotic SR, eukaryotic SR is a heterodimer of an α-subunit (SRα) and a β-subunit (SRβ). SRα is homologous to FtsY in that it also contains a C-terminal NG domain. However, instead of the N-terminal membrane attachment domain, all eukaryotic SRαs contain a conserved domain SRX that directly interacts with SRβ (Fig. 1D).18,19 SRβ is exclusively found in eukaryotes and is permanently anchored in the membrane of the endoplasmic reticulum (ER) via an N-terminal TM helix. SRβ is itself a G protein and it has been shown that stable interaction with SRα is GTP dependent,19 suggesting a regulatory mechanism that involves reversible attachment of SRα to the membrane.
The Protein Conducting Channel
Passage of the nascent chain through the cytoplasmic membrane of prokaryotes or the ER membrane of eukaryotes is mediated by the highly conserved PCC.20 PCC core components are the transmembrane proteins SecY and SecE in the cytoplasmic membrane of bacteria, and the orthologs Sec61α and Sec61γ in the eukaryotic ER membrane. In addition, a nonessential small integral membrane protein is part of the channel, SecG in bacteria or Sec61β in eukaryotes, which appears not to be well conserved.21 The recently solved crystal structure of the archaeal PCC of Methanococcus jannaschii reveals its fundamental architecture22 (Fig. 1E). 10 TM helices of Sec61α arrange as two symmetrical halve rings made of helices 1-5 and 6-10 that together form a cylinder. The two helices of Sec61γ form a clamp that holds both Sec61α halves together, and the single TM helix of Sec61β is at the periphery of the assembly with weak contacts to Sec61α. The center of the structure has an hourglass-like shape with a central constriction made of highly conserved residues. It is proposed that the central constriction seals the closed pore through which the nascent polypeptide would pass in an open conformation.22 The side of the channel not clamped by the γ-subunit (between TM helices 2 and 7) could possibly be pried apart to open the pore allowing membrane proteins to laterally exit the channel into the lipid bilayer. Although the crystal structure suggests that only one Sec61 heterotrimer is sufficient for protein translocation, oligomerization of the PCC has been observed in vitro by single-particle reconstructions using cryo-electron microscopy and also crystallographic studies on 2-D lattices.23-26 With the crystal structure of the disengaged channel at hand, it is now possible to design structure-based experiments to address the remaining mechanistic questions.
The Control of Cotranslational Targeting
Cotranslational protein targeting depends in all organisms on the synchronized interplay of at least two GTPases, namely SRP54 and SRα, that regulate the process and ensure directionality. Eukaryotes in addition use a third G protein, SRβ. The superfamily of G proteins is the largest and most important class of regulatory proteins. Fundamentally, G proteins cycle between GTP and GDP bound states, which result in specific conformational changes in the protein.27 In the GTP bound state, G proteins bind to effector molecules to transmit a signal and are thus commonly referred to as ‘switched on’. Hydrolysis of GTP is the ‘off switch’. Frequently, accessory proteins are necessary to complete the cycle; GTPase activating proteins (GAPs) accelerate GTP hydrolysis whereas guanine nucleotide exchange factors (GEFs) reset the switch by facilitating the release of GDP and the rebinding of GTP. What do we know about the regulation of cotranslational targeting?
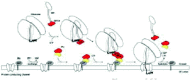
The initial step is the sampling of the emerging signal sequence at the exit tunnel of the ribosome by the M domain of SRP5428,29 (Fig. 2). Conceptually, it is appealing that this interaction triggers a conformational change in the NG GTPase domain of SRP54, loading GTP as a result.30 Despite a considerable repertoire of crystal structures of SRP54 this mechanism is not yet confirmed (see discussion in refs. 4, 31). Concurrent to signal peptide binding, the Alu domain of SRP is positioned into the elongation factor binding site on the ribosome arresting translation.10 GTP-binding of SRP54 enables interaction with the NG domain of SRα, which in turn binds GTP with increased affinity.31 The interaction of SRP54 and SRα is exclusively mediated via a quasi-symmetrical interaction of the homologous NG domains. Crystal structures of this so-called engagement complex have been solved recently and reveal the fascinating atomic details of the GTPase mechanism.32,33 In unprecedented fashion, both GTP molecules are part of the NGSRP54-NGSRα interface and form a composite nucleotide binding site.
