The C-terminal binding proteins (CtBPs) are ubiquitous corepressors that recruit histone-modifying enzymes to a variety of sequence specific DNA-binding proteins and other transcriptional regulators. CtBPs appear to play an important role in mediating repression and transforming activities of a variety of hematopoietic transcription factors such as Basic Krüppel-like Factor/Krüppel-like Factor 3 (BKLF/KLF3), Friend of GATA (FOG), Evi-1 and members of the Ikaros family. Mice lacking CtBPs die during embryonic development and exhibit defects in a wide range of developmental processes, including aberrant heart formation and absence of blood vessels in the yolk sac. The ongoing identification of repressed target genes and interacting transcriptional partners will help to unravel the contributions of CtBP proteins to hematopoiesis.
Introduction
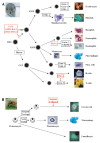
Hematopoiesis is the process through which the various blood lineages (erythrocytic, lymphocytic, monocytic/myelocytic, granulocytic and thrombocytic) develop from self-renewing, pluripotent stem cells.1 This process is tightly regulated by the action of growth factors that signal to lineage restricted or widely expressed transcription factors and their associated coregulators (Figs. 1, 2). These factors then orchestrate lineage commitment by activating and repressing defined sets of target genes. For example, the zinc finger protein GATA-1 and its cofactor FOG are involved in coordinating the expression of genes that drive erythrocytic and megakaryocytic development.2,3
Understanding the transcriptional networks that coordinate such programs of gene expression is an important focus in the study of cell differentiation. Accumulating evidence suggests that the corepressor C-terminal binding protein (CtBP) is an important regulator of hematopoietic homeostasis by virtue of its physical interaction with hematopoietic transcription factors such as BKLF, Evi-1, FOG and Ikaros. This chapter addresses the mechanisms of transcriptional repression and the role of CtBP in development, hematopoiesis and leukemogenesis.
CtBP Proteins during Development
CtBP1 is the founding member of the CtBP family of corepressors. It was first identified as an E1A interacting protein that negatively modulates the oncogenic transformation activity of E1A.4 Subsequently, highly homologous human and mouse proteins termed CtBP2 were identified by analysis of EST data bank sequences and in a yeast two-hybrid screen against the erythroid transcription factor BKLF.5,6
CtBP1 and CtBP2 are widely expressed and are often coexpressed. Knockout studies have revealed that CtBP1-null mice are viable while CtBP2-null embryos die by E10.5.7 Thus the functions of CtBP2 cannot be assumed by CtBP1. Further evidence for distinct functions comes from an examination of expression patterns. CtBP1 is expressed in the thymus and peripheral blood leukocytes, whereas CtBP2 is not readily detected.8,9 In human cancer lines, differences in expression are common with high expression of CtBP1 in chronic myelogenous leukemia K-562 and lymphoblastic leukemia MOLT-4 cell lines.9 Conversely, CtBP2 is readily detected in the forming placenta while CtBP1 is not. CtBP2-null embryos are devoid of blood vessels suggesting that CtBP2 has a specific and essential role in angiogenesis.7
While the two proteins are clearly non redundant, there is evidence that they do have overlapping functions. The phenotypes of compound heterozygotes with various combinations of CtBP1 and CtBP2 alleles provided strong evidence for this.7 Overall, the myriad developmental defects seen in CtBP mutant embryos (axial truncations, delayed neural development, defects in heart morphogenesis) are consistent with the wide diversity of CtBP1 and CtBP2's many interacting partners10,11 (see chapter by Hildebrand of this book for more details).
