NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Proteins provided with unique functional groups such as affinity labels or fluorescence moieties offer high potential in many biotechnological or biomedical investigations, e.g., immobilization studies or high throughput screenings. An attractive alternative to known posttranslational methods of protein modification is the site-directed cotranslational incorporation of unnatural amino acids. Here we point out different aspects of this method with regard to the synthesis of protein conjugates bearing different functionalities. Moreover we describe a cell-free system enabling tRNA-based synthesis of modified proteins even with large functional groups in high yields. This specialized cell-free system contains a substantially decreased level of release factor 1 (RF1) and originates from an E. coli strain encoding a tagged RF1 variant. We present the efficient amber suppressor tRNA-mediated incorporation of the unnatural amino acid biocytin (biotinylated lysine) into stoichiometrically defined protein conjugates, containing the biotin label at the desired position. As potential applications we demonstrate the usefulness of the system for the immobilization of biotinylated proteins directly from a cell-free system and for interaction studies. Finally we show the cotranslational incorporation of a large fluorescent amino acid using our system.
Protein Conjugates
The ability to incorporate a wide array of functional groups at specific sites in proteins provides a powerful tool for characterization of protein function and the development of protein tools or therapeutics. In this context protein conjugates play a key role. Protein conjugates are used in biophysical and functional analytics, they are tools for biomedical research, medical diagnostics or are applied as therapeutically active substances.1,2 Traditionally the production of protein conjugates is an important step on the way to tailor proteins for the analysis of biological processes on a molecular level. The conjugation of proteins with tags such as biotin permits the selective binding of proteins to surfaces. In this way microarrays, beads and nano-particles can be equipped with proteins. Crosslinkers permit linking of proteins among themselves or with other molecules, e.g., nucleic acids. A conjugation of proteins with spectroscopic reporter groups is of significant importance, permitting the study of protein interactions or the localization of proteins within cellular structures and on protein chips. Protein conjugates become increasingly important with the development of innovative products for therapeutics and diagnostics. A prominent example is the equipment of proteins with polyethylenglycol chains, which improves their solubility and pharmacokinetics.3,4
Conventionally protein conjugates are synthesized by posttranslational modification of the purified proteins with functional groups.5 Naturally occurring reactive side chains within the proteins, preferably lysine or cysteine, are treated with commercially available reactive reagents. In certain cases posttranslational modification is a viable tool for the desired purpose but often it is not advantageous to use the natural occurring amino acid side chains for labeling, since the label is introduced at multiple sites and multiple protein derivatives arise. Moreover, typical posttranslational labeling methods may interfere with protein function and solubility, maintaining protein activity only to a minor degree.6 A frequently chosen way to produce site-specifically defined protein conjugates comprises the design of protein variants with a singular cysteine residue that is subsequently used for the introduction of the desired modification.7-9 Typically several variants of the desired protein must be synthesized, purified and modified, until a variant with optimal characteristics is found. The described methodology is complex and laborious and usually hampers the management of a larger number of protein sequences in parallel, thereby rendering it hardly compatible with high throughput concepts required by the pharmaceutical industry. There is a need for a faster and easier to handle methodology that allows the production of stoichiometrically and site-specifically defined protein conjugates maintaining the highest possible degree of protein solubility and activity. An alternative and generally applicable strategy should allow the labeling of proteins site-specifically even in the presence of many identical amino acid side chains. The most reliable method to produce defined site-specific modifications is the combination of tRNAs carrying a functionalized amino acid with a cell-free protein expression system.
Cell-free Systems as a Tool for Protein Conjugate Production
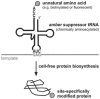
Cell-free protein biosynthesis represents a valuable tool, that allows an economic and parallel synthesis of a large number of proteins in analytical or semipreparative scale.10,11 An outstanding perspective of cell-free protein biosynthesis is the production of proteins containing unnatural amino acids at defined positions.12-16 An advantage of cell-free systems is the fact that they are open systems. Thus, they can easily be supplemented with precharged tRNAs.17,18 A common method to synthesize site-specifically modified proteins containing unnatural amino acids is based on the use of suppressor tRNAs, which are aminoacylated with a desired unnatural amino acid, subsequently added to a cell-free translation system and which insert the amino acid cotranslationally and site-specifically into the growing peptide chain (Fig. 1). With cell-free systems even amino acids bearing large functional side chains such as biotin or fluorescent moieties can be incorporated into proteins. Thus, the production of protein conjugates in one step is possible, avoiding the need of posttranslational modification methods with their caveats. In addition the concentration of precharged tRNA required for the synthesis of adequate amounts of modified proteins fits well to the achievable protein yield in cell-free batch systems. Hence it is highly advantageous to use “tRNA-mediated” incorporation of unnatural amino acids in cell-free protein biosynthesis systems for the production of protein conjugates.
