In this chapter, we outline the tools and techniques available to study the process of host cell invasion by apicomplexan parasites and we provide specific examples of how these methods have been used to further our understanding of apicomplexan invasive mechanisms. Throughout the chapter we focus our discussion on Toxoplasma gondii, because T. gondii is the most experimentally accessible model organism for studying apicomplexan invasion (discussed further in the section, “Toxoplasma as a Model Apicomplexan”) and more is known about invasion in T. gondii than in any other apicomplexan.
Host Cell Invasion by Apicomplexan Parasites
Host cell invasion is a complex, multi-step process that is relatively conserved among apicomplexans (see Fig. 1). T. gondii invasion begins with movement of the parasite over the surface of a host cell, driven by an actin-myosin-based motor within the parasite. This gliding motility is dependent on a solid substrate, and occurs in the absence of flagella, pseudopodia, or other traditional locomotive organelles (reviewed in ref. 2; see also Matuschewski, this volume). As the parasite glides over host cells, a trail of surface proteins and lipids is deposited in the parasite's wake.3 The conoid, a thimble-shaped cytoskeletal structure comprised of tubulin assembled into spiral ribbons,5 repeatedly protrudes and retracts from the apical tip of the gliding parasite (reviewed in ref. 5). Initial attachment of the parasite to the host cell is coupled to the discharge of the micronemes, apical secretory organelles that release adhesins onto the parasite surface6,7 (see also Carruthers, this volume). A more intimate apical interaction then develops between the parasite and host cell8 and a second set of apical organelles, the rhoptries, discharge. Secretion from the micronemes and the rhoptries is tightly controlled, and there is some evidence that secretion from the bulb and neck regions of the rhoptries are distinct, regulated events,8 although the relative order of these various secretory events is not entirely clear. Very early in the process of invasion, perhaps when the micronemes and/or rhoptries are first discharged, a transient change in host cell plasma membrane conductance can be detected,9 suggesting a temporary, localized breach in host plasma membrane integrity. A third set of secretory organelles, the dense granules, are constitutively discharged, and are thought to function primarily in establishing and modifying the parasite's intracellular niche after invasion (reviewed in ref. 1).
Active penetration of the host cell begins at the apical tip of the parasite and is thought to be dependent upon the same actin-myosin machinery that powers gliding motility, since disruption of a single myosin gene, MyoA, disrupts both processes10 and pharmacological agents that disrupt gliding motility also disrupt invasion.11 Due to the duality of function of the actin-myosin motor, it is difficult to determine whether gliding motility is required for invasion; however, video-microscopic analysis of T. gondii suggests that tachyzoites may be probing, perhaps by way of conoid extension, for some specific receptor or membrane microdomain as they glide over the host cell surface before initiating invasion. Both gliding motility and invasion involve receptor-ligand interactions (see Matuschewski, this volume; Carruthers, this volume), resulting in anterior to posterior capping of surface proteins, followed by their protease-mediated shedding7,12 (see also Dowse, this volume). Capping and shedding are thought to be responsible for the trails of surface proteins deposited at the posterior end of gliding parasites.
During penetration, a zone of tight interaction between the host and parasite plasma membranes forms at the site of parasite entry. This “moving junction” translocates from the anterior to posterior end of the parasite concurrent with penetration. Proteins secreted from the necks of the rhoptries, physically complexed with at least one microneme protein, were recently identified as components of the moving junction.13,14 As the tachyzoite penetrates into the host cell it becomes surrounded by a parasitophorous vacuole membrane (PVM), derived from host cell plasma membrane lipids.9 Host plasma membrane proteins are largely excluded from the PVM as it forms, creating a compartment that does not fuse with the endolysosomal system of the host cell15,16 (see also Sinai, this volume). The entire process of invasion takes approximately 20-25 seconds.17 Shortly after invasion, the PV relocates to a position adjacent to the host cell nucleus and becomes tightly associated with host cell mitochondria and endoplasmic reticulum.18
While the various steps in invasion, described above for T. gondii, are broadly conserved among apicomplexan parasites,19-21 several interesting and potentially informative variations are observed in other genera. For example, the conoid appears to be absent from Plasmodium and Babesia22 (reviewed in ref. 5). Parasite reorientation, to bring the apical end in contact with the host cell, is more pronounced in other apicomplexans such as Plasmodium23 and Cryptosporidium21 than it is in T. gondii. An entirely different invasive mechanism, which does not involve formation of a PVM, is employed by Plasmodium sporozoites as they migrate through host cells en route to their preferred site of replication (see Frevert, this volume). Theileria sporozoites are perhaps the most unusual of all the apicomplexans in terms of their invasive mechanisms. The invasive stages of Theileria are not motile, have no recognizable conoid or micronemes, and do not apically reorient prior to invasion. Internalization appears to be a passive process on the part of the parasite, with no role for parasite actin. However, other features of Theileria invasion are similar to those of other apicomplexans, including protease-mediated shedding of surface proteins during internalization, and formation of a PVM from the host cell plasma membrane (reviewed in ref. 24).
Assaying Host Cell Invasion
Given the importance of host cell invasion in the life cycle and pathogenesis of apicomplexan parasites, there is much interest in studying the mechanisms underlying invasion. Many different means of quantitatively assaying invasion have been developed, particularly for T. gondii, as described below.
Plaque Assay
Parasites are added to host cell monolayers and the resulting number of plaques, formed by successive rounds of host cell invasion, lysis, and invasion of surrounding cells by the released daughter parasites, is determined visually.25 Plaque visualization can be facilitated by staining with Giemsa or crystal violet. While the plaque assay is simple to perform and requires no special equipment, it does not separate the process of invasion from the rest of the parasite's intracellular life cycle. A decrease in plaque formation can result from a defect in invasion, but also from defects in intracellular growth, parasite replication, host cell lysis and parasite egress, or any combination of these processes.
Vacuole Assay
The number of mature parasitophorous vacuoles present 24 hours after invasion can be determined by phase microscopy and used as a measure of invasion.10 Caution must be exercised when performing and interpreting this assay since overlapping or adjacent vacuoles can be difficult to count accurately and, like the plaque assay, it may confuse a growth or replication defect with an invasion defect.
3H-Uracil Incorporation Assay
Since many host cells lack a uracil salvage pathway, addition of 3H-uracil to an infected monolayer results in specific incorporation by parasites and can therefore be used to assay the number of intracellular parasites.26,27 However, since both intracellular and extracellular parasites can incorporate 3H-uracil (although extracellular parasites may have a shorter period of incorporation28) it is critical to wash off or kill as many extracellular parasites as possible prior to assaying 3H-uracil incorporation. Extracellular parasites can be killed with pyrrolidine dithiocarbamate (PDTC29), although this adds another potential source of variability to the assay. Like the plaque assay, it can also be difficult to definitively ascribe a decrease in 3H-uracil incorporation to a defect in invasion, rather than some other post-invasion defect that alters 3H-uracil uptake or incorporation. Prelabeling parasites with 3H-uracil and measuring the amount of radioactivity associated with the monolayer after infection30 can circumvent this problem. This approach is best suited for experiments that start with one population of parasites (e.g., comparing the effect of different pharmacological agents on the invasion of a single population of 3H-labeled parasites); it is more problematic to compare two different populations, which may differentially incorporate exogenous uracil. Again, the consistency with which extracellular parasites can be removed or killed will affect the accuracy and variability of this assay.
β-Galactosidase Assay
This method measures the amount of o-nitrophenyl-β-D-galactoside (colorless) that is converted to o-nitrophenol (yellow) by parasites expressing β-galactosidase.11,31 This assay offers the advantages of being easy, fast, and sensitive. However, in addition to the requirement to remove or kill extracellular parasites, the assay can only be used for parasites expressing β-galactosidase. Although such parasites are available for T. gondii32,33 and Neospora,34 the requirement for β-galactosidase expression may complicate further transgenic manipulation of the parasites, due to the limited number of selectable markers available (summarized in Table 1).
Quantitative PCR Assay
Genomic DNA recovered from invaded parasites can be used as a template for quantitative real-time PCR, which is compared to a dilution series of DNA from a known number of parasites.74 This assay again requires removal and/or killing of extracellular parasites to ensure that contaminating DNA from these parasites is not included as template for PCR. A strength of this assay, like the 3H-uracil- and β-galactosidase-based assays, is that it allows large numbers of parasites to be analyzed per sample, compared to microscope-based techniques.
Differential Fluorescent Staining
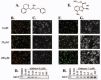
In the most commonly used assay for T. gondii invasion, parasites are labeled with different fluorochromes before and after host cell permeabilization, resulting in dual labeled extracellular parasites and singly labeled intracellular parasites (Fig. 2B, F), which are scored by fluorescence microscopy.11 The use of parasites expressing YFP or GFP further simplifies the assay, by eliminating the need to permeabilize the host cells to visualize intracellular parasites.50,75 This assay isolates invasion from other aspects of the parasite life cycle and does not require removal of extracellular parasites for accurate counting of invaded parasites. However, the assay suffers from significant field-to-field variability, which is compounded by the limited sample size that can be feasibly obtained, given the extensive hands-on time required for manual counting. An automated microscope has been used to semi-quantitatively assay invasion by differential staining in a high-throughput format.50 In an attempt to make the differential staining assay more quantitative and reproducible, the assay was recently adapted for scoring by a laser scanning cytometer (LSC). The LSC-based assay significantly increases the number of parasites that can be counted, overcoming much of the field-to-field variability observed when the assay is scored manually. The major drawbacks to the LSC-based assay are that it requires specialized instrumentation and is not well-suited to analyzing many samples at one time.76 However, it may be possible to adapt these assays for analysis by flow cytometry.77
Other Apicomplexans
In the most common invasion assay for Plasmodium blood stage parasites, mature schizonts are added to a suspension of erythrocytes and the number of ring stages that develop several hours post infection are visualized in blood smears on a glass slide. Staining of rings is typically accomplished with Giemsa,78-81 but DAPI82 and acridine orange83 have also been used. These assays are complicated by the fact that they measure not only invasion, but several pre and post-invasion events as well, including schizont rupture and morphological differentiation to the ring stage. 3H-hypoxanthine uptake assays (e.g., ref. 84) suffer from a similar problem and are further complicated by the possibility that host erythrocytes and leukocytes may also incorporate 3H-hypoxanthine.85,86 An enzymatic assay for Plasmodium lactate dehydrogenase provides a measure of parasitemia, and can be used as an indirect measure of invasion, with the caveats mentioned above.87,88 Assays based on parasite staining have recently been adapted for FACS scoring,89 which significantly reduces operator time but necessitates the quantitative removal of extracellular parasites. A similar FACS assay has been used to measure Eimeria invasion.90 A microscope-based assay that visualizes the incorporation of fluorescent lipid analogs into the PVM during parasite entry can effectively isolate merozoite invasion from other pre and post-invasion events,91 but has thus far been used only for P. knowlesi.
The invasion of Plasmodium sporozoites into hepatocytes and other target cells can be assayed by differential fluorescent staining methods similar to those described above for T. gondii.94-96 Some of the other assays described above for T. gondii are also directly transferable to other species, e.g., RT-PCR assays for assaying invasion in Neospora.74 The invasion of Babesia sporozoites into erythrocytes, which lack a nucleus, can be assayed by nuclear staining.92 Relatively little is known about Babesia invasion, but the recent development of a system that supports efficient in vitro invasion, based upon high voltage release of parasites from previously invaded erythrocytes,93 may increase our understanding of this process.
Assaying the Individual Steps of Invasion
T. gondii invasion can be readily assayed either in its entirety (using the methods described above), or as a sequence of discrete steps, each of which can be assayed independently. A recently developed protocol for synchronous invasion by T. gondii97 further complements these assays. Parasites are allowed to settle onto host monolayers in a high potassium buffer, which inhibits parasite motility and invasion. The medium is then exchanged for low potassium medium, stimulating motility and invasion. This allows for synchronous and robust invasion over a short period of time. A temperature shift can also be used to synchronize invasion of T. gondii.40
Assays for the individual steps of T. gondii invasion are outlined below, and similar assays for other apicomplexans, when available, are described.
Gliding Motility
T. gondii can be observed gliding over host cells or coverglasses using video-50,98 or time lapse-microscopy.10,73 These data can be analyzed either qualitatively8,50 or quantitatively.10,98
Motility can also be assayed by trail deposition; the surface proteins deposited behind T. gondii tachyzoites as they glide over host cells and coverglasses can be readily visualized by immunofluorescence microscopy (Fig. 2 C, G).11, 48 Alternatively, parasites can be prelabeled with fluorescent antibodies or lipids and trail deposition observed in real time or after fixation. 50 Trail deposition can be quantified by measuring the number and length of trails.11 Similar trail deposition assays, using species-specific antibodies, have been used for Cryptosporidium,99,100 Plasmodium78 and Eimeria101,102 sporozoites; trails produced by the latter two species have also been observed by electron microscopy.103,104 A recent study on the motility of Plasmodium berghei ookinetes used the dispersal of aggregated parasites as a quantitative measure of ookinete motility.105
Conoid Extension
Conoid extension can be experimentally induced by calcium ionophores and inhibited by intracellular calcium chelators.106 The percentage of T. gondii tachyzoites with partially or fully extended conoids can be readily determined by phase microscopy.50,106
Microneme Secretion
The secretion of microneme proteins from extracellular parasites occurs constitutively at a basal level and can be upregulated by incubation with calcium ionophores or ethanol.107 Both constitutive and induced secretion can occur in the absence of host cells; the secreted proteins are recovered from the culture supernatant and analyzed by SDS PAGE and Western blotting (Fig. 2D, H). Similar methods have been used to assay microneme secretion in Eimeria,104 Cryptosporidium,99 Sarcocystis,108 and Neospora.109 The recovery and identification of microneme proteins released into Plasmodium culture supernatants was critical in the initial identification and characterization of Plasmodium erythrocyte binding proteins (e.g., refs. 110, 111).
