NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
There are many fundamental similarities in the biology of Drosophila and vertebrates, and Drosophila has become a prominent model organism for studies of animal development. Here the development of the different vertebrate muscle types (skeletal, cardiac and smooth) is compared with their anatomical counterparts in Drosophila (somatic, heart/ cardiac and visceral). The similarities are highlighted and attention is drawn to any differences. The chapter emphasizes, in particular, the impact of Drosophila on the genetic analysis of muscle development. The body of research covered herein also allows an assessment of the extent of the similarity between different aspects of Drosophila and vertebrate muscle development. One outcome of this is an evaluation of the usefulness of Drosophila to inform a variety of studies of clinical significance.
Introduction
There are fundamental similarities between the biology of the fruit fly, Drosophila melanogaster, and that of vertebrate species, including ourselves. While this statement may be readily accepted today, not so many years ago the extent of the similarity was less clear. Similarity at the molecular genetic level has now been brought into sharp focus through the extensive genome sequencing and annotation of the Genome Projects. Such projects have revealed that 60% of Drosophila proteins share sequence similarity with human proteins.1 This genetic conservation is one reason why Drosophila is regarded by many as a super model organism for the analysis of animal development. Other key reasons are the wealth of knowledge accrued in one hundred years of laboratory-based research, the range of available experimental techniques, and the speed and relatively low cost of experimentation. Importantly, genetic analysis of gene function is greatly facilitated in Drosophila because its genome is simpler in composition than its vertebrate counterparts.
A crucial question for muscle biologists, given the experimental advantages of Drosophila, is how similar are the many facets of muscle development between Drosophila and vertebrate species. Fundamental similarities highlight key events and underscore the utility of using a simpler model organism with a view to understanding other animals, and to developing new clinical approaches. Differences indicate how animals use similar gene sets and developmental mechanisms to produce different morphological outcomes. They also caution against simple extrapolation from one species to another.
Vertebrate muscle is categorized into three major muscle types defined by their structural and functional properties: skeletal, cardiac and smooth. The latter includes blood vessel muscle and the visceral muscle surrounding the gut. In this chapter I will restrict myself to these three major muscle types and for this comparison I will equate them to their anatomical counterparts in Drosophila—the somatic (or body wall) muscle, heart, and visceral muscle respectively. There is no Drosophila counterpart of vertebrate blood vessel smooth muscle. The extent to which the different muscle types are physiological equivalents in Drosophila and vertebrates is beyond the scope of this chapter. Nevertheless, and although there are differences in the anatomy and some of the cell biology, it remains apparent that when the development of each muscle type is analyzed there are many similarities between Drosophila and vertebrates.
The coverage of the broad topic of this chapter is necessarily selective. Firstly, it will focus mainly on skeletal/somatic muscle, reflecting the area of most research. Secondly, much of the chapter will focus on the development in the Drosophila embryo of the larval muscles, because this is where most is known. However, references will be made to the Drosophila adult because although some aspects of the development of the adult tissue may essentially be a recapitulation of the embryonic development, other aspects differ and serve as interesting comparisons with vertebrate species. Finally, in making comparisons with Drosophila only specific, illustrative aspects from the wide range of vertebrate species studied are included. The aim here is to highlight some areas of interest, rather than to be comprehensive.
Overview of Drosophila Muscle Development
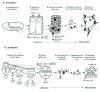
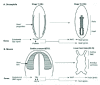
Drosophila somatic muscle, cardiac muscle, and the visceral muscle all develop from the mesoderm,2 which is the layer of cells between the endoderm and ectoderm in the gastrula. It is specified in the ventral region of the syncytial blastula embryo. At gastrulation the mid-ventral cells invaginate and spread in a layer under the ectoderm to form the mesoderm proper. At this stage they are not committed to a specific cell fate. Then, during the next few hours, the embryo becomes segmented and each mesodermal segment becomes subdivided along the anterior-posterior (A/P) and dorsal-ventral (D/V) axes (Fig. 1). The cells proliferate, diversify, and commit to different cell fates that include the three major muscle types together with other mesodermal derivatives (e.g., the fat body, which is equivalent to vertebrate liver). Concurrently, the mesoderm begins to separate into two cell layers, classically known as the somatic and splanchnic mesoderm.3 The somatic muscles derive from the external, somatic mesoderm and the visceral muscles derive from the internal, splanchnic mesoderm.3 The cardiac muscle derives from the most dorsal, external mesodermal cells. Progenitor populations of each of these derivatives develop at specific positions along the A/P and D/V axes in each segment. How the heart and visceral muscle subsequently develop from these cells is described later. The process for the somatic muscle can be summarized as follows.2,4-6
In each abdominal hemisegment of the Drosophila embryo a stereotypic pattern of thirty distinct larval somatic muscles develops. Each muscle has characteristic properties, including its size, shape and innervation. Within the somatic mesoderm individual muscle progenitors are singled out from their neighbors, while the other cells become the “fusion-competent myoblasts”. Progenitors divide asymmetrically to make two “founder” cells, or in certain cases one founder cell and one adult muscle precursor. The adult muscle precursors will later proliferate and differentiate into the adult abdominal muscles. Other adult muscle precursors in the thoracic segments will form the intricate and diverse adult thoracic musculature. The founder cells in the developing embryo have a critical role. Each seeds the development of a specific muscle and endows it with specific characteristics through the expression of “muscle identity” genes. Each founder cell attracts and fuses with fusion-competent myoblasts to form mature individual multinucleate myotubes, the final syncytial muscles, which attach to specific sites on the epidermis.
Overview of Vertebrate Muscle Development
As in Drosophila, vertebrate skeletal, cardiac and visceral muscle develop from the mesoderm. During gastrulation the mesoderm forms by the recruitment and ingression of cells from the epiblast through the primitive streak, and later through the tail bud.7,8 Then, again as in Drosophila, the mesoderm is progressively subdivided and different regions form the progenitors of the different muscle types.
Skeletal muscle arises from the paraxial mesoderm that is present either side of the neural tube in two wide strips of loose unconnected cells or mesenchyme.7 The intermediate mesoderm lies lateral to the paraxial mesoderm and gives rise to the urogenital system (Fig. 1). Lying most laterally is the lateral plate mesoderm. The inner, splanchnic layer of the lateral plate mesoderm becomes the visceral muscle,7 while the cardiac muscle develops from bilaterally symmetrical regions of the lateral plate mesoderm that eventually come together at the midline.9
All skeletal muscles of the vertebrate body, together with some of the head, are derived from the somites.8,10 This chapter will focus on the trunk and limb musculature. The somites are transient structures that form from the paraxial mesoderm. They reveal an underlying segmentation, echoing the overt segmentation of Drosophila. However, unlike Drosophila in which segments appear simultaneously, in vertebrates the somites form sequentially from the anterior end of the paraxial mesoderm at regular time intervals.11
The next step in somitogenesis is the conversion from mesenchymal tissue to an epithelial ball. The cells in this epithelial somite are multipotent and progressively acquire specific fates under the influence of signals received from nearby cells. The somite subdivides into a dorsal dermomyotome and ventral sclerotome (Fig. 1). As the dermomyotome develops, its medial lip deposits myoblasts underneath into the myotome.12 These are the progenitors of the epaxial muscle, the deep dorsal muscles around the backbone, and they differentiate immediately. At the lateral lip muscle precursors are also deposited. These will make the hypaxial muscle, ie. all the ventrally, laterally and superficially located muscles. At inter-limb levels they again immediately differentiate and eventually make the abdominal body wall muscles. However, at limb level, they do not differentiate immediately, but instead migrate to the limbs where they first proliferate and then differentiate.7,13
In the development of the skeletal musculature, the first step is the formation of a scaffold of relatively small primary fibers.10,14-16 This requires that myoblasts fuse to form syncytia. Subsequently, secondary fibers are added alongside the primary fibers as the muscles grow (Fig. 1). A population of cells (satellite cells) is also put aside as a reservoir for subsequent growth and repair. Vertebrate skeletal muscles have many constituent muscle fibers. For example, small eye muscles have hundreds of fibers, whereas limb muscles of large animals may have a thousand times as many.10 Similarly, adult Drosophila somatic muscles are also composed of multiple fibers,17 although in contrast each larval Drosophila muscle fiber is composed of only a single syncytial fiber.
Somatic/Skeletal Muscle Development
The following sections describe and compare the different stages of somatic muscle development in Drosophila and skeletal muscle development in vertebrates.
Subdivision of the Vertebrate Somite and Drosophila Mesoderm
The subdivision of the vertebrate somite has been extensively reviewed elsewhere.8,12,18,19 Here the aim is to compare this process with the subdivision of a mesodermal segment of the Drosophila embryo. For the vertebrate examples, I will focus on the chick, where embryological manipulations have been used to analyze the process, and the mouse where genetic analyses are more readily undertaken. There are some species differences that will not be discussed here, but which are highlighted in other reviews.8,20
Experimental manipulations show that when both the vertebrate somites and the Drosophila mesoderm first form, the constituent cells can contribute to a broad range of mesodermal derivatives.7,8,21 However, cell fate subsequently becomes restricted, and both the somite and a Drosophila mesodermal segment become subdivided into groups of cells that will develop differently.
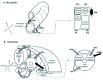
In the vertebrate somite there is a dorsal/ventral difference. The ventral region called the sclerotome will make cartilage and bone, the axial skeleton. The dorsal epithelial region called the dermomyotome underlies the surface ectoderm and gives rise to muscle and dermis (Fig. 2). Within the dermomyotome there is also a medio-lateral difference. The central region makes dermis, the mesenchymal connective tissue of the back skin. The medial region (closest to neural tube) makes epaxial muscle, and the lateral region (furthest from neural tube) makes hypaxial muscle.
A Drosophila mesoderm segment is subdivided too (Fig. 2). Along the A/P axis this is into two domains, one expressing the Even-skipped (Eve) transcription factor, the other expressing the Sloppy paired (Slp) transcription factor. It is also subdivided in the D/V axis. In the Slp domain, the most dorsal cells will develop into the heart, and the remainder will form the somatic musculature. In the Eve domain, the most dorsal cells form visceral muscle, and those more ventrally, the fat body.22-24
In both vertebrates and Drosophila there is a crucial role for the adjacent tissues in the development of these different cell fates. In the chick, ablation experiments and the surgical insertion of barriers have shown the importance of the surface ectoderm, neural tube, notocord and lateral plate mesoderm, and also that these adjacent tissues produce diffusable signals.25-27 These signals may be antagonistic. For example, the specification of the lateral somitic lineage results from the antagonistic actions of a diffusable medializing signal from the neural tube and a diffusable lateralizing signal from the lateral plate mesoderm.25,27
In insects, surgical ablation experiments were also used to indicate the role of the adjacent ectoderm.2,28 More recently, it was found that the adjoining ectoderm provides an important signal(s) for Drosophila mesoderm development in experiments where gastrulation was arrested,29 or mesoderm migration after gastrulation was inhibited.30-32
Signals That Subdivide
Having shown that adjacent tissues provide crucial signals, the next step is to identify them. In vertebrates, the general approach has been to analyze the effect of specific signals selected on the basis of their expression patterns. A substantial body of evidence, mainly from chick and mouse, has produced a model in which Wnt, Bone Morphogenetic Protein (BMP) and Hedgehog (Hh) signals are needed for the development of epaxial muscle, hypaxial muscle and sclerotomal cells (Fig. 2).
The first step in these analyses was to ask what specific signals could do. The combination of BMP4 from lateral plate mesoderm and a Wnt from the surface ectoderm was found to signal hypaxial muscle development.25,27,33 Whereas the combinatorial action of either Wnt 1 or Wnt 3 from the neural tube together with Sonic hedgehog (Shh) from the floor plate/ notocord, can signal epaxial muscle development.12,26,34 Shh alone can signal sclerotome development.35,36 However, there is much complexity to the subdivision of the somite and the subsequent development of different cell types. There are multiple steps and the details of the roles of the different signaling molecules are still unclear. For example, it is unclear if each of these signals is required for the initiation and/or the maintenance of the myogenic pathway of differentiation.8,37
Nevertheless, this body of work shows that BMP4, Wnts 1 and 3, and Shh can signal the development of specific somite compartments. The important question is whether they actually do so in vivo. Answering this requires loss-of-function studies, and is complicated by vertebrates having multiple, closely-related genes with complex, dynamic and often overlapping patterns of expression, e.g., the Wnt family. However, there have been some definitive genetic experiments. For example in the mouse, a double “knock-out” of Wnt 1 and Wnt 3a shows that these signals regulate the formation of the medial compartment of the dermomyotome that makes the epaxial muscle.38 A mouse “knock-out” also shows a role for Shh in epaxial muscle determination.39 In the chick, the introduction of a “dominant negative” construct to “knock down” function shows a role for Wnt 5b in the early steps of myogenesis.37 This is an alternative approach to assessing the in vivo role, and could be an informative general strategy if each dominant negative construct can be shown to be specific for a single signaling molecule.
Drosophila has great advantages for this type of study because genetic analyses can be readily undertaken and there are often only single copies of key genes. Indeed, a series of elegant genetic experiments has identified essential signals in the subdivision of the Drosophila mesoderm. A simplified version of one mesodermal segment is shown in Figure 2. Strikingly, the signals that are important in the development of different fates in the Drosophila mesoderm are similar to those implicated in the vertebrate somite. They are Decapentaplegic (Dpp), which is related to the vertebrate BMP family, Wingless (Wg), which is a Wnt family member, and Hedgehog (Hh), which is related to Shh.
Signaling from ectodermal Dpp is crucial in subdividing the mesoderm in the D/V axis,40,41 and is required for the development of some dorsal somatic muscles, visceral muscle and heart. The subdivision along the A/P axis is more complex. Each mesodermal segment is subdivided into two domains by the two aforementioned transcription factors, Slp and Eve. Somatic muscle and heart develop from the Slp domain where Slp cooperates with Wg signaling in the development of muscle and heart progenitors.24,42 Visceral muscle and fat body develop from the Eve domain. This requires eve function and is partially mediated by Hh signaling.23
Detailed comparisons between the subdivision of a Drosophila mesodermal segment and a vertebrate somite are problematic because of their different developmental anatomy and because signals are used at multiple times and for different events. Nevertheless, there are general similarities in both the molecular players utilized and the developmental strategies adopted.
Comparison of Drosophila and Vertebrates
In Drosophila, as in vertebrates, the development of progenitors for specific cell types is dependent on the activities of more than one signal. For example, dorsal somatic muscle progenitors in Drosophila require Dpp plus Wg, while those for vertebrate hypaxial muscle require BMP4 plus Wnt. In Drosophila, the activity of these signals is also dependent on intersecting with cells expressing specific complements of transcription factors. It is not yet apparent to what extent this is the case in vertebrates too. Some signals have opposite actions. For example, in vertebrates, dorsalizing Wnt signaling can antagonize ventralizing Shh signaling,12 while in the A/P subdivision in Drosophila, Wg signaling has effects opposite to those of Hh.23
Different skeletal/somatic muscles have different signaling requirements. In vertebrates, epaxial muscle develops in response to different signals than hypaxial muscle, while in Drosophila, wg is required for only a subset of muscle founder cells and hence a subset of muscle fibers.43,44 In another parallel, hh has a major role in the development of derivatives other than somatic muscle. In the case of Drosophila, this is for the visceral muscle and fat body, while in vertebrates it is for the sclerotome.