Cells derived from mutant mouse embryos have been used to address more precisely the functions of CtBP proteins and identify their relevant target genes.12 Microarray analysis of CtBPs-knockout versus CtBP-rescued mouse embryo fibroblasts (MEF) revealed that many epithelial and pro-apoptotic genes are de-regulated, suggesting an important role of CtBP proteins during epithelial to mesenchymal transitions, potentially contributing to tumor malignancy.12
Although the impact on the CtBPs on hematopoiesis has not been examined in detail, the microarray study on MEF showed evidence that several hematopoietic genes are up-regulated in the absence of CtBPs (Table 1). Several of the genes that were found to be dys-regulated are erythroid genes that are normally up-regulated by GATA-1 during erythroid differentiation. 13 The GATA cofactors, FOG and FOG-2 are known to bind CtBP (see below). Although GATA-1 and FOG are erythroid proteins and are not likely to be present in MEF, it is likely that other GATA and FOG family proteins, such as the more broadly expressed GATA-2 and FOG-2 are present. Thus the apparent up-regulation of the erythroid genes raises the possibility that GATA and FOG proteins are involved in the repression of ectopic expression of erythroid genes in nonerythroid tissues. It also suggests that GATA-1 and FOG may be involved in repressing some of these genes in early stages of erythroid development. Indeed strong roles for GATA-1 in gene repression have recently been detected in microarray experiments.13 Further experiments, such as the generation of mice harboring blood lineage selective mutations in both CtBP1 and CtBP2, will be required to decipher the full role of these corepressors in hematopoiesis.
Table 1
Hematopoietic genes up-regulated in CtBP -/- cells.
CtBP Partnership with Hematopoietic Factors
CtBP proteins are known to interact with a short sequence motif (PXDLS) present in a variety of transcription factors and cofactors.11,14 Among them, FOG, BKLF and Ikaros are known to play key role during hematopoiesis. The functional interactions with CtBP proteins are reviewed in this section.
GATA and Friend of GATA (FOG) Proteins
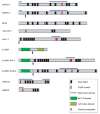
FOG (Friend of GATA-1) was identified in a yeast two-hybrid screen as a GATA-1 cofactor. 15 FOG contains nine zinc fingers of two different types, C2H2 and C2HC, distributed throughout the protein (Fig. 1). When hematopoietic cell lines were examined, it was found that FOG displayed an expression pattern strikingly similar to that of GATA-1. That is, FOG is found in erythrocytes, megakaryocytes and multipotential progenitors. Moreover, FOG-1-/- mice die between E10.5 and E12.5 from severe anemia and exhibit a block in erythroid maturation at a stage similar to that observed in GATA-1-/- mice and also a complete failure of megakaryopoiesis.16 These results provide strong genetic evidence that FOG and GATA-1 function in a coordinate manner in erythroid development (Fig. 2).2
Searches of the murine expressed sequence tag (EST) databases revealed the presence of a second FOG gene named FOG-2.17,18 FOG-2 is expressed highly in heart, brain and liver and mirrors the expression pattern of GATA-4/5/6, suggesting that FOG-2 may serve as a cofactor for these nonhematopoietic GATA factors. The phenotypes of mouse embryos deficient in FOG-2 support this view. They die between E12.5 and E15.5 due to a complex congenital cardiac defect.19 A structurally FOG-related protein, U-shaped (Ush) is present in Drosophila, whereas a single FOG gene has been identified in Xenopus and three in Zebrafish.2
FOG can function as either a transcriptional coactivator or repressor depending on the cell and promoter context.20,21 Although the mechanism of repression by FOG proteins remains elusive, numerous studies suggest that the corepressors CtBP may contribute to repression. Indeed, a common feature among all identified FOG proteins is the presence of a PXDLS motif (Fig. 1) and mutation of this motif consequently abolishes FOG/CtBP interaction.6,21,22
Experimental evidence supporting a role for CtBP proteins in contributing to FOG activity has come from experiments with mFOG and mFOG-2 in Xenopus. Ectopic expression of mFOG and mFOG-2 in Xenopus blocks erythropoiesis and reduces xGata-1 and xSCL levels, suggesting that FOG proteins limit red blood cell formation to prevent depletion of pluripotent cells.23 Conversely, expression of FOGΔCtBP (a FOG mutant unable to interact with CtBP) augments red cell production in whole embryos, arguing that FOG proteins require the CtBP corepressors to regulate lineage commitment in this system.23 In agreement with this, rescue of FOG-1-/- mouse cell line with a FOGΔCtBP mutant resulted in a marked enhancement of erythropoiesis, compared to that achieved by wild-type FOG.22 This result suggests that CtBP proteins play a role in tempering the ability of FOG proteins to drive erythropoiesis. In stark contrast to these results, however, erythropoiesis appears normal in FOGΔCtBP knock-in mice.22 The discrepancy between Xenopus and cell line experiments and the mouse knock-in experiment is puzzling but may be due to compensatory mechanisms in developing mice that are not available in the other systems, or it is also possible that the knock-in mice have subtle defects not yet detected.