After SRP54/SRα binding the SRP-RNC-SR complex is stalled. In prokaryotes, the complex now accumulates on the cytoplasmic membrane until a translocation-competent PCC is found.34 Signal peptide transfer from SRP54 M domain to the PCC triggers mutual hydrolysis of GTP at the NG domains, disengaging SRP and SR and making them available for a new round of targeting.
In eukaryotes the situation is less clear since the role of the additional β-subunit of SR is yet only partially understood. With its N-terminal transmembrane helix SRβ is permanently anchored in the ER membrane. In order to stably interact with SRα, SRβ needs to be GTP bound. Consequently, GTP hydrolysis by SRβ should dissociate the complex. Regulated ER membrane recruitment of SRα might be an additional regulatory mechanism specific for eukaryotes. One important step toward elucidating the potential regulatory role of SRβ is the identification of its GAP, since SRβ itself is catalytically inactive.35 Noteworthy in any case is experimental evidence suggesting that possible SRβ-GTP hydrolysis does not occur prior to signal peptide transfer since in the absence of PCCs stalled SRP-RNC-SR complexes accumulate on the membrane just as is the case for prokaryotes.36
Evolutionary Considerations
Protein translocation across membranes is a basic necessity for all autonomous life forms. Besides the protein synthesizing ribosome the most important component of the translocation system is the PCC. Universal conservation of the essential PCC subunits Sec61α and -γ indicates that the channel evolved from a common ancestor. Coupling translation and translocation is a sophistication of the transport process that is not trivial. As outlined above, it requires a synchronized control mechanism. In fact, a simpler, post-translational transport pathway that converges on the PCC also exists. In this system the synthesized protein is kept in an unfolded, translocation-competent state by chaperones and delivered to the PCC. Post-translational transport is less efficient than cotranslational transport, since it requires additional energy in form of an ATP-driven motor that either pushes (SecA in E.coli) or pulls (BiP in S.cerevisiae) the chain through the PCC.37 Cotranslational transport does not require energy in addition to that required for protein synthesis provided by the translation machinery.38 Interestingly, post-translational transport is a frequently used pathway for secretory proteins in E.coli and S.cerevisiae, yet is only of marginal importance in higher eukaryotes. Unfortunately there is insufficient data for a classification of archaea in this respect,39 but it is tempting to speculate that posttranslational evolved prior to cotranslational transport.
All cotranslational systems share the twin-GTPase mechanism involving the NG domains of SRP54 and SRα or their orthologs. Abundance of structural and genomic information as well as the relative ease with which G proteins can be detected, due to strongly conserved signature motifs, have revealed detailed insight into their phylogeny.9 Based on structural and sequence conservation the NG domain constitutes a unique G protein family. Apart from its role in cotranslational transport the NG domain has not been detected in any other cellular context.
The functional implications of variations in the composition of SRP in different species are not straightforwardly explained. The absence of the Alu domain in E. coli SRP most likely explains why in this organism no translation arrest occurs during targeting. However, the apparent absence of individual architectural SRP proteins in certain parasitic eukaryotes and archaea could have several explanations. Interestingly, experimental evidence suggests that in B. subtilis the SRP9/14 heterodimer in the Alu domain is functional replaced by histone-like HBsu,40 a protein with a substantially different structure. It is conceivable that replacement of SRP proteins by proteins with other functional annotations could extent beyond B.subtilis and SRP9/14. Another possibility is that the architectural SRP proteins do not have detectable sequence signatures and might therefore be very difficult to find by comparative genome analysis. This is not an uncommon phenomenon and has recently been observed many times in crystal structures that turn out to be well-known domain folds, despite having marginal sequence identity.41,42
Phylogenetic analysis of SR is less ambiguous. Apart from the universally conserved NG domain in SRα, a clear distinction can be drawn between the SR of prokaryotes and eukaryotes. Only eukaryotes contain the additional G protein SRβ. This statement can independently be tested by searching for SRαs that contain an SRX domain, which is necessary and sufficient for stable interaction with SRβ.18,19 SRαs with an SRX domain are found exclusively in eukaryotes as well (unpublished data). SRβ belongs to the superfamily of small monomeric G proteins.43 Members of this superfamily are widespread regulators of many cellular functions that characterize eukaryotic cells, i.e., vesicle trafficking, cytoskeleton remodeling and nuclear transport. Because of small G protein diversification especially in eukaryotes, phylogenetic analysis of this protein class might provide clues on the evolutionary history of specific eukaryotic features.44 SRβ is structurally and on the primary sequence level closely related to Arf/Sar-type G proteins, a family of vesicle transport regulators.45 This is in close agreement with the phylogenetic analysis,44 showing that Arf/Sar/SRβ branched off early from all other small G proteins. Despite their similarity, significant functional differences exist between Arf/Sar and SRβ. Arf/Sar are mechanistically characterized by reversible membrane attachment facilitated by an amphiphilic N-terminal helix that can be inserted into one leaflet of a lipid bilayer in nucleotide-controlled manner.46 SRβ instead contains a bona fide TM helix at its N-terminus that permanently anchors the protein in the ER membrane. In addition, SRβ has a characteristic, highly conserved extension of helix 4 of the central G protein fold,19 the function of which is enigmatic. These distinct features show that SRβ constitutes a separate G protein family.
