NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Cells secrete a variety of substances by “regulated exocytosis” (i.e., fusion of secretory vesicles with the plasma membrane) in response to extracellular stimuli. Since two membranes must be apposed with each other prior to the secretion event, phospholipid-binding domains are often found in exocytotic proteins on secretory vesicles, and one of the best characterized phospholipid-binding domains involved in regulated exocytosis is the C2 domain. C2 domains are found in tandem in the C-terminal region of C-type tandem C2 proteins, including synaptotagmins (Syts), synaptotagmin-like proteins (Slps), rabphilin, and Doc2s. In this chapter I provide an overview of the structure and function of the Syt and Slp families, especially focusing on recent advances in research on the molecular mechanisms of secretory vesicle trafficking (e.g., docking, fusion, and recycling of vesicles) mediated by Syt or Slp proteins.
Introduction
Secretion of neurotransmitters, hormones, and enzymes is a fundamental biological activity of the cell, and is achieved by vesicular exocytosis, namely, fusion of secretory vesicles with the plasma membrane. Vesicular exocytosis generally consists of at least three distinct steps: docking/ tethering of a transport vesicle to the plasma membrane, ATP-dependent priming of the vesicle, and actual fusion of the vesicle to the plasma membrane, which is often triggered by extracellular stimuli (e.g., increase in intracellular Ca2+ concentrations).1 Since two membranes (i.e., vesicle membrane and plasma membrane) must be apposed with each other prior to the secretion event, phospholipid-binding domains are often found in exocytotic proteins on secretory vesicles. Among the various phospholipid-binding domains that have been identified, the C2 domain, a putative Ca2+- and phospholipid-binding motif originally identified in Ca2+-dependent protein kinase C,2 is of particular interest, because two C2 domains are often found in tandem in the C-terminal portion of a group of exocytotic proteins (named the C-type tandem C2 protein family).3,4 Tandem C2 domains are also found in the N-terminal portion of GAP1 family proteins and copine family proteins (named the N-type tandem C2 protein family),3 but whether they are involved in secretory vesicle exocytosis is completely unknown. Thus far, 27 different C-type tandem C2 proteins have been identified in mice and humans, and they have been classified into three distinct groups based on their N-terminal structures.3,4 The first, and best characterized group is the synaptotagmin (Syt) family.4-6 Syt is defined as a protein with a single N-terminal transmembrane domain and tandem C-terminal cytoplasmic C2 domains (referred to as the C2A domain and the C2B domain; Fig. 1). The second group consists of rabphilin and Doc2s, both of which share highly homologous tandem C2 domains, although their N-terminal structures are completely different: rabphilin contains an N-terminal Rab-binding domain (RBD),7 whereas Doc2 contains an N-terminal Munc13-1-interacting domain (MID) (Fig. 1).8,9 The final group is the recently identified synaptotagmin-like protein (Slp) family.10-12 Slp family members contain an N-terminal Rab27A/B-binding domain (also called Slp homology domain [SHD] or RBD27),12-14 and have been suggested to control a variety of secretion events.12 B/K,15,16 Strep14 (Syt XIV-related protein),17 and Tac2-N (tandem C2 protein in nucleus)18 also contain tandem C2 domains at the C terminus, but they do not fall into any of the above groups, because they lack a specific N-terminal sequence (Fig. 1). This chapter describes recent studies (specifically after 2001) on the role of the C-type tandem C2 protein family, particularly focusing on the function of the Syt and Slp families in secretory vesicle trafficking.
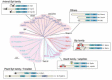
Figure 1
The C-type tandem C2 protein families in mice, plants, and yeasts. The phylogenetic tree and structure of the C-type tandem C2 protein families from mice, plants, and yeasts. The phylogenetic tree has been depicted by using the ClustalW program (http://www.ddbj.nig.ac.jp/search/clustalw-j.html) (more...)
Role of the Synaptotagmin Family Members in Regulated Exocytosis
Syt forms the largest branch in the phylogenetic tree of the C-type tandem C2 protein family (Fig. 1) and is found in a variety of species in different phyla.17,19,20 In principle, Syt family members consist of five different domains, a short extracellular domain (from 0 to less than 70 amino acids), “single transmembrane domain”, a spacer domain of varying length, and tandem C2 domains, but some Syts lack one or several domains as a result of alternative splicing.4,19 All Syt members reported thus far lack a signal peptide sequence, but they are believed to display type I membrane topology (i.e., tandem C2 domains are present in the cytoplasm). To date, 15 distinct syt genes (syts I-XV or syts 1-15) have been identified in mice, rats, and humans (Table 1), and several syt genes have been found in invertebrates.17,19 Mammalian Syt isoforms are further classified into several subfamilies based on their sequence similarities, the Syt I/II/IX subfamily, Syt III/V/VI/X subfamily, and Syt IV/XI subfamily (dotted circles in Fig. 1), and there are no clear sequence similarities between the N-terminal domains of the different Syt subfamilies. Two additional C-type tandem C2 proteins, Strep14 and B/K are sometimes referred to as Syt XVI and Syt XVII, respectively, because they are present in the Syt branch in the phylogenetic tree (Fig. 1). At present, however, there is no evidence that either protein contains an N-terminal transmembrane domain at the protein level or mRNA level,19 indicating that they fall outside the Syt category. Land plants also possess several Syts (e.g., At-Syts A-D), but they from a branch of the phylogenetic tree that is completely distinct from the animal Syt branch (Fig. 1), indicating that the plant Syts evolved from a different source, possibly from yeast Tricalbin proteins21 (three C2 domains), as a result of losing their third C2 domain (Fig. 1).17 In contrast to the animal Syts, the plant Syts lacks a putative fatty-acylation site just downstream of the transmembrane domain that may be responsible for the stable oligomerization,22 and at present nothing is known about the function of the plant Syts in Ca2+-regulated exocytosis. This section describes recent advances in our understanding of the function of mammalian Syt isoforms (or their invertebrate orthologues) in regulated exocytosis.
Table 1
C-type tandem C2 proteins in humans, mice, rats, and invertebrates.