A recent study suggests that FOG may impair the proliferation of hematopoietic cells in a CtBP-dependent and -independent manner, according to the differentiation stages.24 In other words, CtBP proteins appear to repress erythropoiesis only in early stages but are not required in the late stages of cellular maturation. The fact that CtBP is expressed in G1E cells (an immortalized GATA-1 null line derived from gene-targeted embryonic stem cells) and down-regulated during GATA-1 reactivation supports this view and raises the possibility that the regulation of CtBP level is important in the processes leading to erythroid maturation.13 Recent work in Drosophila suggests that the requirement for CtBP reflects the need for overall higher levels of repression, rather than a requirement for an activity unique to CtBP,25 strongly arguing for a quantitative rather than a qualitative CtBP repression function. According to this model, high level of CtBP in multipotent progenitors would limit the activity of GATA-1 but as differentiation and maturation proceeded, increases in GATA-1 levels combined with down regulation of CtBP would allow the activation of erythroid specific genes (Table 1).
As in vertebrates, hematopoiesis in Drosophila can be described as a biphasic developmental process that serves to populate the embryo, larva and adult with mature blood cells.26 There are at least three terminally differentiated hemocyte types (Fig. 2): plasmatocytes that function primarily as phagocytes, crystal cells that function in the process of melanization and facilitate innate immune and wound-healing responses, and lamellocytes that appear to neutralize objects too large to be engulfed by plasmatocytes.26 Interestingly, the Drosophila FOG homolog U-shaped (ush) is down-regulated during crystal cell lineage commitment, which is consistent with a role for the protein as a negative regulator of crystal cell production.27,28 Furthermore ectopically expressed FOG suppresses crystal cell production in a CtBP-dependent manner.29,30 It is noteworthy that CtBP is likely to be expressed during Drosophila hematopoiesis since it was found that a lacZ reporter gene inserted in the enhancer region of the CtBP gene is expressed in the larval plasmatocyte lineage.31 Furthermore, a P-element based genetic screen designed to identify genes that control Drosophila hematopoiesis has also led to the isolation of CtBP.32 Final evidence comes from the fact that mutations in CtBP alter the number of crystal cells. Surprisingly, however, unlike mutations in U-shaped, the mutations in CtBP cause a reduction in the number of crystal cells.
Interestingly, the disruption of eye development and the repression of cardiac cell development by Ush can occur in the absence of CtBP.30 Similarly, mFOG and mFOG-2 can repress GATA-4 activation of cardiac promoters in a CtBP-independent manner.33 Moreover, FOG is also able to repress GATA-3 activity during Th2 cell development34 and C/EBPβ in eosinophil lineage commitment.35 Whereas the repression of GATA-3 appears to be CtBP-independent, the role of CtBP on C/EBPβ has not yet been evaluated. Thus there are now a number of examples suggesting that while FOG proteins can recruit CtBP and CtBP can contribute to their repressive activity, the presence of CtBP is not required in all cases. In short, it is highly likely that FOG proteins bind other corepressors that can subsume CtBP's functions.
Another link between CtBP and GATA factors comes from work on the Mosquito Aedes aegypti.36 The ingestion of blood is required for egg development in mosquitoes. In anautogenous mosquitoes, vitellogenesis is initiated only after a female mosquito ingests vertebrate blood. The blood meal triggers a hormonal cascade which activates yolk protein precursor (YPP) genes. A mosquito GATA factor called AaGATAr has been identified. AaGATAr serves as a transcriptional repressor to prevent the activation of YPP genes in previtellogenic females prior to blood feeding.36 Interestingly, AaGATAr contains a PXDLS motif and thus its transcriptional repression appears to involve the recruitment of CtBP.36,37 Thus, CtBP proteins appear to play a critical role in the repression of GATA-mediated activation through binding to FOG or through direct interaction with GATA, according to the localization of the PXDLS motif.