In both Drosophila and vertebrates, Wnt signaling from outside the mesoderm is critical. However, in chick there is also an early role for Wnt 5a expressed in the presomitic mesoderm before somites have budded off.37 This can be compared with Drosophila where although the major source of Wg is the ectoderm, a contribution from mesodermal wg expression early in mesoderm subdivision cannot be excluded.23,43,45
Wnts are the major dorsalizing signal in vertebrate somites.12 This contrasts with Drosophila where the key signal regulating the D/V subdivision is Dpp. However, there is evidence in the chick that the related signal (BMP) upregulates Wnt 1 and Wnt 3a in the dorsal neural tube.26
In Drosophila, in addition to the A/P subdivision within a mesodermal segment, there are also variations along the A/P axis between different segments in both the pattern of muscles and the characteristics of some muscles.46-48 This is under the control of the Hox genes, which encode the transcription factors required in all animals to establish segment specific differences. This is mirrored in vertebrates, where Hox genes confer the predisposition of somites at limb levels to produce migratory muscle precursors that will populate the limb buds and produce limb muscle.49
Conversion of Mesoderm Subdivision to Muscle Differentiation
The combination of molecular mechanisms described above serves to subdivide the mesoderm into groups of cells that develop into the skeletal musculature of vertebrates and the broadly equivalent somatic muscles of Drosophila. How is this information interpreted to make functional muscle? This issue can be divided into two aspects: (i) the earliest steps in which the patterning events initiate the first steps of muscle development, and (ii) later events that actually produce differentiated muscle. In this section I will address the first aspect. The second will be addressed subsequently.
The Vertebrate Myogenic Regulatory Factor Family
Progress in understanding muscle development was catalyzed by findings in a landmark paper that described the identification of MyoD in mice.50 This single factor can convert many cell types, including those of nonmesodermal origin, into myoblasts.51 The myoblasts can then differentiate into muscle. MyoD is a skeletal muscle-specific, basic helix-loop-helix (bHLH) transcription factor that directly activates gene expression by binding a conserved sequence found in many muscle gene promoters and enhancers. Because MyoD can trigger the muscle program in a different cell type, this gave rise to the idea that it was a master regulatory gene, one that coordinately regulates a cohort of genes to produce a specific phenotype. However, it quickly became apparent that there is more to muscle than MyoD. One aspect to this is that mice have a family of four closely related proteins with similar properties: Myf5, myogenin, MRF4, plus MyoD.51 Together they are often known as myogenic regulatory factors (MRFs).
Myf5 and MyoD are generally the first two MRFs expressed in vertebrate muscle development, around the time of somite formation. Genetic analyses in mice make it clear that Myf5 and MyoD both function in muscle specification. In a Myf5 “knock-out”, muscle development is essentially normal, although MyoD activation is delayed.52-54 Similarly, muscle development is essentially normal in a MyoD “knock-out”, although Myf5 expression is up-regulated.55 However, and strikingly, a Myf5/MyoD double mutant makes no muscle at all.56 Evidently, there is some sort of redundancy between MyoD and Myf5 and/or compensatory mechanisms operate.
In contrast, myogenin functions later in muscle differentiation. myogenin “knock-outs” have a striking phenotype with many mononucleate myoblasts, but very little differentiated muscle.57,58 Muscle progenitors develop, but differentiation does not proceed. MRF4 was also generally considered to function only in the later steps of differentiation, but recent findings in mice indicate an early role in muscle determination too,59 which now awaits further analysis.
It is clear that Myf5 and MyoD occupy pivotal points in muscle development and it is therefore important to understand what is upstream and downstream of these regulators, i.e., how they themselves are regulated and what their targets are. Evidence is accumulating that points of integration of the patterning information that subdivides the mesoderm are the promoters/ enhancers of the MRF gene family. For Myf5, many regulatory elements dispersed throughout a large genomic locus have been described.60-62 For MyoD, enhancers and promoters have also been defined in a number of species,63-65 and upstream regulators identified. For example, analysis of both mouse mutants and chick embryonic manipulations indicates that three of these regulators are Myf5, MRF4, and the paired class transcription factor, Pax3.53,59,66 As well as cross-regulation by other MRFs, a role for autoregulation of MyoD has been suggested by cell culture experiments,67 and has received support from work in Xenopus.68 Patterning signals also regulate MyoD. Notable amongst these in the mouse are the up-regulation of MyoD expression by Wnt signaling,37,69 and the down-regulation of MyoD expression by Notch signaling.70 Another pathway in Xenopus is the direct activation of MyoD by embryonic Fibroblast Growth Factor (eFGF), which can be linked directly to the earliest signals that shape the body plan.71 This differs from the mouse in that MyoD activation precedes somite formation in frogs, as it does in zebrafish. The challenge now is to establish how all these regulatory signals and transcription factors link up with the enhancers/promoters already identified.
Understanding the targets of MyoD and Myf5 is crucial to understanding their profound effects on muscle development. It is now possible to look for and analyze targets on a genome-wide scale. It has already been found that there are subprograms within muscle development, with MyoD regulating different targets at different times during myogenesis and with distinct mechanisms of control.72
What Is a Drosophila MRF?
The seminal MRF work in vertebrates raised the question of which gene(s) in Drosophila plays the role(s) of the MRFs. Drosophila appears to have only one gene whose sequence is closely related to vertebrate MRFs.73,74 It is called nautilus and the encoded protein has a bHLH domain that shares approximately 90% sequence identity with that of vertebrate MRFs. Outside this domain, it is highly divergent. Biochemically, Nautilus shares properties with the MRFs. It can convert 10T1/2 fibroblasts into myoblasts in combination with the ubiquitously expressed bHLH protein Daughterless.75 It is also required for the activation of the myogenic program in response to Daughterless in Drosophila SL2 cultured cells.76 Moreover, conversion of cardiac muscle to somatic muscle is reported in Drosophila embryos in response to Nautilus.77
Despite these physical and functional similarities between Nau and MRFs, genetic analysis has established that nautilus does not play an equivalent role to MRFs in embryonic muscle development. nautilus has a restricted pattern of expression in developing somatic muscle and is not essential for myogenesis. In nautilus mutants, most muscle development is unaffected, with only a small subset of muscle fibers missing.78 Nautilus is an example of how a similar molecule linked to a similar developmental pathway nevertheless has different functions in different species.
The Drosophila gene whose function is most closely related to the vertebrate MRFs is twist. It also encodes a bHLH transcription factor. At gastrulation twist is expressed uniformly throughout the mesoderm. Its expression then modulates. Within each segment there is a domain of low Twist expression that gives rise to visceral muscle, and a domain of high Twist expression that gives rise to somatic muscle. Slp may directly activate twist expression in the latter.6 Genetic analysis of twist function in muscle development is complicated by its essential role in gastrulation and mesoderm formation. However, a subsequent requirement in somatic muscle development was revealed using a temperature-sensitive allelic combination to reduce Twist activity after gastrulation.79 Twist also has a dominant effect on myogenesis, similar to that of the vertebrate MRFs in culture. Ectopic twist expression in the ectoderm or mesoderm induces cells to express muscle marker genes and form small syncytia.79,80 It pushes cells down the somatic muscle differentiation pathway. Twist homodimers have this effect, which contrasts with MRFs, where the major active species is a heterodimer with the ubiquitously expressed bHLH proteins.81-83 Note that possible in vivo roles for MRF homodimers detected in vitro have been suggested.84-86 The effect of Twist in Drosophila embryos appears more striking than that of MRFs in vertebrate embryos, where, in both frog and mouse, ectopic expression of MyoD can activate muscle genes in ectodermal cells, but not the entire myogenic program.87-89
Another parallel between Twist and MyoD is the links to signals that pattern the mesoderm. Notch signaling represses twist expression during mesoderm subdivision,90 as it does MyoD in vertebrates,70 while Wg positively influences twist expression through the slp gene,6,24,42 and Wnts up-regulate MyoD.37,69 Lastly, like the MRFs, which are first expressed in the proliferating, undifferentiated cells of the somite medial wall, Twist is expressed in the subdividing mesoderm in cells that will become the larval somatic muscle and which still divide.2
Mef2—A Key Muscle Differentiation Factor
Although Twist in Drosophila and the MRF family in vertebrates are clearly pivotal to muscle development, other factors are required to make skeletal muscle. The Myocyte enhancer factor- 2 (Mef2) transcription factors, which belong to the MADS family, are one of the other key players in skeletal/somatic muscle differentiation.91,92 This is an area of muscle development where research in vertebrates and in Drosophila has progressed together. Like the MRFs, the initial identification and characterization of Mef2 was through mammalian cell culture studies. Mef2 regulates muscle gene transcription through a binding site found in the promoter of nearly every known muscle specific gene.92 A striking finding is that Mef2 and MRFs synergize in the myogenic conversion of fibroblasts. They also synergistically activate muscle gene expression, either through binding to separate sites on the enhancer, or by physical interaction between Mef2 and an MRF at a single site.93
A general problem in developmental biology after finding that a specific molecule can activate a certain pathway, is to determine whether it actually does so in vivo. This question can often be most clearly and readily addressed in Drosophila. This has been the case for Mef2. Mice have four Mef2 genes with overlapping patterns of expression that complicates the “knock-out” analysis. Mef2c is the earliest of these four genes expressed in skeletal muscle development.94 However, Mef2c mutants are early embryonic lethal, and reported effects on skeletal muscle development are limited to an observation of a differentiation defect indicated by a reporter transgene.95 In contrast, Drosophila has a single mef2 gene. Genetic analysis has revealed a striking phenotype and advanced knowledge significantly. In mef2 mutants, correctly patterned somatic muscle precursors cells are produced, but these cells fail to fuse and differentiate further.91,96-98 The defect is therefore relatively late in the differentiation process, and in some ways this phenotype resembles the myogenin “knock-out” in mice. The role of mef2 during skeletal muscle development is therefore established in Drosophila. At present this remains to be clearly demonstrated in vertebrates, although the use of a “dominant negative” Mef2 construct has shown that Mef2 family function is required for MRFs to convert cultured cells into skeletal muscle.99 Drosophila mef2, like vertebrate Mef2 genes, is expressed in developing skeletal, heart and smooth muscle cells. While the focus here is on skeletal/somatic muscle, mef2 also plays a crucial role in the differentiation of both heart and visceral muscles. In fact, the analysis of Drosophila mef2 mutants identified mef2 as the first gene shown to control differentiation in multiple muscle cell types.91
There are direct links between Mef2 and the earliest steps of muscle specification described in the previous section. A variety of experiments in both Drosophila and vertebrates show that Twist and MRFs activate mef2 gene expression. Thus, in Drosophila, mef2 expression is activated by Twist,100,101 and MRFs directly activate Mef2c expression in skeletal muscle.102,103 There is also accumulating evidence from gene expression studies that Mef2 and MRFs activate and maintain the expression of each other. This picture is incomplete, but it may be very important for maintaining the muscle phenotype. This echoes the possible auto-activation and cross-activation of MyoD described earlier. In Drosophila, maintenance of mef2 expression in developing somatic muscle results from a direct positive feedback mechanism, in which Mef2 activates the mef2 gene.104 In mice, a more complex autoregulation occurs via myogenin, whose expression is controlled by MRFs and Mef2,105,106 together with a Mef3 site that binds Six/sine oculis homeoproteins.107 Taken together, these results point to evolutionarily conserved gene transcription loops to maintain the muscle phenotype.
To understand the place of Mef2 in muscle differentiation, one will need to understand not only how mef2 gene expression is regulated, but also the genes whose expression Mef2 controls. This will shed light on how Mef2 coordinates muscle differentiation. However, so far rather few genes are identified as direct targets of Mef2 in Drosophila somatic muscle development. They include: TmI, β3 tubulin, and Actin57B.108-110 An aspect that remains largely unexplored is that there are effects of Mef2 on gene expression earlier than generally supposed, before overt differentiation is underway. These targets include Dmeso18E and Actin57B.110,111
Lastly, the levels of transcription factors have received little attention when trying to understand how a relatively small number of transcription factors can coordinate all the temporal and spatial aspects of gene expression necessary for proper development. However, the level of transcription factors expressed at a certain time may be critical for normal myogenesis. For example, for the MRFs in mice, skeletal muscle development is sensitive to Myogenin levels,112 while for Mef2, experiments in Drosophila indicate that different levels of Mef2 are required for different aspects of muscle development.113
Muscle Patterning
Different animals have different and characteristic patterned arrays of muscles. In Drosophila, examples include the thirty distinct larval muscles in each abdominal hemisegment,114 and the intricate arrangement of at least seventy-eight muscles in the adult thorax.17 In vertebrates, an example is the forty muscles in the tetrapod limb.115 There are developmental characteristics shared by all these somatic/skeletal muscles. They all have a network of transcription factors to activate the muscle genes that encode the proteins that produce functional syncytial fibers with ordered assemblies of contractile proteins and attachments. However, different muscles also have distinct characteristics. Examples include their size and shape, their points of attachment, and their physiological/biochemical properties, e.g., slow- or fast-contracting.
There are two components to consider in the analysis of the development of these muscle arrays. First, is the pattern itself. How is the reproducible and distinctive pattern of muscles produced? How is a muscle directed to develop at a particular place? Second, how are different elements of this pattern conferred with their specific properties? The best understood example is the development of the abdominal larval muscle pattern during Drosophila embryogenesis. Central to this is the role of so-called “founder cells”.2,114,116-118
Drosophila Embryo Muscle Patterning
Analysis of grasshopper muscle development indicated how a muscle pattern could develop. Grasshoppers have single muscle pioneer cells that prefigure the muscle pattern. They are large cells that span the future muscle territories and act as a scaffold on which the muscle pattern is assembled.119 The pioneers also seed the muscle, that is, if the pioneer is ablated the muscle does not form, even though the myoblasts that would normally contribute are still present.120 In Drosophila, it was also found that the final larval abdominal somatic muscle pattern is prefigured by small muscle precursors, each comprising two or three fused myoblasts.114 Together they form a scaffold for the development of the larval musculature. Each precursor derives from a single founder cell, which itself is produced by the asymmetric division of a muscle progenitor.
Subsequent genetic analysis revealed two crucial properties of these founder cells that propelled Drosophila to prominence in consideration of how muscle patterns develop during animal development. First, in mutant embryos where myoblast fusion does not occur, founder cells differentiate into “mini muscles”, with the characteristics of the full size muscle that it would normally give rise to, including its innervation.118,121 Founder cells are thus revealed as a distinct population of myoblasts with intrinsic muscle pattern information.
Second, it was found that a number of genes, many encoding transcription factors, were expressed in small numbers of founder cells. These include Kruppel, S59/slouch, apterous and ladybird. They are not just convenient markers, their specific expression also translates into a specific function. Thus, muscle identity is specified by the autonomous function of these transcription factors.122-125 In normal development, founder cells fuse with the fusion-competent myoblasts to form the final syncytial muscles, and recruit the incoming nuclei to the characteristic pattern of identity gene expression of that muscle.2,117,118 However, it is not a case of one gene, one founder cell, as identity genes are expressed in overlapping patterns in multiple founder cells. Therefore, some form of combinatorial model for muscle identity is attractive, but how this might operate remains to be established.
Much also remains to be uncovered about how the events that pattern the mesoderm direct the activation of specific identity genes in specific founder cells. However, in the case of a dorsal muscle progenitor, detailed analysis has identified how many of these patterning signals are integrated at an identity gene enhancer to produce precisely localized gene expression.126,127 In summary, founder cells are central to Drosophila somatic muscle development. Each seeds the muscle, directs its fusion, and endows the developing muscle with characteristic properties. It is therefore an intriguing and pressing question whether this conceptually elegant paradigm has parallels in vertebrate muscle development.
Primary and Secondary Fibers in Vertebrate Myogenesis
In vertebrate development, there are two major waves of myogenesis.14,15 First, primary myofibers form from the fusion of mononucleate myoblasts. These primary fibers are the anlage for all future muscles and control the site of the subsequent assembly of secondary myofibers, which adds mass to the muscles.10,16 The primary fibers are small, but extend from tendon to tendon of the embryonic muscle and become innervated.128,129 These characteristics are similar to those of Drosophila embryonic founder cells revealed in the absence of myoblast fusion.118 Furthermore, like founders, the crucial role of primary fibers is to define the type, shape and location of a muscle.16 They serve as a scaffold to organize subsequent secondary fiber formation in a way that recalls Drosophila embryo muscle precursors or grasshopper pioneers. It is not yet known how the number and location of these primary fibers is regulated. However, comparisons with the development of the adult Drosophila musculature are likely to be helpful, because in some ways this more closely resembles the development of vertebrate skeletal muscle than does the development of embryonic Drosophila muscle.