One of the first experiments that provided a basis for engineered tRNA mediated site-directed incorporation of unnatural amino acids was performed in 1957, when the acceptance of a first unnatural amino acid (selenomethionine) by the protein biosynthesis machinery could be shown.19 The fact that a misacylated tRNA could lead to protein production and therefore that codon recognition occurs independent from the amino acid attached to the tRNA verified the “adapter hypothesis”.20 This implied that tRNAs could in principle be charged with arbitrary noncognate amino acids which could then be incorporated into proteins. The first amber suppressor tRNAs were discovered as intergenic suppressors.21 Thus nonsense codons, which are normally not decoded by endogenous tRNAs but serve as a signal for translation termination, were shown to have the potential to be recoded by changing the tRNA anticodon.
Meanwhile natural mechanisms have been elucidated, in which non canonical amino acids are inserted based on nonsense suppressor tRNAs. The cotranslational insertion of selenocysteine in many organisms is directed by a specialized suppressor tRNA, that reads the UGA (opal) codon.22 Another example is the site-directed incorporation of pyrrolysine. In some methyltransferase encoding genes of methanogenic archaea the nonsense codon UAG (amber) serves as a sense codon and is decoded by an amber suppressor tRNA, that inserts pyrrolysine.23,24 A corresponding mechanism is used by the recently developed “orthogonal systems” enabling the in vivo site-directed incorporation of small useful amino acids.25-29 These systems are based on the coexpression of an engineered aminoacyl tRNA synthetase-variant and an appropriate suppressor tRNA in a suitable host cell. P-acetyl-phenylalanine and p-azido-phenylalanine for instance are amino acids bearing chemical reactivities differing from those of the canonical amino acids, allowing the posttranslational site-directed conjugation of the synthesized proteins with appropriate functionalized reagents.30-34
At present in vivo incorporation is restricted to comparatively small unnatural amino acids with structures resembling those of the canonical amino acids, preventing the synthesis of protein conjugates in one step. The ribosome however is able to accept a broad range of unnatural amino acids, among them large amino acids with structures significantly different to the canonical amino acids. The biotin-containing amino acid biocytin for example can be site-directed incorporated into proteins after microinjection of biotinylated amber suppressor tRNAs into Xenopus oocytes.35
In cell-free systems the site-directed incorporation of unnatural amino acids became possible by the development of chemical aminoacylation, which enables the aminoacylation of an amber suppressor tRNA, that is not recognized by naturally occurring aminoacyl tRNA synthetases.36-43 Even amino acids which cannot be taken up by cells or activated by aminoacyl tRNA synthetase, like acids containing biotin35 or large fluorescent side chains can be attached to the tRNA.44 The strategy introduces the unnatural amino acid into the growing peptide chain by a stoichiometric approach, introducing one unnatural amino acid per tRNA and protein. The tRNA is added to the translation system as an aminoacylated molecule and is exhausted during expression of the desired protein. Recharging of the tRNA by canonical amino acids is excluded, since the tRNA does not contain recognition elements for aminoacyl tRNA synthetases present in the respective expression system. In this way, the desired unnatural amino acid is exclusively incorporated at the predicted position of the growing protein chain and a competition of canonical amino acids for this position is excluded. Thus site-specifically defined pure derivatives of the desired modified proteins emerge. In recent years alternative methods for the aminoacylation of tRNAs with the desired amino acids have been developed in addition to chemical aminoacylation. These methods include the use of peptide nucleic acids (PNAs)45,46 and ribozymes.47,48
The most promising approaches for tRNA-mediated site-directed unnatural amino acid incorporation by precharged tRNAs in cell-free systems involve the use of nonsense suppressor tRNAs40-43 and frameshift suppressor tRNAs.44,46 The unnatural amino acids are preferably incorporated at the amber stop codon (UAG) or at four base codons with the possibility to incorporate two or more unnatural amino acids into a single protein.49 Recently a combination of the amber and the four base strategy has been described.50 A special case is the use of the start codon by modified initiator tRNAs.51
Incorporation Efficiencies of Different Unnatural Amino Acids
Meanwhile several useful unnatural amino acids have been incorporated into proteins based on the use of precharged tRNAs in cell-free systems. Among them were amino acids for structural investigations,40,52-54 with selective reactive side chains,55-57 Stable isotope labelled amino acids58,59 and fluorescent amino acids.44,49,50,55,60-64
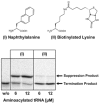
Unnatural amino acids are known to be incorporated with diverse efficiencies.55 For example, the small hydrophobic fluorescent amino acid naphthylalanine is reported to be very well incorporated in cell-free systems, while other unnatural amino acids are hardly incorporated.44,62 We could demonstrate the same in our standard, i.e., untreated E. coli lysate. An almost complete suppression of termination was achieved by an amber suppressor tRNA (tRNAPheCUA from yeast) charged with 2-naphthylalanine without further manipulation of the system, whereas the suppression efficiency with the bulky amino acid biocytin (biotinylated lysine) was very low in this system (Fig. 2).
