NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The β-propeller domain is a widespread protein organizational motif. Typically, β-propeller proteins are encoded by repeated sequences where each repeat unit corresponds to a twisted β-sheet structural motif; these β-sheets are arranged in a circle around a central axis to generate the β-propeller structure. Two superfamilies of β-propeller proteins, the WD-repeat and Kelch-repeat families, exhibit similarities not only in structure, but, remarkably, also in the types of molecular functions they perform. While it is unlikely that WD and Kelch repears evolved from a common ancestor, their evolution into diverse families of similar function may refect the evolutionary advantages of the stable core β-propeller fold. In this chapter, we examine the relationships between these two widespread protein families, emphasizing recently published work relating to the structure and function of both Kelch and WD-repeat proteins.
Introduction
Experimental approaches aimed at understanding the function of proteins are often challenging. An understanding of known protein domains can provide insights into the possible functions of novel proteins. It has been suggested that WD-repeat proteins might often function as coordinators of macromolecular protein complexes, based in part on the finding that the initial WD-repeat proteins characterized were part of large complexes involved in signaling, transcription, or vesicle traffic.1 The Kelch-repeat containing proteins initially appeared to mediate interactions with the cytoskeleton since the first Kelch-repeat proteins characterized bind F-actin.2,3 However, extensive research on proteins with either WD-repeat or Kelch-repeat domains has demonstrated that they perform a very diverse array of molecular functions. Nonetheless, similarities in the structure of these repeat domains and the modular contexts of the β-propellers in both the WD-and Kelch-repeat protein families are useful in predicting the functions of newly discovered members of these families. Several excellent and comprehensive reviews of WD-4-6and Kelch-repeat7,8 proteins have recently been published; here, we focus on recent advances in understanding the structure and function of both families of proteins.
WD and Kelch Repeats: Sequences, Relationship to Structure and Phylogeny
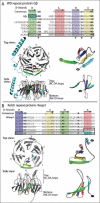
Kelch and WD repeats consist of repeated sequence motifs with hallmark residues spaced at regular intervals (Fig. 1). In each case, only a handful of residues are consistently conserved (bold residues in Fig. 1 sequence alignments) and even these positions can tolerate substitutions, making the identification of all repeats by sequence scanning algorithms alone difficult. In a number of cases, additional repeats present in a protein were only recognized after structural determination9 (Table 1).
Table 1
Structures of WD and Kelch repeat proteins.
The structures of WD-(Gβ) and Kelch- (Keap1) repeat domains are presented in Figure 1 (Fig. 1A,B, respectively). While significant diversity has been observed in both WD-and Kelch-repeat sequences, a large number have repeat lengths and repeat spacing similar to those in Gβ and Keap1, making them good representative models. Indeed, even in the structure of Ski8p, a protein containing an unusually long WD repeat, the β-sheet blades can be superimposed on the blades of Gβ with the extra sequence present in loops that project above the surface of the propeller.9 Overall, both Gβ and Keap1 repeat domains form typical β-propeller folds, with each of the six (Keap1) or seven (Gβ) blades arranged in a circular array around a central axis (Fig. 1A, B). Each propeller blade consists of four β-strands, which by convention are designated A-D, with the A strand the innermost strand and D the outermost. The twisting of the β-strands gives the propeller a tapered toroidal appearance. By convention the “top” of the structure is defined as the narrower surface (Fig. 1).
WD sequence repeats are approximately 40 amino acids in length and the most conserved residues are a Gly-His (GH) dipeptide near the beginning of each repeat, as well as the eponymous Trp-Asp (WD) dipeptide that terminates the repeat.5 The first structure determination of a WD-repeat protein was that of Gβ, the beta subunit of a heterotrimeric G-protein complex10-12and this structure revealed the relationship of the repeat sequence to the β-propeller structure. The β-strands are offset relative to the blade structure of the propeller so that the first β-strand of the sequence repeat is the outermost strand of one blade, while the next three β-strands in the sequence make up the three inner β-strands of the next blade. This is illustrated in Figure 1, where the β-strands of the final sequence repeat (boxed sequence) are displayed in color on the structural model. The RAGVL sequence makes up the outer strand (strand D) of repeat 6, while strands A-C comprise the three inner strands of repeat 7. This offset arrangement of β-strands provides a mechanism to stably close the barrel structure; the seventh and final blade of the propeller is comprised of the three C-terminal β-strands, while the outermost β-strand is the N-terminal sequence at the beginning of the seven repeats. This closure mechanism has been termed N-terminal strand closure and is illustrated in Figure 1A, where the N-terminal β-strand sequence TRRTL is highlighted in Cyan in the sequence and on the structural models. N-terminal strand closure has been observed for all WD-repeat structures determined to date (Table 1).