Syt I, II, and IX Subfamily
Role of Syt I (or II) in Synaptic Vesicle Exocytosis and Endocytosis in Neurons
Syts I, II, and IX are an evolutionarily conserved subfamily of Ca2+-dependent Syts that are involved in the control of exocytosis of secretory vesicles (e.g., synaptic vesicles in neurons and/or dense-core vesicles in neuroendocrine cells).23-25 Both Syt I and Syt II are present on synaptic vesicles, with Syt I being predominant in the rostral region of the brain and Syt II predominating in the caudal region of the brain. The best characterized Syt isoform, Syt I, is now widely believed to be the major low affinity Ca2+-sensor for neurotransmitter release.26-29 Syt I is not a simple Ca2+-sensor, and presumably regulates synaptic vesicle exocytosis itself by modulating the synaptic vesicle docking step,30,31 fusion pore expansion dynamics,32,33 and endocytosis.34-36 A variety of potential ligands of the C2A (or C2B) domain of Syt I (or II), including proteins, divalent cations, phospholipids, and soluble inositol polyphosphates, have been reported thus far (for a review see refs. 4-6), and it has been hypothesized that their binding to the Syt I C2 domains regulates synaptic vesicle exocytosis and endocytosis. For example, Ca2+-dependent binding of Syt I with SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex composed of syntaxin-1a, SNAP-25, and VAMP-2/synaptobrevin-2 or PIP2 (phosphatidylinositol 4,5-bisphosphate) has been hypothesized to promote Ca2+-dependent synaptic vesicle fusion.37-39 Ca2+-independent binding of Syt I with t-SNARE heterodimers to be involved in synaptic vesicle exocytosis.40 Ca2+-dependent self-oligomerization of Syt I to control fusion pore dynamics;41 and Ca2+-independent binding of the Syt I C2B domain with clathrin assembly protein AP-2 and/or stonin-2 to control synaptic vesicle endocytosis.42
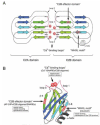
How does Syt I control distinct steps of synaptic vesicle trafficking? Although the precise mechanism remains to be elucidated, the presence of the multiple ligand binding sites in the Syt I C2 domains may ensure binding to a variety of molecules and the control of distinct steps in synaptic vesicle trafficking.4 As shown in Figure 2, both C2 domains are composed of an eight-stranded anti-parallel β-sandwich structure (β1-β8 strands), and two and three Ca2+ ions bind the loop regions formed at the top of the C2A and C2B β-sandwich structure, respectively, and the C2B domain contains an additional α-helix between the β7 and β8 strands.43 The Ca2+-binding loops of the C2A domain and C2B domain are presumed to face with each other, and redundant Ca2+-binding sites that mediate binding of t-SNAREs are thought to be formed (Fig. 2A).44 In contrast to the C2A domain, the C2B domain contains two additional ligand-binding sites (Fig. 2A,B). A polybasic sequence in the β4 strand (known as the “C2B effector domain”) is required for binding of a variety of molecules, including AP-2 and inositol polyphosphates,45 and has been suggested to regulate synaptic vesicle exocytosis and endocytosis. The polybasic sequence is also required for suppression of spontaneous neurotransmitter release.46 The conserved WHXL motif in the β8 strand is required for binding of a plasma membrane protein, neurexin Iα, in vitro, and it has been suggested to be involved in the synaptic vesicle docking to the plasma membrane.30 The WHXL motif is also important for the maintenance of the C2B structure,47 and C-terminal fusion of fluorescent protein (e.g., GFP and CFP) has been shown to impair Syt I function, presumably by abrogating the function of the WHXL motif.48 These three ligand-binding sites in the C2B domain are not totally independent, and ligand-binding of one site presumably affects the function of the other ligand-binding sites.
Syts I and II undergo several posttranslational modifications (e.g., phosphorylation, N- and O-glycosylation at the extracellular domain,49 and fatty-acylation just downstream of the transmembrane domain),22 and the glycosylation or palmitoylation of Syt I has recently been shown to be required for efficient targeting of the Syt I molecule to secretory vesicles.50-52
Role of Syt IX in Dense-Core Vesicle Exocytosis in Neuroendocrine Cells
Although Syt I is also expressed on the dense-core vesicles in certain neuroendocrine cells (e.g., chromaffin cells and pituitary cells) and involved in the control of their exocytosis,53,54 Syt I is dispensable for dense-core vesicle exocytosis by PC12 cells, because Syt I-deficient PC12 cells display normal hormone secretion activity,55,56 indicating the presence of an alternate Ca2+-sensor on dense-core vesicles in PC12 cells. The most likely candidate for the alternate Ca2+-sensor for dense-core vesicle exocytosis is Syt IX (originally described as Syt V),57,58 which is abundantly expressed on the Syt I-containing dense-core vesicles in PC12 cells.23,38,56 Syt IX is also expressed on insulin-containing vesicles in pancreatic β-cell lines59 and islet β-cells60 which do not endogenously express Syt I. Functional ablation of Syt IX has been shown to reduce Ca2+-dependent hormone secretion by PC12 cells and pancreatic β-cells.23,56,59,60 Interestingly, Syt IX is also expressed in a rat basophilic leukemia mast cell line (RBL-2H3) and regulates transport from the perinuclear endocytic recycling compartment (ERC) to the cell surface via interaction with microtubules.61,62
Syt III, V, VI, and X Subfamily
Syts III, V, VI, and X exhibit high sequence similarities throughout the entire proteins, and this subfamily of Syts is characterized by the presence of an N-terminal conserved Cys motif, which is essential for homo-dimer formation through disulfide bonding.63 All members of this subfamily exhibit Ca2+/phospholipid binding activity,25,64,65 and have been suggested to regulate Ca2+-dependent exocytosis of secretory vesicles. This subfamily of Syts has been retained in vertebrates alone, and is not found in invertebrates.4,19
Role of Syt III in Regulated Exocytosis: Plasma Membrane or Vesicular Ca2+-Sensor?
Syt III is abundantly expressed in all brain regions, and its expression increases in parallel with synaptogenesis during postnatal development of the mouse brain. Unlike Syts I and II, Syt III protein is mainly present on the presynaptic plasma membrane, rather than on synaptic vesicles,66 and it has been suggested to function as a plasma membrane high-affinity Ca2+-sensor for neurotransmitter release.64 At present, however, nothing is known about the functional involvement of endogenous Syt III in synaptic vesicle exocytosis, and the expression and subcellular localization of Syt III in neuroendocrine cells (particularly in pancreatic β-cells) are still matters of controversy (dense-core vesicles versus plasma membrane). Recent work indicates that Syt III protein is present just beneath the plasma membrane of pancreatic polypeptide cells, but that it is not present in α-, β-, or δ-cells.60 Syt III is also expressed in RBL-2H3 mast cells and is involved in the formation and delivery of cargo to the perinuclear endocytic recycling compartment.67
Role of Syt V in Dense-Core Vesicle Exocytosis in Specific Types of Neuroendocrine Cells
Syt V has been proposed to function as a Ca2+-sensor for exocytosis of specific populations of dense-core vesicles.68 Syt V is enriched in the dense-core vesicle fraction of specific regions of mouse brain, and it is also expressed in pancreatic islet α-cells that secrete glucagon, but not in β- or δ-cells.59,68
Role of Syt VI in the Acrosome Reaction of Sperm
An alternative splicing isoform of Syt VI lacking a transmembrane domain (named Syt VIDeltaTM)69 is predominantly expressed in selected regions of mouse brain (e.g., olfactory bulb), and it is present in the various membrane fractions.66 Although the function of Syt VI (or Syt VIDeltaTM) in regulated exocytosis in the brain remains completely unknown, Syt VI regulates acrosome reaction, a unique Ca2+-regulated exocytosis, in sperm.70,71 Phosphorylation of the polybasic region of the Syt VI C2 domains, which corresponds to the C2B effector domain of Syt I (see above and Fig. 2), modulates Syt VI function in acrosomal exocytosis in human sperm.72
Syt X Is an Immediate Early Gene Product in Brain Whose Expression Is Induced by Kainic Acid-Induced Seizures
Syt X has been identified as an immediate early gene product in rat brain by differential display between brain exposed and unexposed to kainic acid.73 However, the tissue distribution and subcellular localization of Syt X protein remain completely unknown.
