NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
During cardiomyocyte development, early embryonic ventricular cells show spontaneous activity that disappears at a later stage. Dramatic changes in action potential are mediated by developmental changes in individual ionic currents. Hence, reconstruction of the individual ionic currents into an integrated mathematical model would lead to a better understanding of cardiomyocyte development. To simulate the action potential of the rodent ventricular cell, anecdotally reported developmental changes in individual ionic systems were integrated into two different cardiac electrophysiological models: the Kyoto model and the Luo-Rudy model. Quantitative changes in the ionic currents, pumps, exchangers and sarcoplasmic reticulum Ca2+ kinetics were represented as relative activities, which were multiplied by conductance or conversion factors for individual ionic systems. The integrated models can simulate three representative stages in rodent development: early embryonic, late embryonic and neonatal stages. The simulated action potential of the early embryonic ventricular cell model exhibited spontaneous activity that ceased in the simulated action potential of the late embryonic and neonatal ventricular cell models. The simulations with our models reproduced action potentials consistent with the reported characteristics of the cells in vitro.
Background
Cardiac Electrophysiological Model for Simulation of Action Potential
The cardiac cell membrane contains various ionic channels, exchangers and pumps that allow specific ions to travel or be exchanged through the membrane (Fig. 1). Those ionic components in the cell membrane are utilized to maintain homeostasis of an intracellular and the extracellular environment; such gradient in ionic concentration causes an electrical voltage between the inside and outside of the cell, which is called a “membrane potential.”
The membrane potential and the gradient in ionic concentration both mediate a passive transport of ions through the cell membrane. In addition to the passive force driven by the membrane potential and the gradient in ionic concentration, each ionic channel in the cardiomyocyte opens or closes in response to the shift in the membrane potential, represented as a gating property of the ionic current. All of the current components on the membrane are formulated on the basis of a basic mathematical expression of an ionic current that include three parameters: conductance or a conversion factor, passive transport driven by membrane potential and gradient in ionic concentrations and gating properties.
The traveling of ions through the cell membrane causes either depolarization or hyperpolarization of the cell depending on the transport direction. In adult ventricular cells (Fig. 2), for example, opening of channels for INa (Na+ current) causes the membrane potential to rise to 40 mV; depolarization of the membrane then allows opening of channels for ICaL (L-type Ca2+ current), which maintains the potential around 10 mV even after closing of the Na+ channels; the membrane repolarizes to the resting potential level by opening various channels for outward potassium currents. The entire tracing of the transient change in membrane potential is called “action potential.”
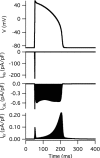
Figure 2
Simulated action potential of an adult ventricular cell with the Kyoto model and changes in INa, ICaL and IKr accompanying the simulated action potential.
Developmental Changes in Electrophysiological Properties of Ventricular Cells
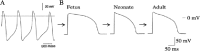
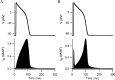
The action potential properties of the ventricular cell have been broadly studied in various species at various stages of development. The early embryonic ventricular cells generally have spontaneous action potential in mouse,1 rat2,3 and chick4; representative in vitro recording of spontaneous action potential in early embryonic rat ventricular cells is shown as an example in Figure 3A.
The late embryonic and postnatal ventricular cells require external stimulation to fire the action potential.5,6 Although several action potential parameters change among different stages, developmental changes in action potential duration (APD) are the most prominent; APD of guinea pig ventricular cells initially decreased in the neonatal stage and then increased until the adult period (Fig. B). Interestingly, the time courses of the changes are different among species; APD continues to decrease in postnatal development of mouse ventricular cells. The developmental changes and the species-specific differences in action potential are mediated by the ion channels of the cells.