Kelch repeats were first identified in the Drosophila cytoskeletal regulatory protein Kelch.3 They possess a consensus sequence distinct from that of WD-repeats and, at 44-56 amino acids in length, are generally slightly longer (Fig. 1B). Signature residues include a diglycine doublet near the beginning, followed by reasonably well-conserved Tyr, Trp and Arg residues at regular spacing throughout the remainder of the repeat. The structures of fungal galactose oxidase10 and the human13 and murine14 Keap1 Kelch-repeat domains have been determined. The overall structural organization of the Keap1 Kelch repeats is quite similar to that of Gβ and other β-propellers. Like Gβ, the sequence repeat is offset relative to the structural repeat and this again provides a mechanism for structurally closing the barrel. However, instead of the N-terminal strand closure mechanism used by WD repeat proteins, Keap1 uses C-terminal strand closure, where the C-terminal β-strand forms the interior-most strand of the first propeller blade (cyan in the sequence alignment and structures in Fig. 1B). In contrast, galactose oxidase uses N-terminal strand closure,10 demonstrating that either mechanism is possible for Kelch repeat proteins. However, sequence analysis revealed that three quarters of the human Kelch-repeat proteins are predicted to use C-terminal strand closure.8
For both WD-and Kelch-repeat proteins, the more highly conserved residues that define the repeat sequences are important for the structural integrity of the β-propeller. In WD-repeat proteins, a conserved tetrad of amino acids (Fig. 1A, asterisks) is hydrogen bonded.12,15 In the Kelch structures, the highly conserved diglycine doublet occurs after the B strand where the backbone adopts conformation that strongly favor glycines.16 The conserved Tyr, Trp and Arg residues in the C-terminal portion of the repeat all participate in hydrogen bonding interactions important for structural stability. The signature residues of WD and Kelch repeats thus are important for structural integrity, while the more variable sequences present in the loops create diverse surfaces that likely determine the functional characteristics of individual WD-and Kelch-repeat proteins.
Despite the remarkable structural similarity, WD-repeat and Kelch-repeat proteins are unlikely to have evolved from a single common ancestor, but instead appear to have evolved along parallel paths.17,18 β-propeller domain proteins likely arose from a single copy of the sequence repeat encoding a four-stranded β-sheet that underwent duplication events to generate a series of tandem repeats. Subsequently, the individual repeats likely diverged through sequence insertions, deletions and polymorphisms, leaving only the relatively few conserved residues important for maintaining the β-propeller fold.18-20 Interestingly, the protein tachylectin-2 is a five-bladed β-propeller made up of highly homologous sequence repeats distinct from WD or Kelch repeats. This suggests that tachylectin-2 is a relatively recent descendant from an ancestral sequence repeat.21 In contrast, the degenerate WD-and Kelch-repeat sequences clearly arose much earlier in evolution.
Both WD-and Kelch-repeat β-propellers are modular in nature. The β-propeller module can be present alone or at the amino- or carboxy-terminus of proteins that contain other structural motifs. The most common accompanying structural motifs mediate dimerization or oligomerization, or association with cullin-based ubiquitin E3 ligases (Table 2). In addition, examples have been found of proteins that contain two (or possibly more) β-propellers connected by linkers. The variety of modular contexts of β-propellers refects the functional diversity of the proteins that contain them.
Table 2
β-propeller domain organization in representative proteins.
Further Insights from Structures of WD-and Kelch-Repeat Proteins
In the past few years the structures of more than 12 WD-repeat proteins have been solved (Table 1), including a number as part of protein complexes or with peptide ligands bound. In addition, the structures of the mammalian Keap1 Kelch-repeat domains represent important additions to the sole previous Kelch-repeat structure, that of the galactose oxidase, which contains seven Kelch repeats. The vast majority of Kelch-repeat proteins in metazoans,8 as well as those that have been functionally characterized in Arabidopsis,22-24 contain six Kelch repeats and exhibit closer overall homology to Keap1 than to galactose oxidase. Thus the large number of WD-repeat protein structures, together with the few Kelch-repeat domain structures available, allows structural comparisons to be made.