Krüppel-Like Factors
BKLF/KLF3 (Basic Krüppel-like factor/Krüppel-like factor 3) belongs to the mammalian Sp/Krüppel-like factor family, of which there are currently 24 members (Sp1-8 and KLF1-16). KLF proteins are characterized by a distinctive DNA binding domain at the C-terminus of the protein that consists of three Krüppel-like C2H2 zinc fingers. Outside this domain there is little homology among the known KLF proteins.38 BKLF is highly abundant in erythroid cells and is known to function as a strong transcriptional repressor on several target promoters.6,39 The repression domain of BKLF has been mapped to the N-terminal region and was found to associate with the transcriptional corepressor CtBP2 through the short CtBP interaction motif PVDLT.6 Disruption of the BKLF-CtBP interaction leads to a significant reduction of the repression potential of BKLF in cellular assays. In particular, BKLF represses GATA-1 activation of the erythroid EpoR and γ-globin promoters in a CtBP-dependent manner in gene reporter assays.6,40,41 However, the functional significance of these interactions during development remains to be assessed in vivo.
Although the various KLF proteins share little overall homology outside their zinc finger regions, sequence alignments have shown that short stretches of homology do exist and the different KLF proteins can be grouped into subfamilies. One subfamily consists of BKLF/ KLF3, AP2rep/KLF12 and KLF8. Although the homology between the repression domains is limited, it is significant that one prominent region of conservation encompasses the PXDLS motif used to contact CtBP corepressors. It has been shown that all three proteins can physically interact with CtBP through this motif and that the interaction is critical for gene repression.6,42,43
Ikaros
Ikaros is the founding member of a ‘Greek’ family of zinc finger DNA binding proteins that includes Aiolos, Helios, Eos and Pegasus.44 Several of these transcription factors are thought to work in concert to promote the proper specification, differentiation and function of lymphocytes. The Ikaros gene encodes a protein with 6 zinc fingers that comply with the Krüppel C2H2 consensus arranged in two domains, the N-terminal domain involved in DNA-binding and the C-terminal domain involved in self-association (Fig. 1). Mice homozygous for an Ikaros mutation had no detectable lymphocytes or lymphocyte precursors (Fig. 2),45 indicating that Ikaros is a critical player during lymphocyte development. Initial studies indicated that Ikaros was a weak activator of transcription but further reports clearly indicate that it is also a strong repressor.46-48 In particular, Ikaros accumulates around clusters of centromeric heterochromatin. 49,50
Ikaros interacts with a plethora of chromatin modifying enzymes including the Mi-2 histone de-acetylase complex.51 HDAC association provided a likely mechanism for repression mediated by Ikaros52 but HDAC-independent effects were also observed.46 Importantly, a CtBP/Ikaros interaction is required for this HDAC-independent Ikaros-mediated repression.46 Moreover, two other members of the family, namely Eos and the Ikaros related GATA protein TRPS1 (tricho-rhino-phalangeal type I) are can also recruit the corepressor CtBP to achieve strong transcriptional repression.53 Taken together, these findings suggest that CtBP association is likely to be one of the mechanisms by which members of the Ikaros family of transcription factors mediate gene repression in vivo. However, establishing formally which genes are targets of either repression or activation by Ikaros family proteins and more precisely which genes are CtBP-dependent, will be important in defining the role of CtBP in lymphocyte commitment.
CtBPs in Leukemogenesis
Early studies indicate that deletions within the C-terminal region of the Adenovirus E1A protein, the region that encompass the conserved CtBP binding motif, confer a hyper-transforming phenotype to E1A. This enhanced activity is seen in assays when E1A is used in cooperation with the activated Ras oncogene to drive transformation.4,54-56 Tumors expressing the E1A mutants are also highly metastatic, further suggesting that CtBP may attenuate the oncogenic potential of E1A. Recent results, however, also suggest that CtBP may contribute to oncogenesis, especially in blood cells.
Evi-1 (Ecotropic viral integration site 1) was initially identified as a common locus of retrovirus integration in myeloid tumors in AKXD mice.57 Evi-1 is a transcriptional regulator that possesses two clusters of C2H2 zinc fingers (Fig. 1) and is implicated in myeloid leukemogenesis. In addition, a t(3;21)(q26;q22) translocation found in chronic myeloid leukemia cells generates a fusion transcript that contains AML1 linked to Evi-1.58 Interestingly, Evi-1 contains a repression domain with two CtBP binding motifs and mutations in these motifs impair transcriptional repression.59-61 Moreover, these mutations also impair transformation of rat fibroblasts in vitro,60 suggesting that CtBP plays a role in Evi-1 mediated leukemogenesis.