Adult Drosophila Myogenesis
During Drosophila metamorphosis almost all larval muscles degenerate and are replaced by a set of adult muscles.2,130 These muscles differ greatly in size and strength according to the number and size of their constituent fibers.17 The fact that some adult Drosophila muscles consist of many fibers makes them more like vertebrate skeletal muscles, and contrasts with the single fiber muscles of the Drosophila embryo. Other similarities that contrast with the Drosophila embryo include the migration of myoblasts to make the muscles and the physiological differences between muscles. In the Drosophila thorax, myoblasts migrate from the imaginal discs to specific positions near the epidermis,131 while in vertebrates subpopulations of progenitor cells undergo long range migrations to form muscle masses in the limbs, diaphragm and tongue. There are also physiological differences between asynchronous and synchronous muscles in Drosophila,132,133 and between fast- and slow-contracting muscles in vertebrates.
The Drosophila adult muscles develop in characteristic positions with a characteristic number of constituent fibers. To reiterate the questions posed at the beginning of this section. How is this patterning regulated and how are the different characteristics of the muscles conferred? The example of the adult Drosophila flight muscles illustrates progress towards answering these questions. These thoracic muscles are grouped into the Direct Flight Muscles (DFMs) and Indirect Flight Muscles (IFMs). The latter have two sub-groups: the Dorso-Ventral Muscles (DVMs), and the Dorsal Longitudinal Muscles (DLMs). The DLMs are the largest adult muscles and are prominent examples of multifiber muscles.
The first muscle organizing features identified were for the DLMs. The DLMs assemble on templates provided by a small set of persistent larval muscles, which, in contrast to the other larval muscles, do not degenerate during metamorphosis.2,130,131,134 Incoming myoblasts fuse with these templates to produce the DLMs. These templates therefore act as a scaffold for DLM assembly, and so in some way this parallels the formation of secondary fibers on the primary fiber scaffold in vertebrates.
In contrast to the DLMs, all the other adult thoracic muscles are thought to develop de novo.2 For both the DVMs and DFMs, cells that prefigure these muscles have been identified through marker gene morphology and expression.135,136 Evidence is accumulating that these cells, and indeed others corresponding to adult abdominal muscles, are founder cells.137,138 The pattern of adult myotubes is prefigured by a pattern of myoblasts at appropriate locations that express the dumbfounded (duf) lacZ transgene.137 duf was characterized in the embryo as a founder cell specific gene involved in myoblast fusion.139 Analysis of the DVMs shows that the number of “founders” corresponds to the number of fibers in a muscle. In the embryo, a key feature of founders is that they can form small muscles in the absence of fusion.118 This is also true of these adult founders. When fusion is compromised they develop into mononucleate myosin-expressing fibers.137 In summary, myotube formation in the adult appears to be initiated by single founders identifiable by duf expression, just as in the embryo, and the number of fibers per muscle is defined by an appropriate number of founders.
Comparison of Muscle Patterning in Adult Drosophila and Vertebrates
It is apparent that vertebrate primary fibers share some characteristics with Drosophila founder cells. This emphasizes the importance of understanding the mechanisms that direct primary fiber formation. There might be a seeding event, which could be through founder-type myoblasts or from an environmental cue. Evidence for the latter comes from the vertebrate limb.140 When muscle cells differentiate here they immediately form a precisely oriented array that prefigures the future muscle pattern. This appears to be in response to a prepattern of the Wnt signaling effector, the TCF transcription factor, in lateral plate-derived cells in the developing limb.
In the Drosophila adult, it is not established how founder cells are selected from the population of muscle precursor cells, but as in vertebrates, it is suggested that the founder cell pattern may be specified by external cues.2,135-138 A second issue is how the number of fibers per muscle is defined. In Drosophila, this appears to be through the number of founders corresponding to the number of fibers,137 but how they are grouped to contribute to a single muscle is not yet known. The situation in vertebrates must be, at least temporally, different, as all muscles are formed in waves with no single period defining the number of fibers in adult muscle.16
How are specific attributes conferred on the muscles? In the Drosophila adult, there is evidence that at least some aspects of identity are specified in groups of myoblasts.141,142 The myoblasts associated with the wing imaginal disc that will make the adult flight muscles are divided into two populations. Those that express the Cut and Apterous transcription factors both vestigial and apterous are founder cell identity genes in the embryo. However, this emerging picture contrasts with the embryo where muscle identity is specified by the founder cell, although even in the embryo there are indications that the fusion-competent myoblast population might be heterogeneous in its gene expression.143
In vertebrates, there is also some evidence for genetic differences in the myoblasts. In the developing mouse limb, one specific subset of muscles is affected in Lbx1 mutants.144-146 Lbx1 is a homologue of Drosophila ladybird, a muscle identity gene in the embryo.124 In the mouse, the myoblast characteristic affected appears to be migration. A different subset of limb muscles is affected in Mox2 mutants, and in this case the effect is not on migration, but on another, not yet defined, aspect of muscle development.147
The specific muscle characteristic most worked on in vertebrates is whether fibers are fast or slow. The broad pattern of slow and fast fibers is defined during development,148,149 although it can be strongly influenced in the adult through nerve activity.10,150 Analysis in zebrafish has revealed that slow fibers are defined through the action of Hh signaling from axial midline tissue inducing the Blimp1 transcriptional repressor. Slow myogenesis can be driven by Blimp1 and ablated by Blimp1 down-regulation.151 In mice, the PPAR δ transcription factor can increase slow fiber number.152
Myoblast Fusion
A characteristic feature of almost all vertebrate skeletal muscles and the analogous somatic muscles in Drosophila is that they are multinucleate syncytia formed by myoblast fusion. There is a difficulty in studying the process in vivo during vertebrate development as fusion is asynchronous and takes place over a protracted period of weeks or longer. Following the finding that myoblast fusion occurs and can be manipulated in vitro, most vertebrate work has therefore been in cell culture. In contrast, in Drosophila much progress has resulted from an in vivo molecular genetic approach to analyzing muscle development. These studies have been driven by the powerful molecular genetics available, coupled to the fact that myoblast fusion to make the larval muscles occurs in a defined period of a few hours of embryonic development.
A consequence of the different experimental approaches is that the study of myoblast fusion in vertebrates and Drosophila has largely remained separate, and the extent of similarities and differences between them is not yet fully apparent. However, because of the underlying conservation of many aspects of muscle development, some significant similarities are anticipated. Already, it is clear that the basic cell biology of myoblast fusion is similar.153 It starts with cell attraction, followed by adhesion, alignment and finally membrane breakdown and fusion itself.
In vertebrate cell culture, molecules that influence these events have been identified.154 Examples include, cadherins and Cell Adhesion Molecules (CAMs) that are implicated in the recognition between newly differentiated myoblasts and fibers, and metalloproteinases called meltrins that are implicated in fusion itself.155-158 However, the microanatomy of these cultures is very different to that in normal muscle development and so it is essential to assess the in vivo role. However, and with some exceptions (for example, see below), the in vivo role of specific molecules in vertebrate myoblast fusion is not yet established. In some cases where it has been explored, e.g., N-cadherin and N-CAM,159,160 no phenotype is apparent and one explanation is genetic redundancy.
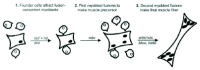
This is where studies of larval muscle development in Drosophila have had a significant impact. Genetic and expression based screens coupled to molecular genetic analysis have identified many molecules that play key roles in myoblast fusion in vivo.5,154,161-163 This work is summarized in the following section. In Drosophila, each larval muscle develops through a specific founder cell fusing with fusion-competent myoblasts (Fig. 3). This requires specific molecules to mediate the initial attraction between founder cell and fusion-competent myoblast, and then others to forge links to the cytoskeletal reorganisation that underlies the cell shape and membrane changes of fusion. There are at least two distinguishable stages in myoblast fusions to produce a syncytial muscle. First, muscle precursors with 2-3 nuclei form, and second these precursors enlarge by further fusions.
Molecular Pathways of Drosophila Myoblast Fusion
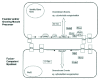
One chain of molecules that links the cell surface to the cytoskeleton has been established on the founder cell side of the process (Fig. 3).5,154,161-163 It starts with Duf, a founder cell-specific transmembrane protein belonging to the Immunoglobulin superfamily, which can function as a myoblast attractant and shares features with adhesion molecules. Duf binds to Rolling pebbles/ Antisocial (Rols/Ants), a founder cell-specific intracellular adaptor protein, which in turn binds Myoblast city (Mbc), an SH3 domain-containing cytoplasmic protein. Mbc interacts with both D-crk, an SH2 and SH3 adaptor protein, and Rac small GTPases that influence both the rearrangement and function of the cytoskeleton. Included in the targets may be D-Titin. There is a second route from Duf to Rac via a guanine-nucleotide exchange factor called Loner and the ARF6 GTPase. The relationship of this second route to the first signaling pathway is not yet understood. For example, do the two routes operate downstream of different cell surface interactions or at different stages of the fusion process?
This body of work has uncovered some of the molecules behind the cell biology of fusion. In addition, it already provides insights into two important characteristics of fusion: its two-step nature and its asymmetry. First the two steps (Fig. 4) differ molecularly. Thus, rols/ants, kette and blown fuse (blow) are not required for (all) initial fusions, but are required for the subsequent enlargement of the muscle precursors.164-166 Second, fusion is asymmetric. Founder cells fuse with fusion-competent myoblasts, but neither myoblast type fuses with itself.123,167 Some of the molecules, both membrane and intracellular, identified as players in Drosophila myoblast fusion, are expressed asymmetrically (Fig. 3). This may lie behind the observed asymmetry of fusion. For example, at the cell surface, Duf is expressed in founder cells, but not in fusion-competent myoblasts. In contrast, two related molecules, Sticks and stones (SNS) and Hibris (Hbs), are expressed in fusion-competent myoblasts, but not in founder cells. Intracellularly, both Rols/Ants and Loner are expressed in founder cells and not fusion-competent myoblasts.
The extent to which the molecular chain of myoblast fusion in Drosophila is recapitulated in vertebrates is not yet determined. However, all the molecules, except Blow, in the above scheme (Fig. 3) have relatives in vertebrates.163 Although it is not yet known whether they function similarly in muscle development, there are some relevant findings. For example, in vertebrate cell culture, there are indications that the roles of Mbc and ARF6 are conserved. Thus, DOCK 180, the vertebrate homologue of Mbc, affects cell morphology,168 and a dominant negative Drosophila ARF6 inhibits myoblast fusion.169 One mouse orthologue of Rols/ Ants is expressed transiently in developing muscle.170 In contrast, this is not the case with mouse SC-1 and Nephrin, the closest mammalian relatives to Drosophila Duf and SNS in the Immunoglobulin superfamily. Neither SC-1 nor Nephrin has been reported in the developing mesoderm. However, CDO and BOC, two other mammalian Immunoglobulin superfamily members that complex with cadherins are expressed during muscle development.171 Moreover, they stimulate myoblast fusion in vitro. Like SNS, Hbs and N-CAM they contain both Immunoglobulin-like and Fibronectin type III-like domains, which suggests a conserved role for this type of cell surface protein in myoblast fusion in Drosophila and vertebrates.
In light of these similarities, it will be of great interest to assess the role of myoblast fusion genes identified in Drosophila in vertebrate development. Also the assessment in vivo of vertebrate genes so far only analyzed in cell culture is clearly important too. Cell culture has revealed much about myoblast fusion and will continue to be important for analyzing the cell biology of the process, and an incisive approach is the combination of developmental genetics and cell culture. Here the phenotype of mouse mutants is assessed alongside the behavior in cell culture of mutant myoblasts isolated from the mice, and this has made for some interesting comparisons with Drosophila.
The Genetics Plus Cell Culture Approach
The first example of this was myogenin, one of the MRFs. Myogenin is a key regulator of muscle differentiation. One aspect of this is the ability to fuse, as revealed by the myogenin “knock-out” phenotype of a large number of unfused myoblasts. However, the nature of the defect is unclear, as myoblasts isolated from these mice will fuse in vitro.57 One can compare its position to Drosophila mef2, which functions after specification to drive differentiation, including fusion. Although myoblasts are specified in mef2 mutants, they do not fuse. Both mef2 in Drosophila and myogenin in mice sit near the top of a hierarchy of muscle differentiation of which fusion is a part, and provide links between the transcriptional network governing muscle differentiation and the process of making a functional muscle itself.
A second example is NFAT2C, a calcium sensitive transcription factor.172 The adult mouse phenotype suggests myofiber formation in embryogenesis is normal, but subsequent growth is altered. There are no embryonic development studies yet, but culture of NFAT2C mutant cells shows that they form smaller myotubes, indicating a muscle intrinsic role for NFAT2C in regulating myotube size. Mutant cells can differentiate and fuse to form the initial multinucleate cell containing 2-4 nuclei, but are defective in recruiting myoblasts or myotubes for subsequent growth. These results echo the Drosophila findings with rols/ants and indicate that there is a two-step process in vertebrate myoblast fusion too, in that fusion of muscle cells with myotubes/myofibers is distinct from the initial fusion of myoblasts to form a multinucleate cell. One player in this is Interleukin-4 (IL-4), which lies downstream of NFAT2C and acts as a secreted myoblast recruitment factor.173 IL-4 is specifically required for myoblast to myotube fusion, not for the distinct myoblast/myoblast fusion. A similar differential role for an attractant/recruitment factor in the two steps of fusion has not yet been attributed to any Drosophila molecule. Whether aspects of IL-4 function are similar to the Drosophila myoblast attractants Duf and Roughest (Rst) remains to be explored.
Mammalian fusion, like that in Drosophila, is also asymmetric and specific. For example, secondary myoblasts do not generally fuse with each other, instead they fuse primarily with the forming secondary myofiber.15,174 Two candidate molecules for a role in this are the potential recognition molecules, vLA-4 and VCAM1.175 They are asymmetrically expressed, with the integrin vLA-4 on secondary myotubes, and its receptor VCAM-1 on secondary myoblasts. This can be compared with the asymmetric distribution of cell surface molecules, and their intracellular links, between founder cells and fusion-competent myoblasts in Drosophila embryonic development.