Direct comparative microneme secretion assays have been developed for T. gondii, using 2D-DIGE (2-D Fluorescence Difference Gel Electrophoresis) techniques that differentially label the secreted proteins from two parasite populations with either red or green fluorochromes.112,113 The samples are mixed in a 1:1 ratio, separated by 2-dimensional gel electrophoresis, and the fluorescent signals analyzed. Proteins that are secreted in equal abundance by both parasite populations appear yellow in a merged image. Spots appear red or green if secretion is up- or down-regulated in one of the populations. The ability to compare two different protein samples on the same gel solves the run-to-run variability problem frequently encountered in 2D gel separations.
Attachment
Both fixed and live host cells have been used for assaying parasite attachment. Parasites cannot invade fixed cells, offering the advantage of having to measure only extracellular parasites. Since fixation may alter the conformation of ligands on the surface of the host cells, it is not known how closely the fixed cell system mimics in vivo attachment and there is some evidence that parasites attach to fixed cells differently than live cells.76 Live cells offer the advantage of more closely mimicking in vivo conditions. However, since invasion can occur in this case, both attached and internalized parasites must be accounted for when measuring attachment to live cells.
Attachment of T. gondii tachyzoites is measured either by counting the number of fluorescently-labeled114 or Giemsa-stained115 parasites or by measuring the amount of β-gal48 or radioactivity30 associated with the monolayer after infection and washing. Similar assays exist for Plasmodium116 and Babesia.117 Generating quantitative and reproducible attachment data can be difficult due to sampling issues and variations in wash stringency within and between coverslips. A LSC-based attachment assay that counts attached parasites, prelabeled with fluorescent antibodies, overcomes much of this inherent variability.76 This is accomplished by comparing two differentially labeled populations of parasites on the same coverslip. While highly reproducible and quantitative, the requirement that populations of parasites be compared pairwise means that the assay is not well suited for scoring numerous samples, e.g., high-throughput screening.
In addition to whole parasites, the ability of putative adhesins from T. gondii, Plasmodium, and Neospora, to bind to host cells or host cell components has been tested using purified,40,109 heterologously expressed,118 or recombinant radiolabeled proteins.119,120
Rhoptry Secretion
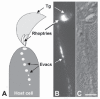
T. gondii tachyzoites pretreated with cytochalasinD are unable to move or invade host cells, but they retain the ability to attach to host cells and to secrete the contents of their rhoptries. The secreted rhoptry proteins can be detected as vesicular clusters (termed evacuoles) within the host cell by immunofluorescence microscopy121 (Fig. 3). The quantity, length, and protein composition of evacuoles can be assessed using different antibodies, as a means of assaying rhoptry secretion.8,122 Evacuole-like structures are also observed within erythrocytes after Plasmodium merozoite invasion is arrested with cytochalasinB, cytochalasinD or staurosporine.91,124,125
Approaches to Studying Invasion
Many different experimental approaches are available for the identification and functional analysis of gene products involved in invasion. These include forward genetic screening, reverse genetic analysis, antibody inhibition, small-molecule-based approaches, alteration of the host cell, and genomic/transcriptomic/proteomic studies.
Forward Genetics
One of the most powerful means to determine which gene products are involved in a particular process is to introduce mutations into the genome of the organism, screen for mutants which no longer carry out the process, and identify the mutated genes. A number of such forward genetic screens have been undertaken to study different aspects of the T. gondii life cycle, utilizing a variety of mutagenic techniques and selection strategies, as described below. In principle, these methods could be applied to the study of invasion. However, in the case of invasion, forward genetic screens must be designed to select for mutations that enhance or decrease invasion, or abolish invasion only under certain conditions, since parasites that are constitutively unable to invade cannot be maintained in culture.
Insertional Mutagenesis
The high frequency of nonhomologous integration in T. gondii,126 coupled with the manageable size of its genome (~65Mbp), have allowed the successful implementation of insertional mutagenesis.127 Mutagenic saturation, i.e., the theoretical disruption of every gene in the T. gondii genome, can be accomplished by mutagenizing approximately 5×106 parasites.127 Sequence from the dihydrofolate reductase (DHFR) locus appears to increase nonhomologous integration into the T. gondii genome (a 10-fold increase compared to non-DHFR sequence42) and is therefore often included in insertional mutagenesis plasmids.128 Interrupting a gene with an insert should alter the function of the disrupted gene, but could also generate toxic truncation product(s) or have unintended effects on adjacent genes, which must be considered when attempting to correlate the interrupted gene with an observed phenotype.
Insertional mutagenesis has been used to study adenosine transport and metabolism,128 as well as parasite differentiation.129,130 In principle, this approach could be used to study invasion given a proper selection or screening strategy, i.e., selection or screening for disruptions that enhance or decrease invasion but do not abolish it completely. For example, insertion into the promoter of a critical adhesin might reduce but not completely abolish invasion. Methods have been developed to identify the interrupted gene either by plasmid recovery129 or by complementing loss of function with a cDNA library that integrates at high frequency and is shuttled by recombination cloning.131 Complementation with an episomal genomic library has also been reported.37
Chemical Mutagenesis
Chemical mutagenesis may be preferable to insertional mutagenesis as a means to genetically modify parasites for invasion screens. Agents such as ethylnitrosourea (ENU)132-134 and N-methyl-N'-nitro-N-nitrosoguanidine25 introduce point mutations, which are less likely than insertional mutagenesis to result in complete loss of protein function, and may therefore be more likely to diminish, but not abolish, invasion. One study has produced temperature sensitive mutants that differ in their infectivity.25 The generation of temperature sensitive mutants should allow for the isolation of parasites with invasion defects, since parasites could be cultured at the permissive temperature and invasion studied at the restrictive temperature.25 Chemically mutagenized, temperature sensitive Neospora have also been screened for invasion and infectivity defects in mouse models.135
The major challenge of chemical mutagenesis is mapping and identifying the site of the mutation. The system for cDNA-based complementation cloning mentioned above131 is one approach to identification of the mutated gene; this approach was recently used to identify a gene involved in cell cycle progression.136
In any forward genetic approach, once the interrupted gene has been identified, the specific mutation should be introduced into wild type parasites to confirm and validate the correlation between the mutation and the observed phenotype.48
Reverse Genetics
While forward genetic screens are useful for identifying new parasite genes and proteins that function in invasion, reverse genetic approaches have been extensively used to study specific proteins postulated to function in invasion based on other criteria, such as antibody inhibition, subcellular localization, sequence homologies, or the forward genetic approaches mentioned above.
Knockout Studies
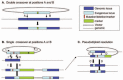
Targeted gene disruption by homologous recombination has been widely used to study gene function in T. gondii. Increasing the size of the targeting sequence seems to increase the frequency of homologous recombination: one study showed that plasmids containing 1.7, 7.6, or 15.7 kb of targeting sequence resulted in 0, 62, and 82% of the clones harboring homologously integrated plasmid DNA, respectively.126 In T. gondii, where genomic sequence data is available, knockout vectors can be constructed using only the noncoding regions flanking the gene of interest. Utilizing coding sequence to target the knockout construct could result in expression of partial proteins, complicating interpretation of the resulting phenotype. Typically, the coding region of the targeted gene is replaced with a selectable marker (Table 1) flanked by several kb of 5' and 3' noncoding sequence. After selection, individual clones can be screened for gene disruption by Western blotting if antibodies are available.71,137-139 Alternatively, after selection, pools of clones can be screened by PCR, utilizing one primer from within the genome and one primer from within the knockout construct to identify those parasites with the targeted disruption.8 In positive clones, gene disruption can be confirmed by Southern blotting, Western blotting and/or immunofluorescence microscopy.8,71,137-139 Knockout constructs are often introduced as linearized plasmids,138,139 to promote double crossover-allelic exchange, as opposed to single crossover events, which result in pseudodiploid formation and the possibility of expression of functional protein (Fig. 4). The transfection efficiency of circular and linear plasmids appears similar;39,41 however, homologous recombination occurs with higher frequency when using circular plasmids, suggesting that pseudodiploid formation is the most prevalent recombination event.39 Since DHFR sequence seems to promote nonhomologous integration in T. gondii (see above), inclusion of DHFR should be avoided in constructs intended for targeted knockouts.
Using a positive selectable marker within the targeting sequence and a negative selectable marker outside the targeting sequence selects against nonhomologous recombination and pseudodiploid formation,141 significantly reducing background. A similar double selection strategy is available for P. falciparum knockouts.62
Attempts to generate a gene knockout could fail either because the gene is essential or because the knockout construct did not properly target the locus of interest. In order to rule out the latter, an exogenous, functional copy of the gene can be randomly integrated into the genome before attempting to knockout the endogenous locus.142,143 Alternatively, an additional copy (mutated or functional) can be inserted by homologous recombination to create a pseudodiploid (Fig. 4B). The pseudodiploid can subsequently be resolved, under negative selection, by intrachromosomal recombination, resulting in either reconstitution of the wild type locus or formation of the mutated locus (Fig. 4C). Since vector sequence, including markers, will be removed by pseudodiploid resolution, a marker which can be used for either positive or negative selection, such as HXGPRT, is ideal for these types of experiments.140 The wild type and the mutated loci should be recovered with equal frequencies; recovery of only the wild type locus suggests that the gene is essential.143 In cases where there is a phenotype associated with a knockout, polar effects can be ruled out by complementing the knockout with an exogenous copy of the gene being studied and observing partial or complete rescue of the affected phenotype.71,137 The choice of promoter and selectable marker should be carefully considered, since either can affect the level of expression during complementation.
Knockouts in a number of T. gondii genes have been generated, some of which result in reduced invasion. For example, MIC2 Associated Protein (M2AP) knockout parasites are significantly defective in invasion, which has been attributed to mistargeting of M2AP's partner protein, MIC2.71 Given that MIC2 itself cannot be knocked out, presumably because it is essential, this work provided valuable information on the function of both M2AP and MIC2. MIC1 knockout parasites are significantly defective in host cell invasion, but only slightly defective in mouse virulence.144 In contrast, MIC3 knockout parasites are not defective in invasion and are only slightly defective in mouse virulence. Interestingly, MIC1/MIC3 double knockouts show similar levels of invasion to the MIC1 knockout but significantly reduced virulence compared to the MIC1 or MIC3 single knockouts, indicating a synergistic role for these two proteins in virulence.144 A number of dense granule proteins have been knocked out,137,139 none of which affect invasion. However, the GRA2 knockout has reduced virulence, consistent with the hypothesis that dense granule proteins are not necessary for active penetration, but are involved in PV modification and possibly immune modulation. The major glycosylphosphatidylinositol (GPI)-anchored surface proteins, SAG1 and SAG3, have both been successfully knocked out. The SAG1 knockout parasites show no defect in invasion; in fact, the knockout parasites were found to invade more quickly than wild type parasites.145 In contrast, the SAG3 knockout showed reduced adhesion, invasion, and virulence, supporting the hypothesis that some members of the SAG family of proteins function as adhesins.138
When comparing knockout parasites to wild type or complemented parasites in invasion assays, it is important to use parasites in equivalent stages of their life cycle. Parasite invasiveness diminishes progressively after release from the host cell; therefore, care must be taken to ensure that release of the two parasite populations to be compared is synchronous (Mital and Ward, unpublished). Growth assays, comparing the intracellular replication of the parasites under study, can help in the synchronization of different parasite populations.8
Knockouts in nonessential genes have been generated and used to study invasion in Plasmodium (e.g., refs. 82, 146-150 and reviewed in ref. 150). A useful variation on the knockoutapproach in Plasmodium has been to disrupt an essential gene in a life cycle stage that does not require the gene (e.g., the blood stages), and then analyze the invasion phenotype in the stage in which the gene is essential (e.g., mosquito salivary gland sporozoites).78,152,153 This approach has provided a great deal of functional information about the proteins involved in sporozoite invasion (reviewed in refs. 154-157).