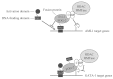
Several mechanisms have been proposed to explain how AML1/Evi-1 fusion proteins bring about the malignant transformation of hematopoietic stem cells.58 For example, the AML1/ Evi-1 fusion proteins are thought to exert a dominant negative effect and repress AML1 target genes by recruiting a CtBP corepressor complex through the Evi-1 part of the chimera62,63 (Fig. 3). Remarkably, studies carried out with other fusion protein such as PML/RARα support the hypothesis that the inappropriate recruitment of corepressors has a causative role in the promotion of leukemia.64,65
Furthermore, CtBP has been recently been implicated in a newly characterized t(X;21)(p22.3;q22.1) translocation found in a patient with myelodysplasia that fuses AML1 with FOG-2.66 Preliminary results suggest that as seen with AML/Evi-1, the AML1/FOG-2 fusion is able to recruit CtBP and thus may repress AML1 and GATA target genes (Fig. 3).66
CtBP proteins also bind the repression domain of MLL (mixed-lineage leukemia), a well known factor involved in more than 30 different fusions in leukemogenesis.67,68 Finally, abnormal expression of CtBP has been proposed to contribute to development of Hodgkin's lymphoma.69 In summary, these studies clearly indicate that CtBPs proteins are implicated in pathways leading to abnormal hematopoeitic growth and differentiation.
Conclusion
CtBP proteins interact with a myriad of transcription factors involved in many key developmental processes. There is now considerable evidence that CtBP physically and functionally interacts with several proteins that play key roles in hematopoietic development. Thus it seems likely that CtBP will play important roles in the control of hematopoietic development. Nevertheless there is also clear evidence that CtBP is not always an obligate partner of hematopoietic regulators. It is possible that it is required to repress certain target genes during specific stages of development but that it is dispensable on other target genes or in other cellular contexts. The CtBP-independence observed may in some cases indicate that it is simply not required and in other cases it may be that another corepressor subsumes the role of CtBP. Further experiments will be required to delineate the precise roles of CtBP during hematopoiesis and to identify the specific subset of genes that it regulates. This information may prove critical to the ability to artificially control hematopoiesis and to treat disorders such as leukemias that arise from inappropriate gene control during blood development.
References
- 1.
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87(10):4025–4039. [PubMed: 8639758]
- 2.
- Cantor AB, Orkin SH. Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins. Semin Cell Dev Biol. 2005;16(1):117–128. [PubMed: 15659346]
- 3.
- Ferreira R, Ohneda K, Yamamoto M. et al. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25(4):1215–1227. [PMC free article: PMC548021] [PubMed: 15684376]
- 4.
- Schaeper U, Boyd JM, Verma S. et al. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc Natl Acad Sci USA. 1995;92(23):10467–10471. [PMC free article: PMC40632] [PubMed: 7479821]
- 5.
- Katsanis N, Fisher EM. A novel C-terminal binding protein (CTBP2) is closely related to CTBP1, an adenovirus E1A-binding protein, and maps to human chromosome 21q21.3. Genomics. 1998;47(2):294–299. [PubMed: 9479502]
- 6.
- Turner J, Crossley M. Cloning and characterization of mCtBP2, a corepressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 1998;17(17):5129–5140. [PMC free article: PMC1170841] [PubMed: 9724649]
- 7.
- Hildebrand JD, Soriano P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol. 2002;22(15):5296–5307. [PMC free article: PMC133942] [PubMed: 12101226]
- 8.
- Furusawa T, Moribe H, Kondoh H. et al. Identification of CtBP1 and CtBP2 as corepressors of zinc finger-homeodomain factor deltaEF1. Mol Cell Biol. 1999;19(12):8581–8590. [PMC free article: PMC84984] [PubMed: 10567582]
- 9.
- Sewalt RG, Gunster MJ, van der Vlag J. et al. C-Terminal binding protein is a transcriptional repressor that interacts with a specific class of vertebrate Polycomb proteins. Mol Cell Biol. 1999;19(1):777–787. [PMC free article: PMC83934] [PubMed: 9858600]
- 10.
- Chinnadurai G. CtBP family proteins: More than transcriptional corepressors. Bioessays. 2003;25(1):9–12. [PubMed: 12508276]
- 11.