Syt IV and XI Subfamily
The Syt IV and XI subfamily is evolutionarily conserved from Caenorhabditis elegans to humans,4,19,74 and shares a point mutation of one of the conserved acidic residues (e.g., Ser-244 of mouse Syt IV, which corresponds to the Asp-232 of mouse Syt I; see Fig. 2A) in the putative Ca2+-binding loop3 of the C2A domain. As a result of this mutation, the isolated C2A domain of Syts IV and XI lacks Ca2+/phospholipid binding activity. Recent structural analysis of the mouse Syt IV C2B domain has shown that it is unlikely to bind Ca2+ ions despite possessing the five conserved acidic residues in the putative Ca2+-binding loops.75 Moreover, “overexpression studies” have suggested that Syt IV functions as a negative regulator of regulated exocytosis. 76,77 Interestingly, however, more recent analysis of “endogenous” Syt IV protein has indicated that Syt IV functions as a positive regulator of some forms of regulated exocytosis (e.g., glutamate release from astrocytes).78,79
Subcellular Localization and Possible Function of Syt IV Protein in Brain and PC12 Cells
The Syt IV isoform has also been identified as an immediate early gene product induced by membrane depolarization (e.g., high-KCl, forskolin, and kainic acid) in brain and PC12 cells.80-82 Syt IV expression is found in all brain regions, but, unlike other Syt isoforms, its protein expression levels in mouse brain are highest during the 1st week of postnatal development.81 Syt IV is mainly present in the Golgi and in the tips of growing neurites in developing neurons, and the signals in the tips of axons and dendrites almost disappear in mature neurons.81 Syt IV is not a synaptic vesicle protein in either mice or Drosophila, and it is mainly localized on certain vesicle/organelle structures in dendrites.74,83 Although the specific cargo of Syt IV-containing vesicles is unknown, it is highly possible that they contain molecules that are directly involved in changes in synaptic plasticity, because Syt IV knockout mice exhibit abnormalities of some forms of memory related to the hippocampus.80 In contrast to neurons, in which Syts I and II are abundantly expressed, the Syt IV isoform, but not Syt I, is specifically expressed in astrocytes, and Syt IV is required for Ca2+-induced glutamate release from astrocytes, strongly indicating that Syt IV is a positive regulator of exocytosis.79 Although Syt IV itself is not a synaptic vesicle protein, if Syt IV is ectopically expressed on synaptic vesicles in neurons, it can rescue the impairment of neurotransmitter release in the Syt I null mutant fly.84
In nerve growth factor (NGF)-differentiated PC12 cells, Syt IV is also present in the Golgi and distal portion of neurites, where dense-core vesicles are accumulated.78 In undifferentiated PC12 cells, however, Syt IV is present in the Golgi and immature secretory vesicles, rather than mature dense-core vesicles. It is very interesting that Syt IV is sorted to newly formed, mature dense-core vesicles that undergo Ca2+-dependent exocytosis in response to NGF.78 The spacer domain of Sty IV, which is not conserved in other Syt isoforms, primarily determines the Golgi localization of Syt IV.85
Syt XI Interaction with Parkin
Syt XI was originally identified as a closely related isoform of Syt IV that is abundantly expressed in brain. The same as Syt IV, Syt XI expressed in PC12 cells is mainly targeted to the BFA (brefeldin A)-sensitive perinuclear region (presumably the Golgi),86 although almost nothing is known about the function and localization of endogenous Syt XI in brain. The only information available is that parkin, an autosomal recessive juvenile Parkinson disease gene product, interacts with and ubiquitinates Syt XI.87
Syt VII
Syt VII is a ubiquitous Syt isoform that is evolutionarily conserved from C. elegans to humans. There are several alternative splicing isoforms of mammalian Syt VII (e.g., Syt VIIα-γ or Syt VIIa-e),88,89 but the shortest form (named Syt VIIα) is predominant in most mouse, rat, and human tissues.89 The mammalian Syt VII isoform binds Ca2+/phopholipids with higher affinity than Syt I does, suggesting that Syt VII functions as a high affinity Ca2+-sensor.64 In addition, both C2 domains of Syt VII contribute to the formation of Ca2+-dependent multimers and hetero-oligomers with other Syt isoforms.90,91
The subcellular localization of Syt VII protein is a matter of some controversy, and it has been shown to be present in three different compartments: presynaptic plasma membranes in neurons,88 dense-core vesicles in endocrine cells,92,93 and lysosomes in fibroblasts.94 Syt VII regulates Ca2+-dependent lysosomal exocytosis in fibroblasts, which contributes to plasma membrane repair.94-96 Syt VII knockout mice also exhibit impaired plasma membrane repair as well as autoimmune myositis, but no neurological abnormalities have been observed.97 While transiently overexpressed Syt VII in PC12 cells is mainly targeted to the plasma membrane, stably expressed Syt VII in PC12 cells is mainly targeted to dense-core vesicles and regulates their exocytosis, suggesting that Syt VII functions as a vesicular Ca2+-sensor, rather than as a plasma membrane Ca2+-sensor.92,93
Other Syt Isoforms (VIII, XII-XV)
The C2 domains of all other Syt isoforms (VIII, XII-XV)17,20,86 lack Ca2+/phospholipid binding activity.98 Syt XII (also known as Syt-related gene 1, Srg1) is specifically expressed in brain, and its expression level is regulated by thyroid hormone.99 Syt VIII is a ubiquitous Syt isoform and is localized at the acrosomal crescent of sperm cells.100 Syt VIII has been proposed to regulate the acrosome reaction through Ca2+-dependent interaction with syntaxin-2.71 Nothing is known about the subcellular localization and function of other Ca2+-independent Syt isoforms (XIII˜XV).
Role of Slp Family Members in Rab27-Dependent Membrane Trafficking
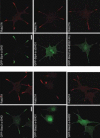
Slp (Syt-like protein) is defined as a protein that consists of an N-terminal “SHD (Slp homology domain)”, a linker domain of varying length, and C-terminal tandem C2 domains. 3,10-12,101 Five different isoforms (Slp1˜5)101,102 are present in mice, rats, and humans, a single isoform (dm-Slp/Btsz)103 in Drosophila, and none in C. elegans (Table 1).12,17 Several alternative splicing isoforms that lack one or several domains have been reported in most Slp members.101 The SHD consists of two potential α-helical regions (named SHD1 and SHD2; see Fig. 1), which are often separated by two zinc finger motifs. The SHD shows weak similarity to the Rab3A-binding domain of rabphilin, and it is now widely believed to function as a specific effector domain for Rab27A (or Rab27B), one of the small GTPase Rab proteins.12-14,104-106 The SHD is also found in the N-terminal region of members of the other protein family, named the Slac2 family (Slac2-a/melanophilin, Slac2-b, and Slac2-c/MyRIP) (for a review see ref. 12). The first α-helical SHD1 alone, and not the SHD2, is capable of interacting with GTP-Rab27A in vitro, and a single point mutation in the SHD1 (e.g., E14A in Slac2-a) completely abrogates Rab27A/B-binding activity.14 In vivo, however, both domains are absolutely required for targeting the SHD to endogenous Rab27. For example, the entire SHD of Slac2-a or Slp4-a, but not the Slac2-a-SHD1, Slac2-a-SHD2, or the Slp4-a-DeltaSHD1, is recruited to Rab27A on dense-core vesicles in the neurites of NGF-differentiated PC12 cells (Fig. 3). By contrast, zinc finger domains are not required for Rab27A/B binding activity, and they are presumably involved in the stability of the SHD structure.14 This section describes the recent discovery of the Rab27 effector function of Slps in Rab27-dependent membrane trafficking, with particular emphasis on the docking of Rab27-containing organelles to the plasma membrane.