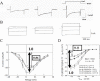
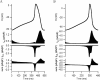
Large amounts of data have been recorded via standard microelectrode techniques to describe electrophysiological properties of the ionic channels; several selected examples are reproduced from literature and shown in Figure 4. The basic property of an ionic current can be obtained by shifting the membrane potential from a given holding potential to an arbitrary potential; tracings of ICaL and IK1 (inward rectifier K+ current) are recorded from ventricular cells at different developmental stages, wherein membrane is depolarized from a holding potential of 50 to 0 mV for recording of ICaL (Fig. 4A) and from a holding potential of –40 to –100, –90, –80 and –60 mV for recording of IK1 (Fig. 4B). Current-voltage (I-V) curves of the currents can be drawn up by plotting selected points in the tracing that is obtained by shifting the membrane potential to different potentials (Fig. 4C, D).
Modeling Developmental Changes in Cardiomyocyte
As shown above, developmental changes in cardiomyocyte have been anecdotally reported at various levels. This chapter summarizes the basic concept presented in a recently published paper7 which aimed to integrate those anecdotally reported data from experiments in vivo or in vitro on the basis of comprehensive cardiac electrophysiological models. I-V curves of ICaL and IK1 (Fig. 4C, D) indicate that the activity level of the ionic current changes among different stages while voltage dependencies of the current do not change. On the basis of these data, we have assumed that developmental changes in the ionic currents can be represented quantitatively as the activities of the channels in the developing rodent relative to those in the adult. Application of the integrated model for simulation of action potential showed that action potential at different developmental stages can be reproduced with common sets of mathematical models, wherein quantitative changes in the ionic currents, pumps, exchangers and sarcoplasmic reticulum (SR) Ca2+ kinetics are expressed as relative activities.
Methods
Implementation of Cardiac Electrophysiological Models to E-cell Simulation Environment
Models for simulating the action potential at different developmental stages were constructed on the basis of the Kyoto and Luo-Rudy models, electrophysiological models of the guinea pig cardiomyocyte. 8 The structures of the Kyoto and Luo-Rudy models are very similar and both models have been developed for simulation of guinea pig ventricular cells. The latest version of the Luo-Rudy model9 was implemented in the E-Cell simulation environment version 3.1. All of the models are constructed on the basis of ElectrophysiologicalBaseProcess.hpp, which had been developed to facilitate further analysis via replacing mathematical equations of ionic currents from one electrophysiological model to the other and the models are available online at http://www.e-cell.org.*
General Approach to Modeling of Different Developmental Stages
All ionic currents, pumps, exchangers and SR Ca2+ kinetics are expressed in mathematical equations; as mentioned above, all of the equations include either a conversion factor (pA/mM) or conductance (pA/mV) as one of the parameters. For instance, ICaL and IK1 in the Kyoto model are expressed as follow:
ICaL = PCaL ·(CFCa + 0.000365·CFK + 0.0000185·CFNa)·p(openCaL) (1)
IK1 = GK1 ·(Vm - EK)·( fo 4 + 8/3· fo 3 fB + 2· fo 2· fB 2)· y (2)
In Equation (1), CFCa, CFK and CFNa represent constant field equations (mM) for Ca2+, K+ and Na+, respectively; the open probability of three gates in the L-type Ca2+ channel is expressed as p(openCaL). Similarly in Equation (2), Vm represents the membrane potential (mV); EK represents the equilibrium potential of K+ (mV); fB and fO represent the fractions of blocked state and those of open state, respectively; y represents the gating variable for a two-state gate. In order to simulate both ventricular cells and sinoatrial (SA) node cells by common mathematical equations, either the conversion factor (PCaL in Eq. 1) or conductance (GK1 in Eq. 2) was adjusted based on electrophysiological experiments of each cell.8
Various in vitro experimental data, including I-V curves and Western blot analyses, were utilized to estimate the relative activities of ionic currents, pumps, exchangers and SR Ca2+ kinetics. Those in vitro experimental studies that used guinea pigs were preferentially adopted, because both models were constructed using the adult guinea pig.8,9 Although the guinea pig was the preferred experimental animal, data from the rat and mouse were also utilized, on the basis of the reported observation that the I-V relationships of the ionic channels are well conserved among different rodents.10,11 In addition, the target stages for simulation of action potentials were set to early embryonic, late embryonic and neonatal, because plenty of literature was available for these stages. The early embryonic stage approximately represents the mouse at 9.5 days postcoitum (dpc) and the rat at 11.5 dpc; the late embryonic and neonatal stages correspond to 1-5 days before and after birth, respectively.