A wide range of repeat numbers has been reported for both WD-and Kelch-repeat proteins. Numbers of WD repeats range from 4 to 14 in characterized proteins and a predicted protein with 18 WD repeats exists in the Pfam database.25 Recent WD-repeat structures provide important insights about how these proteins fold. First, WD proteins with fewer than seven identified repeats likely have additional “hidden” repeats that bring the number of blades to seven. This was the case for six WD-repeat proteins whose structures have been solved (Table 1) and in most cases, once the structure was determined it was possible to identify highly degenerate WD repeats within the protein sequence.9,26-28 Second, a number of WD-repeat proteins have been described containing greater than 10 tandem repeats and it was not clear whether these form single large propellers or fold into multiple smaller discrete propeller domains. The structure of Aip1p, an F-actin interacting protein that was reported to contain 10 WD repeats, revealed a “clamshell” shaped structure consisting of two 7-bladed propellers.27 In this case, the large number of repeats did not indicate a correspondingly large number of blades in a single propeller. These results suggest that there is an inherent stability in, or selection for, seven-bladed WD-repeat β-propellers. However, this is clearly not a rigid requirement: the WD-repeat domain in budding yeast Cdc4p contains eight repeats and forms an 8-bladed propeller.29 In addition, Sec13p, a COPII vesicle coat protein, contains six WD repeats and has no additional sequence that could harbor additional cryptic repeats and so most likely folds into a 6-bladed propeller.30
The number of Kelch repeats that can be identified in a protein also varies, though not to the same extent as WD-repeat proteins. The structures of galactose oxidase and the Kelch-repeat domain of Keap1 have seven and six blades, respectively. The horseshoe crab F-actin crosslinking protein α-scruin has 12 Kelch repeats, but they are in two clusters, each with six tandem repeats at either end of the protein separated by an intervening sequence. Cryo-EM data indicate that α-scruin has a “dumbbell” conformation, apparently with six-bladed β-propellers at either end of the protein.31 Thus, it appears that Kelch repeats form 6 or 7 bladed β-propellers, with 6 being more common. However, there are some examples of predicted proteins with fewer than six Kelch repeats8 for which no structural information is available. In addition, a large family of Kelch-repeat proteins has been identified in the Arabidopsis genome, where the proteins clearly contain only four repeats.32,33 These proteins all contain N-terminal F-boxes and thus are predicted to function as substrate-specific adapter proteins in ubiquitin E3 ligases. However, whether the Kelch repeats fold into typical β-propeller proteins is not clear. The predicted Arabidopsis F-box/4xKelch-repeat proteins also contain two absolutely conserved cysteine residues, raising the possibility that disulfide bridges may play a role in forming a modified Kelch-repeat propeller-like structure.32
Perhaps the most significant differences between the WD-and Kelch-repeat derived β-propellers are the sequences that connect the β-strands. These sequences emerge at the top or bottom of the propeller and therefore determine the characteristics of these surfaces. In many WD-repeat proteins, the loops between β-strands A and B (AB loops; top surface), as well as the BC loops (bottom surface), are short (3-5 amino acids) and show little variability. In contrast, the other two β-strand connecting loops, the CD (bottom) and DA (top) loops, are longer and more variable and create a significant portion of the accessible surface on the top and bottom of the propeller (Fig. 1A).
For Kelch-repeat proteins, the two loops on the bottom of the propeller (the AB and CD loops) are both short and well conserved. Instead, the longer and more variable loops (the BC and DA loops) both extend from the top surface. Thus the longer loops of the Keap1 propeller present a more elaborate top surface when its side view is compared with that of Gβ (Fig. 1B).