Why did eukaryotes develop SRβ so early in their evolution? A plausible scenario is realized if one considers that the development of an endomembrane system may have been the earliest event during eukaryogenesis.44 Before phagocytosis was developed, only small nutrients were transported through the membrane. Secretion of digestive enzymes was likely advantageous under such circumstances, but not necessarily when secreted into the free environment so that neighboring cells would profit equally. Recent evidence suggests that the common ancestor of Arf/Sar/SRβ might have been the protein that facilitated the initial engulfment of the cytoplasmic membrane. In a remarkable study it was shown that simple incubation of liposomes with Sar1-GTP led to deformation of the vesicles into long, tubular structures, strictly dependent on the presence of the exposed, N-terminal membrane-penetrating helix.47 With GDP-bound Sar1, in which the N-terminal helix is retracted and folds into the protein core, no vesicle tubulation was observed. A mechanism to localize protein secretion to these engulfed membranes instead of the outer cytoplasmic membrane becomes necessary if the cell is to benefit from their presence. SRβ may have originated in eukaryotes as a means to target cotranslationally translocated proteins to these engulfed membranes through recruitment of the SRP-RNC complex. Eventually the engulfed membranes separated from the cytoplasmic membrane and formed the endomembrane system of modern eukaryotes. In the extant eukaryotes SRβ is functionally important to ensure targeting of SRP-RNC complexes to the ER, where PCCs are exclusively localized. In contrast to prokaryotes, eukaryotes modify proteins extensively through glycosylation and disulfide bond formation before they leave the cell, processes that only take place in the ER and the Golgi apparatus. Thus, the evolution of SRβ as a mechanism to target protein secretion to the endomembranes may have been a critical step for a defining process in eukaryogenesis.
References
- 1.
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985;184:99–105. [PubMed: 4032478]
- 2.
- Matlin KS. The strange case of the signal recognition particle. Nat Rev Mol Cell Biol. 2002;3:538–542. [PubMed: 12094220]
- 3.
- Doudna JA, Batey RT. Structural insights into the signal recognition particle. Annu Rev Biochem. 2004;73:539–557. [PubMed: 15189152]
- 4.
- Egea PF, Stroud RM, Walter P. Targeting proteins to membranes: Structure of the signal recognition particle. Curr Opin Struct Biol. 2005;15:213–220. [PubMed: 15837181]
- 5.
- Halic M, Beckmann R. The signal recognition particle and its interactions during protein targeting. Curr Opin Struct Biol. 2005;15:116–125. [PubMed: 15718142]
- 6.
- Keenan RJ, Freymann DM, Stroud RM. et al. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. [PubMed: 11395422]
- 7.
- Pool MR. Signal recognition particles in chloroplasts, bacteria, yeast and mammals (review). Mol Membr Biol. 2005;22:3–15. [PubMed: 16092520]
- 8.
- Walter P, Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982;299:691–698. [PubMed: 6181418]
- 9.
- Leipe DD, Wolf YI, Koonin EV. et al. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. [PubMed: 11916378]
- 10.