Conditional Knockouts
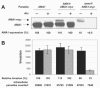
The recent development of an inducible promoter system for T. gondii10 allows the study of genes essential for invasion, and has been successfully used for four such genes (MyoA,10 TgAMA1,8 MIC2,158 and ACP159). This system is based upon an anhydrotetracycline- (Atc-) responsive transactivator protein that drives expression of genes downstream of a minimal SAG1- or SAG4-based promoter containing seven Tet operators. Expression occurs in the absence of Atc, and is repressed in the presence of Atc10 (Fig. 5). Generation of a conditional knockout is accomplished in two steps. First, parasites are transfected with the gene of interest downstream of the regulatable promoter, and clones with regulatable expression of properly localized protein are isolated. These clones are then transfected with a knockout construct to disrupt the endogenous locus of the gene of interest. The resulting parasite's sole source of the essential protein can then be turned off by addition of Atc. Conditional knockout parasites are therefore generated and isolated in the absence of Atc, and phenotypically characterized after incubation with Atc. Since phenotypic characterization is accomplished by comparing conditional knockout parasites in the presence and absence of Atc, all assays begin with the same population of parasites, alleviating the problems associated with comparing two nonequivalent populations of parasites (discussed above), provided that the knockout exhibits no growth defect.8
The success of this approach depends critically on the expression levels of the regulatable gene relative to wild type expression; expression in the absence of Atc must be sufficiently high for parasite viability, and expression after Atc treatment must be sufficiently low to reveal a phenotype (Fig. 5A, B). The optimal expression level will vary with the particular protein being studied, and may be modulated by the number of regulatable copies integrated and the choice of promoter driving regulatable expression. Currently, two versions of the regulatable promoter are available: the SAG1-based promoter gives lower basal expression, but tighter regulation after Atc addition, compared to the SAG4-based construct (personal comm. D. Soldati). In principle, the endogenous gene could be replaced with a regulatable copy in a single step; however, the two step approach enables one to insert multiple copies of the regulatable gene, which may be necessary to achieve the required expression levels prior to disruption of the endogenous gene, and to assess whether the knockout construct can effectively target the locus of interest. Both approaches require that the endogenous gene can be effectively targeted by homologous recombination. Some residual protein may remain after Atc addition, due either to leaky expression or slow protein turnover, which should be considered when interpreting any observed phenotype, or lack thereof.
The conditional promoter system has recently been adapted for use in P. falciparum.160 The development of a conditional mutagenesis system in P. berghei, based upon regulatable sitespecific recombination, presents an alternative means of constructing stage-specific knockouts; the utility of this system will increase as more stage-specific promoters are characterized.161
RNA-based Methods
RNA-based approaches to diminishing, rather than abolishing, expression of a protein are useful for genes that cannot be studied by conditional knockout, either because the required expression levels do not fall within the range of the available conditional promoters, or because the gene cannot be effectively targeted for disruption. In T. gondii, RNA-based techniques such as antisense, double stranded RNA interference (RNAi), and delta ribozymes have been successfully used in a limited number of laboratories to modulate gene expression.162-166 All of these techniques rely on short sequence recognition. The sequence must therefore be carefully chosen to assure knockdown of a specific gene, especially if that gene is part of a gene family or shares significant sequence with other proteins (although this can be an advantage if knockdown of a multigene family is desired.) Optimization of sequence and RNA expression level may be required to achieve a threshold of knockdown that maintains viability of the parasite but results in an invasion phenotype.
As yet, only the antisense RNA technique has been used to study invasion-related genes. Antisense RNA was used to decrease expression of the rhoptry protein ROP2, which was thought to be essential for invasion since previous knockout attempts were unsuccessful.164 A significant decrease in invasion, among other defects, was seen in ROP2-deficient parasites. Antisense RNAs have also been used to study the T. gondii dense granule protein NTPase, which is expressed as two isoforms from different loci167 and is thought to be essential.165 Despite these apparent successes, antisense methods have not yet gained widespread use in T. gondii due to difficulties with reproducibility. Antisense methods have also been used in Plasmodium.168,169
Homologues of many of the genes involved in RNAi have been found in T. gondii,170 but not Plasmodium.171 However, RNAi has been reported to work in both Plasmodium172-174 and T. gondii.162 RNAi has been used to show that decreased expression of two independent lactate dehydrogenase genes in T. gondii results in reduced differentiation and virulence.175 Double-stranded RNA has also been used to knockdown a T. gondii immunophilin.176 Since extensive controls are needed to unambiguously attribute downregulation to the RNAi pathway, there remains some uncertainty about whether the effects observed in these studies result from true RNAi.170
A recent report on the use of catalytically active delta ribozymes to bind and cleave specific targets177 indicates that this may also be a useful technique for studying essential genes.
Transgene Expression
Intra- and inter-species expression of wild type or mutated transgenes has been useful for studying apicomplexan invasion-related proteins. Care must be taken when analyzing data from exogenous expression to ensure that expression levels, post-translational modifications, and trafficking are comparable in the exogenous and native proteins. Additionally, codon bias may need to be considered; whereas T. gondii genes have been expressed in a variety of systems (e.g., refs. 34, 178, 179), codon bias in the A/T rich genome of P. falciparum180 can cause difficult, though not insurmountable problems for heterologous expression.181-184
Expression of various mutated and truncated T. gondii microneme proteins in wild type T. gondii has proven to be a useful approach for elucidating the role of these proteins in invasion and for defining their functional domains. For example, exogenous expression of mutated forms of MIC2 in T. gondii helped to elucidate MIC2's role as an adhesin and to define the mechanisms governing microneme protein cleavage (reviewed in ref. 185). Flow cytometry can be used to identify mutations that cannot be tolerated by the parasite.186
Expression of T. gondii proteins in other apicomplexans, or vice-versa, has provided insights into invasion by both the donor and recipient species. For example, functional homology between the cytoplasmic tails of Toxoplasma MIC2 and Plasmodium TRAP, microneme proteins thought to function in motility and invasion, was demonstrated by the ability of the cytoplasmic tail of Toxoplasma MIC2 to rescue the motility and invasion defects caused by deletion of the TRAP cytoplasmic tail in Plasmodium sporozoites.187 Proper expression and localization of a number of Toxoplasma proteins in the related apicomplexan Neospora34,65,178 indicate that this may also be a useful system to study functional homology and elucidate protein function. Inter-species expression of proteins will be particularly useful for studying protein function and invasion in organisms that are difficult to culture and/or for which molecular genetic tools are not yet available, such as Cryptosporidium.188 Expression of Plasmodium proteins in other species of Plasmodium has proven to be a useful way to study the function of secreted and cell surface proteins and to identify the parasite proteins responsible for host range specificity (Fig. 6A, C).189,190 Finally, expression of apicomplexan genes in both yeast195 and mammalian cells have been useful for determining the role of purported adhesins in host cell attachment (Fig. 6B).118,191-193,196
Antibody Inhibition
Another method to determine a particular protein's role during invasion is to alter its function with specific antibodies or peptides. Blocking active sites or inhibiting important conformational changes of invasion-related proteins, such as adhesins, may decrease or abolish invasion. The use of monoclonal antibodies and Fab fragments rather than whole immunoglobulins can decrease nonspecific cross reactivity and cross-linking effects. However, even well executed antibody inhibition experiments need to be interpreted with caution. For example, SAG1, the major surface protein of T. gondii, was originally thought to be involved in invasion based on the observation that antibodies and Fab fragments against SAG1 block invasion.114,197-199 However, subsequent studies showed that a SAG1 knockout parasite was fully capable of invasion; in fact, these parasites invade more quickly than wild type parasites, indicating that the antibody-mediated effects were probably due to something other than a direct effect on SAG1.145 Despite these caveats, antibody inhibition has proven to be a useful tool for studying invasion in many different apicomplexans, including Neospora,200,201 Eimeria,90,202 Babesia,117 Toxoplasma,27 and Plasmodium.203-206 Because antibodies can affect function by different mechanisms, determining the mode of action of the inhibitory antibody can be informative. For example, certain invasion inhibitory antibodies directed against P. falciparum surface proteins block invasion indirectly, by interfering with the proteolytic processing of these proteins, illustrating the importance of processing for the function of these proteins during invasion.203,207,208
Small-Molecule-based Approaches
The most common way to use small molecules to study a process such as invasion is to determine whether specific pharmacological agents with known targets in other systems affect the process of interest, thereby implicating the known target in the process. For example, the first evidence implicating actin in the process of invasion, both in T. gondii115,209,210 and Plasmodium125 was the inhibition of invasion by cytochalasinB or D. Similarly, inhibition of microneme secretion by the intracellular calcium chelator BAPTA-AM, together with stimulation of microneme secretion by the calcium ionophore A23187, strongly suggested a role for intracellular calcium in microneme secretion.31,211
A more general way to use small molecules, which is not limited to compounds with predefined targets, is “phenotype-based” small molecule screening.212 In such an approach, libraries of structurally diverse small molecules are screened for those that generate a particular phenotype; these compounds are then used to directly identify the target(s) responsible for generating the phenotype (Fig. 7). The approach is essentially the pharmacological analog of classical forward genetics (compounds that disrupt protein function being analogous to mutations); however, in the small molecule approach, perturbation of protein function is under the investigator's control rather than being permanently encoded in the mutant's genome. When applied to invasion, this circumvents the problem of invasion mutants being nonviable. In a recent screen of 12160 small molecules, 24 novel inhibitors and 6 enhancers of T. gondii invasion were identified,50 most of which were fully reversible. Different compounds had distinctly different effects on microneme secretion, motility, and/or conoid extension.
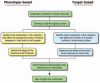
Figure 7
Overview of phenotype- vs. target-based small molecule approaches to studying biological processes. See text for details. Reproduced from: Ward GE et al. Cell Microbiol 4(8):471-482; ©2002, with permission from Blackwell Publishing.
The most difficult part of any small molecule-based screening project is target identification. A number of biochemical and genetic approaches are available for target identification and have been recently reviewed.212-215 “Activity-based probes,” which can be designed to covalently modify specific subsets of enzymatic targets, can facilitate target identification (reviewed in ref. 113). Although such probes sample only a subset of the proteome, if this subset is of interest (e.g., a particular family of proteases), the selectivity is an advantage, and the ability of the probes to covalently modify their targets can greatly facilitate target identification. In all cases, the identified target must be independently validated as the relevant target in vivo.212-215
An alternative way to use small molecules is to screen large collections of structurally diverse small molecules for compounds that affect the activity of an individual recombinant or purified protein (“target-based” screening; Fig. 7), rather than causing a complex, cell-based phenotype. Once a small molecule inhibitor of that protein has been identified, it can be used on whole cells to determine the function of the protein within the context of the cell (the pharmacological analog of reverse genetics). Target-based screening has been used to identify novel inhibitors of T. gondii dense granule NTPases216 and P. falciparum dihydroorotate dehydrogenase.217 One clear benefit of taking a small molecule approach is that it may generate not only new tools for studying invasion, but also new potential lead compounds for drug development.
Altering the Target Cell
Analysis of a parasite's ability to invade genetically or enzymatically altered host cells has been a useful approach to studying invasion. For example, a decrease in the ability of T. gondii tachyzoites to invade either host cells pretreated with enzymes that cleave glycosaminoglycans (GAGs) or GAG biosynthesis mutants,218,219 strongly suggests a role for host cell GAGs in T. gondii invasion, perhaps as receptors for micronemal adhesins. Cell synchronization studies showed that the ability of T. gondii to attach to and invade host cells increases during S-phase of the host cell cycle220,221 suggesting that a critical host cell receptor may be upregulated in S-phase.221,222
Enzyme-treated erythrocytes and mutant erythrocytes lacking specific surface antigens have been tremendously useful experimental tools for studying the receptor-ligand interactions involved in invasion by Plasmodium merozoites. For example, the seminal observation that Duffy(-) erythrocytes are refractory to invasion by P. knowlesi223 ultimately led to the identification of the Duffy-Binding Protein family of parasite receptors.111,193,224 During erythrocyte invasion, the Duffy-binding proteins appear to function in moving junction formation.82,125 Similarly, enzymatic treatment of erythrocytes, together with a variety of naturally occurring erythrocyte mutants, have helped to elucidate the complex and redundant invasion mechanisms used by P. falciparum merozoites.79,193,225-227 More is currently known about the receptor-ligand interactions that regulate invasion in asexual stage Plasmodium parasites than in any other apicomplexan, including T. gondii (reviewed in ref. 228; see also Duraisingh, this volume).
Genomic/Transcriptomic/Proteomic Approaches
Genomics
Comprehensive genome sequencing of many apicomplexans (see Table 1) has facilitated almost every aspect of studying invasion, including identification of mutations generated in forward genetic screens, design and implementation of reverse genetic experiments (e.g., knockout construct design, identification of promoters for exogenous expression, etc.), and identification of unknown proteins by mass spectrometry. The availability of genomic sequence data makes BLAST searching significantly more effective and productive, and allows gene/protein analysis from one apicomplexan to be more broadly interpreted and applied to other apicomplexans (which is particularly important in genetically inaccessible organisms such as Theileria and Cryptosporidium). It also facilitates target identification in small-molecule-based studies, i.e., if a particular compound has a known target in another organism, the apicomplexan homolog(s) of the known target can be identified by BLAST searching. Genome mining can also be used to search for proteins with proven or suspected functional domains, such as adhesive domains or particular protease cleavage sites.229
The discovery of T. gondii rhomboid proteases and their potential substrates exemplifies how genomics can stimulate major discoveries and quickly bring new research areas into experimental focus. Early studies of Drosophila rhomboids used genome mining to search for non-Drosophila proteins that contained consensus rhomboid cleavage recognition motifs, identifying a number of T. gondii microneme proteins.230 Intriguingly, several of these microneme proteins were known to be cleaved intramembranously,112,231 a defining feature of rhomboid cleavage.232 Subsequent studies showed that these proteins could indeed be cleaved by Drosophila rhomboids.230 This led to BLAST searches of the T. gondii EST and genomic databases (http://ToxoDB.org), querying with sequences of known rhomboids,233,234 which ultimately resulted in the discovery, cloning, and localization of T. gondii rhomboid proteases.233,234 Rhomboid-like genes have been found in all available apicomplexan genomes,235 and are currently the focus of numerous studies using reverse genetic techniques to characterize their function during invasion.