Ionic Currents
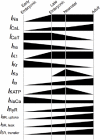
It was assumed that developmental changes in ionic currents are determined mainly by their quantitative changes (Fig. 5), which can be represented as the activities of the current in developing stages relative to that of those in the adult stage.
The relative activities of ionic currents were either computed from I-V curves (Table 1) or estimated on the basis of qualitative observations (Table 2). It was confirmed that the I-V relationship did not change among different developmental stages for ICaL,12 ICaT (T-type Ca2+ current),13 IK1,12,14 IKr (rapid component of delayed rectifier K+ current),15 IKs (slow component of delayed rectifier K+ current) 15 and INa.15
Table 1
Relative activities for ionic currents, as obtained from the literature.
Table 2
Estimated relative activities of ionic currents.
The relative activities were multiplied by the conversion factor or the conductance of the corresponding ionic current. In addition, all currents listed in Table 1 had to be normalized by the ratio of the cell capacitance (Cm) of individual myocytes at the corresponding developmental stages (Table 4) to that of adult ventricular cells (132 pF), because I-V relationships are usually reported as current density (pA/pF) and the Kyoto model presents current in pA. The ratios were 28/132 for the early embryonic ventricular cell model, 35/132 for the late embryonic ventricular cell model and 40/132 for the neonatal ventricular cell model.
Table 4
Cell capacitances and volumes of cell compartments.
The Luo-Rudy model includes all ionic currents listed in Figure 5 except Ito (transient outward current), IKATP (ATP-sensitive K+ current) and Iha (hyperpolarization-activated cation current), all relative activities except those of Ito, IKATP and Iha were thus implemented in the Luo-Rudy model by the same procedure used in the Kyoto model. Unlike the Kyoto model, all of the currents in the Luo-Rudy model are presented as current density (pA/pF), so it was not necessary to normalize the activity of the currents by the ratio of the Cm of individual myocytes at the corresponding developmental stages.
Background Ionic Currents
IbNSC (background nonselective cation current) is known to have a higher current density in SA node cells than in ventricular cells.16 Because we found that IbNSC plays an important role in the spontaneous action potential of both SA node cells and early embryonic ventricular myocytes, we scaled the current amplitudes at different stages according to the cell capacitances of the fetal and neonatal cells (Table 2).
IKACh (ACh-activated K+ current) is known to have negligible effects on the action potential of ventricular cells during the course of development15,17 and is not included in adult ventricular cell models.8 Hence, we excluded IKACh from the models. Other background currents, including IKpl (nonspecific, voltage-dependent outward current), II(Ca) (Ca2+-activated background cation current) and ICab (background Ca2+ current) were assumed to have steady current densities; as such, these currents were normalized to the corresponding cell capacitance by the method described above.
Exchangers, Pumps and SR Ca2+ Kinetics
The relative activities of exchangers, pumps and SR Ca2+ kinetics were computed from a Western blot of SR-related proteins,18,19 as listed in Table 3. Here, we assumed that the relative expression level of the proteins directly reflected the relative activities of Na+/Ca2+ exchange, SR Ca2+ pump, ryanodine receptor (RyR) channel and other SR Ca2+ kinetics. The average relative expression values of SR-related proteins in the early embryonic stage (0.04), late embryonic stage (0.30) and neonatal stage (0.30) were adopted for estimating those values for ISR, transfer and ISR, leak.
Table 3
Relative activities of exchangers, pumps and SR Ca2+ kinetics.