A final example of the combination of developmental genetics and cell culture is integrin β1. The mouse Cre-lox system was used to inactivate integrin β1 specifically in skeletal muscle.176 Unfused myoblasts and small syncytia accumulate during embryogenesis. A cell culture study of the mutant cells shows that integrin β1 is necessary for a step subsequent to myoblast adhesion in myoblast fusion, and also for sarcomere assembly. In contrast, in Drosophila there is no indication of a defect in fusion in integrin mutants, although integrin is required to assemble organized sarcomeres, and for attachment to tendon cells.177
There is much interest in myoblast fusion, both because of various potential clinical applications and because it is a fundamental characteristic of many muscles. However, not all muscle is syncytial. A prominent example from one of the stalwarts of developmental biology is that Xenopus muscle is not fused until late in development.20 The slow muscle of zebrafish is also mononucleate.178
Drosophila Adult Muscle Precursors and Vertebrate Satellite Cells
In both vertebrates and Drosophila there are cells that do not immediately follow the differentiation pathway I have described, but instead remain single and undifferentiated (Fig. 1). In vertebrates, the “satellite cells”, which lie under the basal lamina that surrounds myofibers, are a major population of this type of cell and appear at late fetal and postnatal stages. Satellite cells mediate the post-natal growth of muscle and are the primary means by which the bulk of adult muscle is formed.179 They also have an essential role in both muscle hypertrophy and in muscle regeneration in damage and disease, and are activated by exercise or trauma to up-regulate MyoD or Myf5 expression and reenter the cell cycle. They proliferate and differentiate, and yet the population is maintained, probably through self-renewal.179
Many, but not all, aspects of satellite cell differentiation, including the MRF expression program, recapitulate differentiation during embryogenesis.180,181 However, although their developmental origin is not certain, it appears distinct from the embryonic myogenic lineage.179 The separation of the two pathways of development is illustrated by Pax7 mutant mice in which there are no satellite cells, but embryonic muscle development is relatively normal.182
Partly driven by the possibility of cell-based therapies for degenerative muscle disease, other studies have uncovered different cells that can contribute to muscle regeneration. First, in adult muscle there is a stem cell population distinct from satellite cells and often known as Side Population (SP) cells. In response to Wnt signaling and the activation of Pax7, they can replenish the satellite cell population during muscle regeneration.183 Second, nonmuscle, bone marrow cells can give rise to myogenic cells and repopulate damaged muscle.184
In Drosophila, the adult muscle precursors (AMPs), which arise during embryonic development, have similarities with vertebrate satellite cells. Like satellite cells they are quiescent, undifferentiated cells that are triggered to proliferate and eventually differentiate to make the muscle of the adult fly.2 In both Drosophila and vertebrates the bulk of adult muscle is produced from these cells either through the formation of new fibers or through fusion with existing muscle fibers. In contrast to vertebrates, the developmental origin of the AMPs is established in Drosophila, at least in the abdomen. Abdominal AMPs arise from an asymmetric division of a muscle progenitor cell that produces a founder cell and an AMP.4,123,185
Inhibition of Muscle Development
The molecular analysis of muscle differentiation has moved a long way from a simple model in which the key events were expression of pivotal positive regulators, the MRF family and Mef2, in the right time and place. It is apparent that there are multiple mechanisms to fine tune muscle differentiation. Many of these involve inhibition of differentiation. They are responsible for both spatial and temporal restriction of myogenesis. First, they ensure that the muscle development pathway is restricted to the appropriate group of cells, and second they ensure that some cells fated to become muscle do not differentiate immediately. An example of the latter is the maintenance of cells, e.g., vertebrate satellite cells and Drosophila AMPs, that are required for making or repairing muscle at a later time. There must be mechanisms to maintain these single cells in an undifferentiated state until appropriately triggered. Similarly, vertebrate muscle development occurs in waves.10,14-16 The first wave produces the primary muscle fibers, and the second produces the secondary muscle fibers and uses cells that have avoided earlier differentiation. Another example of escape from premature differentiation is the development of limb muscle. In the somite when some muscle differentiation is underway, other as yet undifferentiated cells have to migrate from the somite into the limb, proliferate and then make muscle there.7,13
These examples indicate that mechanisms to restrain muscle differentiation are required. In vertebrates, in some of the situations one would anticipate that Myf5 and MyoD are likely targets. These two MRFs are expressed while myoblasts are still proliferating and before their target genes are activated, in both cell culture and during development.70,186,187 Similarly, one anticipates mechanisms to down-regulate Drosophila Mef2, which is expressed significantly before muscle differentiation commences.100,111 Much of the early work to implicate specific molecules, e.g., FGF and TGFβ, in this crucial inhibitory function was undertaken in cell culture.186 This revealed that there are multiple levels at which inhibition of muscle differentiation can occur, including the inhibition of both the expression of myogenic genes and the activity of the encoded proteins. However, there are many gaps in knowledge of the in vivo importance and mechanism of action of specific molecules in muscle differentiation inhibition. Here I have selected examples where there is some in vivo information.
Molecules that Inhibit Muscle Development
Twist proteins are bHLH molecules. In cultured cells, mouse Twist inhibits muscle differentiation, and can inhibit the function of both MyoD and Mef2 proteins.188,189 Its in vivo role in muscle development is not established, but included in the complex phenotype of twist mutant mouse embryos there is a somite defect.190 Moreover, its expression pattern is suggestive. Mouse twist is expressed throughout the somite and then is excluded from the myotome at the start of myogenesis when MyoD and Myf5 are up-regulated, persisting only in the dermomyotome and sclerotome.191 A target for Shh in the sclerotome, where myogenesis must be suppressed, may be another inhibitor of MRF protein activity, I-mf.192 Together with Twist, and perhaps other proteins, it may restrict the population of cells that will go on to make muscle.
In Drosophila, twist appears to play a similar inhibitory role in the development of the adult Indirect Flight Muscles (IFMs). First, its expression declines in IFM progenitors prior to their fusion. Second, persistent Twist expression arrests IFM development, indicating that a decline in Twist is a requirement for differentiation of these adult muscles.193 This inhibitory effect appears to contrast with the positive muscle differentiation role for twist in embryonic development described earlier. However, this can be explained by consideration of the Twist dimerization partner. Thus, in the embryo whereas Twist homodimers promote myogenesis, a heterodimer of Twist and Daughterless, the homologue of vertebrate E-proteins, can inhibit it.80 The parsimonious model is that the mesoderm domains that make somatic muscle and which express high levels of Twist favor Twist homodimer formation, whereas the domains that make visceral muscle and fat body and which express low levels of Twist favor Twist/Daughterless heterodimer formation.80
Vertebrate Id proteins and Drosophila Extra macrochaetae (Emc) are related HLH proteins that lack a basic DNA-binding domain. Id can inhibit MyoD function through binding E-proteins, the MyoD heterodimerization partners, both in vitro and in cell culture.84,194 However, its role in vertebrate muscle development is not established. Nevertheless, after early widespread Id expression, Id and MRFs are expressed mutually exclusively in mouse development and Id is lost on myoblast differentiation in culture.195 A more general role for Id is indicated by its suppression of embryonic stem cell differentiation.196 In Drosophila, emc does have a documented role in muscle development, although the mechanism is not established. emc mutants have an extreme disruption of the somatic muscle pattern with muscle losses and detachment.197
In contrast to the proposed mechanism of action of Id, Twist and I-mf, which target the protein, ZEB/zfh1 is a conserved transcriptional regulator that might down-regulate muscle gene expression through binding to promoters/enhancers. Vertebrate ZEB is a zinc finger/homeodomain transcriptional repressor that binds to E-boxes and blocks myotube formation in culture.198Drosophila Zfh1 is also a transcriptional repressor.199 In development its expression declines prior to muscle differentiation and loss-of-function mutants have aberrant muscles.
The Notch signaling pathway is a widely used route to influence differentiation.200 In vertebrate muscle, activation of the Notch pathway keeps cultured cells undifferentiated.187 In development, it may prevent premature differentiation as activation of the Notch pathway down-regulates MyoD and inhibits muscle differentiation in both chick somite and limb bud.70,201 Similarly, in adult Drosophila muscle development, persistent activated Notch expression causes a failure of IFM differentiation.193 There are likely to be multiple targets for Notch in its effects on muscle development. In the IFMs one link may be Twist, which inhibits muscle development at this stage. Persistent Notch signaling causes continued Twist expression, and reduced Notch signaling reduces twist expression.193
Analysis of the effects of Notch on embryonic Drosophila muscle development has revealed a complex situation with effects at different stages of the process.90,202-206 One aspect that has some parallels with vertebrates is that during the subdivision of the mesoderm Notch down-regulates twist expression. In the embryo, it is the domains expressing high Twist levels that go on to make muscle. Notch represses twist both directly through its nuclear effector Suppressor of Hairless (Su(H)) and indirectly through activating emc.90
There is only limited information on the mechanisms that hold Drosophila AMPs and vertebrate satellite cells in an undifferentiated state, but some of the same players are implicated. For example, Notch is activated in satellite cells as they progress from quiescence to active proliferation, and attenuation of Notch signaling leads to MRF expression and commitment to muscle fate.207 In an assessment of gene expression changes in an in vivo muscle regeneration system, both twist and Id were induced at early time-points.181 One striking characteristic of Drosophila AMPs is that they continue to express Twist when quiescent or proliferating, but it declines when they differentiate.2,131,208
An emerging angle likely to be critical for muscle differentiation is the role of chromatin. For example, a specific linker histone, via an interaction with the homeodomain protein Msx1, can repress MyoD expression and inhibit muscle differentiation.209 More generally, there is the role of Histone Acetylases and Deacetylases, which can regulate muscle differentiation through a variety of interactions with MRFs and Mef2 proteins.210 In vivo assessments of their role in muscle development are awaited with interest.
In summary, there is much to learn about the crucial aspect of inhibition of muscle differentiation. It is already apparent that inhibition occurs in many ways and although similar players are used in Drosophila and vertebrates, exactly how they are used may differ as the example of Twist illustrates. Moreover, even in one animal what holds for one muscle group might not hold for another. Thus, although in Drosophila adult muscle development Notch inhibits IFM differentiation, the nearby DFMs appear unaffected.193
Heart Development in Drosophila and Vertebrates
The considerable recent advances in the molecular genetics of heart development in both Drosophila and various vertebrates have been extensively reviewed.211-215 In this comparison of Drosophila and vertebrates I will highlight just some aspects of particular interest. The heart (or dorsal vessel) of Drosophila functions analogously to the vertebrate heart. Both pump in a posterior to anterior direction, although Drosophila is an open circulation without blood vessels, in contrast to the closed circulation of vertebrates. In both the Drosophila and vertebrate heart there are two major cell types. In Drosophila, they are the cardioblasts, which are the contractile muscle cells with a similar ultrastructure to mammalian cardiomyocytes,211 and the pericardial cells, which form a layer outside the muscle cells. In contrast, in vertebrates the second cell type, the endothelial cells, are interior to the muscle. Although the structure of the Drosophila heart at first appears very different to that of vertebrate hearts, which as a group differ substantially themselves, there are considerable similarities in how the heart in all these species develops (Fig. 5).211
Morphogenetic Movements of Heart Development
In both Drosophila and vertebrates, e.g., mice, heart precursors are generated bilaterally in the mesoderm under the influence of inductive signaling from adjacent germ layers (Fig. 5).211 These precursors then move towards the midline where they form a linear, contractile tube. In vertebrates, it is only subsequently that this tubular heart loops and develops into the multi-chambered and physiologically complex organ with which we are familiar. The heart develops dorsally in Drosophila, but ventrally in vertebrates. This is considered to be an illustration of the inversion of the D/V axis between arthropods and vertebrates, which was proposed in 1822,216 and which has received support from more recent studies.217
The similarity between Drosophila and vertebrates extends to the molecular genetic basis of heart development (Fig. 5). This was first analyzed in Drosophila, where studies identified a number of genes with key roles that proved to be similar in Drosophila and vertebrates. They have been assembled into a conserved pathway controlling heart development centered on signaling molecules, for example the BMP family, and several transcription factors, notably the Tinman and GATA families.
Signals in Heart Development
The heart develops from a population of mesodermal cells whose commitment to heart fate depends on signaling from adjacent tissues. In Drosophila, a key signal is Dpp, a member of the BMP superfamily with a major role in D/V patterning in early development and a subsequent role in the subdivision of the mesoderm that refines cell fate. dpp mutants do not form heart progenitor cells.211 The Dpp signal is from the overlying ectoderm. This contrasts with vertebrates where it is signals from the adjacent endoderm that have a key role.213 However, evidence is accumulating that one of these endodermal signals is also a BMP. BMPs are expressed in the right time and place and can ectopically induce heart genes.214 Loss-of-function analyses are, however, less clear-cut. BMP antagonists suppress early heart development in chick,218 and heart mesoderm is not formed in BMP2 mutants in zebrafish,219 nor after expression of dominant negative BMP receptors in Xenopus.220 However, interpretation of the phenotypes is complicated by the signals acting at multiple points in development and the possibility that the phenotypes are a consequence of more general effects.214,221 In mice, BMP2 null mutants have abnormal heart development, but, contrary to the proposed BMP role in early heart development, produce cardiac mesoderm.222 Again, the interpretation of this phenotype of a single gene “knock-out” is compromised by other related genes with overlapping expression patterns and functions.214
Other signals have important roles. One example is the Wnt family. In Drosophila, wg is required at multiple times for heart development.223,224 Its effect, at least for the early phase, is mediated by the canonical wingless signaling pathway.225 Wnt signaling is also important in vertebrates. However, here it is a noncanonical Wnt signaling pathway that promotes cardiogenesis, whereas canonical Wnt signaling inhibits it.211,213,214,226
Transcription Factors in Heart Specification
A conserved target for Dpp/BMP signaling in both Drosophila and vertebrates is the homeobox containing transcription factor Tinman/Nkx2-5. The tinman (tin) gene was discovered in Drosophila. It is expressed in heart progenitors, and mutants show it is required for the specification of all heart cells.211,227,228 However, Tin is not sufficient to promote cardiogenesis, in contrast to MRFs and Twist in skeletal muscle. Dpp directly activates tin expression via its transcription factor effectors, the Smads, which bind directly to a tin enhancer.229 Here they synergize with Tin itself, bound to adjacent sites. This recalls the auto-regulation in the transcriptional control of skeletal muscle development. A second gene essential for Drosophila heart development is pannier, which encodes a GATA family transcription factor. Pannier together with Tin can induce expanded heart gene expression.230,231
Transcription factors related to Tin and Pannier also have roles in vertebrate heart formation, illustrating the underlying similarity of the transcriptional regulatory circuits of heart development in Drosophila and vertebrates (Fig. 5). In vertebrates, the Nkx2 family are the tin-related genes. Analysis is again complicated by the number of genes and their overlapping expression patterns. Moreover, different combinations of Nkx genes are expressed in the developing heart in different species.232 However, Nkx2-5 stands out as the gene expressed in all vertebrate hearts. The mouse “knock-out” of Nkx2-5 is embryonic lethal. However, in contrast to Drosophila tin, Nkx2-5 is not required for heart specification, instead mutants have severe early disruption of heart tube morphogenesis.232-234 Is there redundancy obscuring an earlier Nkx2-5 role? It is not clear yet, although in mouse, the only other Nkx gene expressed in the myocardium is Nkx2-6, and Nkx2-6 mutants have no heart phenotype. Nevertheless, an earlier role for tin homologues in vertebrate development is suggested, but not proven, in Xenopus, where dominant negative or repressor Nkx constructs block heart formation.235,236 Another, later role is indicated by the association of defects in heart valve and septal development with Nkx2.5 mutations in humans.237,238
Despite the conserved role of tin and Nkx2-5 in cardiogenesis, the mouse Nkx2-5 protein does not have all the same functions as Tin. When tested for its ability to rescue the tin mutant phenotype in Drosophila, although it can rescue some aspects, it cannot rescue the heart phenotype.239,240 In contrast, zebrafish Nkx2-3 can.239 Nkx2-5, like tin, is a direct target for transcriptional activation by Smad proteins.241 Despite this similarity, the independence of this Nkx2-5 enhancer from Nkx2-5 binding indicates a difference in the transcriptional control of Nkx2-5 and tin expression during heart development in vertebrates and Drosophila. In summary, Tin has many parallels with its vertebrate counterparts, but there are clearly differences too.
GATA transcription factors are implicated in vertebrate heart development. Three GATA genes, GATA 4, 5 and 6, are expressed in the developing heart,214 and they can activate a range of heart genes in cultured cells.221,242 Mouse “knock-outs” of each of GATA4, 5 and 6 have been analyzed to make an in vivo assessment of their role in heart development.214 However, interpretation is again complicated by the overlapping expression and possible function of different family members, and also by their roles in other events and tissues, for example in endoderm differentiation. Apart from extrapolating from the documented role of Drosophila pannier, there are two striking reasons for pursuing the analysis of the precise role of GATA factors in vertebrate heart development. Firstly, overexpression of GATA4 in Xenopus and of GATA5 in zebrafish can induce ectopic, beating tissue.221,243 Secondly, GATA4 mutations are responsible for a class of congenital heart defects in humans.244
In both Drosophila and vertebrates heart development, there is evidence for a mutually reinforcing regulatory network centered on Tin and GATA transcription factors. This has some parallels with the relationship between MRFs and MEF2 in skeletal muscle. For example, in Drosophila, pannier is regulated by Tin.245 In mice, the GATA6 promoter contains functionally important Nkx2-5 binding sites, and the Nkx2-5 promoter contains GATA sites involved in early heart field expression.214
Heart Differentiation
In Drosophila, the Mef2 transcription factor is required for proper differentiation of the heart, but not for its specification and basic morphogenesis.96,97 Many, but not all, genes encoding components of the contractile apparatus are not expressed in mef2 mutants.212 Drosophila mef2 is a target of both Tin and Pannier and so lies downstream of these regulators in the genetic pathway controlling heart development.230,246,247
Mef2 genes are also important in vertebrate heart development, although the picture of its role is less complete. In mouse Mef2c mutants, there is a failure in normal cardiogenesis and differentiation. For example, looping does not occur and some cardiac muscle genes are down-regulated.248 In part of the developing heart, one GATA factor target is Mef2c.249
It is now apparent that cardiac gene transcription is regulated by a number of transcription factors acting in various combinations.212,250 This includes members of the Nkx2, GATA and Mef2 families, together with SRF, Myocardin and the T-box factor, Tbx5. Many of these transcription factors form complexes through protein/protein interactions.212,250 Prominent amongst these is Myocardin, which binds to SRF to regulate gene transcription and is implicated in both Xenopus heart development and in cardiomyocyte differentiation.251,252
Patterning
Some progress has been made into the control of development of different regions of the heart. In Drosophila, the most obvious subdivision of its linear heart tube is into a posterior “heart proper” and a more anterior aorta (Fig. 5). This heart proper is specified by the Hox gene, AbdA.253-255 The aorta region itself is also subdivided along the A/P axis, and Hox genes function in this too.256 In vertebrates at the beating heart stage, there is polarity along the A/P axis. The regions that will form the different subdivisions of the final heart structure, e.g., right ventricle, atria, are found at defined positions along this axis.211 There are suggestive, but not compelling, indications of Hox gene involvement in A/P subdivisions of the developing vertebrate heart.253,254,257
One gene with a clear region-specific role is that encoding the dHAND bHLH transcription factor. In mouse “knock-outs” for this gene, development of the right ventricle fails.258 This phenotype reflects the specific expression of dHAND in the developing ventricle. A related factor, eHAND, is expressed in the developing left ventricle. Drosophila has a single Hand gene, which is expressed throughout the heart, but no functional information is available yet.259
Visceral Muscle Development
In general, much less is known about vertebrate smooth muscle than about skeletal and heart muscle. Nevertheless, smooth muscle is very important clinically and detailed knowledge of its development and differentiation is likely to have significant applications in many diseases. It is the muscle of the blood vessel wall and the digestive tract, and also of the respiratory and urogenital systems. Here I will compare vertebrate digestive tract muscle with the visceral muscle surrounding the Drosophila midgut (Table 1). Drosophila hindgut visceral muscle is described elsewhere.260,261
Table 1
A comparison of visceral muscle development in Drosophila and vertebrates.