- Turner J, Crossley M. The CtBP family: Enigmatic and enzymatic transcriptional corepressors. Bioessays. 2001;23(8):683–690. [PubMed: 11494316]
- 12.
- Grooteclaes M, Deveraux Q, Hildebrand J. et al. C-terminal-binding protein corepresses epithelial and proapoptotic gene expression programs. Proc Natl Acad Sci USA. 2003;100(8):4568–4573. [PMC free article: PMC153596] [PubMed: 12676992]
- 13.
- Welch JJ, Watts JA, Vakoc CR. et al. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood. 2004;104(10):3136–3147. [PubMed: 15297311]
- 14.
- Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9(2):213–224. [PubMed: 11864595]
- 15.
- Tsang AP, Visvader JE, Turner CA. et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90(1):109–119. [PubMed: 9230307]
- 16.
- Tsang AP, Fujiwara Y, Hom DB. et al. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes Dev. 1998;12(8):1176–1188. [PMC free article: PMC316724] [PubMed: 9553047]
- 17.
- Svensson EC, Tufts RL, Polk CE. et al. Molecular cloning of FOG-2: A modulator of transcription factor GATA-4 in cardiomyocytes. Proc Natl Acad Sci USA. 1999;96(3):956–961. [PMC free article: PMC15332] [PubMed: 9927675]
- 18.
- Tevosian SG, Deconinck AE, Cantor AB. et al. FOG-2: A novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc Natl Acad Sci USA. 1999;96(3):950–955. [PMC free article: PMC15331] [PubMed: 9927674]
- 19.
- Tevosian SG, Deconinck AE, Tanaka M. et al. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101(7):729–739. [PubMed: 10892744]
- 20.
- Fox AH, Liew C, Holmes M. et al. Transcriptional cofactors of the FOG family interact with GATA proteins by means of multiple zinc fingers. EMBO J. 1999;18(10):2812–2822. [PMC free article: PMC1171362] [PubMed: 10329627]
- 21.
- Holmes M, Turner J, Fox A. et al. hFOG-2, a novel zinc finger protein, binds the corepressor mCtBP2 and modulates GATA-mediated activation. J Biol Chem. 1999;274(33):23491–23498. [PubMed: 10438528]
- 22.
- Katz SG, Cantor AB, Orkin SH. Interaction between FOG-1 and the corepressor C-terminal binding protein is dispensable for normal erythropoiesis in vivo. Mol Cell Biol. 2002;22(9):3121–3128. [PMC free article: PMC133767] [PubMed: 11940669]
- 23.
- Deconinck AE, Mead PE, Tevosian SG. et al. FOG acts as a repressor of red blood cell development in Xenopus. Development. 2000;127(10):2031–2040. [PubMed: 10769228]
- 24.
- Tanaka M, Zheng J, Kitajima K. et al. Differentiation status dependent function of FOG-1. Genes Cells. 2004;9(12):1213–1226. [PubMed: 15569153]
- 25.
- Struffi P, Corado M, Kulkarni M. et al. Quantitative contributions of CtBP-dependent and -independent repression activities of Knirps. Development. 2004;131(10):2419–2429. [PubMed: 15128671]
- 26.
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: Conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5(5):673–690. [PubMed: 14602069]
- 27.
- Fossett N, Schulz RA. Functional conservation of hematopoietic factors in Drosophila and vertebrates. Differentiation. 2001;69(2-3):83–90. [PubMed: 11798069]
- 28.
- Sorrentino RP, Gajewski KM, Schulz RA. GATA factors in Drosophila heart and blood cell development. Semin Cell Dev Biol. 2005;16(1):107–116. [PubMed: 15659345]
- 29.
- Fossett N, Hyman K, Gajewski K. et al. Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc Natl Acad Sci USA. 2003;100(20):11451–11456. [PMC free article: PMC208778] [PubMed: 14504400]
- 30.
- Fossett N, Tevosian SG, Gajewski K. et al. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci USA. 2001;98(13):7342–7347. [PMC free article: PMC34670] [PubMed: 11404479]
- 31.
- Braun A, Lemaitre B, Lanot R. et al. Drosophila immunity: Analysis of larval hemocytes by P-element-mediated enhancer trap. Genetics. 1997;147(2):623–634. [PMC free article: PMC1208184] [PubMed: 9335599]
- 32.