Transcriptomics
The up- or down-regulation of gene expression during certain processes (e.g., differentiation or response to specific stimuli) can be used to infer the involvement of those genes in the processes. Expression from a wide spectrum of genes can be examined by determining the change in the amount of transcript (transcriptome) as analyzed by microarray binding or Serial Analysis of Gene Expression (SAGE). These technologies have been used alone236 and in combination with either insertional129 or chemical134 mutagenesis to identify genes involved in T. gondii differentiation. A more accurate method of analyzing gene transcription/regulation utilizes a T. gondii uracil salvage enzyme (UPRT) to measure only the amount of RNA synthesized during a given interval (via incorporation of thio-substituted uridines), rather than the total amount of transcript present. This technique was initially developed for T. gondii,237 but can be used in any organism or cell in which the salvage enzyme can be expressed.
Because invasion happens so quickly (~20 sec), it is unlikely that changes in transcription during this time frame play a direct role in driving the process. However, changes in the Plasmodium transcriptome throughout its life cycle have been used to identify genes that are upregulated in the invasive stages and therefore may be involved in Plasmodium invasion.238-240
Analogous studies of the host cell's response to invasion has identified host cell transcripts upregulated following T. gondii invasion.241 Transcriptome studies have also recently been performed in Theileria.242
Proteomics
Changes in expression can also be analyzed at the protein level, and proteins that reside in a particular subcellular compartment thought to be involved in invasion can be identified by cell fractionation and proteomic analysis (reviewed in ref. 113). For example, the recent proteomic analysis of purified T. gondii rhoptries243 identified 38 novel proteins, many of which were subsequently confirmed to be rhoptry proteins by fluorescence microscopy. These proteins include a novel subset of rhoptry proteins that localize to the thin, anterior “neck” portion of the rhoptries,243 and may be discharged differentially from rhoptry “bulb” proteins.8,13 This study also identified the first T. gondii rhoptry proteins with homologs in Plasmodium. Proteomic analysis of the excreted/secreted antigens (ESA) of T. gondii was recently reported, identifying a number of novel microneme proteins.244 Proteomic analysis of purified Eimeria tenella micronemes has also recently been accomplished.245 These newly identified rhoptry and microneme proteins can be further studied using the reverse genetic techniques described above.
A combination of proteomic and computational techniques has recently been used to identify novel, GPI-anchored proteins of asexual stage malaria parasites.246 As GPI-anchored proteins dominate the surface of merozoites, identification and characterization of GPI-anchored proteins will likely be critical to understanding the molecular mechanisms of erythrocyte invasion by Plasmodium merozoites.
Combined Approaches
In practice, the most powerful strategy for studying invasion has been to employ a combination of the approaches described above. We will briefly review three of the examples that best illustrate this principle: AMA1, actin/myosin, and PKG.
AMA1
Inhibition of erythrocyte invasion by antibodies and Fab fragments directed against Plasmodium AMA1 provided the first clue that this protein might be involved in invasion.247,248 The P. falciparum AMA1 gene could be disrupted by homologous recombination, but viable parasites with the disruption could only be isolated when accompanied by transgenic AMA1 expression, demonstrating that the gene is essential.189 Remarkably, the introduction of AMA1 from P. chabaudi (a mouse malaria parasite) into P. falciparum (a human malaria parasite) generated transgenic parasites that invade mouse erythrocytes more efficiently than wild type P. falciparum.189 Expression of portions of Plasmodium AMA1 in COS and CHO cells increased cell binding to erythrocytes, suggesting that AMA1 may act as an adhesin,118 although neither shed fragments of AMA1 nor properly folded recombinant AMA1 show similar binding. Plasmodium invasion was shown to be inhibited by peptides either derived from, or with affinity for, AMA1.121,249,250 Furthermore, Plasmodium merozoites whose invasion was arrested by an anti-AMA1 antibody were shown by electron microscopy to be attached at a distance to the erythrocyte, prior to apical reorientation.205
The T. gondii homolog of AMA1 (TgAMA1) was identified both by BLAST searches of the T. gondii EST database27 and through a large-scale monoclonal antibody screen.251 Early failed attempts to knock out TgAMA127 suggested it was essential, which was confirmed when the gene was knocked out in the presence of exogenous copies of TgAMA1.8 The development of the tetracycline-regulatable promoter in T. gondii enabled the generation of a conditional TgAMA1 knockout. Using these parasites, it was shown that TgAMA1-depleted parasites are significantly impaired in invasion (Fig. 5B). Further analysis showed them to be defective both in forming close interactions with host cell membranes and in rhoptry secretion.8
Actin/Myosin
As described above, the inhibition of invasion by cytochalasinB and D was the first indication that actin plays a role in invasion. Invasion studies using cytochalasinD-resistant host cells demonstrated that actin of the parasite, but not host cell actin, was involved.48 This same study used chemically mutagenized parasites to isolate cytochalasinD-resistant parasites that can invade host cells in the presence of cytochalasinD. A point mutation in the single copy actin gene (ACT1) of these mutant parasites correlated with cytochalasinD resistance. Actin's role in invasion was confirmed when exogenous expression of the mutated allele was shown to confer cytochalasinD resistance in wild type parasites.48 A central role for T. gondii myosinA in motility and invasion was subsequently demonstrated by the creation and phenotypic analysis of a MyoA conditional knockout parasite.10
Protein Kinase G (PKG)
“Compound 1,” a trisubstituted pyrrole that was originally identified as an inhibitor of Eimeria tenella growth in vitro, was subsequently shown to have activity against other apicomplexan parasites, including T. gondii.252 A tritiated analog was used to purify the major Compound 1-binding protein from extracts of E. tenella.252 This protein was identified as PKG, and PKG was also shown to also be a target for Compound 1 in T. gondii.252 Computer modeling was used to predict the catalytic site residues in T. gondii PKG that interact with Compound 1, which were subsequently mutated. An elegant series of molecular genetic experiments, in which wild type T. gondii PKG was replaced with a mutant form that was not inhibited by Compound 1, proved that PKG was the relevant target of Compound 1 both in vitro and in animal models of infection.143 Having validated PKG as the primary molecular target of Compound 1, the compound was used to demonstrate a role for PKG in parasite motility and invasion.102
Do We Have All the Tools We Need?
While the approaches available to study invasion are now numerous and powerful, the toolbox is not yet complete. The currently available conditional promoters only work for proteins whose expression levels fall within a certain range; a more complete set of inducible promoters would be very useful. Methods for enhancing the frequency with which genes can be homologously targeted in T. gondii would facilitate reverse genetic studies. A method of culturing T. gondii in the absence of host cells would also greatly enhance the study of invasion (and many other aspects of the parasite's life cycle) by allowing knockouts or mutations in essential invasion genes to be isolated; the resulting invasive phenotype could then be analyzed in cell culture. Such a cell-free system would also expand the type of screens that could be designed for studying apicomplexan invasion. In this regard, it is encouraging that partial, cell-free development of Plasmodium blood stage parasites has been reported.253
Toxoplasma as a Model Apicomplexan
The overall process of invasion appears to be relatively well conserved among apicomplexan parasites, even if the particular molecules mediating the process vary. For example, the individual microneme proteins of Toxoplasma and Plasmodium are different, but many are organized along a similar theme, i.e., modules of adhesive motifs.254 In fact, portions of some microneme proteins are functionally interchangeable between Toxoplasma and Plasmodium, despite limited sequence identity.187 These and other examples8,50,157,205 clearly demonstrate that findings from one species of apicomplexan parasite can often be extended to others.
One of the main reasons why T. gondii has become a model for studies of apicomplexan invasion (reviewed in ref. 255) is its amenability to molecular genetic manipulation (see previous section on “Approaches to Studying Invasion”). Several selectable markers are available, exogenous DNA integrates with high frequency and is readily expressed, and a variety of approaches can be used for gene knockouts, conditional gene expression, and chemical or insertional mutagenesis (Table 1). The genome is completely sequenced and readily accessible (ToxoDB.org). In addition, the T. gondii tachyzoite (2 × 7 μm) is considerably bigger than, e.g., the Plasmodium merozoite (1 × 1 μm), facilitating subcellular localization of proteins and a variety of other cell biological techniques. T. gondii is easily cultured, and the ability of T. gondii tachyzoites to invade virtually any nucleated mammalian cell creates powerful opportunities for studying the host cell requirements for invasion.218,219 As described above, assays have been developed that allow each step of T. gondii invasion to be examined in isolation, and a well-developed mouse model is available for in vivo studies. The ability to express non-T. gondii genes in T. gondii188,256,257 (see above) also makes it an excellent model for studying genes from less experimentally tractable parasites.
Outstanding Questions
While a great deal of progress has been made in recent years in our understanding of the invasive mechanisms of apicomplexan parasites, many important questions remain. These include:
How Is T. gondii Able to Invade Such a Wide Variety of Host Cells, When the Host Range of Other Apicomplexans (Such as Malaria Merozoites) Is So Limited?
T. gondii's promiscuity might reflect parasite recognition of a ubiquitous host cell ligand, or the presence of redundant receptors on the parasite surface. Alternatively, T. gondii might insert its own receptor into the host cell, analogous to translocation of the enteropathogenic E. coli TIR protein into the host cell during invasion.258 One way to begin to address these questions would be to express potential T. gondii receptors in other apicomplexans, and determine whether this expands the host range of the recipient species; a similar approach has proven useful in the analysis of AMA1 function in Plasmodium.189
What Is the Function of the Conoid and Its Repeated Cycles of Extension and Retraction during Parasite Motility and Invasion?
Identification of small molecule inhibitors of conoid extension/retraction50 and the proteomic analysis of isolated conoids259 are two potentially powerful approaches to studying conoid function.
How Do the Components of the “Moving Junction” Physically Connect the Parasite to the Host Cell during Invasion and Block Host Cell Transmembrane Proteins from Being Incorporated into the Developing Parasitophorous Vacuole?
Recent studies identifying components of the T. gondii moving junction13,14 represent an important first step towards understanding the function of the moving junction and the molecular basis of host transmembrane protein exclusion from the PVM.
What Are the Signaling Mechanisms That Underlie Rhoptry Secretion?
The recent analysis of the rhoptry proteome,243 combined with the generation of a conditional knockout parasite that is defective in rhoptry secretion,8 will be useful for studying the regulation of rhoptry secretion. The development of a method for assaying rhoptry secretion in the absence of host cells, analogous to the microneme secretion assay, would be extremely helpful, and could be combined with screens designed to identify small molecules or mutants that enhance or inhibit rhoptry secretion.
What Is the Function of Microneme Protein “Shedding” from the Parasite Surface during Invasion?
While several models have been proposed,55 these models remain to be experimentally tested. One possible approach to this question would be to determine the functional consequences of inhibiting the rhomboid proteases thought to be responsible for microneme protein shedding, using mutagenesis, targeted gene disruption, or small molecules.
Conclusion
Given the powerful combination of experimental tools currently available, it is our hope that we will soon more fully understand the molecular mechanisms of host cell invasion by apicomplexan parasites. Our efforts can then be directed towards developing effective ways of blocking these processes, as a means of preventing and treating the devastating diseases caused by these important human pathogens.
Acknowledgements
We thank Vern Carruthers, Chetan Chitnis, Stacey Gilk, and members of our laboratory for helpful comments on the manuscript. We also gratefully acknowledge the support of the Burroughs-Wellcome Fund and Public Health Service grants AI054961 and AI063276.
References
- 1.
- Mercier C, Adjogble KD, Daubener W. et al. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int J Parasitol. 2005;35(8):829–849. [PubMed: 15978597]
- 2.
- Kappe SH, Buscaglia CA, Bergman LW. et al. Apicomplexan gliding motility and host cell invasion: overhauling the motor model. Trends Parasitol. 2004;20(1):13–16. [PubMed: 14700584]
- 3.
- Hakansson S, Morisaki H, Heuser J. et al. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell. 1999;10(11):3539–3547. [PMC free article: PMC25631] [PubMed: 10564254]
- 4.
- Hu K, Roos DS, Murray JM. A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J Cell Biol. 2002;156(6):1039–1050. [PMC free article: PMC2173456] [PubMed: 11901169]
- 5.
- Morrissette NS, Sibley LD. Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev. 2002;66(1):21–38. [PMC free article: PMC120781] [PubMed: 11875126]
- 6.
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73(2):114–123. [PubMed: 9208224]
- 7.
- Dowse T, Soldati D. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr Opin Microbiol. 2004;7(4):388–396. [PubMed: 15358257]
- 8.
- Mital J, Meissner M, Soldati D. et al. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell. 2005;16(9):4341–4349. [PMC free article: PMC1196342] [PubMed: 16000372]
- 9.
- Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci USA. 1996;93(16):8413–8418. [PMC free article: PMC38685] [PubMed: 8710885]
- 10.