Cell Capacitance and Volume of Cell Compartments
Cm (cell capacitance) and volumes (Vi, Vrel, Vup) were computed on the basis of quantitative data obtained from the literature (Table 4). No significant differences were observed between the Cm of mouse ventricular cells (31 ± 3.3 pF) and that of guinea pig ventricular cells (34.5 ± 2.72 pF) in the late embryonic stage;1,12 as such, the Cm values for mouse early embryonic ventricular cells (28 pF), guinea pig late embryonic ventricular cells (35 pF) and guinea pig neonatal ventricular cells (40 pF) were adopted.
The developmental change in Vi (cell volume accessible for ion diffusion) in rabbit ventricular cells is roughly proportional to that of cell capacitance.20 In addition, a positive linear correlation has been found between membrane capacitance and cell volume in several species.21 Hence, cell volume was estimated by multiplying the adult Vi (8.0 × 10–3 μL) by the corresponding Cm (28, 35 or 40 pF) over the adult Cm (132 pF).
In the Kyoto model, the volume fractions of Vrel (volume of SR release site) and Vup (volume of SR uptake site) were set to 2% and 5% of Vi, respectively.8 The SR-mediated Ca2+ transient is modeled by multiplying an estimated value called the “SA factor” by Vrel, Vup and SR-related currents in the Kyoto model.8 The same approach has been adopted for estimating Vrel and Vup in different developmental stages of ventricular cells; on the basis of quantitative changes in SR-related proteins, 18 the average relative expression values of those proteins in the early embryonic stage (0.04), late embryonic stage (0.30) and neonatal stage (0.30) were utilized for the estimation.
Simulation of Action Potential at Three Different Developmental Stages
On the basis of the assumption that developmental changes in ionic currents are determined mainly by their quantitative changes (Fig. 5), the developmental changes in ionic components were represented as the activities of the components in the developing rodent relative to those in the adult; the relative activities were multiplied by either the conversion factor or conductance in corresponding mathematical equations. All of the models were simulated for 200 s to confirm that the spontaneous action potentials were stable or that the membrane potential had reached a quasi-steady state. Hence, the simulation results presented in this chapter were recorded after simulating the corresponding models for 200 s. In addition, an external current (Iext) was applied in the late embryonic and neonatal ventricular cell models in order to fire the action potential of the cells. Because the Luo-Rudy model requires “pacing” of the action potential, the model was simulated for 600 s as instructed in the report.9
Results
Simulated Action Potential at Three Representative Developmental Stages
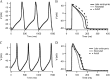
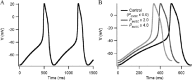
The implementation of relative activities of ionic components at early embryonic stage to both the Kyoto and Luo-Rudy models exhibited a spontaneous action potential (Fig. 6A,C). In the simulated action potential with the Kyoto model, the membrane slowly depolarized from the maximum diastolic potential (MDP) at –62.86 mV until it reached approximately –40 mV when spontaneous action potential was triggered. The membrane then started to repolarize after overshoot potential at 3.13 mV and completed the repolarization in less than 100 ms. The whole action potential was completed in a basic cycle length (BCL) of 492 ms. On the other hand, the membrane overshot to 13.74 mV from MDP at –71.16 mV in the simulation with the Luo-Rudy model; the whole action potential was completed in a BCL of 414 ms, which resulted from faster depolarization and repolarization of the membrane.
The spontaneous action potential ceased in the later stages of development in simulation of the corresponding stages with both the Kyoto model (Fig. 6B) and the Luo-Rudy model (Fig. 6D). In simulation with the Kyoto model, both late embryonic and neonatal ventricular cells showed resting membrane potentials that were more negative (–83.60 mV) than the MDP of the early embryonic ventricular cell. Repolarization of the membrane occurred more slowly in the late embryonic ventricular cell than in the neonatal ventricular cell; the APD was 140 ms in the late embryonic ventricular cell and 117 ms in the neonatal ventricular cell. The qualitative characteristics of the cells at those stages were well reproduced in the simulation with the Luo-Rudy model as well; the late embryonic and neonatal ventricular cells showed more negative resting membrane potentials than the MDP of the early embryonic ventricular cell and shorter APD in neonatal ventricular cells than in late embryonic ventricular cells.