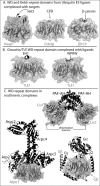
The observation that WD-repeat proteins have variable loops on both the top and bottom surfaces suggests an ability to use both surfaces for protein-protein interaction; indeed, some WD-repeat proteins that have co-evolved in multi-protein complexes do have interactions on both surfaces and even on the sides (see below). However, existing data do not support this idea as a general rule. Several structures have been solved with a WD-repeat protein bound to a known interacting protein in which the protein-protein binding interfaces are exclusively on the top face of the β-propeller (Fig. 2A,B). For example, the WD-repeat domains of Cdc4p and β-TrCP are substrate recruitment domains for ubiquitin E3 ligases and in each case, the bound peptide makes extensive contacts with residues in the variable loops that extend from the top surface. In addition, the WD-repeat domain of the Groucho/TLE transcriptional repressor protein TLE1 has been crystallized with peptides whose sequences are derived from two distinct classes of Groucho/ TLE interacting proteins.34 Both peptides bind on the top surface in the pocket created where the central channel of the propeller opens and both peptides form binding interactions with residues from each of the seven propeller blades. LIS1 has a well-characterized function regulating the microtubule motor dynein and in vertebrates is also known to associate with the dimeric enzyme Platelet-Activating Factor acetylhydrolase (PAF-AH). Murine LIS1 was crystallized in complex with PAF-AH and this structure revealed that the LIS1 WD-repeat domain makes extensive contacts with PAF-AH exclusively through its top face (Fig. 2C).35 Binding competition studies and mapping of conserved surface-exposed residues suggest that other LIS1 ligands compete with PAF-AH for binding to the top surface of the LIS1 β-propeller.
Two WD-repeat proteins, Gβ and ARPC1, were crystallized as parts of large multiprotein complexes (Fig. 2C).15,36 ARPC 1 is a component of the F-actin nucleating Arp2/3 complex and Gβ is an integral component of the G-protein heterotrimer. Both of these protein complexes are evolutionarily ancient, being present throughout all eukaryotic phyla. These structures reveal that within these complexes, WD-repeat proteins are indeed multivalent proteins making simultaneous contacts with multiple proteins. The Gα and Gγ proteins make extensive contacts with the sides and top of the Gβ propeller (Fig. 2C). The Arp2/3 components that bind ARPC1 make extensive contacts with top surface of the propeller; only the actin-related protein Arp2 is associated with the side of the propeller (Fig. 2C). However, additional known Arp2/3 interactions likely involve the bottom surface of the β-propeller. These include F-actin and Arp2/3 activating proteins of the Scar/Wasp family (discussed in reference 37). Indeed, there is a large patch of highly conserved surface-exposed residues on the bottom face of the ARPC1 β-propeller that likely forms an interaction surface.37
The structures of the Kelch-repeat domains from the mammalian Keap1 proteins were solved with peptides derived from Nrf2 (Fig. 2A).14,38 Like Cdc4p and β-TrCP, Keap1 is also a substrate-targeting component for a cullin-based ubiquitin E3 ligase and the only known target is the transcription factor Nrf2.39-41 Like the WD-repeat based E3 ligase substrate adaptors, the Nrf2 peptide is present in a pocket created by the channel on the top surface of the propeller. The Nrf2 peptide makes contacts with residues in the CB and DA loops from all but the third blade.
The structural information currently available suggests that β-propellers can act as a scaffold for multiple protein interactions or form a monovalent interaction surface. In cases where the β-propellers are components of ancient protein complexes such as the Arp2/3 complex or heterotrimeric G-proteins, multiple interactions have evolved. However, other WD-and Kelch-repeat proteins, notably the ubiquitin E3 ligase substrate adaptors, bind only one or several similar sequences. This may explain the expansion of WD-and Kelch-repeat proteins in higher eukaryotes; the β-propellers form a rigid structural domain, perhaps providing a stable platform on which the surface loops can evolve to form distinct protein-protein interaction domains.
Major Functional Classes of WD-and Kelch-Repeat Proteins
Both WD-and Kelch-repeat proteins have been demonstrated to participate in a wide variety of cellular and biochemical functions and new functions for these proteins will certainly be ascribed as additional proteins are functionally characterized. Comprehensive enumerations of functional classes for both WD-and Kelch-repeat proteins have been presented for both classes previously.1,6,7However, an examination of the molecular functions of WD-and Kelch-repeat proteins reveals particular functional classes that are common for both and other classes that are represented only by WD-repeat proteins but not Kelch-repeat proteins (Table 3).
Table 3
Representative functional classes of WD-and Kelch-repeat proteins.
Most prominent among the functional classes that are common between WD-and Kelch-repeat proteins are substrate adapters for ubiquitin E3 ligases. Ubiquitin E3 ligases mediate the conjugation of ubiquitin onto specific target proteins, typically to signal their destruction by the ubiquitin-proteasome pathway. Cullin Ring Ligases (CRLs) are a large class of E3 ligases that are assembled on a cullin scaffold protein. Cullin is an elongated protein that binds a substrate targeting adaptor protein at its N-terminus and a RING domain protein at its C-terminus.42 The RING domain protein recruits the ubiquitin E2 enzyme that catalyzes the transfer of ubiquitin to a substrate bound by the substrate-targeting component. In animals, there are multiple cullin proteins and they associate with distinct classes of substrate targeting components.