- Milchanowski AB, Henkenius AL, Narayanan M. et al. Identification and characterization of genes involved in embryonic crystal cell formation during Drosophila hematopoiesis. Genetics. 2004;168(1):325–339. [PMC free article: PMC1448098] [PubMed: 15454546]
- 33.
- Svensson EC, Huggins GS, Dardik FB. et al. A functionally conserved N-terminal domain of the friend of GATA-2 (FOG-2) protein represses GATA4-dependent transcription. J Biol Chem. 2000;275(27):20762–20769. [PubMed: 10801815]
- 34.
- Kurata H, Lee HJ, McClanahan T. et al. Friend of GATA is expressed in naive Th cells and functions as a repressor of GATA-3-mediated Th2 cell development. J Immunol. 2002;168(9):4538–4545. [PubMed: 11971000]
- 35.
- Querfurth E, Schuster M, Kulessa H. et al. Antagonism between C/EBPbeta and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors. Genes Dev. 2000;14(19):2515–2525. [PMC free article: PMC316981] [PubMed: 11018018]
- 36.
- Martin D, Piulachs MD, Raikhel AS. A novel GATA factor transcriptionally represses yolk protein precursor genes in the mosquito Aedes aegypti via interaction with the CtBP corepressor. Mol Cell Biol. 2001;21(1):164–174. [PMC free article: PMC88790] [PubMed: 11113191]
- 37.
- Attardo GM, Higgs S, Klingler KA. et al. RNA interference-mediated knockdown of a GATA factor reveals a link to anautogeny in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2003;100(23):13374–13379. [PMC free article: PMC263821] [PubMed: 14595016]
- 38.
- Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4(2):206. [PMC free article: PMC151296] [PubMed: 12620113]
- 39.
- Crossley M, Whitelaw E, Perkins A. et al. Isolation and characterization of the cDNA encoding BKLF/TEF-2, a major CACCC-box-binding protein in erythroid cells and selected other cells. Mol Cell Biol. 1996;16(4):1695–1705. [PMC free article: PMC231156] [PubMed: 8657145]
- 40.
- Perdomo J, Verger A, Turner J. et al. Role for SUMO modification in facilitating transcriptional repression by BKLF. Mol Cell Biol. 2005;25(4):1549–1559. [PMC free article: PMC548027] [PubMed: 15684403]
- 41.
- Turner J, Nicholas H, Bishop D. et al. The LIM protein FHL3 binds basic Kruppel-like factor/ Kruppel-like factor 3 and its corepressor C-terminal-binding protein 2. J Biol Chem. 2003;278(15):12786–12795. [PubMed: 12556451]
- 42.
- Schuierer M, Hilger-Eversheim K, Dobner T. et al. Induction of AP-2alpha expression by adenoviral infection involves inactivation of the AP-2rep transcriptional corepressor CtBP1. J Biol Chem. 2001;276(30):27944–27949. [PubMed: 11373277]
- 43.
- van Vliet J, Turner J, Crossley M. Human Kruppel-like factor 8: A CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000;28(9):1955–1962. [PMC free article: PMC103308] [PubMed: 10756197]
- 44.
- Rebollo A, Schmitt C. Ikaros, Aiolos and Helios: Transcription regulators and lymphoid malignancies. Immunol Cell Biol. 2003;81(3):171–175. [PubMed: 12752680]
- 45.
- Georgopoulos K, Bigby M, Wang JH. et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–156. [PubMed: 7923373]
- 46.
- Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem. 2000;275(26):19594–19602. [PubMed: 10766745]
- 47.
- Koipally J, Georgopoulos K. Ikaros-CtIP interactions do not require CtBP and participate in a deacetylase-independent mode of repression. J Biol Chem. 2002;277(26):23143–23149. [PubMed: 11959865]
- 48.
- Koipally J, Georgopoulos K. A molecular dissection of the repression circuitry of Ikaros. J Biol Chem. 2002;277:27697–27705. [PubMed: 12015313]
- 49.
- Brown KE, Guest SS, Smale ST. et al. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91(6):845–854. [PubMed: 9413993]
- 50.
- Cobb BS, Morales-Alcelay S. et al. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14(17):2146–2160. [PMC free article: PMC316893] [PubMed: 10970879]
- 51.