There are many factors influencing the ability of a certain amino acid to be incorporated into a protein, including binding of the aminoacylated tRNA to elongation factor Tu (EF-Tu), competition of the tRNA for codon recognition with Release Factor 1 (RF1), binding of the tRNA to the ribosome by cognate codon-anticodon interaction, behavior of the amino acid in the peptidyl transferase center, and channeling of the amino acid within the growing peptide through the ribosome tunnel. Suppression efficiencies of different amber suppressor tRNAs have been traced back to structural features of the tRNAs, mainly to the anticodon arm.65-69 One possibility to improve suppression efficiency is to screen for more efficient suppressor tRNAs.64,70 Screening different amber suppressor tRNAs in order to increase the efficiency of biocytin incorporation, we could detect considerable differences in suppression efficiencies even among tRNAs with optimized anticodon arms (will be published elsewhere).
Depletion of RF1 from a Cell-free System Results in Efficient Incorporation of Biocytin
The major factor regarding the suppression efficiency of an amber suppressor tRNA is its ability to compete with release factor 1 (RF1) for decoding the amber (UAG) codon. Preference for the charged suppressor tRNA by the ribosome results in the synthesis of full-length protein with the desired unnatural amino acid incorporated, while selection of RF1 results in the synthesis of truncated protein. Therefore, a decrease in the RF1 content of the expression system will increase the suppression efficiency of added tRNAs.
Different approaches have been described to increase suppression efficiency of amber suppressor tRNAs via a manipulation of RF1 activity, e.g., RF1-inhibitory aptamers71 or antibodies72 have been used in in vitro translation systems. Another approach has been the use of E. coli strains with thermosensitive RF1 variants.73 In lysates of these strains the activity of RF1 could be diminished considerably by heat inactivation which led to a significant increase in suppression rate. However, overall protein synthesis rates were relatively low in this system. In principle, the “pure system” which is synthetically reconstituted from all single translation factors provides another possibility to lower termination rates by just omitting RF1 alltogether.74
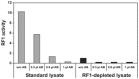
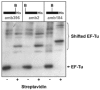
In our laboratory we developed an alternative strategy to produce RF1-depleted lysates, based on genetic engineering of a suitable Escherichia coli strain. The starting point was one of our proven high-producer E. coli strains to guarantee optimal lysate activity. Since the termination factor RF1 is essential for cell growth and a construction of knock out mutants is not possible,75 we created a strategy involving the construction of an E. coli high-producer strain containing the chromosomal sequence of an RF1 variant with a C-terminal tag instead of the wild type RF1 gene (Fig. 3). Due to the tag an almost complete removal of RF1 using affinity chromatography during lysate production was achieved.
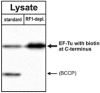
Using such an RF1-depleted lysate, we could demonstrate an 8-fold increase of Biotin-incorporation into a model protein (Fig. 4). A slight additional reduction of RF1 activity was observed with a polyclonal RF1-antibody (Fig. 5), but this had only a marginal auxiliary effect on biotin incorporation. A complete switch-off of RF1 activity led to a reduction of protein synthesis (data not shown). These results suggest, that our “depletion of RF1-strategy” is comparable to an inactivation of RF1 by antibodies in its effects on the incorporation of unnatural amino acids into proteins (Fig. 5). RF1-depleted lysates however are easier to handle, more cost effective and show a better reproducibility. Furthermore, our lysate preparation is associated with removal of biotinylated endogenous E. coli proteins (Fig. 6), which otherwise may interfere with subsequent biotin-based applications.
For most applications not only site-specifically defined, but also stoichiometrically uniform derivatives of the desired protein conjugates with biotin need to be synthesized. The incorporation of the desired unnatural amino acid by a supplemented tRNA is not only in competition to a termination by RF1, but also to a spontaneous hydrolysis of the aminoacylation and to an unwanted read-through due to misreading by endogenous tRNA. It is therefore necessary to harmonise the RF1 content of the lysates, the velocity of protein synthesis and the duration of the reaction. Synthesis of full length protein due to unwanted read-through is negligible as long as some RF1 is present. However, at a complete inhibition of RF1 by antibodies, the synthesis of full length protein due to read-through can reach a significant level of its corresponding wild type protein even in the absence of suppressor tRNA. In our RF1-depleted system as well, we could detect some read-through in the absense of suppressor tRNA. For this reason it is highly advisable to arrest the protein biosynthesis reaction before the added tRNA is completely hydrolyzed and becomes unable to compete with endogenous read-through. This is easily achieved by limiting incubation time or amino acid concentration. Using optimized conditions we could demonstrate that the biotinylated tRNA is able to repress read-through by misreading endogenous tRNA completely (Fig. 7).