Role of Slp2-a in Melanosome Transport in Melanocytes
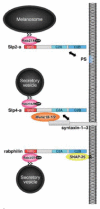
Slp2-a is abundantly expressed on the melanosomes of cultured melanocytes and is involved in intracellular melanosome transport.13 Slp2-a simultaneously interacts with Rab27A on the melanosome via the N-terminal SHD and with phosphatidylserine (PS) in the plasma membrane via the C2A domain, and thereby promotes melanosome anchoring to the plasma membrane in melanocytes (Fig. 4, top). Knockdown of Slp2-a by the specific siRNA (small interfering RNA) results in a reduction in the numbers of peripheral melanosomes in melanocytes (referred to as “peripheral dilution phenotype”) and induction of a rounded cell shape, rather than their normal elongated (or dendritic) shape.107 Although the Slp2-a C2A domain is required for maintenance of the melanocyte morphology, the exact mechanism by which Slp2-a regulates cell morphology remains unknown. Since Slp2-a is also expressed in other cell types besides melanocytes,101 Slp2-a may be involved in the docking step of Rab27A-containing organelles to the plasma membrane in these cells.
Role of Slp4-a/granuphilin-a in Secretory Vesicle Docking to the Plasma Membrane
Slp4-a was originally identified as granuphilin-a, which is specifically localized on insulin-containing vesicles in pancreatic β-cells, however, recent studies have shown that Slp4-a is also expressed in dense-core vesicles in PC12 cells108 and amylase-containing vesicles in parotid acinar cells.109 In contrast to the other Slp members, Slp4-a exhibits several unique biochemical features. First, the SHD of Slp4-a is capable of interacting with Rab3 and Rab8 isoforms in addition to Rab27 isoforms in vitro, whereas others specifically interact with Rab27 isoforms.13,106,110,111 Second, the SHD of Slp4-a is capable of interacting with Rab27A(T23N), which mimics the GDP-bound form of Rab27A, and the others are not.110 Third, the linker domain of Slp4-a, which is not conserved among the Slp family members, interacts with Munc18-1, Munc18-1/syntaxin-1a complex, and Munc18-2/syntaxin-2/3 complex.110,112 Syntaxin-1a has been reported to interact with the SHD in a Rab27A-dependent manner,113 but this interaction has not been observed in other studies.110,111 Finally, expression of Slp4-a, but not other Slps, in pancreatic β-cells or PC12 cells strongly attenuates Ca2+-dependent hormone secretion,104,108,110-113 although Slp4-a expression increases the number of vesicles docked to the plasma membrane.114 Consistent with these in vitro cell culture findings, Slp4-a knockout mice contain fewer insulin-containing vesicles docked to the plasma membrane in islet β-cells but possess increased insulin secretion activity.115 It is of great interest that the level of expression of both syntaxin-1a and Munc18-1 is also reduced in Slp4-a knockout mice.115 All these observations suggest that Slp4-a simultaneously interacts with Rab27A/B on the secretory vesicles via the N-terminal SHD and with a certain syntaxin/Munc18 complexes in the plasma membrane via the linker domain, and thereby promotes the docking of the dense-core vesicle to the plasma membrane in secretory cells (Fig. 4, middle). Consistent with this notion, Rab27A or Munc18-1 itself has been shown to control the docking step of secretory vesicles in certain cell types.116-118
Role of Other Slps in Secretory Vesicle Exocytosis
Slp1 (also called JFC1) is characterized as a phosphatidylinositol 3,4,5-trisphosphate (PIP3)-binding protein119 and as an Akt substrate.120 Slp1 has been shown to control secretion of prostate-specific antigen (PSA) by prostate cell lines,121 although the mechanism by which Slp1 regulates PSA secretion is largely unknown. Nevertheless, it is tempting to speculate that Slp1 simultaneously interacts with Rab27A on the granules via the SHD, and with PIP3 in the plasma membrane via the C2A domain, and promotes granule docking to the plasma membrane, the same as Slp2-a does.
Almost nothing is known about the expression and localization of Slp3-a and Slp5, two Ca2+-dependent type Slps98,102 that promote dense-core vesicle exocytosis when expressed in PC12 cells.110 It is currently unknown whether Slp3-a and Slp5 are involved in the docking step of secretory vesicles.
Role of Rabphilin in Rab27-Dependent Membrane Trafficking
The Doc2/rabphilin family in mice, rats, and humans consists of three Doc2 isoforms (α, β, and γ)122,123 and one rabphilin (Table 1), and only the rabphilin has been retained during evolution (from C. elegans to humans).17 Doc2α and rabphilin are mainly expressed in neural tissues and certain neuroendocrine cells, whereas Doc2β and Doc2γ are expressed ubiquitously. Involvement of Doc2α/β or rabphilin in regulated exocytosis was intensively investigated by biochemical, structural, and genetic analyses before 2001,9,124-126 and there have been only a few extensions of research on this family since 2002. One of the most important findings concerning this family is that rabphilin functions as a Rab27 effector, rather than as a Rab3 effector, especially in invertebrates.127,128 In the final section, I describe the recent discovery of the Rab27 effector function of rabphilin in regulated exocytosis.
Genetic analysis of rabphilin mutant animals125,126 and biochemical studies129,130 have indicated that rabphilin is targeted to secretory vesicles independently of the function of Rab3. In early 2002, our group discovered that the Rab-binding domain of rabphilin also interacts in vitro with Rab27, the closest subfamily of Rab3 in the phylogenetic tree.13,106 Although the endogenous expression level of Rab27A in PC12 cells is lower than that of Rab3A, rabphilin preferentially interacts with Rab27A, because rabphilin binds Rab27A with much higher affinity than Rab3A.127,128 It should be noted that invertebrate rabphilin specifically binds Rab27, but does not bind Rab3 or Rab8, indicating that rabphilin has functioned as a Rab27 effector during evolution.127 It has recently been proposed that rabphilin promotes docking of dense-core vesicles to the plasma membrane in PC12 cells through simultaneous interaction with Rab3/27 on the dense-core vesicle via the RBD and with SNAP-25 at the plasma membrane via the C2B domain (Fig. 4, bottom).131 In contrast to Slp4-a described above, expression of rabphilin significantly increases the depolarization-induced exocytotic fusion events in PC12 cells.110,131
Concluding Remarks
The C-type tandem C2 protein family is a large family of putative membrane trafficking proteins found in a variety of species from invertebrates, to vertebrates, and to plants (Fig. 1 and Table 2). Although more than 1000 of papers on the C-type tandem C2 proteins have been published in the literature, this chapter has mainly dealt with recent advances in research on the molecular mechanism of membrane trafficking mediated by the C-type tandem C2 protein family. If the reader is interested in learning more about this family and would like to know of other outstanding studies on the role of the C-type tandem C2 protein family that have been published before 2001, the following reviews provide the details on individual subfamilies of C-type tandem C2 proteins: for the Syt family (see refs. 4-6), for the Doc2/rabphilin family (see refs. 9,132), and for the Slp family (see refs. 10-12). Tremendous advances have been made in research on C-type tandem C2 protein during the past five years, including on the role of Syt I in multiple stages of synaptic vesicle trafficking (Fig. 2), involvement of other Syt isoforms in specific types of Ca2+-regulated exocytosis (e.g., lysosomal exocytosis in fibroblasts, acrosome reaction in sperm, and glutamate release from astrocytes), and novel docking machinery consisting of Rab27 and its effectors (Slp and rabphilin; Fig. 4). By contrast, the tissue distribution, subcellular localization, and function of more than half of the C-type tandem C2 proteins remain unknown. Therefore, one important direction of future research will be the elucidation of the localization and function of individual C-type tandem C2 proteins of unknown function. Another important direction of research will be the determination of functional relationships between the distinct families of C-type tandem C2 proteins during regulated exocytosis, because most secreting cells express members of several families of C-type tandem C2 proteins (e.g., Syt, Slp, and Doc2/rabphilin family) in a single cell type. Intensive studies will be required to achieve full understanding of the roles of the C-type tandem C2 proteins in membrane trafficking at the molecular level.