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298(5594):837–840. [PubMed: 12399593]
- 11.
- Dobrowolski JM, Carruthers VB, Sibley LD. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997;26(1):163–173. [PubMed: 9383198]
- 12.
- Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304(5668):248–253. [PubMed: 15073368]
- 13.
- Alexander DL, Mital J, Ward GE. et al. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathol. 2005;1(2):e17. [PMC free article: PMC1262624] [PubMed: 16244709]
- 14.
- Lebrun M, Michelin A, El Hajj H. et al. The rhoptry neck protein RON4 relocalizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol. 2005;7(12):1823–1833. [PubMed: 16309467]
- 15.
- Mordue DG, Hakansson S, Niesman I. et al. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol. 1999;92(2):87–99. [PubMed: 10366534]
- 16.
- Lingelbach K, Joiner KA. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J Cell Sci. 1998;111(Pt 11):1467–1475. [PubMed: 9580555]
- 17.
- Morisaki JH, Heuser JE, Sibley LD. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108(Pt 6):2457–2464. [PubMed: 7673360]
- 18.
- Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110(Pt 17):2117–2128. [PubMed: 9378762]
- 19.
- Augustine PC. Cell:sporozoite interactions and invasion by apicomplexan parasites of the genus Eimeria. Int J Parasitol. 2001;31(1):1–8. [PubMed: 11286188]
- 20.
- Buxton D, McAllister MM, Dubey JP. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18(12):546–552. [PubMed: 12482540]
- 21.
- Smith HV, Nichols RA, Grimason AM. Cryptosporidium excystation and invasion: getting to the guts of the matter. Trends Parasitol. 2005;21(3):133–142. [PubMed: 15734661]
- 22.
- Moltmann UG, Mehlhorn H, Schein E. et al. Fine structure of Babesia equi Laveran, 1901 within lymphocytes and erythrocytes of horses: an in vivo and in vitro study. J Parasitol. 1983;69(1):111–120. [PubMed: 6827432]
- 23.
- Miller LH, Aikawa M, Johnson JG. et al. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J Exp Med. 1979;149(1):172–84. [PMC free article: PMC2184746] [PubMed: 105074]
- 24.
- Shaw MK. Cell invasion by Theileria sporozoites. Trends Parasitol. 2003;19(1):2–6. [PubMed: 12488213]
- 25.
- Pfefferkorn ER, Pfefferkorn LC. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976;39(3):365–376. [PubMed: 1269580]
- 26.
- Saffer LD, Long Krug SA, Schwartzman JD. The role of phospholipase in host cell penetration by Toxoplasma gondii. Am J Trop Med Hyg. 1989;40(2):145–149. [PubMed: 2919724]
- 27.
- Hehl AB, Lekutis C, Grigg ME. et al. Toxoplasma gondii homologue of Plasmodium apical membrane antigen 1 is involved in invasion of host cells. Infect Immun. 2000;68(12):7078–7086. [PMC free article: PMC97818] [PubMed: 11083833]
- 28.
- Schwartzman JD, Pfefferkorn ER. Pyrimidine synthesis by intracellular Toxoplasma gondii. J Parasitol. 1981;67(2):150–158. [PubMed: 7241272]
- 29.
- Camps M, Boothroyd JC. Toxoplasma gondii: selective killing of extracellular parasites by oxidation using pyrrolidine dithiocarbamate. Exp Parasitol. 2001;98(4):206–214. [PubMed: 11560413]
- 30.
- Soldati D, Kim K, Kampmeier J. et al. Complementation of a Toxoplasma gondii ROP1 knock-out mutant using phleomycin selection. Mol Biochem Parasitol. 1995;74(1):87–97. [PubMed: 8719248]
- 31.
- Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol. 1999;1(3):225–235. [PubMed: 11207555]
- 32.
- Seeber F, Boothroyd JC. Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene. 1996;169(1):39–45. [PubMed: 8635747]
- 33.
- Sibley LD, Howe DK, Wan KL. et al. Toxoplasma as a model genetic system. In: Smith DF, Parsons M, eds. Molecular Biology of Parasitic Protozoa. New York: Oxford University Press. 1996:55–74.
- 34.
- Howe DK, Mercier C, Messina M. et al. Expression of Toxoplasma gondii genes in the closely-related apicomplexan parasite Neospora caninum. Mol Biochem Parasitol. 1997;86(1):29–36. [PubMed: 9178265]
- 35.
- Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260(5106):349–352. [PubMed: 8469986]
- 36.
- Donald RG, Roos DS. Stable molecular transformation of Toxoplasma gondii: A selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci USA. 1993;90(24):11703–11707. [PMC free article: PMC48052] [PubMed: 8265612]
- 37.
- Black MW, Boothroyd JC. Development of a stable episomal shuttle vector for Toxoplasma gondii. J Biol Chem. 1998;273(7):3972–3979. [PubMed: 9461585]
- 38.
- Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262(5135):911–914. [PubMed: 8235614]
- 39.
- Roos DS, Donald RG, Morrissette NS. et al. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994:27–63. [PubMed: 7707991]
- 40.
- Garcia-Reguet N, Lebrun M, Fourmaux M. et al. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell Microbiol. 2000;2(4):353–364. [PubMed: 11207591]
- 41.
- Messina M, Niesman I, Mercier C. et al. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene. 1995;165(2):213–217. [PubMed: 8522178]
- 42.
- Donald RGK, Carter D, Ullman B. et al. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271(24):14010–14019. [PubMed: 8662859]
- 43.
- Donald RG, Roos DS. Insertional mutagenesis and marker rescue in a protozoan parasite: Cloning of the uracil phosphoribosyltransferase locus from Toxoplasma gondii. Proc Natl Acad Sci USA. 1995;92(12):5749–5753. [PMC free article: PMC41774] [PubMed: 7777580]
- 44.
- Radke JR, White MW. A cell cycle model for the tachyzoite of Toxoplasma gondii using the Herpes simplex virus thymidine kinase. Mol Biochem Parasitol. 1998;94(2):237–247. [PubMed: 9747974]
- 45.
- Sibley LD, Messina M, Niesman IR. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. Proc Natl Acad Sci USA. 1994;91(12):5508–5512. [PMC free article: PMC44025] [PubMed: 8202518]
- 46.
- Van Tam T, Rooney PJ, Knoll LJ. Nourseothricin acetyltransferease: a positive selectable marker for Toxoplasma gondii. J Parasitol. 2006;92(3):668–670. [PubMed: 16884024]
- 47.
- Mercier C, Lefebvre-Van Hende S, Garber GE. et al. Common cis-acting elements critical for the expression of several genes of Toxoplasma gondii. Mol Microbiol. 1996;21(2):421–428. [PubMed: 8858595]
- 48.
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84(6):933–939. [PubMed: 8601316]
- 49.
- Black M, Seeber F, Soldati D. et al. Restriction enzyme-mediated integration elevates transformation frequency and enables cotransfection of Toxoplasma gondii. Mol Biochem Parasitol. 1995;74(1):55–63. [PubMed: 8719245]
- 50.
- Carey KL, Westwood NJ, Mitchison TJ. et al. A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc Natl Acad Sci USA. 2004;101(19):7433–7438. [PMC free article: PMC409936] [PubMed: 15123807]
- 51.
- Striepen B, He CY, Matrajt M. et al. Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92(2):325–338. [PubMed: 9657336]
- 52.
- Matrajt M, Nishi M, Fraunholz MJ. et al. Amino-terminal control of transgenic protein expression levels in Toxoplasma gondii. Mol Biochem Parasitol. 2002;120(2):285–289. [PubMed: 11897133]
- 53.
- Wu Y, Sifri CD, Lei HH. et al. Transfection of Plasmodium falciparum within human red blood cells. Proc Natl Acad Sci USA. 1995;92(4):973–977. [PMC free article: PMC42619] [PubMed: 7862676]
- 54.
- van Dijk MR, Waters AP, Janse CJ. Stable transfection of malaria parasite blood stages. Science. 1995;268(5215):1358–1362. [PubMed: 7761856]
- 55.
- Carruthers VB, Blackman MJ. A new release on life: emerging concepts in proteolysis and parasite invasion. Mol Microbiol. 2005;55(6):1617–1630. [PubMed: 15752188]
- 56.
- Crabb BS, Cowman AF. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93(14):7289–7294. [PMC free article: PMC38976] [PubMed: 8692985]
- 57.
- Kadekoppala M, Cheresh P, Catron D. et al. Rapid recombination among transfected plasmids, chimeric episome formation and trans gene expression in Plasmodium falciparum. Mol Biochem Parasitol. 2001;112(2):211–218. [PubMed: 11223128]
- 58.
- Carvalho TG, Menard R. Manipulating the Plasmodium genome. Curr Issues Mol Biol. 2005;7(1):39–55. [PubMed: 15580779]
- 59.
- de Koning-Ward TF, Fidock DA, Thathy V. et al. The selectable marker human dihydrofolate reductase enables sequential genetic manipulation of the Plasmodium berghei genome. Mol Biochem Parasitol. 2000;106(2):199–212. [PubMed: 10699250]
- 60.
- Mamoun CB, Gluzman IY, Goyard S. et al. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96(15):8716–8720. [PMC free article: PMC17582] [PubMed: 10411941]
- 61.
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124(4):755–766. [PubMed: 16497586]
- 62.
- Duraisingh MT, Triglia T, Cowman AF. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int J Parasitol. 2002;32(1):81–89. [PubMed: 11796125]
- 63.
- Deitsch K, Driskill C, Wellems T. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res. 2001;29(3):850–853. [PMC free article: PMC30384] [PubMed: 11160909]
- 64.
- Waller RF, Reed MB, Cowman AF. et al. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 2000;19(8):1794–1802. [PMC free article: PMC302007] [PubMed: 10775264]
- 65.
- Howe DK, Sibley LD. Development of molecular genetics for Neospora caninum: a complementary system to Toxoplasma gondii. Methods. 1997;13(2):123–133. [PubMed: 9405196]
- 66.
- Kelleher M, Tomley FM. Transient expression of beta-galactosidase in differentiating sporozoites of Eimeria tenella. Mol Biochem Parasitol. 1998;97(1-2):21–31. [PubMed: 9879884]
- 67.
- Adamson R, Lyons K, Sharrard M. et al. Transient transfection of Theileria annulata. Mol Biochem Parasitol. 2001;114(1):53–61. [PubMed: 11356513]
- 68.
- Gardner MJ, Bishop R, Shah T. et al. Genome sequence of Theileria parva, a bovine pathogen that transforms lymphocytes. Science. 2005;309(5731):134–137. [PubMed: 15994558]
- 69.
- Suarez CE, Palmer GH, LeRoith T. et al. Intergenic regions in the rhoptry associated protein-1 (rap-1) locus promote exogenous gene expression in Babesia bovis. Int J Parasitol. 2004;34(10):1177–1184. [PubMed: 15380689]
- 70.
- Vaishnava S, Morrison DP, Gaji RY. et al. Plastid segregation and cell division in the apicomplexan parasite Sarcocystis neurona. J Cell Sci. 2005;118(Pt 15):3397–3407. [PubMed: 16079283]
- 71.
- Huynh MH, Rabenau KE, Harper JM. et al. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22(9):2082–2090. [PMC free article: PMC156089] [PubMed: 12727875]
- 72.
- Gaji RY, Zhang D, Breathnach CC. et al. Molecular genetic transfection of the coccidian parasite Sarcocystis neurona. Mol Biochem Parasitol. 2006;150(1):1–9. [PubMed: 16844242]
- 73.
- Lovett JL, Sibley LD. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J Cell Sci. 2003;116(Pt 14):3009–3016. [PubMed: 12783987]
- 74.
- Naguleswaran A, Muller N, Hemphill A. Neospora caninum and Toxoplasma gondii: a novel adhesion/invasion assay reveals distinct differences in tachyzoite-host cell interactions. Exp Parasitol. 2003;104(3-4):149–158. [PubMed: 14552862]
- 75.
- Barragan A, Brossier F, Sibley LD. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005;7(4):561–568. [PubMed: 15760456]
- 76.
- Mital J, Schwarz J, Taatjes DJ. et al. Laser scanning cytometer-based assays for measuring host cell attachment and invasion by the human pathogen Toxoplasma gondii. Cytometry A. 2006;69(1):13–19. [PMC free article: PMC1428790] [PubMed: 16342112]
- 77.
- Gay-Andrieu F, Cozon GJ, Ferrandiz J. et al. Flow cytometric quantification of Toxoplasma gondii cellular infection and replication. J Parasitol. 1999;85(3):545–549. [PubMed: 10386451]
- 78.
- Sultan AA, Thathy V, Frevert U. et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90(3):511–522. [PubMed: 9267031]
- 79.
- Reed MB, Caruana SR, Batchelor AH. et al. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci USA. 2000;97(13):7509–7514. [PMC free article: PMC16576] [PubMed: 10861015]
- 80.
- Bharara R, Singh S, Pattnaik P. et al. Structural analogs of sialic acid interfere with the binding of erythrocyte binding antigen-175 to glycophorin A, an interaction crucial for erythrocyte invasion by Plasmodium falciparum. Mol Biochem Parasitol. 2004;138(1):123–129. [PubMed: 15500923]
- 81.