The half-life of the aminoacyl-bond of the tRNA due to spontaneous deacylation has been determined to be between 10 and 30 minutes in our translation system.69 In order to avoid the deprivation of aminoacyl-tRNA due to deacylation, it is necessary to use lysates with fast kinetics of synthesis. We could show for different model proteins, that a stoichiometric incorporation of biocytin (one residue per one protein molecule) was provided at a tRNA concentration of 12 μM in a 30 minutes reaction (Fig. 7). Using these conditions up to 7 μM and over 200 μg/ml of site-specifically biotinylated protein was synthesized, i.e., up to 60% of the biotin residues attached to the tRNA were incorporated into protein.
Applications of Site Specifically and Stoichiometrically Defined Protein Conjugates
Biotin moieties in proteins are widely used for the detection and immobilization of protein conjugates, since corresponding biotin-based methods are robust and readily available. In one of our application examples the usefulness of our cell-free system was demonstrated to detect protein protein interactions efficiently. The interaction of elongation factor Tu (EF-Tu) and elongation factor Ts (EF-Ts) was probed using immobilized EF-Tu and biotinylated EF-Ts as the interaction partner (Fig. 8). Different sites of EF-Ts were site-specifically biotinylated with the EasyXpress Site-Specific Biotin Kit (QIAGEN GmbH, Hilden, Germany) to allow its detection in the LiquiChip system (QIAGEN GmbH, Hilden, Germany) by using a Streptavidin-Phycoerythrin conjugate as a reporter. We could show that the detection signal was strongly dependent on the site of biotinylation. Our identification of the best site (position 47) for biotinylation to achieve a strong signal correlated well with a site in the published structure of the EF-Tu*EF-Ts complex,76 for which a modification would probably not interfere with complex formation. Accordingly a biotinylation of position 94 protruding into the same direction as the immobilization tag of EF-Tu or a C-terminal biotinylation did not lead to a detectable signal in our experiments. N- or C-terminal labeling positions, which are typically favored for tag addition, did not lead to a satisfying signal strength, instead an internal position was best for prominent signal production.
In a second example we present the usefulness of our system to site-specifically biotinylate a wide range of different proteins for protein immobilization applications. Various proteins of different size, including prokaryotic translation factors, an aminoacyl tRNA synthetase, single chain antibodies and different human proteins, were immobilized on magnetic beads directly from the reaction mixtures (Fig. 9). In our system there is no need for removal of free biotin because we use precharged biotinylated suppressor tRNA as the sole source of biotin, which is almost totally incorporated into protein. The negligible amount of free biotin due to spontaneous hydrolysis of the amino acyl bond at the tRNA does not perturb following applications. Therefore an immobilization of proteins is possible without prior purification. This allows the immobilization even of proteins that are difficult or impossible to purify. Cyclophilin A, for example, could not be immobilized after purification, presumably due to aggregation of the purified protein. Figure 9 exemplifies that proteins of different size can be biotinylated in considerable amounts in our system. Up to 150 pmol of protein could be immobilized from a 25 μl synthesis reaction, using only 250 pmol biotinylated tRNA as the source for biotin.
We can show in our system that site-specific labeling of each of two interacting proteins is possible, rendering labeling more flexible for subsequent microarray or phage display applications. As an example two single chain antibodies and their corresponding antigens (Cyclophilin A and Ubiquitin) were expressed in the system and immobilized (Fig. 9). The immobilized antigens could be subsequently detected with high sensitivity by corresponding nonbiotinylated single chain antibodies (data not shown).
A template encoding an amber stop codon in an appropriate position can be used for the incorporation of biocytin as well as for other useful unnatural amino acids. In our opinion the site-directed incorporation of fluorescent amino acids provides an outstanding benefit allowing the direct and sensitive detection of synthesized proteins, e.g., in single molecule studies and interaction studies including FRET-based investigations. We have charged an amber suppressor tRNA with the fluorescent group BODIPY TMR (Molecular Probes, Eugene, OR, U.S.A.) attached to the e-aminogroup of lysine. The resulting large fluorescent amino acid could be incorporated into proteins within the RF1 depleted system (Fig. 10).
Conclusions
We have demonstrated the high yield synthesis of site-specifically and stoichiometrically defined protein conjugates with biotin using precharged tRNAs in an improved cell-free protein biosynthesis system. Since biocytin is difficult to incorporate due to its size, our success advocates the assumption that the system will allow the site-directed incorporation also of other valuable pharmaceutically desired groups or dyes. This could be verified by the site-directed incorporation of the fluorescent BODIPY TMR.
The most important advantage of our cotranslational strategy for the production of sitespecifically defined protein conjugates with biotin is that protein conjugate synthesis is possible in one step, enabling a rapid synthesis of a wide range of biotinylated proteins in parallel. Thus the use of posttranslational modification procedures, which may interfere with solubility and activity of proteins, can be avoided. The usefulness of the system is further advanced by the fact that an immobilization of biotinylated proteins is possible directly from translation reactions without the need for purification. Additionally the site-directed incorporation of biocytin allows a flexible and gentle handling maintaining the highest possible activity of the biotinylated proteins.