Acknowledgement
This work was supported in part by the Ministry of Education, Culture, Sports, and Technology of Japan (Grants 15689006, 16044248, 17024065, and 17657067), by the Kato Memorial Bioscience Foundation (to M. Fukuda), by the NOVARTIS Foundation (Japan) for the Promotion of Science, and by the Life Science Foundation of Japan. I thank members of the Fukuda Initiative Research Unit in RIKEN for preparing the manuscripts and for helpful discussions.
References
- 1.
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83(2):581–632. [PubMed: 12663867]
- 2.
- Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci. 1996;5(12):2375–2390. [PMC free article: PMC2143302] [PubMed: 8976547]
- 3.
- Fukuda M, Mikoshiba K. Synaptotagmin-like protein 1-3: A novel family of C-terminal-type tandem C2 proteins. Biochem Biophys Res Commun. 2001;281(5):1226–1233. [PubMed: 11243866]
- 4.
- Fukuda M. Synaptotagmins, Ca2+- and phospholipid-binding proteins that control Ca2+-regulated membrane trafficking. Recent Res Dev Chem Phys Lipids. 2003;1:15–51.
- 5.
- Südhof TC. Synaptotagmins: Why so many? J Biol Chem. 2002;277(10):7629–7632. [PubMed: 11739399]
- 6.
- Chapman ER. Synaptotagmin: A Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3(7):498–508. [PubMed: 12094216]
- 7.
- Shirataki H, Kaibuchi K, Sakoda T. et al. Rabphilin-3A, a putative target protein for smg p25A/ rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13(4):2061–2068. [PMC free article: PMC359527] [PubMed: 8384302]
- 8.
- Orita S, Naito A, Sakaguchi G. et al. Physical and functional interactions of Doc2 and Munc13 in Ca2+-dependent exocytotic machinery. J Biol Chem. 1997;272(26):16081–16084. [PubMed: 9195900]
- 9.
- Duncan RR, Shipston MJ, Chow RH. Double C2 protein: A review. Biochimie. 2000;82(5):421–426. [PubMed: 10865129]
- 10.
- Fukuda M. Slp and Slac2, novel families of Rab27 effectors that control Rab27-dependent membrane traffic. Recent Res Dev Neurochem. 2002;5:297–309.
- 11.
- Cheviet S, Waselle L, Regazzi R. Noc-king out exocrine and endocrine secretion. Trends Cell Biol. 2004;14(10):525–528. [PubMed: 15450973]
- 12.
- Fukuda M. Versatile role of Rab27 in membrane trafficking: Focus on the Rab27 effector families. J Biochem (Tokyo). 2005;137(1):9–16. [PubMed: 15713878]
- 13.
- Kuroda TS, Fukuda M, Ariga H. et al. The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem. 2002;277(11):9212–9218. [PubMed: 11773082]
- 14.
- Fukuda M. Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J Biol Chem. 2002;277(42):40118–40124. [PubMed: 12189142]
- 15.
- Kwon OJ, Gainer H, Wray S. et al. Identification of a novel protein containing two C2 domains selectively expressed in the rat brain and kidney. FEBS Lett. 1996;378(2):135–139. [PubMed: 8549819]
- 16.
- Fukuda M, Mikoshiba K. The N-terminal cysteine cluster is essential for membrane targeting of B/K protein. Biochem J. 2001;360(2):441–448. [PMC free article: PMC1222245] [PubMed: 11716773]
- 17.
- Fukuda M. Molecular cloning, expression, and characterization of a novel class of synaptotagmin (Syt XIV) conserved from Drosophila to humans. J Biochem (Tokyo). 2003;133(5):641–649. [PubMed: 12801916]
- 18.
- Fukuda M, Mikoshiba K. Tac2-N, an atypical C-type tandem C2 protein localized in the nucleus. FEBS Lett. 2001;503(2-3):217–218. [PubMed: 11526914]
- 19.
- Craxton M. Synaptotagmin gene content of the sequenced genomes. BMC Genomics. 2004;5(1):43. [PMC free article: PMC471550] [PubMed: 15238157]
- 20.
- Fukuda M. Molecular cloning and characterization of human, rat, and mouse synaptotagmin XV. Biochem Biophys Res Commun. 2003;306(1):64–71. [PubMed: 12788067]
- 21.
- Schulz TA, Creutz CE. The tricalbin C2 domains: Lipid-binding properties of a novel, synaptotagmin-like yeast protein family. Biochemistry. 2004;43(13):3987–3995. [PubMed: 15049706]
- 22.
- Fukuda M, Kanno E, Ogata Y. et al. Mechanism of the SDS-resistant synaptotagmin clustering mediated by the cysteine cluster at the interface between the transmembrane and spacer domains. J Biol Chem. 2001;276(43):40319–40325. [PubMed: 11514560]
- 23.
- Fukuda M, Kowalchyk JA, Zhang X. et al. Synaptotagmin IX regulates Ca2+-dependent secretion in PC12 cells. J Biol Chem. 2002;277(7):4601–4604. [PubMed: 11751925]
- 24.
- Shin OH, Maximov A, Lim BK. et al. Unexpected Ca2+-binding properties of synaptotagmin 9. Proc Natl Acad Sci USA. 2004;101(8):2554–2559. [PMC free article: PMC356988] [PubMed: 14983047]
- 25.
- Hui E, Bai J, Wang P. et al. Three distinct kinetic groupings of the synaptotagmin family: Candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci USA. 2005;102(14):5210–5214. [PMC free article: PMC556003] [PubMed: 15793006]
- 26.
- Mackler JM, Drummond JA, Loewen CA. et al. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418(6895):340–344. [PubMed: 12110842]
- 27.
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36(5):897–908. [PubMed: 12467593]
- 28.
- Stevens CF, Sullivan JM. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron. 2003;39(2):299–308. [PubMed: 12873386]
- 29.
- Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24(39):8542–8550. [PMC free article: PMC6729890] [PubMed: 15456828]
- 30.
- Fukuda M, Moreira JE, Liu V. et al. Role of the conserved WHXL motif in the C terminus of synaptotagmin in synaptic vesicle docking. Proc Natl Acad Sci USA. 2000;97(26):14715–14719. [PMC free article: PMC18984] [PubMed: 11114192]
- 31.
- Chieregatti E, Witkin JW, Baldini G. SNAP-25 and synaptotagmin 1 function in Ca2+-dependent reversible docking of granules to the plasma membrane. Traffic. 2002;3(7):496–511. [PubMed: 12047557]
- 32.
- Tsuboi T, Rutter GA. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr Biol. 2003;13(7):563–567. [PubMed: 12676086]
- 33.
- Bai J, Wang CT, Richards DA. et al. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 2004;41(6):929–942. [PubMed: 15046725]
- 34.
- Poskanzer KE, Marek KW, Sweeney ST. et al. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426(6966):559–563. [PubMed: 14634669]
- 35.