CRLs assembled with Cullin1 (CUL1) use F-Box proteins as substrate targeting components; the N-terminal F-Box mediates the association with Cul1 via the Skp1 adaptor protein and the C-terminus of the F-Box protein contains a substrate-binding domain.42 WD-and Kelch-repeat domains, among others, form the substrate binding domains for F-Box substrate adaptor proteins. Among the best characterized F-Box adaptor proteins are the WD-repeat proteins β-TrCP and Cdc4.43 In addition, more than 20 additional F-Box/WD-repeat proteins are predicted in the human genome (Table 2) and these are likely to also function as CRL substrate adaptors.42 In contrast, animal genomes appear to only contain one or two F-Box proteins with Kelch-repeat domains and these predicted proteins have not been characterized. Curiously, the prevalence of WD-and Kelch-repeat domains in Arabidopsis F-Box proteins is essentially the opposite as that in animal genomes. Only two F-Box proteins are paired with WD-repeat substrate binding domains,33 but over 40 F-Box proteins have a C-terminal Kelch-repeat domain.32,33 Several of these have been functionally characterized and are involved in degradation of circadian rhythm proteins.44-46
Cullin3 (CUL3) based E3 ligases use BTB proteins instead of F-Box proteins as substrate adaptors.47,48 The BTB domain binds directly with Cullin3 and an additional domain mediates substrate targeting, the most common of which in vertebrates is the Kelch-repeat domain.47 More than 40 genes encoding BTB-Kelch proteins have been identified in the human genome and most or all of these are likely to function as CUL3-associated substrate adaptor proteins. Thus far, no WD-repeat domains have been identified within BTB domain proteins (Table 2). The Arabidopsis genome, which appears to rely heavily on the Kelch-repeat domain as the substrate-binding domain in F-Box proteins, appears to contain only a few genes that contain a BTB domain paired with a Kelch-repeat domain.
The budding and fission yeast genomes contain only five F-Box/WD-repeat proteins and no BTB/Kelch proteins.42 Most of the CRL substrate adaptors from higher eukaryotes that have been characterized have only one known substrate (e.g., Keap1) or target several distinct proteins through a similar recognition motif in several proteins (e.g., Cdc4p). It thus appears that the WD-and Kelch-repeat domain CRL families underwent expansion as the additional regulatory requirements associated with multicellular life emerged. Presumably, genes encoding WD-and Kelch-repeat domain CRL substrate adaptors underwent duplication and divergence to evolve new target specifcities. The large number of WD-and Kelch-repeat domains in CRL substrate adaptors may be a consequence of their ability to rapidly evolve new binding affinities.
Another functional class of proteins containing many WD-and Kelch-repeat domains is cytoskeletal regulatory proteins (Table 3). WD-repeat domain proteins have diverse roles in regulating both the actin and microtubules. In some cases, the WD-domain makes direct contact with cytoskeletal filaments, as appears to be the case for the F-actin regulators of the Coronin-family49,50 and ARPC137and probably also Aip1p.51 Each of these WD-domain proteins makes contacts with other cytoskeletal proteins as well; thus the WD-repeat regulators of the actin cytoskeleton may coordinate the action of multiple F-actin regulatory proteins. Similarly, WD-repeat proteins that regulate the microtubule cytoskeleton appear to function in conjunction with other microtubule interacting proteins. These include LIS1, which interacts with both dynein and proteins associated with the growing ends of microtubules52 and the p80 subunit of the microtubule severing protein katanin.53 Of note, the Coronin protein has also been reported to bind microtubules and so may represent a protein that regulates both of these filament systems simultaneously.49 The common functional theme involves coordinating the action of cytoskeletal interacting proteins.