Biocytin and fluorescent amino acids represent functionalities with exquisite potential for interaction-based applications. We have shown that both modifications can be incorporated deploying the same template encoding an amber stop codon in an appropriate position.
In conjunction with Expression-PCR,77 the technology for rapid generation of ready to express templates, our system enables the parallel synthesis and immobilization of a huge number of cotranslationally modified proteins. In the future this technology will facilitate proteomics-related applications such as protein-protein interaction studies, drug screening, antibody selection, FRET analyses and single molecule detection.
Acknowledgements
We thank Prof. Matthias Sprinzl (University of Bayreuth) for providing the antibody against RF1 and Dr. Zoltán Konthur (MPI for Molecular Genetics, Berlin) for plasmids encoding the two single chain antibodies and the corresponding antigens.
This work was supported by the German ministry of research and education (BMBF) and the Senate of Berlin.
References
- 1.
- Kapanidis AN, Weiss S. Fluorescent probes and bioconjugation chemistries for single-molecule fluorescence analysis of biomolecules. J Chem Phys. 2002;117(24):10953–64.
- 2.
- Elvira C, Gallardo A, Roman JS. et al. Covalent polymer-drug conjugates. Molecules. 2005;10:114–125. [PMC free article: PMC6147706] [PubMed: 18007281]
- 3.
- Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54(4):531–45. [PubMed: 12052713]
- 4.
- Duncan R. Nanomedicines in action. Pharmaceutical J. 2004;273:485–488.
- 5.
- Brunner J. New photolabeling and crosslinking methods. Annu Rev Biochem. 1993;62:483–514. [PubMed: 8352595]
- 6.
- Service RF. Unnatural amino acid could prove boon for protein therapeutics. Science. 2005;308(5718):44. [PubMed: 15802581]
- 7.
- Spruijt RB, Wolfs CJ, Verver JW. et al. Accessibility and environment probing using cysteine residues introduced along the putative transmembrane domain of the major coat protein of bacteriophage M13. Biochemistry. 1996;35(32):10383–91. [PubMed: 8756694]
- 8.
- Kumita JR, Jain L, Safroneeva E. et al. A cysteine-free firefly luciferase retains luminescence activity. Biochem Biophys Res Commun. 2000;267(1):394–7. [PubMed: 10623630]
- 9.
- Rosendahl MS, Doherty DH, Smith DJ. et al. Site-specific protein PEGylation: application to cysteine analogs of recombinant human granulocyte colony-stimulating factor. BioProcess International. 2005;3(52-62) [PMC free article: PMC5995563] [PubMed: 29899681]
- 10.
- Katzen F, Chang G, Kudlicki W. The past, present and future of cell-free protein synthesis. Trends Biotechnol. 2005;23(3):150–6. [PubMed: 15734558]
- 11.
- Swartz J. Developing cell-free biology for industrial applications. J Ind Microbiol Biotechnol. 2006;33(7):476–85. [PubMed: 16761165]
- 12.
- Hohsaka T, Sisido M. Incorporation of nonnatural amino acids into proteins. Curr Opin Chem Biol. 2002;6(6):809–15. [PubMed: 12470735]
- 13.
- Hendrickson TL, de Crecy-Lagard V, Schimmel P. Incorporation of nonnatural amino acids into proteins. Annu Rev Biochem. 2004;73:147–76. [PubMed: 15189139]
- 14.
- Wang L, Schultz PG. Expanding the genetic code. Angew Chem Int Ed Engl. 2004;44(1):34–66. [PubMed: 15599909]
- 15.
- Budisa N. Prolegomena to future experimental efforts on genetic code engineering by expanding its amino acid repertoire. Angew Chem Int Ed Engl. 2004;43(47):6426–63. [PubMed: 15578784]
- 16.
- Magliery TJ. Unnatural protein engineering: producing proteins with unnatural amino acids. Med Chem Rev. 2005;2:303–323.
- 17.
- Kurzchalia TV, Wiedmann M, Breter H. et al. tRNA-mediated labeling of proteins with biotin. A nonradioactive method for the detection of cell-free translation products. Eur J Biochem. 1988;172(3):663–8. [PubMed: 3350017]
- 18.
- Stiege W, Erdmann VA. The potentials of the in vitro protein biosynthesis system. J Biotech. 1995;41:81–90. [PubMed: 7654353]
- 19.
- Cowie DB, Cohen GN. Biosynthesis by Escherichia coli of active altered proteins containing selenium instead of sulfur. Biochim Biophys Acta. 1957;26(2):252–61. [PubMed: 13499359]
- 20.