- Nicholson-Tomishima K, Ryan TA. Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc Natl Acad Sci USA. 2004;101(47):16648–16652. [PMC free article: PMC534526] [PubMed: 15492212]
- 36.
- Llinás RR, Sugimori M, Moran KA. et al. Vesicular reuptake inhibition by a synaptotagmin I C2B domain antibody at the squid giant synapse. Proc Natl Acad Sci USA. 2004;101(51):17855–17860. [PMC free article: PMC539760] [PubMed: 15591349]
- 37.
- Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304(5669):435–438. [PubMed: 15044754]
- 38.
- Zhang X, Kim-Miller MJ, Fukuda M. et al. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34(4):599–611. [PubMed: 12062043]
- 39.
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11(1):36–44. [PubMed: 14718921]
- 40.
- Rickman C, Archer DA, Meunier FA. et al. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J Biol Chem. 2004;279(13):12574–12579. [PubMed: 14709554]
- 41.
- Wu Y, He Y, Bai J. et al. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc Natl Acad Sci USA. 2003;100(4):2082–2087. [PMC free article: PMC149962] [PubMed: 12578982]
- 42.
- Walther K, Diril MK, Jung N. et al. Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc Natl Acad Sci USA. 2004;101(4):964–969. [PMC free article: PMC327125] [PubMed: 14726597]
- 43.
- Fernandez I, Arac D, Ubach J. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32(6):1057–1069. [PubMed: 11754837]
- 44.
- Earles CA, Bai J, Wang P. et al. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J Cell Biol. 2001;154(6):1117–1123. [PMC free article: PMC2150817] [PubMed: 11551981]
- 45.
- Fukuda M, Mikoshiba K. The function of inositol high polyphosphate binding proteins. Bioessays. 1997;19(7):593–603. [PubMed: 9230692]
- 46.
- Mackler JM, Reist NE. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J Comp Neurol. 2001;436(1):4–16. [PubMed: 11413542]
- 47.
- Fukuda M, Yamamoto A, Mikoshiba K. Formation of crystalloid endoplasmic reticulum induced by expression of synaptotagmin lacking the conserved WHXL motif in the C terminus: Structural importance of the WHXL motif in the C2B domain. J Biol Chem. 2001;276(44):41112–41119. [PubMed: 11533032]
- 48.
- Han W, Rhee JS, Maximov A. et al. C-terminal ECFP fusion impairs synaptotagmin 1 function: Crowding out synaptotagmin 1. J Biol Chem. 2005;280(6):5089–5100. [PubMed: 15561725]
- 49.
- Fukuda M. Vesicle-associated membrane protein-2/synaptobrevin binding to synaptotagmin I promotes O-glycosylation of synaptotagmin I. J Biol Chem. 2002;277(33):30351–30358. [PubMed: 12048209]
- 50.
- Han W, Rhee JS, Maximov A. et al. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 2004;41(1):85–99. [PubMed: 14715137]
- 51.
- Kang R, Swayze R, Lise MF. et al. Presynaptic trafficking of synaptotagmin I is regulated by protein palmitoylation. J Biol Chem. 2004;279(48):50524–50536. [PubMed: 15355980]
- 52.
- Atiya-Nasagi Y, Cohen H, Medalia O. et al. O-glycosylation is essential for intracellular targeting of synaptotagmins I and II in nonneuronal specialized secretory cells. J Cell Sci. 2005;118(7):1363–1372. [PubMed: 15755799]
- 53.
- Voets T, Moser T, Lund PE. et al. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc Natl Acad Sci USA. 2001;98(20):11680–11685. [PMC free article: PMC58789] [PubMed: 11562488]
- 54.
- Kreft M, Kuster V, Grilc S. et al. Synaptotagmin I increases the probability of vesicle fusion at low [Ca2+] in pituitary cells. Am J Physiol Cell Physiol. 2003;284(2):C547–554. [PubMed: 12388083]
- 55.
- Shoji-Kasai Y, Yoshida A, Sato K. et al. Neurotransmitter release from synaptotagmin-deficient clonal variants of PC12 cells. Science. 1992;256(5065):1821–1823. [PubMed: 1352065]
- 56.
- Fukuda M. RNA interference-mediated silencing of synaptotagmin IX, but not synaptotagmin I, inhibits dense-core vesicle exocytosis in PC12 cells. Biochem J. 2004;380(3):875–879. [PMC free article: PMC1224215] [PubMed: 15015935]
- 57.
- Craxton M, Goedert M. Synaptotagmin V: A novel synaptotagmin isoform expressed in rat brain. FEBS Lett. 1995;361(2-3):196–200. [PubMed: 7698322]
- 58.
- Hudson AW, Birnbaum MJ. Identification of a nonneuronal isoform of synaptotagmin. Proc Natl Acad Sci USA. 1995;92(13):5895–5899. [PMC free article: PMC41608] [PubMed: 7597049]
- 59.
- Iezzi M, Kouri G, Fukuda M. et al. Synaptotagmin V and IX isoforms control Ca2+-dependent insulin exocytosis. J Cell Sci. 2004;117(15):3119–3127. [PubMed: 15190121]
- 60.
- Iezzi M, Eliasson L, Fukuda M. et al. Adenovirus-mediated silencing of synaptotagmin 9 inhibits Ca2+-dependent insulin secretion in islets. FEBS Lett. 2005;579(23):5241–5246. [PubMed: 16165130]
- 61.
- Haberman Y, Grimberg E, Fukuda M. et al. Synaptotagmin IX, a possible linker between the perinuclear endocytic recycling compartment and the microtubules. J Cell Sci. 2003;116(21):4307–4318. [PubMed: 12966166]
- 62.
- Haberman Y, Ziv I, Gorzalczany Y. et al. Classical protein kinase C(s) regulates targeting of synaptotagmin IX to the endocytic recycling compartment. J Cell Sci. 2005;118(8):1641–1649. [PubMed: 15784685]
- 63.
- Fukuda M, Kanno E, Mikoshiba K. Conserved N-terminal cysteine motif is essential for homoand heterodimer formation of synaptotagmins III, V, VI, and X. J Biol Chem. 1999;274(44):31421–31427. [PubMed: 10531343]
- 64.
- Sugita S, Shin OH, Han W. et al. Synaptotagmins form a hierarchy of exocytotic Ca2+ sensors with distinct Ca2+ affinities. EMBO J. 2002;21(3):270–280. [PMC free article: PMC125835] [PubMed: 11823420]
- 65.
- Rickman C, Craxton M, Osborne S. et al. Comparative analysis of tandem C2 domains from the mammalian synaptotagmin family. Biochem J. 2004;378(2):681–686. [PMC free article: PMC1223993] [PubMed: 14713287]
- 66.
- Butz S, Fernandez-Chacon R, Schmitz F. et al. The subcellular localizations of atypical synaptotagmins III and VI: Synaptotagmin III is enriched in synapses and synaptic plasma membranes but not in synaptic vesicles. J Biol Chem. 1999;274(26):18290–18296. [PubMed: 10373432]
- 67.
- Grimberg E, Peng Z, Hammel I. et al. Synaptotagmin III is a critical factor for the formation of the perinuclear endocytic recycling compartment and determination of secretory granules size. J Cell Sci. 2003;116(1):145–154. [PubMed: 12456724]
- 68.
- Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem. 2002;277(27):24499–24505. [PubMed: 12006594]
- 69.