Evaluation of Individual Ionic Currents in Two Different Models
Comparison of the action potential tracings of early embryonic ventricular cell simulated with the Kyoto (Fig. 6A) and the Luo-Rudy model (Fig. 6C) clearly indicate that the Kyoto model reproduced the action potential recording in vitro (Fig. 3A) more consistently than did the Luo-Rudy model; the most prominent differences are the faster repolarization phase (RP) and diastolic slow depolarization (DSD) phase, both of which cause overall shortening of BCL. The differences in simulated action potential were determined by differences in mathematical equations of ionic currents, particularly IKr that plays a predominant role in repolarization of the membrane during action potential. The dynamic behaviors of IKr underlying the action potential were compared between the Kyoto and the Luo-Rudy models along with ICaL and sums of ICaL and IKr (Fig. 7). Apparently, activation of IKr in RP was faster in the LuoRudy model (Fig. 7B) than in the Kyoto model (Fig. 7A); the difference in ICaL in the inactivation phase is canceled out by the fast activation of IKr, illustrated as sum of ICaL and IKr. Hence, we made a working hypothesis that the difference in the mathematical equations of IKr in the two models undertakes more consistent simulated action potentials in the Kyoto model.
In order to assess the working hypothesis, mathematical equations of IKr in the Luo-Rudy model was replaced with those of IKr in the Kyoto model. Figure 8 illustrates simulated results of adult ventricular cell with original Luo-Rudy model (A) and the Luo-Rudy model whose IKr is replaced with those of the Kyoto model (B); the conductance amplitude of IKr (GKr) was adjusted to achieve approximately the same APD. The replaced model was then utilized for simulation of spontaneous action potential in early embryonic ventricular cell (Fig. 9A). Apparently, the replacement of the mathematical equations made all quantitative characteristics of the action potential less consistent with those of the cells in vitro; such characteristics are more negative MDP (–75.94 mV), more positive overshoot (21.03 mV) and longer BCL (697 ms).
Another difference between the Kyoto and Luo-Rudy models is that the Kyoto model successfully incorporated five known types of background current components. One of the defined background current is called IbNSC (background nonselective cation current), which shows ion selectivity of both K+ and Na+ and is determined by constant field equations of each ions. Because the balance of IKr and IbNSC determines the MDP of the simulated spontaneous action potential of SA node cell with the Kyoto model24 and the Luo-Rudy model lacks such nonselective current, model equations of IbNSC were augmented in addition to replacing IK equations (Fig. 9B). Varying the amplitude of IbNSC (PbNSC) arbitrarily showed that IbNSC indeed plays an important role in both determining MDP and rate of DSD phase and all three quantitative characteristics, MDP (–71.15 mV), overshoot (18.20 mV) and BCL (510 ms), were thus improved compared to the IKr-replaced model without augmentation of IbNSC.
Discussion
Spontaneous Action Potential in Vitro Was Well Simulated with the Early Embryonic Models
The early embryonic ventricular cell model, which was constructed on the basis of the Kyoto model (Fig. 6A), reproduced well the spontaneous action potential that is generated by ventricular cells in 12-dpc rats.2 Species-specific differences in spontaneous action potential waveforms have been observed between ventricular cells in 9.5-dpc mice1 and those in 12-dpc rats.2 The ventricular cells in 9.5-dpc mice generate a more hyperpolarized MDP (–71.2 ± 0.4 mV) than those in 12-dpc rats (–66.7 ± 3.6 mV). A spontaneous action potential is triggered when the membrane potential reaches approximately –60 mV in 9.5-dpc mice and approximately –40 mV in 12-dpc rats.1,2 The simulated action potential in our early embryonic ventricular cell model constructed on the basis of the Kyoto model (Fig. 6A) was very similar to the action potential waveforms generated by the automatically beating cells in 12-dpc rats (Fig. 3A).2 In addition, the MDP of the simulated action potential (–62.86 mV) was approximately consistent with that of the ventricular cells in 12-dpc rats. Hence, our early embryonic ventricular cell model could reproduce an action potential that was in reasonable agreement with those of previous studies.