- Casey JL, Coley AM, Anders RF. et al. Antibodies to malaria peptide mimics inhibit Plasmodium falciparum invasion of erythrocytes. Infect Immun. 2004;72(2):1126–1134. [PMC free article: PMC321628] [PubMed: 14742560]
- 82.
- Singh AP, Ozwara H, Kocken CH. et al. Targeted deletion of Plasmodium knowlesi Duffy binding protein confirms its role in junction formation during invasion. Mol Microbiol. 2005;55(6):1925–1934. [PubMed: 15752210]
- 83.
- Xu L, Chaudhuri A. Plasmodium yoelii: a differential fluorescent technique using Acridine Orange to identify infected erythrocytes and reticulocytes in Duffy knockout mouse. Exp Parasitol. 2005;110(1):80–87. [PubMed: 15804382]
- 84.
- Kocken CH, Withers-Martinez C, Dubbeld MA. et al. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect Immun. 2002;70(8):4471–4476. [PMC free article: PMC128198] [PubMed: 12117958]
- 85.
- Kocken CH, Ozwara H, van der Wel A. et al. Plasmodium knowlesi provides a rapid in vitro and in vivo transfection system that enables double-crossover gene knockout studies. Infect Immun. 2002;70(2):655–660. [PMC free article: PMC127703] [PubMed: 11796595]
- 86.
- Malagon F, Castillo O. On the labeling of malaria parasites In Vivo with 3H-hipoxanthine while developing an infection in the mouse. Acta Protozool. 2000;39:281–288.
- 87.
- Makler MT, Ries JM, Williams JA. et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48(6):739–741. [PubMed: 8333566]
- 88.
- Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48(2):205–210. [PubMed: 8447524]
- 89.
- Drew DR, O'Donnell RA, Smith BJ. et al. A common cross-species function for the double epidermal growth factor-like modules of the highly divergent Plasmodium surface proteins MSP-1 and MSP-8. J Biol Chem. 2004;279(19):20147–20153. [PubMed: 14976193]
- 90.
- Labbe M, de Venevelles P, Girard-Misguich F. et al. Eimeria tenella microneme protein EtMIC3: identification, localisation and role in host cell infection. Mol Biochem Parasitol. 2005;140(1):43–53. [PubMed: 15694485]
- 91.
- Ward GE, Fujioka H, Aikawa M. et al. Staurosporine inhibits invasion of erythrocytes by malarial merozoites. Exp Parasitol. 1994;79(3):480–487. [PubMed: 7957765]
- 92.
- Gaffar FR, Yatsuda AP, Franssen FF. et al. Erythrocyte invasion by Babesia bovis merozoites is inhibited by polyclonal antisera directed against peptides derived from a homologue of Plasmodium falciparum apical membrane antigen 1. Infect Immun. 2004;72(5):2947–2955. [PMC free article: PMC387893] [PubMed: 15102807]
- 93.
- Franssen FF, Gaffar FR, Yatsuda AP. et al. Characterisation of erythrocyte invasion by Babesia bovis merozoites efficiently released from their host cell after high-voltage pulsing. Microbes Infect. 2003;5(5):365–372. [PubMed: 12737991]
- 94.
- Rathore D, Hrstka SC, Sacci Jr JB. et al. Molecular mechanism of host specificity in Plasmodium falciparum infection: role of circumsporozoite protein. J Biol Chem. 2003;278(42):40905–40910. [PubMed: 12904297]
- 95.
- Silvie O, Franetich JF, Charrin S. et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279(10):9490–9496. [PubMed: 14676185]
- 96.
- Brahimi K, Badell E, Sauzet JP. et al. Human antibodies against Plasmodium falciparum liver-stage antigen 3 cross-react with Plasmodium yoelii preerythrocytic-stage epitopes and inhibit sporozoite invasion in vitro and in vivo. Infect Immun. 2001;69(6):3845–3852. [PMC free article: PMC98406] [PubMed: 11349050]
- 97.
- Kafsack BF, Beckers C, Carruthers VB. Synchronous invasion of host cells by Toxoplasma gondii. Mol Biochem Parasitol. 2004;136(2):309–311. [PubMed: 15478810]
- 98.
- Wetzel DM, Hakansson S, Hu K. et al. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol Biol Cell. 2003;14(2):396–406. [PMC free article: PMC149980] [PubMed: 12589042]
- 99.
- Chen XM, O'Hara SP, Huang BQ. et al. Apical organelle discharge by Cryptosporidium parvum is temperature, cytoskeleton, and intracellular calcium dependent and required for host cell invasion. Infect Immun. 2004;72(12):6806–6816. [PMC free article: PMC529161] [PubMed: 15557601]
- 100.
- Wetzel DM, Schmidt J, Kuhlenschmidt MS. et al. Gliding motility leads to active cellular invasion by Cryptosporidium parvum sporozoites. Infect Immun. 2005;73(9):5379–5387. [PMC free article: PMC1231075] [PubMed: 16113253]
- 101.
- Entzeroth R, Zgrzebski G, Dubremetz JF. Secretion of trials during gliding motility of Eimeria (Apicomplexa, Coccidia) sporozoites visualized by a monoclonal antibody and immuno-gold-silver enhancement. Parasitol Res. 1989;76(2):174–175. [PubMed: 2616569]
- 102.
- Wiersma HI, Galuska SE, Tomley FM. et al. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int J Parasitol. 2004;34(3):369–380. [PubMed: 15003497]
- 103.
- Stewart MJ, Vanderberg JP. Electron microscopic analysis of circumsporozoite protein trail formation by gliding malaria sporozoites. J Protozool. 1992;39(6):663–671. [PubMed: 1453354]
- 104.
- Bumstead J, Tomley F. Induction of secretion and surface capping of microneme proteins in Eimeria tenella. Mol Biochem Parasitol. 2000;110(2):311–321. [PubMed: 11071285]
- 105.
- Siden-Kiamos I, Ecker A, Nyback S. et al. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol Microbiol. 2006;60(6):1355–1363. [PMC free article: PMC1513514] [PubMed: 16796674]
- 106.
- Mondragon R, Frixione E. Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbiol. 1996;43(2):120–127. [PubMed: 8720941]
- 107.
- Carruthers VB, Moreno SN, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999;342(Pt 2):379–386. [PMC free article: PMC1220475] [PubMed: 10455025]
- 108.
- Hoane JS, Carruthers VB, Striepen B. et al. Analysis of the Sarcocystis neurona microneme protein SnMIC10: Protein characteristics and expression during intracellular development. Int J Parasitol. 2003;33(7):671–679. [PubMed: 12814647]
- 109.
- Keller N, Riesen M, Naguleswaran A. et al. Identification and characterization of a Neospora caninum microneme-associated protein (NcMIC4) that exhibits unique lactose-binding properties. Infect Immun. 2004;72(8):4791–4800. [PMC free article: PMC470650] [PubMed: 15271941]
- 110.
- Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230(4725):553–556. [PubMed: 3901257]
- 111.
- Adams JH, Hudson DE, Torii M. et al. The duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–153. [PubMed: 2170017]
- 112.
- Zhou XW, Blackman MJ, Howell SA. et al. Proteomic analysis of cleavage events reveals a dynamic two-step mechanism for proteolysis of a key parasite adhesive complex. Mol Cell Proteomics. 2004;3(6):565–576. [PubMed: 14982962]
- 113.
- Phillips CI, Bogyo M. Proteomics meets microbiology: Technical advances in the global mapping of protein expression and function. Cell Microbiol. 2005;7(8):1061–1076. [PubMed: 16008574]
- 114.
- Mineo JR, Kasper LH. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp Parasitol. 1994;79(1):11–20. [PubMed: 8050521]
- 115.
- Silva SR, Meirelles SS, De Souza W. Mechanism of entry of Toxoplasma gondii into vertebrate cells. J Submicrosc Cytol. 1982;14(3):471–482. [PubMed: 7175984]
- 116.
- Friedman MJ, Blankenberg T, Sensabaugh G. et al. Recognition and invasion of human erythrocytes by malarial parasites: Contribution of sialoglycoproteins to attachment and host specificity. J Cell Biol. 1984;98(5):1672–1677. [PMC free article: PMC2113184] [PubMed: 6373782]
- 117.
- Mosqueda J, McElwain TF, Stiller D. et al. Babesia bovis merozoite surface antigen 1 and rhoptry-associated protein 1 are expressed in sporozoites, and specific antibodies inhibit sporozoite attachment to erythrocytes. Infect Immun. 2002;70(3):1599–1603. [PMC free article: PMC127786] [PubMed: 11854249]
- 118.
- Fraser TS, Kappe SH, Narum DL. et al. Erythrocyte-binding activity of Plasmodium yoelii apical membrane antigen-1 expressed on the surface of transfected COS-7 cells. Mol Biochem Parasitol. 2001;117(1):49–59. [PubMed: 11551631]
- 119.
- Jacquet A, Coulon L, De Neve J. et al. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol. 2001;116(1):35–44. [PubMed: 11463464]
- 120.
- Urquiza M, Suarez JE, Cardenas C. et al. Plasmodium falciparum AMA-1 erythrocyte binding peptides implicate AMA-1 as erythrocyte binding protein. Vaccine. 2000;19(4-5):508–513. [PubMed: 11027815]
- 121.
- Hakansson S, Charron AJ, Sibley LD. Toxoplasma evacuoles: A two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 2001;20(12):3132–3144. [PMC free article: PMC150190] [PubMed: 11406590]
- 122.
- Carey KL, Jongco AM, Kim K. et al. The Toxoplasma gondii rhoptry protein ROP4 is secreted into the parasitophorous vacuole and becomes phosphorylated in infected cells. Eukaryot Cell. 2004;3(5):1320–1330. [PMC free article: PMC522600] [PubMed: 15470260]
- 123.
- Carey KL, Donahue CG, Ward GE. Identification and molecular characterization of GRA8, a novel, proline- rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol. 2000;105(1):25–37. [PubMed: 10613696]
- 124.
- Aikawa M, Miller LH, Rabbege JR. et al. Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J Cell Biol. 1981;91(1):55–62. [PMC free article: PMC2111932] [PubMed: 7298726]
- 125.
- Miller LH, Aikawa M, Johnson JG. et al. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J Exp Med. 1979;149(1):172–184. [PMC free article: PMC2184746] [PubMed: 105074]
- 126.
- Donald RG, Roos DS. Homologous recombination and gene replacement at the dihydrofolate reductase-thymidylate synthase locus in Toxoplasma gondii. Mol Biochem Parasitol. 1994;63(2):243–253. [PubMed: 8008022]
- 127.
- Roos DS, Sullivan WJ, Striepen B. et al. Tagging genes and trapping promoters in Toxoplasma gondii by insertional mutagenesis. Methods. 1997;13(2):112–122. [PubMed: 9405195]
- 128.
- Sullivan Jr WJ, Chiang CW, Wilson CM. et al. Insertional tagging of at least two loci associated with resistance to adenine arabinoside in Toxoplasma gondii, and cloning of the adenosine kinase locus. Mol Biochem Parasitol. 1999;103(1):1–14. [PubMed: 10514076]
- 129.
- Matrajt M, Donald RG, Singh U. et al. Identification and characterization of differentiation mutants in the protozoan parasite Toxoplasma gondii. Mol Microbiol. 2002;44(3):735–747. [PubMed: 11994154]
- 130.
- Vanchinathan P, Brewer JL, Harb OS. et al. Disruption of a locus encoding a nucleolar zinc finger protein decreases tachyzoite-to-bradyzoite differentiation in Toxoplasma gondii. Infect Immun. 2005;73(10):6680–6688. [PMC free article: PMC1230886] [PubMed: 16177345]
- 131.
- Striepen B, White MW, Li C. et al. Genetic complementation in apicomplexan parasites. Proc Natl Acad Sci USA. 2002;16:16. [PMC free article: PMC122944] [PubMed: 11959921]
- 132.
- Black MW, Arrizabalaga G, Boothroyd JC. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol Cell Biol. 2000;20(24):9399–9408. [PMC free article: PMC102196] [PubMed: 11094090]
- 133.
- Radke JR, Guerini MN, White MW. Toxoplasma gondii: Characterization of temperature-sensitive tachyzoite cell cycle mutants. Exp Parasitol. 2000;96(3):168–177. [PubMed: 11162367]
- 134.
- Singh U, Brewer JL, Boothroyd JC. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol Microbiol. 2002;44(3):721–733. [PubMed: 11994153]
- 135.
- Lindsay DS, Lenz SD, Blagburn BL. et al. Characterization of temperature-sensitive strains of Neospora caninum in mice. J Parasitol. 1999;85(1):64–67. [PubMed: 10207365]
- 136.
- White MW, Jerome ME, Vaishnava S. et al. Genetic rescue of a Toxoplasma gondii conditional cell cycle mutant. Mol Microbiol. 2005;55(4):1060–1071. [PubMed: 15686554]
- 137.
- Mercier C, Howe DK, Mordue D. et al. Targeted disruption of the GRA2 locus in Toxoplasma gondii decreases acute virulence in mice. Infect Immun. 1998;66(9):4176–4182. [PMC free article: PMC108503] [PubMed: 9712765]
- 138.