- Fukuda M, Mikoshiba K. A novel alternatively spliced variant of synaptotagmin VI lacking a transmembrane domain: Implications for distinct functions of the two isoforms. J Biol Chem. 1999;274(44):31428–31434. [PubMed: 10531344]
- 70.
- Michaut M, De Blas G, Tomes CN. et al. Synaptotagmin VI participates in the acrosome reaction of human spermatozoa. Dev Biol. 2001;235(2):521–529. [PubMed: 11437455]
- 71.
- Hutt DM, Baltz JM, Ngsee JK. Synaptotagmin VI and VIII and syntaxin 2 are essential for the mouse sperm acrosome reaction. J Biol Chem. 2005;280(21):20197–20203. [PubMed: 15774481]
- 72.
- Roggero CM, Tomes CN, De Blas GA. et al. Protein kinase C-mediated phosphorylation of the two polybasic regions of synaptotagmin VI regulates their function in acrosomal exocytosis. Dev Biol. 2005;285(2):422–435. [PubMed: 16111671]
- 73.
- Babity JM, Armstrong JN, Plumier JC. et al. A novel seizureinduced synaptotagmin gene identified by differential display. Proc Natl Acad Sci USA. 1997;94(6):2638–2641. [PMC free article: PMC20141] [PubMed: 9122248]
- 74.
- Adolfsen B, Saraswati S, Yoshihara M. et al. Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment. J Cell Biol. 2004;166(2):249–260. [PMC free article: PMC2172321] [PubMed: 15263020]
- 75.
- Dai H, Shin OH, Machius M. et al. Structural basis for the evolutionary inactivation of Ca2+ binding to synaptotagmin 4. Nat Struct Mol Biol. 2004;11(9):844–849. [PubMed: 15311271]
- 76.
- Wang CT, Grishanin R, Earles CA. et al. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294(5544):1111–1115. [PubMed: 11691996]
- 77.
- Machado HB, Liu W, Vician LJ. et al. Synaptotagmin IV overexpression inhibits depolarization-induced exocytosis in PC12 cells. J Neurosci Res. 2004;76(3):334–341. [PubMed: 15079862]
- 78.
- Fukuda M, Kanno E, Ogata Y. et al. Nerve growth factor-dependent sorting of synaptotagmin IV protein to mature dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem. 2003;278(5):3220–3226. [PubMed: 12446703]
- 79.
- Zhang Q, Fukuda M, Van Bockstaele E. et al. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA. 2004;101(25):9441–9446. [PMC free article: PMC438995] [PubMed: 15197251]
- 80.
- Ferguson GD, Vician L, Herschman HR. Synaptotagmin IV: Biochemistry, genetics, behavior, and possible links to human psychiatric disease. Mol Neurobiol. 2001;23(2-3):173–185. [PubMed: 11817218]
- 81.
- Ibata K, Fukuda M, Hamada T. et al. Synaptotagmin IV is present at the Golgi and distal parts of neurites. J Neurochem. 2000;74(2):518–526. [PubMed: 10646502]
- 82.
- Fukuda M, Yamamoto A. Effect of forskolin on synaptotagmin IV protein trafficking in PC12 cells. J Biochem (Tokyo). 2004;136(2):245–253. [PubMed: 15496596]
- 83.
- Ibata K, Hashikawa T, Tsuboi T. et al. Nonpolarized distribution of synaptotagmin IV in neurons: Evidence that synaptotagmin IV is not a synaptic vesicle protein. Neurosci Res. 2002;43(4):401–406. [PubMed: 12135783]
- 84.
- Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C2A domain. Nature. 2002;418(6895):336–340. [PubMed: 12110845]
- 85.
- Fukuda M, Ibata K, Mikoshiba K. A unique spacer domain of synaptotagmin IV is essential for Golgi localization. J Neurochem. 2001;77(3):730–740. [PubMed: 11331402]
- 86.
- Fukuda M, Mikoshiba K. Characterization of KIAA1427 protein as an atypical synaptotagmin (Syt XIII). Biochem J. 2001;354(2):249–257. [PMC free article: PMC1221650] [PubMed: 11171101]
- 87.
- Huynh DP, Scoles DR, Nguyen D. et al. The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet. 2003;12(20):2587–2597. [PubMed: 12925569]
- 88.
- Sugita S, Han W, Butz S. et al. Synaptotagmin VII as a plasma membrane Ca2+ sensor in exocytosis. Neuron. 2001;30(2):459–473. [PubMed: 11395007]
- 89.
- Fukuda M, Ogata Y, Saegusa C. et al. Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem J. 2002;365(1):173–180. [PMC free article: PMC1222667] [PubMed: 12071850]
- 90.
- Fukuda M, Mikoshiba K. Mechanism of the calcium-dependent multimerization of synaptotagmin VII mediated by its first and second C2 domains. J Biol Chem. 2001;276(29):27670–27676. [PubMed: 11373279]
- 91.
- Fukuda M, Katayama E, Mikoshiba K. The calcium-binding loops of the tandem C2 domains of synaptotagmin VII cooperatively mediate calcium-dependent oligomerization. J Biol Chem. 2002;277(32):29315–29320. [PubMed: 12034723]
- 92.
- Fukuda M, Kanno E, Satoh M. et al. Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem. 2004;279(50):52677–52684. [PubMed: 15456748]
- 93.
- Wang P, Chicka MC, Bhalla A. et al. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol. 2005;25(19):8693–8702. [PMC free article: PMC1265757] [PubMed: 16166648]
- 94.
- Martinez I, Chakrabarti S, Hellevik T. et al. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148(6):1141–1149. [PMC free article: PMC2174306] [PubMed: 10725327]
- 95.
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106(2):157–169. [PubMed: 11511344]
- 96.
- Andrews NW, Chakrabarti S. There's more to life than neurotransmission: The regulation of exocytosis by synaptotagmin VII. Trends Cell Biol. 2005;15(11):626–631. [PubMed: 16168654]
- 97.
- Chakrabarti S, Kobayashi KS, Flavell RA. et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162(4):543–549. [PMC free article: PMC2173791] [PubMed: 12925704]
- 98.
- Fukuda M. The C2A domain of synaptotagmin-like protein 3 (Slp3) is an atypical calcium-dependent phospholipid-binding machine: Comparison with the C2A domain of synaptotagmin I. Biochem J. 2002;366(2):681–687. [PMC free article: PMC1222805] [PubMed: 12049610]
- 99.
- Potter GB, Facchinetti F, Beaudoin IIIrd GM. et al. Neuronal expression of synaptotagmin-related gene 1 is regulated by thyroid hormone during cerebellar development. J Neurosci. 2001;21(12):4373–4380. [PMC free article: PMC6762732] [PubMed: 11404423]
- 100.
- Hutt DM, Cardullo RA, Baltz JM. et al. Synaptotagmin VIII is localized to the mouse sperm head and may function in acrosomal exocytosis. Biol Reprod. 2002;66(1):50–56. [PubMed: 11751263]
- 101.
- Fukuda M, Saegusa C, Mikoshiba K. Novel splicing isoforms of synaptotagmin-like proteins 2 and 3: Identification of the Slp homology domain. Biochem Biophys Res Commun. 2001;283(2):513–519. [PubMed: 11327731]
- 102.
- Kuroda TS, Fukuda M, Ariga H. et al. Synaptotagmin-like protein 5: A novel Rab27A effector with C-terminal tandem C2 domains. Biochem Biophys Res Commun. 2002;293(3):899–906. [PubMed: 12051743]
- 103.