- Chapeville F, Lipmann F, Von Ehrenstein G. et al. On the role of soluble ribonucleic acid in coding for amino acids. Proc Natl Acad Sci USA. 1962;48:1086–92. [PMC free article: PMC220908] [PubMed: 13878159]
- 21.
- Garen A, Garen S, Wilhelm RC. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965;14(1):167–78. [PubMed: 5327650]
- 22.
- Leinfelder W, Zehelein E, Mandrand-Berthelot MA. et al. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331(6158):723–5. [PubMed: 2963963]
- 23.
- Srinivasan G, James CM, Krzycki JA. Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science. 2002;296(5572):1459–62. [PubMed: 12029131]
- 24.
- Hao B, Gong W, Ferguson TK. et al. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science. 2002;296(5572):1462–6. [PubMed: 12029132]
- 25.
- Liu DR, Magliery TJ, Pastrnak M. et al. Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc Natl Acad Sci USA. 1997;94(19):10092–7. [PMC free article: PMC23315] [PubMed: 9294168]
- 26.
- Furter R. Expansion of the genetic code: site-directed p-fluoro-phenylalanine incorporation in Escherichia coli. Protein Sci. 1998;7(2):419–26. [PMC free article: PMC2143905] [PubMed: 9521119]
- 27.
- Ohno S, Yokogawa T, Nishikawa K. Changing the amino acid specificity of yeast tyrosyl-tRNA synthetase by genetic engineering. J Biochem (Tokyo). 2001;130(3):417–23. [PubMed: 11530018]
- 28.
- Kowal AK, Kohrer C, RajBhandary UL. Twenty-first aminoacyl-tRNA synthetase-suppressor tRNA pairs for possible use in site-specific incorporation of amino acid analogues into proteins in eukaryotes and in eubacteria. Proc Natl Acad Sci USA. 2001;98(5):2268–73. [PMC free article: PMC30127] [PubMed: 11226228]
- 29.
- Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomol Struct. 2006;35:225–49. [PubMed: 16689635]
- 30.
- Behrens C, Nielsen JN, Fan X-J. et al. Development of strategies for the site-specific in vivo incorporation of photoreactive amino acids. Tetrahedron. 2000;56:9443–9.
- 31.
- Chin JW, Santoro SW, Martin AB. et al. Addition of p-azido-L-phenylalanine to the genetic code of Escherichia coli. J Am Chem Soc. 2002;124(31):9026–7. [PubMed: 12148987]
- 32.
- Deiters A, Cropp TA, Summerer D. et al. Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg Med Chem Lett. 2004;14(23):5743–5. [PubMed: 15501033]
- 33.
- Tsao ML, Tian F, Schultz PG. Selective Staudinger modification of proteins containing p-azidophenylalanine. Chem Bio Chem. 2005;6(12):2147–9. [PubMed: 16317766]
- 34.
- Ryu Y, Schultz PG. Efficient incorporation of unnatural amino acids into proteins in Escherichia coli. Nat Methods. 2006;3(4):263–5. [PubMed: 16554830]
- 35.
- Gallivan JP, Lester HA, Dougherty DA. Site-specific incorporation of biotinylated amino acids to identify surface-exposed residues in integral membrane proteins. Chem Biol. 1997;4(10):739–49. [PubMed: 9375252]
- 36.
- Hecht SM, Alford BL, Kuroda Y. et al. Chemical aminoacylation of tRNAs. J Biol Chem. 1978;253(13):4517–20. [PubMed: 248056]
- 37.
- Bruce AG, Atkins JF, Wills N. et al. Replacement of anticodon loop nucleotides to produce functional tRNAs: amber suppressors derived from yeast tRNAPhe. Proc Natl Acad Sci USA. 1982;79(23):7127–31. [PMC free article: PMC347291] [PubMed: 6961400]
- 38.
- Shih LB, Bayley H. A carbene-yielding amino acid for incorporation into peptide photoaffinity reagents. Anal Biochem. 1985;144(1):132–41. [PubMed: 3985309]
- 39.
- Baldini G, Martoglio B, Schachenmann A. et al. Mischarging Escherichia coli tRNAPhe with L-4'-[3-(trifluoromethyl)-3H-diazirin-3-yl]phenylalanine, a photoactivatable analogue of phenylalanine. Biochemistry. 1988;27(20):7951–9. [PubMed: 3061465]
- 40.
- Roesser JR, Xu C, Payne RC. et al. Preparation of misacylated aminoacyl-tRNA(Phe)s useful as probes of the ribosomal acceptor site. Biochemistry. 1989;28(12):5185–95. [PubMed: 2569893]
- 41.
- Noren CJ, Anthony-Cahill SJ, Griffith MC. et al. A general method for site-specific incorporation of unnatural amino acids into proteins. Science. 1989;244(4901):182–8. [PubMed: 2649980]
- 42.
- Ellman J, Mendel D, Anthony-Cahill S. et al. Biosynthetic method for introducing unnatural amino acids site-specifically into proteins. Methods Enzymol. 1991;202:301–336. [PubMed: 1784180]
- 43.