Gut muscle has analogous developmental origins in vertebrates and Drosophila. In vertebrates, precursors are lateral plate mesoderm cells from the splanchnic, or inner, layer,7 and in Drosophila, visceral muscle precursors also derive from the splanchnic layer of the mesoderm.3 The differentiated muscle has similarities too, and in both Drosophila and vertebrates there is a lattice of circular and longitudinal musculature.3,262 There are also differences. Drosophila midgut visceral muscle is syncytial,263,264 whereas vertebrate visceral muscle is generally regarded as unicellular. Also, in Drosophila, both larval and adult visceral muscle is striated,2,17 although other aspects are like vertebrate smooth muscle.265
In Drosophila, the circular visceral muscle develops from clusters of cells, one per hemisegment along each side of the embryo. These clusters derive from part of the eve domain of each hemi-segment defined by the intersecting A/P and D/V cues described earlier and express the homeodomain transcription factor Bagpipe (Bap).23 Hh and Dpp signals from the ectoderm, together with Bap are sufficient for the development of the circular muscle primordia.23 Although few details are established, similar signals are implicated in vertebrates. For example, there is Hh family signaling between the epithelium and muscle in the intestinal wall, and both Shh and Indian hedgehog mutants have reduced smooth muscle.266 Furthermore, BMP signaling lateralizes developing mesoderm and lateral plate may require high BMP4.267
The Role of Bagpipe and FoxF Family Transcription Factors
Bagpipe (bap) is required for Drosophila visceral mesoderm development,228 and is part of a transcriptional hierarchy. It is a target of Tin whose expression is in turn dependent on Twist. bap expression is not maintained during visceral muscle development and so differentiation itself must occur through downstream genes, e.g., vimar and β3 tubulin.268,269 A key downstream target is biniou (bin), which is specifically expressed in all visceral muscle and encodes a protein that belongs to the FoxF subfamily of Forkhead transcription factors. In its absence, differentiation of visceral muscle fails, and ectopic Bin expression in the somatic mesoderm ectopically activates visceral mesoderm genes.265
Two vertebrate Bin orthologues, FoxF1 and Fox F2, are expressed in the splanchnic mesoderm and the derived intestinal smooth muscle (see ref. 265). Consistent with this, foxF1 has a role in lateral plate differentiation. In mouse foxF1 mutants, the separation into somatic and splanchnic layers is often absent or incomplete,270 while in Xenopus foxF1 “knock downs” the visceral mesoderm does not differentiate normally.271 Another point of similarity between vertebrates and Drosophila is the relationship between BMP family signals and the bin orthologues. First, in Drosophila, Dpp regulates bin expression,261 and in vertebrates there appear to be many links between BMP4 signaling and foxF1.270,271 Second, a specific enhancer of dpp is a direct target of Bin,265 and normal BMP4 expression in the vertebrate lateral plate requires foxF1.270 Moreover, in the mouse Bin is coexpressed with the bap orthologue bapx1 in the splanchnic mesoderm, although bapx1 is expressed elsewhere too.272 These and other findings have led to the suggestion that the “splanchnic mesoderm layers in Drosophila and vertebrates are homologous structures whose development into gut muscle and other visceral organs is critically dependent on FoxF genes”.265 There are some differences of course, such as in Xenopus where bap is not expressed until after foxF1.271
There have been significant advances in understanding the gene expression pathways that govern determination and differentiation in skeletal and cardiac muscle. However, much less is known about gene expression in smooth muscle differentiation. Notwithstanding this, targets of Bap and Bin and their orthologues are clearly going to be important. Moreover, in vertebrates, many, but not all, smooth muscle genes have functionally important CArG boxes in their promoter/enhancer regions.273 These bind the widely expressed SRF transcription factor together with the smooth and cardiac muscle restricted coactivator, Myocardin.251,274 It is not known whether this regulation has parallels in Drosophila.
Mef2, which is structurally related to SRF, is important in differentiation. In Drosophila, and as with somatic muscle and the heart, mef2 is required for the differentiation, but not the specification, of visceral muscle.91 In vertebrates, I am not aware of any functional analysis of mef2 on digestive tract muscle development, although in mice, mef2c mutants have defects in smooth muscle differentiation in the vasculature,95 and in humans, mef2a mutations are linked to coronary artery disease.275
Visceral Muscle Founder Cells
As with Drosophila larval somatic muscle, in Drosophila visceral muscle development there are two types of cell, founder cells and fusion-competent myoblasts, that specifically express duf and sns respectively. There are two classes of visceral founders: those for the circular musculature develop from clusters in each trunk hemisegment and then form a continuous ordered file of cells along the A/P axis of the embryo; those for the longitudinal muscles develop at the posterior end of the germ band and subsequently migrate. There appears to be a common pool of fusion-competent myoblasts for both classes of muscle in the trunk.264,276 It has been argued that the founder cells really are true founders, not simply pioneers.264 Thus, in the absence of fusion they make mononucleate circular or longitudinal fibers, as appropriate, and fusion-competent myoblasts remain undifferentiated. Moreover, localized gene expression is initiated in subsets of the founders and spreads throughout the muscles arising from them by fusion.264 The finding of founder cells in another example of the development of syncytial muscles makes it clear that founder cells are not unique to Drosophila somatic muscle.
There is a novel signal implicated in the development of visceral founder cells. It is called Jelly belly (Jeb). It contains a LDL receptor motif, and is secreted by the nearby somatic mesoderm. 277 In jeb mutants, visceral muscle is not produced because all precursors become fusion-competent myoblasts and founder cells do not develop.278,279 The Jeb receptor is Alk, the Drosophila version of the tyrosine kinase receptor encoded by the human proto-oncogene Anaplastic Lymphoma Kinase.278-280 It is unknown whether there are vertebrate Jeb-like proteins that bind to Alk to affect muscle development, although there are other known ligands for vertebrate Alk.281,282
Analysis of jeb highlights the closeness of the relationship between visceral and somatic muscle and raises the question of how distinct the myoblast populations of somatic and visceral muscle are. Thus, in jeb mutants, with visceral founders not specified, the visceral fusion-competent myoblasts become incorporated into the somatic musculature.278-280 There is a close relationship in vertebrates too. Thus, transdifferentiation of smooth muscle cells into skeletal muscle is reported, for example in the mouse oesophagus,283,284 and there are cell lines with characteristics of both smooth and skeletal muscle.285 A counter example from Drosophila is in eve mutants where there is no midgut visceral mesoderm of the trunk. Longitudinal muscle founder cells still migrate and yet remain mononucleate despite the nearby somatic fusion-competent myoblasts.264
Concluding Remarks
In comparing diverse aspects of muscle development in Drosophila and in vertebrates it is apparent that there are many similarities. These include the developmental strategies, some of the cell biology, and the underlying transcriptional networks. Of course there are differences too, and I have highlighted some examples. Nevertheless, the overriding conclusion is that muscle development in Drosophila and in vertebrates shares fundamental similarities. This suggests that findings in one organism will continue to advance understanding in others. This can happen in many ways. For example, the orthologues of key genes identified in screens in Drosophila can be analyzed in vertebrates to provide new entry points into studies in these species. The direction of information transfer is certainly not just from Drosophila to vertebrates. Genes identified in vertebrates may be difficult and/or expensive to study in vertebrate systems and so a helpful strategy is to analyze orthologues in Drosophila. Mef2, which was first described in mouse, is an example of this. One major outcome of comparative analyses of different aspects of muscle development in different species is to gain insights into how different animals develop their distinct morphologies and functional attributes. Another important outcome is the opening of new approaches to the treatment of muscle-linked diseases.
A prominent example of how studies of muscle development in both Drosophila and vertebrates have advanced clinical understanding is the heart.286 Mutations in some of the key transcription factors involved in heart development, Nkx2-5, TBX5 and GATA4, are linked to human congenital heart disease.215 The Nkx2-5 story illustrates the synergism between Drosophila research and clinical investigations.286 This greatly accelerated the normally lengthy procedure of discovering the genetic basis of a disease, in this case, atrial septal defects and conduction abnormalities. Conventional linkage analysis identified a chromosomal region associated with these heart abnormalities. Of the many genes in the region, Nkx2-5 quickly became the focus of attention, as it was highly related to tin, which was already known to have a pivotal role in Drosophila heart development. In another aspect of heart disease, hypertrophy of the adult heart, it has also become apparent that some transcription factors that function in heart development, e.g., GATA4 and Mef2, play a central role here too.215,286
One possible route for the future repair of damaged and diseased skeletal, cardiac or smooth muscle tissue is the use of stem cell based therapies.184,287 Central to this will be a detailed knowledge of muscle cell differentiation both to monitor and to control the differentiation of stem cells. Approaches might involve introducing cells by transplantation or systemic injection, or signaling to endogenous stem cell populations. In skeletal muscle, for example, the latter approach might exploit the natural, but limited, repair capacity of adult muscle via its satellite cells, although much remains to be understood about this regenerative ability, including how close the parallels are with embryonic development. A final specific aspect of skeletal muscle differentiation of great clinical interest is myoblast fusion. This will be critical to the repair of damaged or diseased muscle tissue, and may also be a novel route for stable expression of molecules to treat various nonmuscle conditions.288,289
Whether your interest is in development, in comparative zoology, in the basic biology of muscle or the clinical treatment of muscle disease, there is much still to be uncovered from future studies of muscle development in Drosophila and the variety of vertebrate species.
References
- 1.
- Rubin GM. The draft sequences. Comparing species. Nature. 2001;409(6822):820–821. [PubMed: 11236995]
- 2.
- Bate M. The mesoderm and its derivatives. In: Bate M, Martinez-Arias A, eds. The Development of Drosophila melanogaster. New York: Cold Spring Harbor Press. 1993:1013–1090.
- 3.
- Poulson DF. 1950. Histogensis, organogenesis and differentiation in the embryo of Drosophila melanogaster. In: Demerec M, ed. Biology of Drosophila. Facsimile ed. New York: Cold Spring Harbor Press; pp. 168–274.
- 4.
- Ruiz-Gomez M. Muscle patterning and specification in Drosophila. Int J Dev Biol. 1998;42(3):283–290. [PubMed: 9654010]
- 5.
- Taylor MV. Muscle differentiation: How two cells become one. Curr Biol. 2002;12(6):R224–228. [PubMed: 11909553]
- 6.
- Carmena A, Baylies M. The development of the Drosophila larval somatic musculature. In: Sink H, ed. Muscle Development in Drosophila. Georgetown: Landes Bioscience. 2006:79–91.
- 7.
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl). 1995;191(5):381–396. [PubMed: 7625610]
- 8.
- Marcelle C, Lesbros C, Linker C. Somite patterning: A few more pieces of the puzzle. Results Probl Cell Differ. 2002;38:81–108. [PubMed: 12132400]
- 9.
- Redkar A, Montgomery M, Litvin J. Fate map of early avian cardiac progenitor cells. Development. 2001;128(12):2269–2279. [PubMed: 11493546]
- 10.
- Wigmore PM, Evans DJ. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int Rev Cytol. 2002;216:175–232. [PubMed: 12049208]
- 11.
- Pourquie O. The segmentation clock: Converting embryonic time into spatial pattern. Science. 2003;301(5631):328–330. [PubMed: 12869750]
- 12.
- Brent AE, Tabin CJ. Developmental regulation of somite derivatives: Muscle, cartilage and tendon. Curr Opin Genet Dev. 2002;12(5):548–557. [PubMed: 12200160]
- 13.
- Buckingham M, Bajard L, Chang T. et al. The formation of skeletal muscle: From somite to limb. J Anat. 2003;202(1):59–68. [PMC free article: PMC1571050] [PubMed: 12587921]
- 14.
- Kelly AM, Zacks SI. The histogenesis of rat intercostal muscle. J Cell Biol. 1969;42(1):135–153. [PMC free article: PMC2107573] [PubMed: 5786979]
- 15.
- Harris AJ, Duxson MJ, Fitzsimons RB. et al. Myonuclear birthdates distinguish the origins of primary and secondary myotubes in embryonic mammalian skeletal muscles. Developments. 1989;107(4):771–784. [PubMed: 2698800]
- 16.
- Patel K, Christ B, Stockdale FE. Control of muscle size during embryonic, fetal, and adult life. Results Probl Cell Differ. 2002;38:163–186. [PubMed: 12132394]
- 17.
- Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, ed. Biology of Drosophila. Facsimile ed. New York: Cold Spring Harbor Press. 1950:420–534.
- 18.
- Lassar AB, Munsterberg AE. The role of positive and negative signals in somite patterning. Curr Opin Neurobiol. 1996;6(1):57–63. [PubMed: 8794041]
- 19.
- Tajbakhsh S, Cossu G. Establishing myogenic identity during somitogenesis. Curr Opin Genet Dev. 1997;7(5):634–641. [PubMed: 9388780]
- 20.
- Chanoine C, Hardy S. Xenopus muscle development: From primary to secondary myogenesis. Dev Dyn. 2003;226(1):12–23. [PubMed: 12508220]
- 21.
- Beer J, Technau G, Campos Ortega J. Lineage analysis of transplanted individual cells in embryos of Drosophila melanogaster. IV. Commitment and proliferative capabilities of individual mesodermal cells. Roux's Arch Dev Biol. 1987;196:222–230. [PubMed: 28305697]
- 22.
- Borkowski OM, Brown NH, Bate M. Anterior-posterior subdivision and the diversification of the mesoderm in Drosophila. Development. 1995;121(12):4183–4193. [PubMed: 8575318]
- 23.
- Azpiazu N, Lawrence PA, Vincent JP. et al. Segmentation and specification of the Drosophila mesoderm. Genes Dev. 1996;10(24):3183–3194. [PubMed: 8985186]
- 24.
- Riechmann V, Irion U, Wilson R. et al. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124(15):2915–2922. [PubMed: 9247334]
- 25.
- Pourquie O, Fan CM, Coltey M. et al. Lateral and axial signals involved in avian somite patterning: A role for BMP4. Cell. 1996;84(3):461–471. [PubMed: 8608600]
- 26.
- Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124(20):3955–3963. [PubMed: 9374393]
- 27.
- Dietrich S, Schubert FR, Healy C. et al. Specification of the hypaxial musculature. Development. 1998;125(12):2235–2249. [PubMed: 9584123]
- 28.