The speed of the spontaneous action potential in the early embryonic stage has been a controversial issue. Unfortunately, the action potential of early embryonic guinea pig ventricular cells has not been reported. Early embryonic hearts have shown a large range of heart rates, from 61 to 219 min–1 in 11.5-dpc rats.22 The BCL of the simulated action potential of our early embryonic ventricular cell model (492 ms) was roughly consistent with that of ventricular cells in 9.5-dpc mice, which is 510.8 ± 32.8 ms.1 Although the BCL of the action potential of our model was approximately consistent with those of previous studies, it should be noted that the BCL of the simulated action potential might not be quantitatively accurate, because early embryonic hearts have a large range of rates in vivo.
Developmental Changes in APD Were Reproduced Q ualitatively with the Model
APD changes over the course of rodent development. Although the duration and shape of the action potential in the adult rat are totally different from those in the adult guinea pig because of differences in the Ito, shortening of the APD between the late embryonic stage and the neonatal stage has been observed in both guinea pigs2 and rats.3 The rapid component (IKr) and slow component (IKs) of the delayed rectifier K+ current play important roles in repolarization and thus control the length of the APD; both IKr and IKs undergo very complex changes in their activities between the late embryonic and neonatal stages (Fig. 5). As described in the Methods sections, I estimated the relative activities of both IKr and IKs on the basis of the qualitative characteristics of these currents, including the changes in APD in response to a selective IKr blocker. Although qualitatively consistent, APD may not be quantitatively accurate because several relative activities were estimated on the basis of qualitative characteristics.
Evaluating the Role of Individual Ionic Currents for Better Simulation
All of the simulated results with the Luo-Rudy model indicate that the Kyoto model reproduced the action potential in the early embryonic stage more consistently than the LuoRudy model. The role of individual ionic currents in the better simulation with the Kyoto model was evaluated by replacing mathematical models of specific ionic currents between the Kyoto model and the Luo-Rudy model.
Dynamic behavior was improved by replacement of the mathematical models for IKr. This result indicated that the fast repolarization in the LuoRudy model is determined by fast activation of IKr. The formulations of IKr in the Luo-Rudy and Kyoto models are as follow:
IKr = GKr · (Vm - EK) · Xr · R (3)
IKr = GKr · Cm · (Vm - EK) · (0.6 · y1 + 0.4 · y2) · y3 (4)
Whereas IKr in the LuoRudy model (Eq. 3) is described by a time-dependent activation gate (Xr) and a time-independent inactivation gate (R),9 IKr in the Kyoto model (Eq. 4) is described by two activation gates (y1, y2) and by one inactivation gate (y3)8. In the Kyoto model, the equations were intentionally developed for two cell types, ventricular cells as well as SA node cells,23 because no obvious difference has been observed in the electrophysiological properties of the currents in terms of their kinetics. In addition, the original paper24 specifically mentioned that the balance of IKr and IbNSC determines the MDP of the simulated spontaneous action potential of SA node cell with the Kyoto model; as such, the difference in background currents between the Kyoto and the Luo-Rudy models is one of the important differences contributing to the increased speed of the DSD phase. The Kyoto model may thus have been suitable for this study, because most currents in ventricular cells change quantitatively with similar kinetics throughout the stages of development.