- Bain JD, Diala ES, Glabe CG. et al. Site-specific incorporation of nonnatural residues during in vitro protein biosynthesis with semisynthetic aminoacyl-tRNAs. Biochemistry. 1991;30(22):5411–21. [PubMed: 2036409]
- 44.
- Hohsaka T, Kajihara D, Ashizuka Y. et al. Efficient incorporation of nonnatural amino acids with large aromatic groups into streptavidin in in vitro protein synthesizing systems. J Am Chem Soc. 1999;121:34–40.
- 45.
- Ninomiya K, Minohata T, Nishimura M. et al. In situ chemical aminoacylation with amino acid thioesters linked to a peptide nucleic acid. J Am Chem Soc. 2004;126(49):15984–9. [PubMed: 15584731]
- 46.
- Sisido M, Ninomiya K, Ohtsuki T. et al. Four-base codon/anticodon strategy and non-enzymatic aminoacylation for protein engineering with nonnatural amino acids. Methods. 2005;36(3):270–8. [PubMed: 16076453]
- 47.
- Bessho Y, Hodgson DR, Suga H. A tRNA aminoacylation system for nonnatural amino acids based on a programmable ribozyme. Nat Biotechnol. 2002;20(7):723–8. [PubMed: 12089559]
- 48.
- Kourouklis D, Murakami H, Suga H. Programmable ribozymes for mischarging tRNA with nonnatural amino acids and their applications to translation. Methods. 2005;36(3):239–44. [PubMed: 16076449]
- 49.
- Taki M, Hohsaka H, Taira K. et al. Position-specific incorporation of a fluorophore-quencher pair into a single streptavidin through orthogonal four-base codon/anticodon pairs. J Am Chem Soc. 2002;124:14568–90. [PubMed: 12465968]
- 50.
- Muranaka N, Hohsaka T, Sisido M. Four-base codon mediated mRNA display to construct peptide libraries that contain multiple nonnatural amino acids. Nucleic Acids Res. 2006;34(1):e7. [PMC free article: PMC1325208] [PubMed: 16397292]
- 51.
- Gite S, Mamaev S, Olejnik J. et al. Ultrasensitive fluorescence-based detection of nascent proteins in gels. Anal Biochem. 2000;279(2):218–25. [PubMed: 10706791]
- 52.
- Cornish VW, Mendel D, Schultz PG. Probing protein-structure and function with an expanded genetic-code. Angew Chem Int Ed Engl. 1995;34(6):621–633.
- 53.
- Dougherty DA. Unnatural amino acids as probes of protein structure and function. Curr Opin Chem Biol. 2000;4(6):645–52. [PubMed: 11102869]
- 54.
- Dedkova LM, Fahmi NE, Golovine SY. et al. Enhanced D-amino acid incorporation into protein by modified ribosomes. J Am Chem Soc. 2003;125(22):6616–7. [PubMed: 12769555]
- 55.
- Cornish VW, Benson DR, Altenbach CA. et al. Site-specific incorporation of biophysical probes into proteins. Proc Natl Acad Sci USA. 1994;91(8):2910–4. [PMC free article: PMC521698] [PubMed: 8159678]
- 56.
- Cornish VW, Hahn KM, Schultz PG. Site-specific protein modification using a ketone handle. J Am Chem Soc. 1996;118(34):8150–8151.
- 57.
- Schmidt RR, Castro-Palomino JC, Retz O. New aspects of glycoside bond formation. 1999.
- 58.
- Ellman JA, Volkman BF, Mendel D. Site-specific isotopic labeling of proteins for NMR-studies. 1992.
- 59.
- Yabuki T, Kigawa T, Dohmae N. et al. Dual amino acid-selective and site-directed stable-isotope labeling of the human c-Ha-Ras protein by cell-free synthesis. J Biomol NMR. 1998;11(3):295–306. [PubMed: 9691277]
- 60.
- Turcatti G, Nemeth K, Edgerton MD. et al. Probing the structure and function of the tachykinin neurokinin-2 receptor through biosynthetic incorporation of fluorescent amino acids at specific sites. J Biol Chem. 1996;271(33):19991–8. [PubMed: 8702716]
- 61.
- Karginov VA, Mamaev SV, Hecht SM. In vitro suppression as a tool for the investigation of translation initiation. Nucleic Acids Res. 1997;25(19):3912–6. [PMC free article: PMC146976] [PubMed: 9380516]
- 62.
- Sisido M, Hohsaka T. Extension of protein functions by the incorporation of nonnatural amino acids. Bull Chem Soc Jpn. 1999;72(7):1409–25.
- 63.
- Murakami H, Hohsaka T, Ashizuka Y. et al. Site-directed incorporation of fluorescent nonnatural amino acids into streptavidin for highly sensitive detection of biotin. Biomacromolecules. 2000;1(1):118–25. [PubMed: 11709833]
- 64.