- Bock E. Wechselbeziehungen zwischen den Keimblattern bei der Organbildung von Chrysopa perla L. Die Entwicklung des Ektoderms in mesodermdefekten Keimteilen. Wilhelm Roux' Arch Entwicklungsmech Org. 1941;141:159–247. [PubMed: 28354999]
- 29.
- Baker R, Schubiger G. Ectoderm induces muscle-specific gene expression in Drosophila embryos. Development. 1995;121(5):1387–1398. [PubMed: 7789269]
- 30.
- Maggert K, Levine M, Frasch M. The somatic-visceral subdivision of the embryonic mesoderm is initiated by dorsal gradient thresholds in Drosophila. Development. 1995;121(7):2107–2116. [PubMed: 7635056]
- 31.
- Gisselbrecht S, Skeath JB, Doe CQ. et al. Heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10(23):3003–3017. [PubMed: 8957001]
- 32.
- Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10(23):2993–3002. [PubMed: 8957000]
- 33.
- Fan CM, Lee CS, Tessier-Lavigne M. A role for WNT proteins in induction of dermomyotome. Dev Biol. 1997;191(1):160–165. [PubMed: 9356179]
- 34.
- Munsterberg AE, Lassar AB. Combinatorial signals from the neural tube, floor plate and notochord induce myogenic bHLH gene expression in the somite. Development. 1995;121(3):651–660. [PubMed: 7720573]
- 35.
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: Evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79(7):1175–1186. [PubMed: 8001153]
- 36.
- Johnson RL, Laufer E, Riddle RD. et al. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79(7):1165–1173. [PubMed: 8001152]
- 37.
- Linker C, Lesbros C, Stark MR. et al. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130(20):4797–4807. [PubMed: 12917295]
- 38.
- Ikeya M, Takada S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development. 1998;125(24):4969–4976. [PubMed: 9811581]
- 39.
- Borycki AG, Brunk B, Tajbakhsh S. et al. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999;126(18):4053–4063. [PubMed: 10457014]
- 40.
- Staehling-Hampton K, Hoffmann FM, Baylies MK. et al. Dpp induces mesodermal gene expression in Drosophila. Nature. 1994;372(6508):783–786. [PubMed: 7997266]
- 41.
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374(6521):464–467. [PubMed: 7700357]
- 42.
- Lee HH, Frasch M. Wingless effects mesoderm patterning and ectoderm segmentation events via induction of its downstream target sloppy paired. Development. 2000;127(24):5497–5508. [PubMed: 11076769]
- 43.
- Baylies MK, Martinez Arias A, Bate M. Wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development. 1995;121(11):3829–3837. [PubMed: 8582292]
- 44.
- Ranganayakulu G, Schulz RA, Olson EN. Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev Biol. 1996;176(1):143–148. [PubMed: 8654890]
- 45.
- Lawrence PA, Bodmer R, Vincent JP. Segmental patterning of heart precursors in Drosophila. Development. 1995;121(12):4303–4308. [PubMed: 8575330]
- 46.
- Greig S, Akam M. Homeotic genes autonomously specify one aspect of pattern in the Drosophila mesoderm. Nature. 1993;362(6421):630–632. [PubMed: 8096627]
- 47.
- Michelson AM. Muscle pattern diversification in Drosophila is determined by the autonomous function of homeotic genes in the embryonic mesoderm. Development. 1994;120(4):755–768. [PubMed: 7600955]
- 48.
- Capovilla M, Kambris Z, Botas J. Direct regulation of the muscle-identity gene apterous by a Hox protein in the somatic mesoderm. Development. 2001;128(8):1221–1230. [PubMed: 11262224]
- 49.
- Alvares LE, Schubert FR, Thorpe C. et al. Intrinsic, Hox-dependent cues determine the fate of skeletal muscle precursors. Dev Cell. 2003;5(3):379–390. [PubMed: 12967558]
- 50.
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. [PubMed: 3690668]
- 51.
- Weintraub H, Davis R, Tapscott S. et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science. 1991;251(4995):761–766. [PubMed: 1846704]
- 52.
- Braun T, Rudnicki MA, Arnold HH. et al. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71(3):369–382. [PubMed: 1423602]
- 53.
- Tajbakhsh S, Rocancourt D, Cossu G. et al. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89(1):127–138. [PubMed: 9094721]
- 54.
- Kaul A, Koster M, Neuhaus H. et al. Myf-5 revisited: Loss of early myotome formation does not lead to a rib phenotype in homozygous Myf-5 mutant mice. Cell. 2000;102(1):17–19. [PubMed: 10929709]
- 55.
- Rudnicki MA, Braun T, Hinuma S. et al. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71(3):383–390. [PubMed: 1330322]
- 56.
- Rudnicki MA, Schnegelsberg PN, Stead RH. et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75(7):1351–1359. [PubMed: 8269513]
- 57.
- Nabeshima Y, Hanaoka K, Hayasaka M. et al. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364(6437):532–535. [PubMed: 8393146]
- 58.
- Hasty P, Bradley A, Morris JH. et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364(6437):501–506. [PubMed: 8393145]
- 59.
- Kassar-Duchossoy L, Gayraud-Morel B, Gomes D. et al. Mrf4 determines skeletal muscle identity in Myf5: Myod double-mutant mice. Nature. 2004;431(7007):466–471. [PubMed: 15386014]
- 60.
- Hadchouel J, Tajbakhsh S, Primig M. et al. Modular long-range regulation of Myf5 reveals unexpected heterogeneity between skeletal muscles in the mouse embryo. Development. 2000;127(20):4455–4467. [PubMed: 11003844]
- 61.
- Summerbell D, Ashby PR, Coutelle O. et al. The expression of Myf5 in the developing mouse embryo is controlled by discrete and dispersed enhancers specific for particular populations of skeletal muscle precursors. Development. 2000;127(17):3745–3757. [PubMed: 10934019]
- 62.
- Buchberger A, Nomokonova N, Arnold HH. Myf5 expression in somites and limb buds of mouse embryos is controlled by two distinct distal enhancer activities. Development. 2003;130(14):3297–3307. [PubMed: 12783799]
- 63.
- Dechesne CA, Wei Q, Eldridge J. et al. E-box- and MEF-2-independent muscle-specific expression, positive autoregulation, and cross-activation of the chicken MyoD (CMD1) promoter reveal an indirect regulatory pathway. Mol Cell Biol. 1994;14(8):5474–5486. [PMC free article: PMC359067] [PubMed: 8035824]
- 64.
- Asakura A, Lyons GE, Tapscott SJ. The regulation of MyoD gene expression: Conserved elements mediate expression in embryonic axial muscle. Dev Biol. 1995;171(2):386–398. [PubMed: 7556922]
- 65.
- Kucharczuk KL, Love CM, Dougherty NM. et al. Fine-scale transgenic mapping of the MyoD core enhancer: MyoD is regulated by distinct but overlapping mechanisms in myotomal and nonmyotomal muscle lineages. Development. 1999;126(9):1957–1965. [PubMed: 10101129]
- 66.
- Maroto M, Reshef R, Munsterberg AE. et al. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89(1):139–148. [PubMed: 9094722]
- 67.
- Thayer MJ, Tapscott SJ, Davis RL. et al. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58(2):241–248. [PubMed: 2546677]
- 68.
- Lun Y, Sawadogo M, Perry M. Autoactivation of Xenopus MyoD transcription and its inhibition by USF. Cell Growth Differ. 1997;8(3):275–282. [PubMed: 9056669]
- 69.
- Tajbakhsh S, Borello U, Vivarelli E. et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125(21):4155–4162. [PubMed: 9753670]
- 70.
- Hirsinger E, Malapert P, Dubrulle J. et al. Notch signalling acts in postmitotic avian myogenic cells to control MyoD activation. Development. 2001;128(1):107–116. [PubMed: 11092816]
- 71.
- Fisher ME, Isaacs HV, Pownall ME. eFGF is required for activation of XmyoD expression in the myogenic cell lineage of Xenopus laevis. Development. 2002;129(6):1307–1315. [PubMed: 11880340]
- 72.
- Bergstrom DA, Penn BH, Strand A. et al. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell. 2002;9(3):587–600. [PubMed: 11931766]
- 73.
- Michelson AM, Abmayr SM, Bate M. et al. Expression of a MyoD family member prefigures muscle pattern in Drosophila embryos. Genes Dev. 1990;4(12A):2086–2097. [PubMed: 2176634]
- 74.
- Paterson BM, Walldorf U, Eldridge J. et al. The Drosophila homologue of vertebrate myogenic-determination genes encodes a transiently expressed nuclear protein marking primary myogenic cells. Proc Natl Acad Sci USA. 1991;88(9):3782–3786. [PMC free article: PMC51537] [PubMed: 1902570]
- 75.
- Zhang JM, Chen L, Krause M. et al. Evolutionary conservation of MyoD function and differential utilization of E proteins. Dev Biol. 1999;208(2):465–472. [PubMed: 10191059]
- 76.
- Wei Q, Marchler G, Edington K. et al. RNA interference demonstrates a role for nautilus in the myogenic conversion of Schneider cells by daughterless. Dev Biol. 2000;228(2):239–255. [PubMed: 11112327]
- 77.
- Keller CA, Erickson MS, Abmayr SM. Misexpression of nautilus induces myogenesis in cardioblasts and alters the pattern of somatic muscle fibers. Dev Biol. 1997;181(2):197–212. [PubMed: 9013930]
- 78.
- Balagopalan L, Keller CA, Abmayr SM. Loss-of-function mutations reveal that the Drosophila nautilus gene is not essential for embryonic myogenesis or viability. Dev Biol. 2001;231(2):374–382. [PubMed: 11237466]
- 79.
- Baylies MK, Bate M. Twist: A myogenic switch in Drosophila. Science. 1996;272(5267):1481–1484. [PubMed: 8633240]
- 80.
- Castanon I, Von Stetina S, Kass J. et al. Dimerization partners determine the activity of the Twist bHLH protein during Drosophila mesoderm development. Development. 2001;128(16):3145–3159. [PubMed: 11688563]
- 81.
- Murre C, McCaw PS, Vaessin H. et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58(3):537–544. [PubMed: 2503252]
- 82.
- Lassar AB, Davis RL, Wright WE. et al. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66(2):305–315. [PubMed: 1649701]
- 83.
- Neuhold LA, Wold B. HLH forced dimers: Tethering MyoD to E47 generates a dominant positive myogenic factor insulated from negative regulation by Id. Cell. 1993;74(6):1033–1042. [PubMed: 7691411]
- 84.
- Benezra R, Davis RL, Lockshon D. et al. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61(1):49–59. [PubMed: 2156629]
- 85.
- Mitsui K, Shirakata M, Paterson BM. Phosphorylation inhibits the DNA-binding activity of MyoD homodimers but not MyoD-E12 heterodimers. J Biol Chem. 1993;268(32):24415–24420. [PubMed: 8226992]
- 86.
- Li FQ, Coonrod A, Horwitz M. Preferential MyoD homodimer formation demonstrated by a general method of dominant negative mutation employing fusion with a lysosomal protease. J Cell Biol. 1996;135(4):1043–1057. [PMC free article: PMC2133387] [PubMed: 8922385]
- 87.
- Hopwood ND, Gurdon JB. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990;347(6289):197–200. [PubMed: 1697650]
- 88.
- Rashbass J, Taylor MV, Gurdon JB. The DNA-binding protein E12 cooperates with XMyoD in the activation of muscle-specific gene expression in Xenopus embryos. EMBO J. 1992;11(8):2981–2990. [PMC free article: PMC556780] [PubMed: 1322293]
- 89.
- Faerman A, Pearson-White S, Emerson C. et al. Ectopic expression of MyoD1 in mice causes prenatal lethalities. Dev Dyn. 1993;196(3):165–173. [PubMed: 8400402]
- 90.
- Tapanes-Castillo A, Baylies MK. Notch signaling patterns Drosophila mesodermal segments by regulating the bHLH transcription factor twist. Development. 2004;131(10):2359–2372. [PubMed: 15128668]
- 91.
- Taylor MV. Muscle development. Making Drosophila muscle. Curr Biol. 1995;5(7):740–742. [PubMed: 7583119]
- 92.
- Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor- 2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. [PubMed: 9891782]
- 93.
- Molkentin JD, Black BL, Martin JF. et al. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83(7):1125–1136. [PubMed: 8548800]
- 94.
- Edmondson DG, Lyons GE, Martin JF. et al. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120(5):1251–1263. [PubMed: 8026334]
- 95.
- Lin Q, Lu J, Yanagisawa H. et al. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development. 1998;125(22):4565–4574. [PubMed: 9778514]
- 96.
- Bour BA, O'Brien MA, Lockwood WL. et al. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9(6):730–741. [PubMed: 7729689]
- 97.
- Lilly B, Zhao B, Ranganayakulu G. et al. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267(5198):688–693. [PubMed: 7839146]
- 98.
- Ranganayakulu G, Zhao B, Dokidis A. et al. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev Biol. 1995;171(1):169–181. [PubMed: 7556894]
- 99.
- Ornatsky OI, Andreucci JJ, McDermott JC. A dominant-negative form of transcription factor MEF2 inhibits myogenesis. J Biol Chem. 1997;272(52):33271–33278. [PubMed: 9407117]
- 100.
- Taylor MV, Beatty KE, Hunter HK. et al. Drosophila MEF2 is regulated by twist and is expressed in both the primordia and differentiated cells of the embryonic somatic, visceral and heart musculature. Mech Dev. 1995;50(1):29–41. [PubMed: 7605749]
- 101.
- Cripps RM, Black BL, Zhao B. et al. The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev. 1998;12(3):422–434. [PMC free article: PMC316486] [PubMed: 9450935]
- 102.
- Wang DZ, Valdez MR, McAnally J. et al. The Mef2c gene is a direct transcriptional target of myogenic bHLH and MEF2 proteins during skeletal muscle development. Development. 2001;128(22):4623–4633. [PubMed: 11714687]
- 103.
- Dodou E, Xu SM, Black BL. Mef2c is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech Dev. 2003;120(9):1021–1032. [PubMed: 14550531]
- 104.
- Cripps RM, Lovato TL, Olson EN. Positive autoregulation of the Myocyte enhancer factor-2 myogenic control gene during somatic muscle development in Drosophila. Dev Biol. 2004;267(2):536–547. [PubMed: 15013812]
- 105.
- Cheng TC, Wallace MC, Merlie JP. et al. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science. 1993;261(5118):215–218. [PubMed: 8392225]
- 106.
- Yee SP, Rigby PW. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993;7(7A):1277–1289. [PubMed: 8391506]
- 107.
- Spitz F, Demignon J, Porteu A. et al. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci USA. 1998;95(24):14220–14225. [PMC free article: PMC24354] [PubMed: 9826681]
- 108.
- Lin MH, Nguyen HT, Dybala C. et al. Myocyte-specific enhancer factor 2 acts cooperatively with a muscle activator region to regulate Drosophila tropomyosin gene muscle expression. Proc Natl Acad Sci USA. 1996;93(10):4623–4628. [PMC free article: PMC39328] [PubMed: 8643453]
- 109.
- Damm C, Wolk A, Buttgereit D. et al. Independent regulatory elements in the upstream region of the Drosophila beta 3 tubulin gene (beta Tub60D) guide expression in the dorsal vessel and the somatic muscles. Dev Biol. 1998;199(1):138–149. [PubMed: 9676198]
- 110.
- Kelly KK, Meadows SM, Cripps RM. Drosophila MEF2 is a direct regulator of Actin57B transcription in cardiac, skeletal, and visceral muscle lineages. Mech Dev. 2002;110(1-2):39–50. [PubMed: 11744367]
- 111.
- Taylor MV. A novel Drosophila, mef2-regulated muscle gene isolated in a subtractive hybridization- based molecular screen using small amounts of zygotic mutant RNA. Dev Biol. 2000;220(1):37–52. [PubMed: 10720429]
- 112.
- Vivian JL, Gan L, Olson EN. et al. A hypomorphic myogenin allele reveals distinct myogenin expression levels required for viability, skeletal muscle development, and sternum formation. Dev Biol. 1999;208(1):44–55. [PubMed: 10075840]
- 113.