- Halic M, Becker T, Pool MR. et al. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature. 2004;427:808–814. [PubMed: 14985753]
- 11.
- Van Nues RW, Brown JD. Saccharomyces SRP RNA secondary structures: A conserved S-domain and extended Alu-domain. RNA. 2004;10:75–89. [PMC free article: PMC1370520] [PubMed: 14681587]
- 12.
- Zwieb C, Eichler J. Getting on target: The archaeal signal recognition particle. Archaea. 2002;1:27–34. [PMC free article: PMC2685543] [PubMed: 15803656]
- 13.
- Powers T, Walter P. Cotranslational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 1997;16:4880–4886. [PMC free article: PMC1170123] [PubMed: 9305630]
- 14.
- Gilmore R, Walter P, Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982;95:470–477. [PMC free article: PMC2112977] [PubMed: 6292236]
- 15.
- de Leeuw E, Poland D, Mol O. et al. Membrane association of FtsY, the E. coli SRP receptor. FEBS Lett. 1997;416:225–229. [PubMed: 9373157]
- 16.
- Bibi E, Herskovits AA, Bochkareva ES. et al. Putative integral membrane SRP receptors. Trends Biochem Sci. 2001;26:15–16. [PubMed: 11252252]
- 17.
- Zelazny A, Seluanov A, Cooper A. et al. The NG domain of the prokaryotic signal recognition particle receptor, FtsY, is fully functional when fused to an unrelated integral membrane polypeptide. Proc Natl Acad Sci USA. 1997;94:6025–6029. [PMC free article: PMC20994] [PubMed: 9177162]
- 18.
- Young JC, Ursini J, Legate KR. et al. An amino-terminal domain containing hydrophobic and hydrophilic sequences binds the signal recognition particle receptor alpha subunit to the beta subunit on the endoplasmic reticulum membrane. J Biol Chem. 1995;270:15650–15657. [PubMed: 7797564]
- 19.
- Schwartz T, Blobel G. Structural basis for the function of the beta subunit of the eukaryotic signal recognition particle receptor. Cell. 2003;112:793–803. [PubMed: 12654246]
- 20.
- Simon SM, Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991;65:371–380. [PubMed: 1902142]
- 21.
- Hartmann E, Sommer T, Prehn S. et al. Evolutionary conservation of components of the protein translocation complex. Nature. 1994;367:654–657. [PubMed: 8107851]
- 22.
- Van den Berg B, Clemons Jr WM, Collinson I. et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. [PubMed: 14661030]
- 23.
- Beckmann R, Spahn CM, Eswar N. et al. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 2001;107:361–372. [PubMed: 11701126]
- 24.
- Collinson I, Breyton C, Duong F. et al. Projection structure and oligomeric properties of a bacterial core protein translocase. EMBO J. 2001;20:2462–2471. [PMC free article: PMC125464] [PubMed: 11350935]
- 25.
- Menetret JF, Hegde RS, Heinrich SU. et al. Architecture of the ribosome-channel complex derived from native membranes. J Mol Biol. 2005;348:445–457. [PubMed: 15811380]
- 26.
- Morgan DG, Menetret JF, Neuhof A. et al. Structure of the mammalian ribosome-channel complex at 17A resolution. J Mol Biol. 2002;324:871–886. [PubMed: 12460584]
- 27.
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. [PubMed: 11701921]
- 28.
- Romisch K, Webb J, Lingelbach K. et al. The 54-kD protein of signal recognition particle contains a methionine-rich RNA binding domain. J Cell Biol. 1990;111:1793–1802. [PMC free article: PMC2116322] [PubMed: 1699948]
- 29.
- Zopf D, Bernstein HD, Johnson AE. et al. The methionine-rich domain of the 54 kd protein subunit of the signal recognition particle contains an RNA binding site and can be crosslinked to a signal sequence. Embo J. 1990;9:4511–4517. [PMC free article: PMC552245] [PubMed: 1702385]
- 30.
- Bacher G, Lutcke H, Jungnickel B. et al. Regulation by the ribosome of the GTPase of the signal-recognition particle during protein targeting. Nature. 1996;381:248–251. [PubMed: 8622769]
- 31.
- Powers T, Walter P. Reciprocal stimulation of GTP hydrolysis by two directly interacting GTPases. Science. 1995;269:1422–1424. [PubMed: 7660124]
- 32.