- Dzierszinski F, Mortuaire M, Cesbron-Delauw MF. et al. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol. 2000;37(3):574–582. [PubMed: 10931351]
- 139.
- Mercier C, Rauscher B, Lecordier L. et al. Lack of expression of the dense granule protein GRA5 does not affect the development of Toxoplasma tachyzoites. Mol Biochem Parasitol. 2001;116(2):247–251. [PubMed: 11522359]
- 140.
- Donald RG, Roos DS. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol Biochem Parasitol. 1998;91(2):295–305. [PubMed: 9566522]
- 141.
- Arrizabalaga G, Ruiz F, Moreno S. et al. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J Cell Biol. 2004;165(5):653–662. [PMC free article: PMC2172388] [PubMed: 15173192]
- 142.
- Wichroski MJ, Ward GE. Biosynthesis of glycosylphosphatidylinositol is essential to the survival of the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2003;2(5):1132–1136. [PMC free article: PMC219362] [PubMed: 14555496]
- 143.
- Donald RG, Allocco J, Singh SB. et al. Toxoplasma gondii cyclic GMP-dependent kinase: Chemotherapeutic targeting of an essential parasite protein kinase. Eukaryot Cell. 2002;1(3):317–328. [PMC free article: PMC118020] [PubMed: 12455981]
- 144.
- Cerede O, Dubremetz JF, Soete M. et al. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J Exp Med. 2005;201(3):453–463. [PMC free article: PMC2213027] [PubMed: 15684324]
- 145.
- Lekutis C, Ferguson DJ, Grigg ME. et al. Surface antigens of Toxoplasma gondii: Variations on a theme. Int J Parasitol. 2001;31(12):1285–1292. [PubMed: 11566296]
- 146.
- Duraisingh MT, Triglia T, Ralph SA. et al. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003;22(5):1047–1057. [PMC free article: PMC150330] [PubMed: 12606570]
- 147.
- Khater EI, Sinden RE, Dessens JT. A malaria membrane skeletal protein is essential for normal morphogenesis, motility, and infectivity of sporozoites. J Cell Biol. 2004;167(3):425–432. [PMC free article: PMC2172497] [PubMed: 15533999]
- 148.
- Sijwali PS, Rosenthal PJ. Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodium falciparum. Proc Natl Acad Sci USA. 2004;101(13):4384–4389. [PMC free article: PMC384756] [PubMed: 15070727]
- 149.
- Sijwali PS, Kato K, Seydel KB. et al. Plasmodium falciparum cysteine protease falcipain-1 is not essential in erythrocytic stage malaria parasites. Proc Natl Acad Sci USA. 2004;101(23):8721–8726. [PMC free article: PMC423262] [PubMed: 15166288]
- 150.
- Tewari R, Ogun SA, Gunaratne RS. et al. Disruption of Plasmodium berghei merozoite surface protein 7 gene modulates parasite growth in vivo. Blood. 2005;105(1):394–396. [PubMed: 15339842]
- 151.
- Cowman AF, Baldi DL, Healer J. et al. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Lett. 2000;476(1-2):84–88. [PubMed: 10878256]
- 152.
- Eksi S, Haile Y, Furuya T. et al. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143(1):90–99. [PubMed: 15996767]
- 153.
- Mueller AK, Camargo N, Kaiser K. et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102(8):3022–3027. [PMC free article: PMC548321] [PubMed: 15699336]
- 154.
- Yuda M, Ishino T. Liver invasion by malarial parasites—how do malarial parasites break through the host barrier? Cell Microbiol. 2004;6(12):1119–1125. [PubMed: 15527492]
- 155.
- Kappe SH, Buscaglia CA, Nussenzweig V. Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol. 2004;20:29–59. [PubMed: 15473834]
- 156.
- Mota MM, Rodriguez A. Invasion of mammalian host cells by Plasmodium sporozoites. Bioessays. 2002;24(2):149–156. [PubMed: 11835279]
- 157.
- Menard R. Gliding motility and cell invasion by Apicomplexa: Insights from the Plasmodium sporozoite. Cell Microbiol. 2001;3(2):63–73. [PubMed: 11207621]
- 158.
- Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2(8) [PMC free article: PMC1550269] [PubMed: 16933991]
- 159.
- Mazumdar JE, Wilson H, Masek K. et al. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103(35):13192–13197. [PMC free article: PMC1559775] [PubMed: 16920791]
- 160.
- Meissner M, Krejany E, Gilson PR. et al. Tetracycline analogue-regulated transgene expression in Plasmodium falciparum blood stages using Toxoplasma gondii transactivators. Proc Natl Acad Sci USA. 2005;102(8):2980–2985. [PMC free article: PMC548799] [PubMed: 15710888]
- 161.
- Carvalho TG, Thiberge S, Sakamoto H. et al. Conditional mutagenesis using site-specific recombination in Plasmodium berghei. Proc Natl Acad Sci USA. 2004;101(41):14931–14936. [PMC free article: PMC522007] [PubMed: 15465918]
- 162.
- Al-Anouti F, Ananvoranich S. Comparative analysis of antisense RNA, double-stranded RNA, and delta ribozyme-mediated gene regulation in Toxoplasma gondii. Antisense Nucleic Acid Drug Dev. 2002;12(4):275–281. [PubMed: 12238816]
- 163.
- Nakaar V, Ngo EO, Joiner KA. Selection based on the expression of antisense hypoxanthine-xanthineguanine- phosphoribosyltransferase RNA in Toxoplasma gondii. Mol Biochem Parasitol. 2000;110(1):43–51. [PubMed: 10989144]
- 164.
- Nakaar V, Ngo HM, Aaronson EP. et al. Pleiotropic effect due to targeted depletion of secretory rhoptry protein ROP2 in Toxoplasma gondii. J Cell Sci. 2003;116(Pt 11):2311–2320. [PubMed: 12711703]
- 165.
- Nakaar V, Samuel BU, Ngo EO. et al. Targeted reduction of nucleoside triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J Biol Chem. 1999;274(8):5083–5087. [PubMed: 9988756]
- 166.
- Ngo HM, Yang M, Paprotka K. et al. AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J Biol Chem. 2003;278(7):5343–5352. [PubMed: 12446678]
- 167.
- Bermudes D, Peck KR, Afifi MA. et al. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J Biol Chem. 1994;269(46):29252–29260. [PubMed: 7961894]
- 168.
- Gardiner DL, Holt DC, Thomas EA. et al. Inhibition of Plasmodium falciparum clag9 gene function by antisense RNA. Mol Biochem Parasitol. 2000;110(1):33–41. [PubMed: 10989143]
- 169.
- Noonpakdee W, Pothikasikorn J, Nimitsantiwong W. et al. Inhibition of Plasmodium falciparum proliferation in vitro by antisense oligodeoxynucleotides against malarial topoisomerase II. Biochem Biophys Res Commun. 2003;302(4):659–664. [PubMed: 12646219]
- 170.
- Ullu E, Tschudi C, Chakraborty T. RNA interference in protozoan parasites. Cell Microbiol. 2004;6(6):509–519. [PubMed: 15104593]
- 171.
- Aravind L, Iyer LM, Wellems TE. et al. Plasmodium biology: Genomic gleanings. Cell. 2003;115(7):771–785. [PubMed: 14697197]
- 172.
- McRobert L, McConkey GA. RNA interference (RNAi) inhibits growth of Plasmodium falciparum. Mol Biochem Parasitol. 2002;119(2):273–278. [PubMed: 11814579]
- 173.
- Malhotra P, Dasaradhi PV, Kumar A. et al. Double-stranded RNA-mediated gene silencing of cysteine proteases (falcipain-1 and -2) of Plasmodium falciparum. Mol Microbiol. 2002;45(5):1245–1254. [PubMed: 12207693]
- 174.
- Mohmmed A, Dasaradhi PV, Bhatnagar RK. et al. In vivo gene silencing in Plasmodium berghei— a mouse malaria model. Biochem Biophys Res Commun. 2003;309(3):506–511. [PubMed: 12963018]
- 175.
- Al-Anouti F, Tomavo S, Parmley S. et al. The expression of lactate dehydrogenase is important for the cell cycle of Toxoplasma gondii. J Biol Chem. 2004 [PubMed: 15459194]
- 176.
- Adams B, Musiyenko A, Kumar R. et al. A novel class of dual-family immunophilins. J Biol Chem. 2005;280(26):24308–24314. [PMC free article: PMC2270415] [PubMed: 15845546]
- 177.
- Sheng J, Al-Anouti F, Ananvoranich S. Engineered delta ribozymes can simultaneously knock down the expression of the genes encoding uracil phosphoribosyltransferase and hypoxanthine-xanthine-guanine phosphoribosyltransferase in Toxoplasma gondii. Int J Parasitol. 2004;34(3):253–263. [PubMed: 15003487]
- 178.
- Beckers CJ, Wakefield T, Joiner KA. The expression of Toxoplasma proteins in Neospora caninum and the identification of a gene encoding a novel rhoptry protein. Mol Biochem Parasitol. 1997;89(2):209–223. [PubMed: 9364966]
- 179.
- Kim K, Bulow R, Kampmeier J. et al. Conformationally appropriate expression of the Toxoplasma antigen SAG1 (p30) in CHO cells. Infect Immun. 1994;62(1):203–209. [PMC free article: PMC186087] [PubMed: 8262628]
- 180.
- Gardner MJ, Hall N, Fung E. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. [PMC free article: PMC3836256] [PubMed: 12368864]
- 181.
- Withers-Martinez C, Carpenter EP, Hackett F. et al. PCR-based gene synthesis as an efficient approach for expression of the A+T-rich malaria genome. Protein Eng. 1999;12(12):1113–1120. [PubMed: 10611405]
- 182.
- Flick K, Ahuja S, Chene A. et al. Optimized expression of Plasmodium falciparum erythrocyte membrane protein 1 domains in Escherichia coli. Malar J. 2004;3(1):50. [PMC free article: PMC544839] [PubMed: 15601471]
- 183.
- Yadava A, Ockenhouse CF. Effect of codon optimization on expression levels of a functionally folded malaria vaccine candidate in prokaryotic and eukaryotic expression systems. Infect Immun. 2003;71(9):4961–4969. [PMC free article: PMC187353] [PubMed: 12933838]
- 184.
- Mehlin C, Boni E, Buckner FS. et al. Heterologous expression of proteins from Plasmodium falciparum: Results from 1000 genes. Mol Biochem Parasitol. 2006;148(2):144–160. [PubMed: 16644028]
- 185.
- Brossier F, David Sibley L. Toxoplasma gondii: Microneme protein MIC2. Int J Biochem Cell Biol. 2005;37(11):2266–2272. [PubMed: 16084754]
- 186.
- Jewett TJ, Sibley LD. The Toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J Biol Chem. 2004;279(10):9362–9369. [PubMed: 14670959]
- 187.
- Kappe S, Bruderer T, Gantt S. et al. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol. 1999;147(5):937–944. [PMC free article: PMC2169348] [PubMed: 10579715]
- 188.
- O'Connor RM, Kim K, Khan F. et al. Expression of Cpgp40/15 in Toxoplasma gondii: A surrogate system for the study of Cryptosporidium glycoprotein antigens. Infect Immun. 2003;71(10):6027–6034. [PMC free article: PMC201096] [PubMed: 14500524]
- 189.
- Triglia T, Healer J, Caruana SR. et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol Microbiol. 2000;38(4):706–718. [PubMed: 11115107]
- 190.
- Tewari R, Rathore D, Crisanti A. Motility and infectivity of Plasmodium berghei sporozoites expressing avian Plasmodium gallinaceum circumsporozoite protein. Cell Microbiol. 2005;7(5):699–707. [PubMed: 15839899]
- 191.
- Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180(2):497–506. [PMC free article: PMC2191600] [PubMed: 8046329]
- 192.
- Chattopadhyay R, Taneja T, Chakrabarti K. et al. Molecular analysis of the cytoadherence phenotype of a Plasmodium falciparum field isolate that binds intercellular adhesion molecule-1. Mol Biochem Parasitol. 2004;133(2):255–265. [PubMed: 14698437]
- 193.
- Sim BK, Chitnis CE, Wasniowska K. et al. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264(5167):1941–1944. [PubMed: 8009226]
- 194.
- Ward GE, Chitnis CE, Miller LH. The invasion of erythrocytes by malarial merozoites. In: Russell DG, ed. Clinical Infectious Diseases: International Practice and Research, Strategies for Intracellular Survival of Microbes. 1st ed. London: Bailliere Tindall. 1994:155–190.
- 195.
- Saouros S, Edwards-Jones B, Reiss M. et al. A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J Biol Chem. 2005;280(46):38583–38591. [PubMed: 16166092]
- 196.
- Kato K, Mayer DC, Singh S. et al. Domain III of Plasmodium falciparum apical membrane antigen 1 binds to the erythrocyte membrane protein Kx. Proc Natl Acad Sci USA. 2005;102(15):5552–5557. [PMC free article: PMC556269] [PubMed: 15805191]
- 197.
- Grimwood J, Smith JE. Toxoplasma gondii: The role of a 30-kDa surface protein in host cell invasion. Exp Parasitol. 1992;74(1):106–111. [PubMed: 1730266]
- 198.