- Gunthorpe D, Beatty KE, Taylor MV. Different levels, but not different isoforms, of the Drosophila transcription factor DMEF2 affect distinct aspects of muscle differentiation. Dev Biol. 1999;215(1):130–145. [PubMed: 10525355]
- 114.
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110(3):791–804. [PubMed: 2100994]
- 115.
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125(20):4019–4032. [PubMed: 9735363]
- 116.
- Leiss D, Hinz U, Gasch A. et al. Beta 3 tubulin expression characterizes the differentiating mesodermal germ layer during Drosophila embryogenesis. Development. 1988;104(4):525–531. [PubMed: 3077351]
- 117.
- Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4(12A):2098–2111. [PubMed: 1980118]
- 118.
- Rushton E, Drysdale R, Abmayr SM. et al. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121(7):1979–1988. [PubMed: 7635046]
- 119.
- Ho RK, Ball EE, Goodman CS. Muscle pioneers: Large mesodermal cells that erect a scaffold for developing muscles and motoneurones in grasshopper embryos. Nature. 1983;301(5895):66–69. [PubMed: 6337338]
- 120.
- Ball EE, Ho RK, Goodman CS. Muscle development in the grasshopper embryo. I. Muscles, nerves, and apodemes in the metathoracic leg. Dev Biol. 1985;111(2):383–398. [PubMed: 4043524]
- 121.
- Prokop A, Landgraf M, Rushton E. et al. Presynaptic development at the Drosophila neuromuscular junction: Assembly and localization of presynaptic active zones. Neuron. 1996;17(4):617–626. [PubMed: 8893020]
- 122.
- Bourgouin C, Lundgren SE, Thomas JB. Apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron. 1992;9(3):549–561. [PubMed: 1524829]
- 123.
- Ruiz-Gomez M, Romani S, Hartmann C. et al. Specific muscle identities are regulated by Kruppel during Drosophila embryogenesis. Development. 1997;124(17):3407–3414. [PubMed: 9310335]
- 124.
- Jagla T, Bellard F, Lutz Y. et al. Ladybird determines cell fate decisions during diversification of Drosophila somatic muscles. Development. 1998;125(18):3699–3708. [PubMed: 9716535]
- 125.
- Knirr S, Azpiazu N, Frasch M. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development. 1999;126(20):4525–4535. [PubMed: 10498687]
- 126.
- Halfon MS, Carmena A, Gisselbrecht S. et al. Ras pathway specificity is determined by the integration of multiple signal-activated and tissue-restricted transcription factors. Cell. 2000;103(1):63–74. [PubMed: 11051548]
- 127.
- Knirr S, Frasch M. Molecular integration of inductive and mesoderm-intrinsic inputs governs even-skipped enhancer activity in a subset of pericardial and dorsal muscle progenitors. Dev Biol. 2001;238(1):13–26. [PubMed: 11783990]
- 128.
- Ross JJ, Duxson MJ, Harris AJ. Formation of primary and secondary myotubes in rat lumbrical muscles. Development. 1987;100(3):383–394. [PubMed: 3652976]
- 129.
- Duxson MJ, Usson Y. Cellular insertion of primary and secondary myotubes in embryonic rat muscles. Development. 1989;107(2):243–251. [PubMed: 2632223]
- 130.
- Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, ed. Biology of Drosophila. Facsimile ed. New York: Cold Spring Harbor Press. 1950:275–367.
- 131.
- Fernandes J, Bate M, Vijayraghavan K. Development of the indirect flight muscles of Drosophila. Development. 1991;113(1):67–77. [PubMed: 1765009]
- 132.
- Peckham M, Molloy JE, Sparrow JC. et al. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J Muscle Res Cell Motil. 1990;11(3):203–215. [PubMed: 2119393]
- 133.
- Silva R, Sparrow JC, Geeves MA. Isolation and kinetic characterisation of myosin and myosin S1 from the Drosophila indirect flight muscles. J Muscle Res Cell Motil. 2003;24(8):489–498. [PubMed: 14870964]
- 134.
- Farrell ER, Fernandes J, Keshishian H. Muscle organizers in Drosophila: The role of persistent larval fibers in adult flight muscle development. Dev Biol. 1996;176(2):220–229. [PubMed: 8660863]
- 135.
- Rivlin PK, Schneiderman AM, Booker R. Imaginal pioneers prefigure the formation of adult thoracic muscles in Drosophila melanogaster. Dev Biol. 2000;222(2):450–459. [PubMed: 10837132]
- 136.
- Kozopas KM, Nusse R. Direct flight muscles in Drosophila develop from cells with characteristics of founders and depend on DWnt-2 for their correct patterning. Dev Biol. 2002;243(2):312–325. [PubMed: 11884040]
- 137.
- Dutta D, Anant S, Ruiz-Gomez M. et al. Founder myoblasts and fibre number during adult myogenesis in Drosophila. Development. 2004;131(15):3761–3772. [PubMed: 15262890]
- 138.
- Dutta D, VijayRaghavan K. Metamorphosis and the formation of the adult musculature. In: Sink H, ed. Muscle development in Drosophila. Georgetown: Landes Bioscience. 2006:125–142.
- 139.
- Ruiz-Gomez M, Coutts N, Price A. et al. Drosophila dumbfounded: A myoblast attractant essential for fusion. Cell. 2000;102(2):189–198. [PubMed: 10943839]
- 140.
- Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell. 2003;5(6):937–944. [PubMed: 14667415]
- 141.
- Ghazi A, Anant S, VijayRaghavan K. Apterous mediates development of direct flight muscles autonomously and indirect flight muscles through epidermal cues. Development. 2000;127(24):5309–5318. [PubMed: 11076753]
- 142.
- Sudarsan V, Anant S, Guptan P. et al. Myoblast diversification and ectodermal signaling in Drosophila. Dev Cell. 2001;1(6):829–839. [PubMed: 11740944]
- 143.
- Artero R, Furlong EE, Beckett K. et al. Notch and Ras signaling pathway effector genes expressed in fusion competent and founder cells during Drosophila myogenesis. Development. 2003;130(25):6257–6272. [PubMed: 14602676]
- 144.
- Schafer K, Braun T. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat Genet. 1999;23(2):213–216. [PubMed: 10508520]
- 145.
- Gross MK, Moran-Rivard L, Velasquez T. et al. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development. 2000;127(2):413–424. [PubMed: 10603357]
- 146.
- Brohmann H, Jagla K, Birchmeier C. The role of Lbx1 in migration of muscle precursor cells. Development. 2000;127(2):437–445. [PubMed: 10603359]
- 147.
- Mankoo BS, Collins NS, Ashby P. et al. Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature. 1999;400(6739):69–73. [PubMed: 10403250]
- 148.
- Stockdale FE. Myogenic cell lineages. Dev Biol. 1992;154(2):284–298. [PubMed: 1426639]
- 149.
- Nikovits Jr W, Cann GM, Huang R. et al. Patterning of fast and slow fibers within embryonic muscles is established independently of signals from the surrounding mesenchyme. Development. 2001;128(13):2537–2544. [PubMed: 11493570]
- 150.
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23(12):569–575. [PubMed: 12457775]
- 151.
- Baxendale S, Davison C, Muxworthy C. et al. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat Genet. 2004;36(1):88–93. [PubMed: 14702044]
- 152.
- Wang YX, Zhang CL, Yu RT. et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2(10):e294. [PMC free article: PMC509410] [PubMed: 15328533]
- 153.
- Doberstein SK, Fetter RD, Mehta AY. et al. Genetic analysis of myoblast fusion: Blown fuse is required for progression beyond the prefusion complex. J Cell Biol. 1997;136(6):1249–1261. [PMC free article: PMC2132517] [PubMed: 9087441]
- 154.
- Abmayr SM, Balagopalan L, Galletta BJ. et al. Cell and molecular biology of myoblast fusion. Int Rev Cytol. 2003;225:33–89. [PubMed: 12696590]
- 155.
- Knudsen KA, Myers L, McElwee SA. A role for the Ca2(+)-dependent adhesion molecule, N-cadherin, in myoblast interaction during myogenesis. Exp Cell Res. 1990;188(2):175–184. [PubMed: 2335185]
- 156.
- Dickson G, Peck D, Moore SE. et al. Enhanced myogenesis in NCAM-transfected mouse myoblasts. Nature. 1990;344(6264):348–351. [PubMed: 2179732]
- 157.
- Mege RM, Goudou D, Diaz C. et al. N-cadherin and N-CAM in myoblast fusion: Compared localisation and effect of blockade by peptides and antibodies. J Cell Sci. 1992;103(Pt 4):897–906. [PubMed: 1487503]
- 158.
- Yagami-Hiromasa T, Sato T, Kurisaki T. et al. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377(6550):652–656. [PubMed: 7566181]
- 159.
- Charlton CA, Mohler WA, Radice GL. et al. Fusion competence of myoblasts rendered genetically null for N-cadherin in culture. J Cell Biol. 1997;138(2):331–336. [PMC free article: PMC2138190] [PubMed: 9230075]
- 160.
- Charlton CA, Mohler WA, Blau HM. Neural cell adhesion molecule (NCAM) and myoblast fusion. Dev Biol. 2000;221(1):112–119. [PubMed: 10772795]
- 161.
- Paululat A, Holz A, Renkawitz-Pohl R. Essential genes for myoblast fusion in Drosophila embryogenesis. Mech Dev. 1999;83(1-2):17–26. [PubMed: 10507836]
- 162.
- Dworak HA, Sink H. Myoblast fusion in Drosophila. Bioessays. 2002;24(7):591–601. [PubMed: 12111720]
- 163.
- Chen EH, Olson EN. Towards a molecular pathway for myoblast fusion in Drosophila. Trends Cell Biol. 2004;14(8):452–460. [PubMed: 15308212]
- 164.
- Rau A, Buttgereit D, Holz A. et al. Rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development. 2001;128(24):5061–5073. [PubMed: 11748142]
- 165.
- Menon SD, Chia W. Drosophila rolling pebbles: A multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast attractant Dumbfounded. Dev Cell. 2001;1(5):691–703. [PubMed: 11709189]
- 166.
- Schroter RH, Lier S, Holz A. et al. Kette and blown fuse interact genetically during the second fusion step of myogenesis in Drosophila. Development. 2004;131(18):4501–4509. [PubMed: 15342475]
- 167.
- Landgraf M, Baylies M, Bate M. Muscle founder cells regulate defasciculation and targeting of motor axons in the Drosophila embryo. Curr Biol. 1999;9(11):589–592. [PubMed: 10359699]
- 168.
- Hasegawa H, Kiyokawa E, Tanaka S. et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol. 1996;16(4):1770–1776. [PMC free article: PMC231163] [PubMed: 8657152]
- 169.
- Chen EH, Pryce BA, Tzeng JA. et al. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114(6):751–762. [PubMed: 14505574]
- 170.
- Chen EH, Olson EN. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev Cell. 2001;1(5):705–715. [PubMed: 11709190]
- 171.
- Kang JS, Feinleib JL, Knox S. et al. Promyogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc Natl Acad Sci USA. 2003;100(7):3989–3994. [PMC free article: PMC153035] [PubMed: 12634428]
- 172.
- Horsley V, Friday BB, Matteson S. et al. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol. 2001;153(2):329–338. [PMC free article: PMC2169453] [PubMed: 11309414]
- 173.
- Horsley V, Jansen KM, Mills ST. et al. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113(4):483–494. [PubMed: 12757709]
- 174.
- Duxson MJ, Usson Y, Harris AJ. The origin of secondary myotubes in mammalian skeletal muscles: Ultrastructural studies. Development. 1989;107(4):743–750. [PubMed: 2483685]
- 175.
- Rosen GD, Sanes JR, LaChance R. et al. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69(7):1107–1119. [PubMed: 1377605]
- 176.
- Schwander M, Leu M, Stumm M. et al. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4(5):673–685. [PubMed: 12737803]
- 177.
- Bokel C, Brown NH. Integrins in development: Moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3(3):311–321. [PubMed: 12361595]
- 178.
- Roy S, Wolff C, Ingham PW. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001;15(12):1563–1576. [PMC free article: PMC312718] [PubMed: 11410536]
- 179.
- Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218(2):115–124. [PubMed: 10656756]
- 180.
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191(2):270–283. [PubMed: 9398440]
- 181.
- Zhao P, Hoffman EP. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229(2):380–392. [PubMed: 14745964]
- 182.
- Seale P, Sabourin LA, Girgis-Gabardo A. et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. [PubMed: 11030621]
- 183.
- Seale P, Ishibashi J, Scime A. et al. Pax7 is necessary and sufficient for the myogenic specification of CD45(+): Sca1(+) stem cells from injured muscle. PLoS Biol. 2004;2(5):E130. [PMC free article: PMC406392] [PubMed: 15138500]
- 184.
- Cossu G, Mavilio F. Myogenic stem cells for the therapy of primary myopathies: Wishful thinking or therapeutic perspective? J Clin Invest. 2000;105(12):1669–1674. [PMC free article: PMC378519] [PubMed: 10862780]
- 185.
- Carmena A, Bate M, Jimenez F. Lethal of scute, a proneural gene, participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev. 1995;9(19):2373–2383. [PubMed: 7557389]
- 186.
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154(2):261–272. [PubMed: 1330787]
- 187.
- Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: A constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120(9):2385–2396. [PubMed: 7956819]
- 188.
- Hebrok M, Wertz K, Fuchtbauer EM. M-twist is an inhibitor of muscle differentiation. Dev Biol. 1994;165(2):537–544. [PubMed: 7958419]
- 189.
- Spicer DB, Rhee J, Cheung WL. et al. Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein Twist. Science. 1996;272(5267):1476–1480. [PubMed: 8633239]
- 190.
- Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9(6):686–699. [PubMed: 7729687]
- 191.
- Fuchtbauer EM. Expression of M-twist during postimplantation development of the mouse. Dev Dyn. 1995;204(3):316–322. [PubMed: 8573722]
- 192.
- Chen CM, Kraut N, Groudine M. et al. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell. 1996;86(5):731–741. [PubMed: 8797820]
- 193.
- Anant S, Roy S, VijayRaghavan K. Twist and Notch negatively regulate adult muscle differentiation in Drosophila. Development. 1998;125(8):1361–1369. [PubMed: 9502718]
- 194.
- Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: In vivo association of Id with E2A proteins. Genes Dev. 1992;6(8):1466–1479. [PubMed: 1644289]
- 195.
- Wang Y, Benezra R, Sassoon DA. Id expression during mouse development: A role in morphogenesis. Dev Dyn. 1992;194(3):222–230. [PubMed: 1361374]
- 196.
- Ying QL, Nichols J, Chambers I. et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115(3):281–292. [PubMed: 14636556]
- 197.
- Cubas P, Modolell J, Ruiz-Gomez M. The helix-loop-helix extramacrochaetae protein is required for proper specification of many cell types in the Drosophila embryo. Development. 1994;120(9):2555–2566. [PubMed: 7956831]
- 198.
- Postigo AA, Dean DC. ZEB, a vertebrate homolog of Drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J. 1997;16(13):3935–3943. [PMC free article: PMC1170017] [PubMed: 9233803]
- 199.
- Postigo AA, Ward E, Skeath JB. et al. Zfh-1, the Drosophila homologue of ZEB, is a transcriptional repressor that regulates somatic myogenesis. Mol Cell Biol. 1999;19(10):7255–7263. [PMC free article: PMC84718] [PubMed: 10490660]
- 200.
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284(5415):770–776. [PubMed: 10221902]
- 201.
- Delfini M, Hirsinger E, Pourquie O. et al. Delta 1-activated notch inhibits muscle differentiation without affecting Myf5 and Pax3 expression in chick limb myogenesis. Development. 2000;127(23):5213–5224. [PubMed: 11060246]
- 202.
- Corbin V, Michelson AM, Abmayr SM. et al. A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell. 1991;67(2):311–323. [PubMed: 1913825]
- 203.
- Bate M, Rushton E, Frasch M. A dual requirement for neurogenic genes in Drosophila myogenesis. Dev Suppl. 1993:149–161. [PubMed: 8049469]
- 204.