- Taira H, Hohsaka T, Sisido M. In vitro selection of tRNAs for efficient four-base decoding to incorporate nonnatural amino acids into proteins in an Escherichia coli cell-free translation system. Nucleic Acids Res. 2006;34(5):1653–62. [PMC free article: PMC1405820] [PubMed: 16549877]
- 65.
- Yarus M. Translational efficiency of transfer RNAs: uses of an extended anticodon. Science. 1982;218(4573):646–52. [PubMed: 6753149]
- 66.
- Yarus M, Cline SW, Wier P. et al. Actions of the anticodon arm in translation on the phenotypes of RNA mutants. J Mol Biol. 1986;192(2):235–55. [PubMed: 2435916]
- 67.
- Yarus M, Cline S, Raftery L. et al. The translational efficiency of tRNA is a property of the anticodon arm. J Biol Chem. 1986;261(23):10496–505. [PubMed: 3525546]
- 68.
- Kleina LG, Masson JM, Normanly J. et al. Construction of Escherichia coli amber suppressor tRNA genes. II. Synthesis of additional tRNA genes and improvement of suppressor efficiency. J Mol Biol. 1990;213(4):705–17. [PubMed: 2193162]
- 69.
- Gerrits M. Funktion und Effizienz von amber-Suppressor-tRNAs in der zellfreien Proteinbiosynthese, PhD Thesis. Berlin: Freie Universität. 2001
- 70.
- Cload ST, Liu DR, Froland WA. et al. Development of improved tRNAs for in vitro biosynthesis of proteins containing unnatural amino acids. Chem Biol. 1996;3(12):1033–8. [PubMed: 9000011]
- 71.
- Szkaradkiewicz K, Nanninga M, Nesper-Brock M. et al. RNA aptamers directed against release factor 1 from Thermus thermophilus. FEBS Lett. 2002;514(1):90–5. [PubMed: 11904188]
- 72.
- Agafonov DE, Huang Y, Grote M. et al. Efficient suppression of the amber codon in E. coli in vitro translation system. FEBS Lett. 2005;579(10):2156–60. [PubMed: 15811334]
- 73.
- Short GF, 3rd, Golovine SY, Hecht SM. Effects of release factor 1 on in vitro protein translation and the elaboration of proteins containing unnatural amino acids. Biochemistry. 1999;38(27):8808–19. [PubMed: 10393557]
- 74.
- Shimizu Y, Inoue A, Tomari Y. et al. Cell-free translation reconstituted with purified components. Nat Biotechnol. 2001;19(8):751–5. [PubMed: 11479568]
- 75.
- Ryden M, Murphy J, Martin R. et al. Mapping and complementation studies of the gene for release factor 1. J Bacteriol. 1986;168(3):1066–9. [PMC free article: PMC213603] [PubMed: 3782033]
- 76.
- Kawashima T, Berthet-Colominas C, Wulff M. et al. The structure of the Escherichia coli EF-Tu.EF-Ts complex at 2.5 A resolution. Nature. 1996;379(6565):511–8. [PubMed: 8596629]
- 77.
- Merk H, Meschkat D, Stiege W. Expression-PCR: from gene pools to purified proteins within 1 day. In: Swartz JR, editor. Cell-free protein expression. Berlin, Heidelberg, New York: Springer Verlag. 2003:p15–23.
- Protein Conjugates
- Cell-free Systems as a Tool for Protein Conjugate Production
- Incorporation Efficiencies of Different Unnatural Amino Acids
- Depletion of RF1 from a Cell-free System Results in Efficient Incorporation of Biocytin
- Applications of Site Specifically and Stoichiometrically Defined Protein Conjugates
- Conclusions
- Acknowledgements
- References
- Cell-Free Synthesis of Defined Protein Conjugates by Site-directed Cotranslation...Cell-Free Synthesis of Defined Protein Conjugates by Site-directed Cotranslational Labeling - Madame Curie Bioscience Database
- Regulation of Cytoskeletal Dynamics and Cell Morphogenesis by Abl Family Kinases...Regulation of Cytoskeletal Dynamics and Cell Morphogenesis by Abl Family Kinases - Madame Curie Bioscience Database
- Dynamic Interaction between PARP-1, PCNA and p21waf1/cip1 - Madame Curie Bioscie...Dynamic Interaction between PARP-1, PCNA and p21waf1/cip1 - Madame Curie Bioscience Database
- DNA: Alternative Conformations and Biology - Madame Curie Bioscience DatabaseDNA: Alternative Conformations and Biology - Madame Curie Bioscience Database
- NMD in Drosophila: A Snapshot into the Evolution of a Conserved mRNA Surveillanc...NMD in Drosophila: A Snapshot into the Evolution of a Conserved mRNA Surveillance Pathway - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...