Conclusion
In the present chapter, developmental changes of the ion channels were represented quantitatively as the activities of the channels in the developing rodent relative to those in the adult. Multiplication of the relative activities by the corresponding mathematical equations reproduced the developmental changes in the action potential of the rodent ventricular cell. Although both the Kyoto and LuoRudy models represented various characteristics, the Kyoto model reproduced action potentials in the early embryonic stage more consistently than did the LuoRudy model, because of differences in mathematical model of IKr and background currents.
References
- 1.
- Yasui K, Liu W, Opthof T. et al. I(f ) current and spontaneous activity in mouse embryonic ventricular myocytes. Circ Res. 2001;88(5):536–542. [PubMed: 11249878]
- 2.
- Nagashima M, Tohse N, Kimura K. et al. Alternation of inwardly rectifying background K+ channel during development of rat fetal cardiomyocytes. J Mol Cell Cardiol. 2001;33(3):533–543. [PubMed: 11181021]
- 3.
- Kojima M, Sada H, Sperelakis N. Developmental changes in beta-adrenergic and cholinergic interactions on calcium-dependent slow action potentials in rat ventricular muscles. Br J Pharmacol. 1990;99(2):327–333. [PMC free article: PMC1917385] [PubMed: 2158374]
- 4.
- Satoh H, Sada H, Tohse N. et al. Developmental aspects of electrophysiology in cardiac muscle. Nippon Yakurigaku Zasshi. 1996;107(5):213–223. [PubMed: 8690303]
- 5.
- Wang L, Feng ZP, Kondo CS. et al. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res. 1996;79(1):79–85. [PubMed: 8925572]
- 6.
- Agata N, Tanaka H, Shigenobu K. Developmental changes in action potential properties of the guinea-pig myocardium. Acta Physiol Scand. 1993;149(3):331–337. [PubMed: 8310838]
- 7.
- Itoh H, Naito Y, Tomita M. Simulation of developmental changes in action potentials with ventricular cell models. Systems and Synthetic Biology. 2007;1(1):11–23. [PMC free article: PMC2533146] [PubMed: 19003434]
- 8.
- Matsuoka S, Sarai N, Kuratomi S. et al. Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol. 2003;53(2):105–123. [PubMed: 12877767]
- 9.
- Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78(5):2392–2404. [PMC free article: PMC1300828] [PubMed: 10777735]
- 10.
- Linz KW, Meyer R. Profile and kinetics of L-type calcium current during the cardiac ventricular action potential compared in guinea-pigs, rats and rabbits. Pflugers Arch. 2000;439(5):588–599. [PubMed: 10764219]
- 11.
- Zhang ZJ, Jurkiewicz NK, Folander K. et al. K+ currents expressed from the guinea pig cardiac IsK protein are enhanced by activators of protein kinase C. Proc Natl Acad Sci USA. 1994;91(5):1766–1770. [PMC free article: PMC43244] [PubMed: 7510407]
- 12.
- Kato Y, Masumiya H, Agata N. et al. Developmental changes in action potential and membrane currents in fetal, neonatal and adult guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1996;28(7):1515–1522. [PubMed: 8841938]
- 13.
- Ferron L, Capuano V, Deroubaix E. et al. Functional and molecular characterization of a T-type Ca(2+) channel during fetal and postnatal rat heart development. J Mol Cell Cardiol. 2002;34(5):533–546. [PubMed: 12056857]
- 14.
- Masuda H, Sperelakis N. Inwardly rectifying potassium current in rat fetal and neonatal ventricular cardiomyocytes. Am J Physiol. 1993;265(4 Pt 2):H1107–1111. [PubMed: 8238396]
- 15.
- Davies MP, An RH, Doevendans P. et al. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res. 1996;78(1):15–25. [PubMed: 8603498]
- 16.
- Kiyosue T, Spindler AJ, Noble SJ. et al. Background inward current in ventricular and atrial cells of the guinea-pig. Proc Biol Sci. 1993;252(1333):65–74. [PubMed: 7684843]
- 17.