- Serano J, Rubin GM. The Drosophila synaptotagmin-like protein bitesize is required for growth and has mRNA localization sequences within its open reading frame. Proc Natl Acad Sci USA. 2003;100(23):13368–13373. [PMC free article: PMC263820] [PubMed: 14581614]
- 104.
- Yi Z, Yokota H, Torii S. et al. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22(6):1858–1867. [PMC free article: PMC135591] [PubMed: 11865063]
- 105.
- Strom M, Hume AN, Tarafder AK. et al. A family of Rab27-binding proteins: Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277(28):25423–25430. [PubMed: 11980908]
- 106.
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2: Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278(17):15373–15380. [PubMed: 12578829]
- 107.
- Kuroda TS, Fukuda M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol. 2004;6(12):1195–1203. [PubMed: 15543135]
- 108.
- Fukuda M, Kanno E, Saegusa C. et al. Slp4-a/granuphilin-a regulates dense-core vesicle exocytosis in PC12 cells. J Biol Chem. 2002;277(42):39673–39678. [PubMed: 12176990]
- 109.
- Imai A, Yoshie S, Nashida T. et al. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J Cell Sci. 2004;117(10):1945–1953. [PubMed: 15039459]
- 110.
- Fukuda M. Slp4-a/granuphilin-a inhibits dense-core vesicle exocytosis through interaction with the GDP-bound form of Rab27A in PC12 cells. J Biol Chem. 2003;278(17):15390–15396. [PubMed: 12590134]
- 111.
- Coppola T, Frantz C, Perret-Menoud V. et al. Pancreatic β-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell. 2002;13(6):1906–1915. [PMC free article: PMC117613] [PubMed: 12058058]
- 112.
- Fukuda M, Imai A, Nashida T. et al. Slp4-a/granuphilin-a interacts with syntaxin-2/3 in a Munc18-2-dependent manner. J Biol Chem. 2005;280(47):39175–39184. [PubMed: 16186111]
- 113.
- Torii S, Zhao S, Yi Z. et al. Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a. Mol Cell Biol. 2002;22(15):5518–5526. [PMC free article: PMC133943] [PubMed: 12101244]
- 114.
- Torii S, Takeuchi T, Nagamatsu S. et al. Rab27 effector granuphilin promotes the plasma membrane targeting of insulin granules via interaction with syntaxin 1a. J Biol Chem. 2004;279(21):22532–22538. [PubMed: 15028737]
- 115.
- Gomi H, Mizutani S, Kasai K. et al. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol. 2005;171(1):99–109. [PMC free article: PMC2171228] [PubMed: 16216924]
- 116.
- Trambas CM, Griffiths GM. Delivering the kiss of death. Nat Immunol. 2003;4(5):399–403. [PubMed: 12719728]
- 117.
- Kasai K, Ohara-Imaizumi M, Takahashi N. et al. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. 2005;115(2):388–396. [PMC free article: PMC546426] [PubMed: 15690086]
- 118.
- Korteweg N, Maia AS, Thompson B. et al. The role of Munc18-1 in docking and exocytosis of peptide hormone vesicles in the anterior pituitary. Biol Cell. 2005;97(6):445–455. [PubMed: 15898951]
- 119.
- Catz SD, Johnson JL, Babior BM. The C2A domain of JFC1 binds to 3'-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc Natl Acad Sci USA. 2002;99(18):11652–11657. [PMC free article: PMC129324] [PubMed: 12189202]
- 120.
- Johnson JL, Pacquelet S, Lane WS. et al. Akt regulates the subcellular localization of the Rab27a-binding protein JFC1 by phosphorylation. Traffic. 2005;6(8):667–681. [PubMed: 15998322]
- 121.
- Johnson JL, Ellis BA, Noack D. et al. The Rab27a-binding protein, JFC1, regulates androgen-dependent secretion of prostate-specific antigen and prostatic-specific acid phosphatase. Biochem J. 2005;391(3):699–710. [PMC free article: PMC1276972] [PubMed: 16004602]
- 122.
- Fukuda M, Mikoshiba K. Doc2γ, a third isoform of double C2 protein, lacking calcium-dependent phospholipid binding activity. Biochem Biophys Res Commun. 2000;276(2):626–632. [PubMed: 11027523]
- 123.
- Fukuda M, Saegusa C, Kanno E. et al. The C2A domain of double C2 protein γ contains a functional nuclear localization signal. J Biol Chem. 2001;276(27):24441–24444. [PubMed: 11371549]
- 124.
- Sakaguchi G, Manabe T, Kobayashi K. et al. Doc2α is an activity-dependent modulator of excitatory synaptic transmission. Eur J Neurosci. 1999;11(12):4262–4268. [PubMed: 10594652]
- 125.
- Schluter OM, Schnell E, Verhage M. et al. Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release. J Neurosci. 1999;19(14):5834–5846. [PMC free article: PMC6783077] [PubMed: 10407024]
- 126.
- Staunton J, Ganetzky B, Nonet ML. Rabphilin potentiates soluble N-ethylmaleimide sensitive factor attachment protein receptor function independently of rab3. J Neurosci. 2001;21(23):9255–9264. [PMC free article: PMC6763921] [PubMed: 11717359]
- 127.
- Fukuda M, Kanno E, Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem. 2004;279(13):13065–13075. [PubMed: 14722103]
- 128.
- Fukuda M. Rabphilin and Noc2 function as Rab27 effectors that control Ca2+-regulated exocytosis. Recent Res Dev Neurochem. 2004;7:57–69.
- 129.
- Chung SH, Joberty G, Gelino EA. et al. Comparison of the effects on secretion in chromaffin and PC12 cells of Rab3 family members and mutants: Evidence that inhibitory effects are independent of direct interaction with Rabphilin3. J Biol Chem. 1999;274(25):18113–18120. [PubMed: 10364266]
- 130.
- Joberty G, Stabila PF, Coppola T. et al. High affinity Rab3 binding is dispensable for Rabphilin-dependent potentiation of stimulated secretion. J Cell Sci. 1999;112(20):3579–3587. [PubMed: 10504306]
- 131.
- Tsuboi T, Fukuda M. The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense-core vesicle exocytosis in PC12 cells. J Biol Chem. 2005;280(47):39253–39259. [PubMed: 16203731]
- 132.
- Sasaki T, Shirataki H, Nakanishi H. et al. Rab3A-rabphilin-3A system in neurotransmitter release. Adv Second Messenger Phosphoprotein Res. 1997;31:279–294. [PubMed: 9344258]
- 133.
- Li C, Ullrich B, Zhang JZ. et al. Ca2+-dependent and -independent activities of neural and nonneural synaptotagmins. Nature. 1995;375(6532):594–599. [PubMed: 7791877]
- The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exoc...The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exocytosis - Madame Curie Bioscience Database
- Circadian Organization in the Algal Flagellate Euglena - Madame Curie Bioscience...Circadian Organization in the Algal Flagellate Euglena - Madame Curie Bioscience Database
- Pro-Inflammatory Responses in Macrophages during Toxoplasma gondii Infection - M...Pro-Inflammatory Responses in Macrophages during Toxoplasma gondii Infection - Madame Curie Bioscience Database
- Periodic Selection and Ecological Diversity in Bacteria - Madame Curie Bioscienc...Periodic Selection and Ecological Diversity in Bacteria - Madame Curie Bioscience Database
- Brain Metastasis - Madame Curie Bioscience DatabaseBrain Metastasis - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...