Most Kelch-repeat proteins involved in cytoskeletal regulation interact with F-actin. Indeed, a number of proteins, including Drosophila Kelch,54 Limulus α-Scruin55 and the mammalian proteins Mayven,56 IPP,57 ENC-1,58 actin-fragmin kinase59,60 and Calicin61 have all been shown to bind F-actin, but interactions with other cytoskeletal regulatory proteins have not been reported. Interestingly, Kelch, Mayven, IPP and ENC1 all have N-terminal BTB domains, raising the question of whether these proteins also associate with CUL3 to form E3 CRLs. We have found that in Drosophila, Kelch does in fact associate with CUL3 and that loss of CUL3 leads to a similar phenotype as that of Kelch (AH and LC, manuscript in preparation). However, we have seen no evidence that actin is a target for ubiquitination by a Kelch/CUL3 CRL, so understanding the distinct functions of F-actin binding and ubiquitin substrate targeting will await further work. Tea1p is the one Kelch repeat protein that appears to regulate the microtubule cytoskeleton.62 This protein contains N-terminal Kelch repeats and a C-terminal coiled-coil and is required for proper microtubule organization during polarized growth in S. pombe. Presently, it is not known whether Tea1p interacts directly with microtubules or microtubule regulatory proteins.
Functional groupings of WD-and Kelch-repeat proteins also reveal that WD-repeats are prominent in a number of functional classes where Kelch-repeats are poorly represented. The WD-repeat family contains a significant number of proteins involved in transcriptional regulation, RNA metabolism, vesicle trafficking and signal transduction; in contrast, there are few or no known examples of Kelch-repeat proteins that function in these pathways.
Conclusions
Recent advances on structures and functions of WD-and Kelch-repeat proteins have provided a better general understanding of these protein families. While folding into similar core structures, the active binding surfaces are distinct for the two classes of β-propellers. Variable surface loops are restricted to the top surface of Kelch-repeat β-propellers, while WD-repeat proteins have variable loops on both surfaces. This may explain some of the trends emerging from functional experiments. Kelch-repeat domains studied to date bind to only one partner and structural work on Keap1 suggests that this occurs on the top face of the propeller. In contrast, WD-repeats can have one or more binding partners, using binding sites on both the top and bottom of the β-propeller.
Acknowledgements
Our work on Kelch-related protiens is supported by NIH grant GM052702.
References
- 1.
- Neer E, Schmidt C, Nambudripad R. et al. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371(6495):297–300. [PubMed: 8090199]
- 2.
- Way M, Sanders M, Garcia C. et al. Sequence and domain organization of scruin, an actin-cross-linking protein in the acrosomal process of Limulus sperm. J Cell Biol. 1995;128(1-2):51–60. [PMC free article: PMC2120335] [PubMed: 7822422]
- 3.
- Xue F, Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72(5):681–693. [PubMed: 8453663]
- 4.
- Li D, Roberts R. WD-repeat proteins: structure characteristics, biological function and their involvement in human diseases. Cell Mol Life Sci. 2001;58(14):2085–2097. [PubMed: 11814058]
- 5.
- Smith T, Gaitatzes C, Saxena K. et al. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24(5):181–185. [PubMed: 10322433]
- 6.
- Yu L, Gaitatzes C, Neer E. et al. Thirty-plus functional families from a single motif. Protein Sci. 2000;9(12):2470–2476. [PMC free article: PMC2144505] [PubMed: 11206068]
- 7.
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10(1):17–24. [PubMed: 10603472]
- 8.
- Prag S, Adams J. Molecular phylogeny of the kelch-repeat superfamily reveals an expansion of BTB/kelch proteins in animals. BMC Bioinformatics. 2003;4(42) [PMC free article: PMC222960] [PubMed: 13678422]
- 9.
- Madrona A, Wilson D. The structure of Ski8p, a protein regulating mRNA degradation: Implications for WD protein structure. Protein Sci. 2004;13(6):1557–1565. [PMC free article: PMC2279974] [PubMed: 15152089]
- 10.
- Ito N, Phillips SE, Stevens C. et al. Novel thioether bond revealed by a 1.7 A crystal structure of galactose oxidase. Nature. 1991;350(6313):87–90. [PubMed: 2002850]
- 11.
- Lambright DG, Sondek J, Bohm A. et al. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379(6563):311–319. [PubMed: 8552184]
- 12.
- Sondek J, Bohm A, Lambright DG. et al. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379(6563):369–374. [PubMed: 8552196]
- 13.
- Li X, Zhang D, Hannink M. et al. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004;279(52):54750–54758. [PubMed: 15475350]
- 14.
- Padmanabhan B, Tong K, Ohta T. et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21(5):689–700. [PubMed: 16507366]
- 15.
- Wall M, Coleman D, Lee E. et al. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell. 1995;83(6):1047–1058. [PubMed: 8521505]
- 16.