- Xie LH, Takano M, Noma A. Development of inwardly rectifying K+ channel family in rat ventricular myocytes. Am J Physiol. 1997;272(4 Pt 2):H1741–1750. [PubMed: 9139958]
- 18.
- Liu W, Yasui K, Opthof T. et al. Developmental changes of Ca(2+) handling in mouse ventricular cells from early embryo to adulthood. Life Sci. 2002;71(11):1279–1292. [PubMed: 12106593]
- 19.
- Chen F, Ding S, Lee BS. et al. Sarcoplasmic reticulum Ca(2+)ATPase and cell contraction in developing rabbit heart. J Mol Cell Cardiol. 2000;32(5):745–755. [PubMed: 10775480]
- 20.
- Huynh TV, Chen F, Wetzel GT. et al. Developmental changes in membrane Ca2+ and K+ currents in fetal, neonatal, and adult rabbit ventricular myocytes. Circ Res. 1992;70(3):508–515. [PubMed: 1537088]
- 21.
- Satoh H, Delbridge LM, Blatter LA. et al. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J. 1996;70(3):1494–1504. [PMC free article: PMC1225076] [PubMed: 8785306]
- 22.
- Couch JR, West TC, Hoff HE. Development of the action potential of the prenatal rat heart. Circ Res. 1969;24(1):19–31. [PubMed: 5763736]
- 23.
- Ono K, Ito H. Role of rapidly activating delayed rectifier K+ current in sinoatrial node pacemaker activity. Am J Physiol. 1995;269(2 Pt 2):H453–462. [PubMed: 7653609]
- 24.
- Sarai N, Matsuoka S, Kuratomi S. et al. Role of individual ionic current systems in the SA node hypothesized by a model study. Jpn J Physiol. 2003;53(2):125–134. [PubMed: 12877768]
- 25.
- Chun KR, Koenen M, Katus HA. et al. Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp Mol Med. 2004;36(4):367–371. [PubMed: 15365256]
- 26.
- Spence SG, Vetter C, Hoe CM. Effects of the class III antiarrhythmic, dofetilide (UK-68,798) on the heart rate of midgestation rat embryos, in vitro. Teratology. 1994;49(4):282–292. [PubMed: 8073367]
- 27.
- Kilborn MJ, Fedida D. A study of the developmental changes in outward currents of rat ventricular myocytes. J Physiol. 1990;430:37–60. [PMC free article: PMC1181726] [PubMed: 2086767]
- 28.
- Artman M. Sarcolemmal Na(+)-Ca2+ exchange activity and exchanger immunoreactivity in developing rabbit hearts. Am J Physiol. 1992;263(5 Pt 2):H1506–1513. [PubMed: 1443202]
- 29.
- Artman M, Ichikawa H, Avkiran M. et al. Na+/Ca2+ exchange current density in cardiac myocytes from rabbits and guinea pigs during postnatal development. Am J Physiol. 1995;268(4 Pt 2):H1714–1722. [PubMed: 7733375]
- Electrophysiological Simulation of Developmental Changes in Action Potentials of...Electrophysiological Simulation of Developmental Changes in Action Potentials of Cardiomyocytes - Madame Curie Bioscience Database
- A Guide to Modeling Reaction-Diffusion of Molecules with the E-Cell System - Mad...A Guide to Modeling Reaction-Diffusion of Molecules with the E-Cell System - Madame Curie Bioscience Database
- Therapeutic Utilities of SOD Mimetics: Cancer, Radiotherapy and SOD Mimetics - M...Therapeutic Utilities of SOD Mimetics: Cancer, Radiotherapy and SOD Mimetics - Madame Curie Bioscience Database
- TRAFs in RANK Signaling - Madame Curie Bioscience DatabaseTRAFs in RANK Signaling - Madame Curie Bioscience Database
- Keratins As Targets in and Modulators of Liver Diseases - Madame Curie Bioscienc...Keratins As Targets in and Modulators of Liver Diseases - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...