- Grimwood J, Smith JE. Toxoplasma gondii: The role of parasite surface and secreted proteins in host cell invasion. Int J Parasitol. 1996;26(2):169–173. [PubMed: 8690540]
- 199.
- Mineo JR, McLeod R, Mack D. et al. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol. 1993;150(9):3951–3964. [PubMed: 7682587]
- 200.
- Uchida Y, Ike K, Kurotaki T. et al. Monoclonal antibodies preventing invasion of Neospora caninum tachyzoites into host cells. J Vet Med Sci. 2004;66(11):1355–1358. [PubMed: 15585948]
- 201.
- Augustine PC, Jenkins MC, Dubey JP. Effect of polyclonal antisera developed against dense granule-associated Neospora caninum proteins on cell invasion and development in vitro by N. caninum tachyzoites. Parasitology. 1999;119(Pt 5):441–445. [PubMed: 10599076]
- 202.
- Augustine PC. Reduced invasion of cultured cells pretreated with a monoclonal antibody elicited against refractile body antigens of avian coccidial sporozoites. J Eukaryot Microbiol. 1999;46(3):254–258. [PubMed: 10377986]
- 203.
- Dutta S, Haynes JD, Moch JK. et al. Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. Proc Natl Acad Sci USA. 2003;100(21):12295–12300. [PMC free article: PMC218752] [PubMed: 14526103]
- 204.
- Singh AP, Puri SK, Chitnis CE. Antibodies raised against receptor-binding domain of Plasmodium knowlesi Duffy binding protein inhibit erythrocyte invasion. Mol Biochem Parasitol. 2002;121(1):21–31. [PubMed: 11985860]
- 205.
- Mitchell GH, Thomas AW, Margos G. et al. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun. 2004;72(1):154–158. [PMC free article: PMC343990] [PubMed: 14688092]
- 206.
- Ramasamy R, Ramasamy M, Yasawardena S. Antibodies and Plasmodium falciparum merozoites. Trends Parasitol. 2001;17(4):194–197. [PubMed: 11282510]
- 207.
- Dutta S, Haynes JD, Barbosa A. et al. Mode of action of invasion-inhibitory antibodies directed against apical membrane antigen 1 of Plasmodium falciparum. Infect Immun. 2005;73(4):2116–2122. [PMC free article: PMC1087451] [PubMed: 15784553]
- 208.
- Blackman MJ, Scott-Finnigan TJ, Shai S. et al. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180(1):389–393. [PMC free article: PMC2191569] [PubMed: 7516416]
- 209.
- Ryning FW, Remington JS. Effect of cytochalasin D on Toxoplasma gondii cell entry. Infect Immun. 1978;20(3):739–743. [PMC free article: PMC421921] [PubMed: 669821]
- 210.
- Schwartzman JD, Pfefferkorn ER. Immunofluorescent localization of myosin at the anterior pole of the coccidian, Toxoplasma gondii. J Protozool. 1983;30(4):657–661. [PubMed: 6363679]
- 211.
- Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31(2):421–428. [PubMed: 10027960]
- 212.
- Ward GE, Carey KL, Westwood NJ. Using small molecules to study big questions in cellular microbiology. Cell Microbiol. 2002;4(8):471–482. [PubMed: 12174082]
- 213.
- Morgan RE, Evans KM, Patterson S. et al. Targeting invasion and egress: From tools to drugs? Current Drug Targets 2006 . (In Press) [PubMed: 17266531]
- 214.
- Mayer TU. Chemical genetics: Tailoring tools for cell biology. Trends Cell Biol. 2003;13(5):270–277. [PubMed: 12742171]
- 215.
- Stockwell BR. Chemical genetics: Ligand-based discovery of gene function. Nat Rev Genet. 2000;1(2):116–125. [PMC free article: PMC3171795] [PubMed: 11253651]
- 216.
- Asai T, Takeuchi T, Diffenderfer J. et al. Identification of small-molecule inhibitors of nucleoside triphosphate hydrolase in Toxoplasma gondii. Antimicrob Agents Chemother. 2002;46(8):2393–2399. [PMC free article: PMC127337] [PubMed: 12121910]
- 217.
- Baldwin J, Michnoff CH, Malmquist NA. et al. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem. 2005;280(23):21847–21853. [PubMed: 15795226]
- 218.
- Carruthers VB, Hakansson S, Giddings OK. et al. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect Immun. 2000;68(7):4005–4011. [PMC free article: PMC101681] [PubMed: 10858215]
- 219.
- Ortega-Barria E, Boothroyd JC. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J Biol Chem. 1999;274(3):1267–1276. [PubMed: 9880495]
- 220.
- Grimwood J, Mineo JR, Kasper LH. Attachment of Toxoplasma gondii to host cells is host cell cycle dependent. Infect Immun. 1996;64(10):4099–4104. [PMC free article: PMC174343] [PubMed: 8926075]
- 221.
- Dvorak JA, Crane MS. Vertebrate cell cycle modulates infection by protozoan parasites. Science. 1981;214(4524):1034–1036. [PubMed: 7029713]
- 222.
- Dutta C, Grimwood J, Kasper LH. Attachment of Toxoplasma gondii to a specific membrane fraction of CHO cells. Infect Immun. 2000;68(12):7198–7201. [PMC free article: PMC97841] [PubMed: 11083856]
- 223.
- Miller LH, Mason SJ, Dvorak JA. et al. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189(4202):561–563. [PubMed: 1145213]
- 224.
- Su XZ, Heatwole VM, Wertheimer SP. et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82(1):89–100. [PubMed: 7606788]
- 225.
- Miller LH, Haynes JD, McAuliffe FM. et al. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977;146(1):277–281. [PMC free article: PMC2180731] [PubMed: 327014]
- 226.
- Kaneko O, Fidock DA, Schwartz OM. et al. Disruption of the C-terminal region of EBA-175 in the Dd2/Nm clone of Plasmodium falciparum does not affect erythrocyte invasion. Mol Biochem Parasitol. 2000;110(1):135–146. [PubMed: 10989151]
- 227.
- Klotz FW, Orlandi PA, Reuter G. et al. Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol Biochem Parasitol. 1992;51(1):49–54. [PMC free article: PMC7173321] [PubMed: 1565137]
- 228.
- Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol. 2004;34(13-14):1413–1429. [PubMed: 15582519]
- 229.
- Wu Y, Wang X, Liu X. et al. Data-mining approaches reveal hidden families of proteases in the genome of malaria parasite. Genome Res. 2003;13(4):601–616. [PMC free article: PMC430172] [PubMed: 12671001]
- 230.
- Urban S, Freeman M. Substrate specificity of rhomboid intramembrane proteases is governed by helix-breaking residues in the substrate transmembrane domain. Mol Cell. 2003;11(6):1425–1434. [PubMed: 12820957]
- 231.
- Opitz C, Di Cristina M, Reiss M. et al. Intramembrane cleavage of microneme proteins at the surface of the apicomplexan parasite Toxoplasma gondii. EMBO J. 2002;21(7):1577–1585. [PMC free article: PMC125952] [PubMed: 11927542]
- 232.
- Urban S, Lee JR, Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107(2):173–182. [PubMed: 11672525]
- 233.
- Brossier F, Jewett TJ, Sibley LD. et al. A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc Natl Acad Sci USA. 2005;102(11):4146–4151. [PMC free article: PMC554800] [PubMed: 15753289]
- 234.
- Dowse TJ, Pascall JC, Brown KD. et al. Apicomplexan rhomboids have a potential role in microneme protein cleavage during host cell invasion. Int J Parasitol. 2005;35(7):747–756. [PubMed: 15913633]
- 235.
- Dowse TJ, Soldati D. Rhomboid-like proteins in Apicomplexa: Phylogeny and nomenclature. Trends Parasitol. 2005;21(6):254–258. [PubMed: 15922242]
- 236.
- Cleary MD, Singh U, Blader IJ. et al. Toxoplasma gondii asexual development: Identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryotic Cell. 2002;1(3):329–340. [PMC free article: PMC118016] [PubMed: 12455982]
- 237.
- Cleary MD, Meiering CD, Jan E. et al. Biosynthetic labeling of RNA with uracil phosphoribosyltransferase allows cell-specific microarray analysis of mRNA synthesis and decay. Nat Biotechnol. 2005;23(2):232–237. [PubMed: 15685165]
- 238.
- Kappe SH, Gardner MJ, Brown SM. et al. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci USA. 2001;98(17):9895–9900. [PMC free article: PMC55549] [PubMed: 11493695]
- 239.
- Le Roch KG, Zhou Y, Blair PL. et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–1508. [PubMed: 12893887]
- 240.
- Bozdech Z, Llinas M, Pulliam BL. et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1(1):E5. [PMC free article: PMC176545] [PubMed: 12929205]
- 241.
- Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem. 2001;276(26):24223–24231. [PubMed: 11294868]
- 242.
- Bishop R, Shah T, Pelle R. et al. Analysis of the transcriptome of the protozoan Theileria parva using MPSS reveals that the majority of genes are transcriptionally active in the schizont stage. Nucleic Acids Res. 2005;33(17):5503–5511. [PMC free article: PMC1236717] [PubMed: 16186131]
- 243.
- Bradley PJ, Ward C, Cheng SJ. et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280(40):34245–34258. [PubMed: 16002398]
- 244.
- Zhou XW, Kafsack BF, Cole RN. et al. The opportunistic pathogen Toxoplasma gondii deploys a diverse legion of invasion and survival proteins. J Biol Chem. 2005;280(40):34233–34244. [PMC free article: PMC1360232] [PubMed: 16002397]
- 245.
- Bromley E, Leeds N, Clark J. et al. Defining the protein repertoire of microneme secretory organelles in the apicomplexan parasite Eimeria tenella. Proteomics. 2003;3(8):1553–1561. [PubMed: 12923781]
- 246.
- Gilson PR, Nebl T, Vukcevic D. et al. Identification and stoichiometry of glycosylphosphatidylinositolanchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2006;5(7):1286–1299. [PubMed: 16603573]
- 247.
- Thomas AW, Deans JA, Mitchell GH. et al. The Fab fragments of monoclonal IgG to a merozoite surface antigen inhibit Plasmodium knowlesi invasion of erythrocytes. Mol Biochem Parasitol. 1984;13(2):187–199. [PubMed: 6513992]
- 248.
- Deans JA. Protective antigens of bloodstage Plasmodium knowlesi parasites. Philos Trans R Soc Lond B Biol Sci. 1984;307(1131):159–169. [PubMed: 6151680]
- 249.
- Li F, Dluzewski A, Coley AM. et al. Phage-displayed peptides bind to the malarial protein apical membrane antigen-1 and inhibit the merozoite invasion of host erythrocytes. J Biol Chem. 2002;277(52):50303–50310. [PubMed: 12381731]
- 250.
- Keizer DW, Miles LA, Li F. et al. Structures of phage-display peptides that bind to the malarial surface protein, apical membrane antigen 1, and block erythrocyte invasion. Biochemistry. 2003;42(33):9915–9923. [PubMed: 12924940]
- 251.
- Donahue CG, Carruthers VB, Gilk SD. et al. The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol Biochem Parasitol. 2000;111(1):15–30. [PubMed: 11087913]
- 252.
- Gurnett AM, Liberator PA, Dulski PM. et al. Purification and molecular characterization of cGMP-dependent protein kinase from Apicomplexan parasites: a novel chemotherapeutic target. J Biol Chem. 2002;277(18):15913–15922. [PubMed: 11834729]
- 253.
- Trager W, Zung J, Tershakovec M. Initial extracellular development in vitro of erythrocytic stages of malaria parasites (Plasmodium falciparum). Proc Natl Acad Sci USA. 1990;87(15):5618–5622. [PMC free article: PMC54378] [PubMed: 2198568]
- 254.
- Tomley FM, Soldati DS. Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 2001;17(2):81–88. [PubMed: 11228014]
- 255.
- Kim K, Weiss LM. Toxoplasma gondii: the model apicomplexan. Int J Parasitol. 2004;34(3):423–432. [PMC free article: PMC3086386] [PubMed: 15003501]
- 256.
- Di Cristina M, Ghouze F, Kocken CH. et al. Transformed Toxoplasma gondii tachyzoites expressing the circumsporozoite protein of Plasmodium knowlesi elicit a specific immune response in rhesus monkeys. Infect Immun. 1999;67(4):1677–1682. [PMC free article: PMC96513] [PubMed: 10085003]
- 257.
- Charest H, Sedegah M, Yap GS. et al. Recombinant attenuated Toxoplasma gondii expressing the Plasmodium yoelii circumsporozoite protein provides highly effective priming for CD8+ T cell-dependent protective immunity against malaria. J Immunol. 2000;165(4):2084–2092. [PubMed: 10925293]
- 258.
- Kenny B, DeVinney R, Stein M. et al. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91(4):511–520. [PubMed: 9390560]
- 259.
- Hu K, Johnson J, Florens L. et al. Cytoskeletal components of an invasion machine-the apical complex of Toxoplasma gondii. PLoS Pathol. 2006;2(2):e13. [PMC free article: PMC1383488] [PubMed: 16518471]
Publication Details
Author Information and Affiliations
Authors
Jeffrey Mital and Gary E Ward*.Affiliations
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Mital J, Ward GE. Current and Emerging Approaches to Studying Invasion in Apicomplexan Parasites. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.