- Egea PF, Shan SO, Napetschnig J. et al. Substrate twinning activates the signal recognition particle and its receptor. Nature. 2004;427:215–221. [PubMed: 14724630]
- 33.
- Focia PJ, Shepotinovskaya IV, Seidler JA. et al. Heterodimeric GTPase core of the SRP targeting complex. Science. 2004;303:373–377. [PMC free article: PMC3546161] [PubMed: 14726591]
- 34.
- Herskovits AA, Shimoni E, Minsky A. et al. Accumulation of endoplasmic membranes and novel membrane-bound ribosome-signal recognition particle receptor complexes in Escherichia coli. J Cell Biol. 2002;159:403–410. [PMC free article: PMC2173083] [PubMed: 12417577]
- 35.
- Legate KR, Andrews DW. The beta-subunit of the signal recognition particle receptor is a novel GTP-binding protein without intrinsic GTPase activity. J Biol Chem. 2003;278:27712–27720. [PubMed: 12759365]
- 36.
- Song W, Raden D, Mandon EC. et al. Role of Sec61alpha in the regulated transfer of the ribosome-nascent chain complex from the signal recognition particle to the translocation channel. Cell. 2000;100:333–343. [PubMed: 10676815]
- 37.
- Stephenson K. Sec-dependent protein translocation across biological membranes: Evolutionary conservation of an essential protein transport pathway (review). Mol Membr Biol. 2005;22:17–28. [PubMed: 16092521]
- 38.
- Gorlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. [PubMed: 8242738]
- 39.
- Bolhuis A. The archaeal Sec-dependent protein translocation pathway. Philos Trans R Soc Lond B Biol Sci. 2004;359:919–927. [PMC free article: PMC1693384] [PubMed: 15306407]
- 40.
- Nakamura K, Yahagi S, Yamazaki T. et al. Bacillus subtilis histone-like protein, HBsu, is an integral component of a SRP-like particle that can bind the Alu domain of small cytoplasmic RNA. J Biol Chem. 1999;274:13569–13576. [PubMed: 10224127]
- 41.
- Caetano-Anolles G, Caetano-Anolles D. Universal sharing patterns in proteomes and evolution of protein fold architecture and life. J Mol Evol. 2005;60:484–498. [PubMed: 15883883]
- 42.
- Chothia C, Gough J, Vogel C. et al. Evolution of the protein repertoire. Science. 2003;300:1701–1703. [PubMed: 12805536]
- 43.
- Sprang SR. G protein mechanisms: Insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. [PubMed: 9242920]
- 44.
- Jekely G. Small GTPases and the evolution of the eukaryotic cell. Bioessays. 2003;25:1129–1138. [PubMed: 14579253]
- 45.
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. [PubMed: 12892779]
- 46.
- Pasqualato S, Renault L, Cherfils J. Arf, Arl, Arp and Sar proteins: A family of GTP-binding proteins with a structural device for ‘front-back’ communication. EMBO Rep. 2002;3:1035–1041. [PMC free article: PMC1307594] [PubMed: 12429613]
- 47.
- Lee MC, Orci L, Hamamoto S. et al. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. [PubMed: 16122427]
- Origins and Evolution of Cotranslational Transport to the ER - Madame Curie Bios...Origins and Evolution of Cotranslational Transport to the ER - Madame Curie Bioscience Database
- Thermo-Chemo-Radiotherapy Association: Biological Rationale, Preliminary Observa...Thermo-Chemo-Radiotherapy Association: Biological Rationale, Preliminary Observations on Its Use on Malignant Brain Tumors - Madame Curie Bioscience Database
- IgA and Mucosal Homeostasis - Madame Curie Bioscience DatabaseIgA and Mucosal Homeostasis - Madame Curie Bioscience Database
- Epithelial-Mesenchymal Transformation in the Embryonic Heart - Madame Curie Bios...Epithelial-Mesenchymal Transformation in the Embryonic Heart - Madame Curie Bioscience Database
- Inferring Evolutionary History through Inter- and Intraspecific DNA Sequence Com...Inferring Evolutionary History through Inter- and Intraspecific DNA Sequence Comparison: The Drosophila janus and ocnus Genes - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...