- Kim J, Sif S, Jones B. et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10(3):345–355. [PubMed: 10204490]
- 52.
- Koipally J, Renold A, Kim J. et al. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18(11):3090–3100. [PMC free article: PMC1171390] [PubMed: 10357820]
- 53.
- Perdomo J, Crossley M. The Ikaros family protein Eos associates with C-terminal-binding protein corepressors. Eur J Biochem. 2002;269(23):5885–5892. [PubMed: 12444977]
- 54.
- Boyd JM, Subramanian T, Schaeper U. et al. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993;12(2):469–478. [PMC free article: PMC413230] [PubMed: 8440238]
- 55.
- Chinnadurai G. Modulation of oncogenic transformation by the human adenovirus E1A C-terminal region. Curr Top Microbiol Immunol. 2004;273:139–161. [PubMed: 14674601]
- 56.
- Subramanian T, La Regina M, Chinnadurai G. Enhanced ras oncogene mediated cell transformation and tumorigenesis by adenovirus 2 mutants lacking the C-terminal region of E1a protein. Oncogene. 1989;4(4):415–420. [PubMed: 2524023]
- 57.
- Morishita K, Parker DS, Mucenski ML. et al. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell. 1988;54(6):831–840. [PubMed: 2842066]
- 58.
- Mitani K. Molecular mechanisms of leukemogenesis by AML1/EVI-1. Oncogene. 2004;23(24):4263–4269. [PubMed: 15156182]
- 59.
- Izutsu K, Kurokawa M, Imai Y. et al. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor beta signaling. Blood. 2001;97(9):2815–2822. [PubMed: 11313276]
- 60.
- Palmer S, Brouillet JP, Kilbey A. et al. Evi-1 transforming and repressor activities are mediated by CtBP corepressor proteins. J Biol Chem. 2001;276(28):25834–25840. [PubMed: 11328817]
- 61.
- Hirai H, Izutsu K, Kurokawa M. et al. Oncogenic mechanisms of Evi-1 protein. Cancer Chemother Pharmacol. 2001;48(Suppl 1):S35–40. [PubMed: 11587364]
- 62.
- Izutsu K, Kurokawa M, Imai Y. et al. The t(3;21) fusion product, AML1/Evi-1 blocks AML1-induced transactivation by recruiting CtBP. Oncogene. 2002;21(17):2695–2703. [PubMed: 11965542]
- 63.
- Senyuk V, Chakraborty S, Mikhail FM. et al. The leukemia-associated transcription repressor AML1/ MDS1/EVI1 requires CtBP to induce abnormal growth and differentiation of murine hematopoietic cells. Oncogene. 2002;21(20):3232–3240. [PubMed: 12082639]
- 64.
- Lin RJ, Evans RM. Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol Cell. 2000;5(5):821–830. [PubMed: 10882118]
- 65.
- Minucci S, Maccarana M, Cioce M. et al. Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol Cell. 2000;5(5):811–820. [PubMed: 10882117]
- 66.
- Chan EM, Comer EM, Brown FC. et al. AML1-FOG2 fusion protein in myelodysplasia. Blood. 2005;105(11):4523–4526. [PubMed: 15705784]
- 67.
- Daser A, Rabbitts TH. Extending the repertoire of the mixed-lineage leukemia gene MLL in leukemogenesis. Genes Dev. 2004;18(9):965–974. [PubMed: 15132992]
- 68.
- Xia ZB, Anderson M, Diaz MO. et al. MLL repression domain interacts with histone deacetylases, the polycomb group proteins HPC2 and BMI-1, and the corepressor C-terminal-binding protein. Proc Natl Acad Sci USA. 2003;100(14):8342–8347. [PMC free article: PMC166231] [PubMed: 12829790]
- 69.
- Dukers DF, van Galen JC, Giroth C. et al. Unique polycomb gene expression pattern in Hodgkin's lymphoma and Hodgkin's lymphoma-derived cell lines. Am J Pathol. 2004;164(3):873–881. [PMC free article: PMC1613333] [PubMed: 14982841]
Publication Details
Author Information and Affiliations
Authors
Alexis Verger, Jose Perdomo,* and Merlin Crossley.Affiliations
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Verger A, Perdomo J, Crossley M. CtBP and Hematopoietic Transcriptional Regulators. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.