- Rusconi JC, Corbin V. Evidence for a novel Notch pathway required for muscle precursor selection in Drosophila. Mech Dev. 1998;79(1-2):39–50. [PubMed: 10349619]
- 205.
- Fuerstenberg S, Giniger E. Multiple roles for notch in Drosophila myogenesis. Dev Biol. 1998;201(1):66–77. [PubMed: 9733574]
- 206.
- Brennan K, Baylies M, Arias AM. Repression by Notch is required before Wingless signalling during muscle progenitor cell development in Drosophila. Curr Biol. 1999;9(13):707–710. [PubMed: 10395544]
- 207.
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409. [PubMed: 12361602]
- 208.
- Bate M, Rushton E, Currie DA. Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development. 1991;113(1):79–89. [PubMed: 1765010]
- 209.
- Lee H, Habas R, Abate-Shen C. MSX1 cooperates with histone H1b for inhibition of transcription and myogenesis. Science. 2004;304(5677):1675–1678. [PubMed: 15192231]
- 210.
- McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev. 2001;11(5):497–504. [PubMed: 11532390]
- 211.
- Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91(6):457–469. [PubMed: 12242263]
- 212.
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246(1):14–28. [PubMed: 12027431]
- 213.
- Solloway MJ, Harvey RP. Molecular pathways in myocardial development: A stem cell perspective. Cardiovasc Res. 2003;58(2):264–277. [PubMed: 12757862]
- 214.
- Brand T. Heart development: Molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258(1):1–19. [PubMed: 12781678]
- 215.
- Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10(5):467–474. [PubMed: 15122248]
- 216.
- Saint-Hilaire EG. Mem, du Mus Hist Nat. 1822. pp. 89–119.
- 217.
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380(6569):37–40. [PubMed: 8598900]
- 218.
- Schlange T, Andree B, Arnold HH. et al. BMP2 is required for early heart development during a distinct time period. Mech Dev. 2000;91(1-2):259–270. [PubMed: 10704850]
- 219.
- Kishimoto Y, Lee KH, Zon L. et al. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124(22):4457–4466. [PubMed: 9409664]
- 220.
- Shi Y, Katsev S, Cai C. et al. BMP signaling is required for heart formation in vertebrates. Dev Biol. 2000;224(2):226–237. [PubMed: 10926762]
- 221.
- Latinkic BV, Kotecha S, Mohun TJ. Induction of cardiomyocytes by GATA4 in Xenopus ectodermal explants. Development. 2003;130(16):3865–3876. [PubMed: 12835401]
- 222.
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122(10):2977–2986. [PubMed: 8898212]
- 223.
- Wu X, Golden K, Bodmer R. Heart development in Drosophila requires the segment polarity gene wingless. Dev Biol. 1995;169(2):619–628. [PubMed: 7781903]
- 224.
- Jagla K, Frasch M, Jagla T. et al. Ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development. 1997;124(18):3471–3479. [PubMed: 9342040]
- 225.
- Park M, Wu X, Golden K. et al. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol. 1996;177(1):104–116. [PubMed: 8660881]
- 226.
- Pandur P, Lasche M, Eisenberg LM. et al. Wnt-11 activation of a noncanonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418(6898):636–641. [PubMed: 12167861]
- 227.
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118(3):719–729. [PubMed: 7915669]
- 228.
- Azpiazu N, Frasch M. Tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7(7B):1325–1340. [PubMed: 8101173]
- 229.
- Xu X, Yin Z, Hudson JB. et al. Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev. 1998;12(15):2354–2370. [PMC free article: PMC317052] [PubMed: 9694800]
- 230.
- Gajewski K, Fossett N, Molkentin JD. et al. The zinc finger proteins Pannier and GATA4 function as cardiogenic factors in Drosophila. Development. 1999;126(24):5679–5688. [PubMed: 10572044]
- 231.
- Klinedinst SL, Bodmer R. Gata factor Pannier is required to establish competence for heart progenitor formation. Development. 2003;130(13):3027–3038. [PubMed: 12756184]
- 232.
- Evans SM. Vertebrate tinman homologues and cardiac differentiation. Semin Cell Dev Biol. 1999;10(1):73–83. [PubMed: 10355031]
- 233.
- Biben C, Harvey RP. Homeodomain factors Nkx2-5 controls left/right asymmetric expression of bHLH gene eHand during murine heart development. Genes Dev. 1997;11(11):1357–1369. [PubMed: 9192865]
- 234.
- Tanaka M, Chen Z, Bartunkova S. et al. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126(6):1269–1280. [PubMed: 10021345]
- 235.
- Fu Y, Yan W, Mohun TJ. et al. Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development. 1998;125(22):4439–4449. [PubMed: 9778503]
- 236.
- Grow MW, Krieg PA. Tinman function is essential for vertebrate heart development: Elimination of cardiac differentiation by dominant inhibitory mutants of the tinman-related genes, XNkx2-3 and XNkx2-5. Dev Biol. 1998;204(1):187–196. [PubMed: 9851852]
- 237.
- Benson DW, Silberbach GM, Kavanaugh-McHugh A. et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104(11):1567–1573. [PMC free article: PMC409866] [PubMed: 10587520]
- 238.
- Schott JJ, Benson DW, Basson CT. et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281(5373):108–111. [PubMed: 9651244]
- 239.
- Park M, Lewis C, Turbay D. et al. Differential rescue of visceral and cardiac defects in Drosophila by vertebrate tinman-related genes. Proc Natl Acad Sci USA. 1998;95(16):9366–9371. [PMC free article: PMC21344] [PubMed: 9689086]
- 240.
- Ranganayakulu G, Elliott DA, Harvey RP. et al. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development. 1998;125(16):3037–3048. [PubMed: 9671578]
- 241.
- Lien CL, McAnally J, Richardson JA. et al. Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev Biol. 2002;244(2):257–266. [PubMed: 11944935]
- 242.
- Evans T. Regulation of cardiac gene expression by GATA-4/5/6. Trends in Cardiovascular Medicine. 1997;7(3):75–83. [PubMed: 21235868]
- 243.
- Reiter JF, Alexander J, Rodaway A. et al. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13(22):2983–2995. [PMC free article: PMC317161] [PubMed: 10580005]
- 244.
- Garg V, Kathiriya IS, Barnes R. et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424(6947):443–447. [PubMed: 12845333]
- 245.
- Gajewski K, Zhang Q, Choi CY. et al. Pannier is a transcriptional target and partner of Tinman during Drosophila cardiogenesis. Dev Biol. 2001;233(2):425–436. [PubMed: 11336505]
- 246.
- Gajewski K, Kim Y, Lee YM. et al. D-mef2 is a target for Tinman activation during Drosophila heart development. EMBO J. 1997;16(3):515–522. [PMC free article: PMC1169655] [PubMed: 9034334]
- 247.
- Gajewski K, Kim Y, Choi CY. et al. Combinatorial control of Drosophila mef2 gene expression in cardiac and somatic muscle cell lineages. Dev Genes Evol. 1998;208(7):382–392. [PubMed: 9732552]
- 248.
- Lin Q, Schwarz J, Bucana C. et al. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276(5317):1404–1407. [PMC free article: PMC4437729] [PubMed: 9162005]
- 249.
- Dodou E, Verzi MP, Anderson JP. et al. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131(16):3931–3942. [PubMed: 15253934]
- 250.
- Bruneau BG. Transcriptional regulation of vertebrate cardiac morphogenesis. Circ Res. 2002;90(5):509–519. [PubMed: 11909814]
- 251.
- Wang D, Chang PS, Wang Z. et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105(7):851–862. [PubMed: 11439182]
- 252.
- Ueyama T, Kasahara H, Ishiwata T. et al. Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol Cell Biol. 2003;23(24):9222–9232. [PMC free article: PMC309615] [PubMed: 14645532]
- 253.
- Lo PC, Skeath JB, Gajewski K. et al. Homeotic genes autonomously specify the anteroposterior subdivision of the Drosophila dorsal vessel into aorta and heart. Dev Biol. 2002;251(2):307–319. [PubMed: 12435360]
- 254.
- Lovato TL, Nguyen TP, Molina MR. et al. The Hox gene abdominal-A specifies heart cell fate in the Drosophila dorsal vessel. Development. 2002;129(21):5019–5027. [PubMed: 12397110]
- 255.
- Ponzielli R, Astier M, Chartier A. et al. Heart tube patterning in Drosophila requires integration of axial and segmental information provided by the Bithorax Complex genes and hedgehog signaling. Development. 2002;129(19):4509–4521. [PubMed: 12223408]
- 256.
- Perrin L, Monier B, Ponzielli R. et al. Drosophila cardiac tube organogenesis requires multiple phases of Hox activity. Dev Biol. 2004;272(2):419–431. [PubMed: 15282158]
- 257.
- Lo PC, Frasch M. Establishing A-P polarity in the embryonic heart tube: A conserved function of Hox genes in Drosophila and vertebrates? Trends Cardiovasc Med. 2003;13(5):182–187. [PubMed: 12837580]
- 258.
- Srivastava D. HAND proteins: Molecular mediators of cardiac development and congenital heart disease. Trends Cardiovasc Med. 1999;9(1-2):11–18. [PubMed: 10189962]
- 259.
- Kolsch V, Paululat A. The highly conserved cardiogenic bHLH factor Hand is specifically expressed in circular visceral muscle progenitor cells and in all cell types of the dorsal vessel during Drosophila embryogenesis. Dev Genes Evol. 2002;212(10):473–485. [PubMed: 12424518]
- 260.
- San Martin B, Bate M. Hindgut visceral mesoderm requires an ectodermal template for normal development in Drosophila. Development. 2001;128(2):233–242. [PubMed: 11124118]
- 261.
- Lee H-H, Zaffran S, Frasch M. Development of the larval visceral musculature. In: Sink H, ed. Muscle Development in Drosophila. Georgetown: Landes Bioscience. 2006:62–78.
- 262.
- Masumoto K, Nada O, Suita S. et al. The formation of the chick ileal muscle layers as revealed by alpha-smooth muscle actin immunohistochemistry. Anat Embryol (Berl). 2000;201(2):121–129. [PubMed: 10672364]
- 263.
- Klapper R, Heuser S, Strasser T. et al. A new approach reveals syncytia within the visceral musculature of Drosophila melanogaster. Development. 2001;128(13):2517–2524. [PubMed: 11493568]
- 264.
- Martin BS, Ruiz-Gomez M, Landgraf M. et al. A distinct set of founders and fusion-competent myoblasts make visceral muscles in the Drosophila embryo. Development. 2001;128(17):3331–3338. [PubMed: 11546749]
- 265.
- Zaffran S, Kuchler A, Lee HH. et al. Biniou (FoxF), a central component in a regulatory network controlling visceral mesoderm development and midgut morphogenesis in Drosophila. Genes Dev. 2001;15(21):2900–2915. [PMC free article: PMC312807] [PubMed: 11691840]
- 266.
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127(12):2763–2772. [PubMed: 10821773]
- 267.
- Tonegawa A, Funayama N, Ueno N. et al. Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development. 1997;124(10):1975–1984. [PubMed: 9169844]
- 268.
- Lo PC, Frasch M. Bagpipe-Dependent expression of vimar, a novel Armadillo-repeats gene, in Drosophila visceral mesoderm. Mech Dev. 1998;72(1-2):65–75. [PubMed: 9533953]
- 269.
- Zaffran S, Frasch M. The beta 3 tubulin gene is a direct target of bagpipe and biniou in the visceral mesoderm of Drosophila. Mech Dev. 2002;114(1-2):85–93. [PubMed: 12175492]
- 270.
- Mahlapuu M, Ormestad M, Enerback S. et al. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128(2):155–166. [PubMed: 11124112]
- 271.
- Tseng HT, Shah R, Jamrich M. Function and regulation of FoxF1 during Xenopus gut development. Development. 2004;131(15):3637–3647. [PubMed: 15229177]
- 272.
- Tribioli C, Frasch M, Lufkin T. Bapx1: An evolutionary conserved homologue of the Drosophila bagpipe homeobox gene is expressed in splanchnic mesoderm and the embryonic skeleton. Mech Dev. 1997;65(1-2):145–162. [PubMed: 9256352]
- 273.
- Miano JM. Serum response factor: Toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35(6):577–593. [PubMed: 12788374]
- 274.
- Wang Z, Wang DZ, Hockemeyer D. et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428(6979):185–189. [PubMed: 15014501]
- 275.
- Wang L, Fan C, Topol SE. et al. Mutation of MEF2A in an inherited disorder with features of coronary artery disease. Science. 2003;302(5650):1578–1581. [PMC free article: PMC1618876] [PubMed: 14645853]
- 276.
- Klapper R, Stute C, Schomaker O. et al. The formation of syncytia within the visceral musculature of the Drosophila midgut is dependent on duf, sns and mbc. Mech Dev. 2002;110(1-2):85–96. [PubMed: 11744371]
- 277.
- Weiss JB, Suyama KL, Lee HH. et al. Jelly belly: A Drosophila LDL receptor repeat-containing signal required for mesoderm migration and differentiation. Cell. 2001;107(3):387–398. [PubMed: 11701128]
- 278.
- Lee HH, Norris A, Weiss JB. et al. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425(6957):507–512. [PubMed: 14523446]
- 279.
- Englund C, Loren CE, Grabbe C. et al. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425(6957):512–516. [PubMed: 14523447]
- 280.
- Stute C, Schimmelpfeng K, Renkawitz-Pohl R. et al. Myoblast determination in the somatic and visceral mesoderm depends on Notch signalling as well as on milliways(mili(Alk)) as receptor for Jeb signalling. Development. 2004;131(4):743–754. [PubMed: 14757637]
- 281.
- Stoica GE, Kuo A, Aigner A. et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276(20):16772–16779. [PubMed: 11278720]
- 282.
- Stoica GE, Kuo A, Powers C. et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277(39):35990–35998. [PubMed: 12122009]
- 283.
- Patapoutian A, Wold BJ, Wagner RA. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science. 1995;270(5243):1818–1821. [PubMed: 8525375]
- 284.
- Stratton CJ, Bayguinov Y, Sanders KM. et al. Ultrastructural analysis of the transdifferentiation of smooth muscle to skeletal muscle in the murine esophagus. Cell Tissue Res. 2000;301(2):283–298. [PubMed: 10955724]
- 285.
- Miano JM, Thomas S, Disteche CM. Expression and chromosomal mapping of the mouse smooth muscle calponin gene. Mamm Genome. 2001;12(3):187–191. [PubMed: 11252166]
- 286.
- Epstein JA. Developmental cardiology comes of age. Circ Res. 2000;87(10):833–834. [PubMed: 11073875]
- 287.
- Rudnicki MA. Marrow to muscle, fission versus fusion. Nat Med. 2003;9(12):1461–1462. [PubMed: 14647520]
- 288.
- Partridge TA, Davies KE. Myoblast-based gene therapies. Br Med Bull. 1995;51(1):123–137. [PubMed: 7767639]
- 289.
- Blau HM. A twist of fate. Nature. 2002;419(6906):437. [PubMed: 12374136]
- 290.
- Baylies MK, Bate M, Ruiz Gomez M. Myogenesis: A view from Drosophila. Cell. 1998;93(6):921–927. [PubMed: 9635422]
- Comparison of Muscle Development in Drosophila and Vertebrates - Madame Curie Bi...Comparison of Muscle Development in Drosophila and Vertebrates - Madame Curie Bioscience Database
- Cancer Invasion and Metastasis: Molecular and Cellular Perspective - Madame Curi...Cancer Invasion and Metastasis: Molecular and Cellular Perspective - Madame Curie Bioscience Database
- Lipoxins as an Immune-Escape Mechanism - Madame Curie Bioscience DatabaseLipoxins as an Immune-Escape Mechanism - Madame Curie Bioscience Database
- Slits and Their Receptors - Madame Curie Bioscience DatabaseSlits and Their Receptors - Madame Curie Bioscience Database
- FOXP Genes, Neural Development, Speech and Language Disorders - Madame Curie Bio...FOXP Genes, Neural Development, Speech and Language Disorders - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...