- Beamer L, Li X, Bottoms C. et al. Conserved solvent and side-chain interactions in the 1.35 Angstrom structure of the Kelch domain of Keap1. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 10):1335–1342. [PubMed: 16204884]
- 17.
- Bork P, Doolittle R. Drosophila kelch motif is derived from a common enzyme fold. J Mol Biol. 1994;236(5):1277–1282. [PubMed: 8126718]
- 18.
- Paoli M. Protein folds propelled by diversity. Prog Biophys Mol Biol. 2001;76(1-2):103–130. [PubMed: 11389935]
- 19.
- Jawad-Alami Z, Paoli M. Novel sequences propel familiar folds. Structure. 2002;10(4):447–454. [PubMed: 11937049]
- 20.
- Murzin A. Structural principles for the propeller assembly of beta-sheets: the preference for seven-fold symmetry. Proteins. 1992;14(2):191–201. [PubMed: 1409568]
- 21.
- Beisel H, Kawabata S, Iwanaga S. et al. Tachylectin-2: crystal structure of a specific GlcNAc/GalNAc-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus. EMBO J. 1999;18(9):2313–2322. [PMC free article: PMC1171314] [PubMed: 10228146]
- 22.
- Kiyosue T, Wada M. LKP1 (LOV kelch protein 1): a factor involved in the regulation of flowering time in arabidopsis. Plant J. 2000;23(6):807–815. [PubMed: 10998191]
- 23.
- Nelson D, Lasswell J, Rogg L. et al. FKF1 a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell. 2000;101(3):331–340. [PubMed: 10847687]
- 24.
- Somers DE, Schultz TF, Milnamow M. et al. ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell. 2000;101(3):319–329. [PubMed: 10847686]
- 25.
- Finn RD, Mistry J, Schuster-Bockler B. et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34(Database issue):D247–251. [PMC free article: PMC1347511] [PubMed: 16381856]
- 26.
- Larsen N, Harrison S. Crystal structure of the spindle assembly checkpoint protein Bub3. J Mol Biol. 2004;344(4):885–892. [PubMed: 15544799]
- 27.
- Voegtli W, Madrona A, Wilson D. The structure of Aip1p, a WD repeat protein that regulates Cofilin-mediated actin depolymerization. J Biol Chem. 2003;278(36):34373–34379. [PubMed: 12807914]
- 28.
- Wilson D, Cerna D, Chew E. The 1.1-angstrom structure of the spindle checkpoint protein Bub3p reveals functional regions. J Biol Chem. 2005;280(14):13944–13951. [PubMed: 15644329]
- 29.
- Orlicky S, Tang X, Willems A. et al. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112(2):243–256. [PubMed: 12553912]
- 30.
- Garcia-Higuera I, Gaitatzes C, Smith T. et al. Folding a WD repeat propeller. Role of highly conserved aspartic acid residues in the G protein beta subunit and Sec13. J Biol Chem. 1998;273(15):9041–9049. [PubMed: 9535892]
- 31.
- Owen C, DeRosier D. A 13-A map of the actin-scruin filament from the limulus acrosomal process. J Cell Biol. 1993;123(2):337–344. [PMC free article: PMC2119840] [PubMed: 8408217]
- 32.
- Andrade M, González-Guzmán M, Serrano R. et al. A combination of the F-box motif and kelch repeats defines a large Arabidopsis family of F-box proteins. Plant Mol Biol. 2001;46(5):603–614. [PubMed: 11516153]
- 33.
- Kuroda H, Takahashi N, Shimada H. et al. Classification and expression analysis of Arabidopsis F-box-containing protein genes. Plant Cell Physiol. 2002;43(10):1073–1085. [PubMed: 12407186]
- 34.
- Jennings B, Pickles L, Wainwright S. et al. Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol Cell. 2006;22(5):645–655. [PubMed: 16762837]
- 35.
- Tarricone C, Perrina F, Monzani S. et al. Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44(5):809–821. [PubMed: 15572112]
- 36.
- Robinson R, Turbedsky K, Kaiser D. et al. Crystal structure of Arp2/3 complex. Science. 2001;294(5547):1679–1684. [PubMed: 11721045]
- 37.
- Beltzner C, Pollard T. Identification of functionally important residues of Arp2/3 complex by analysis of homology models from diverse species. J Mol Biol. 2004;336(2):551–565. [PubMed: 14757065]
- 38.
- Lo S, Li X, Henzl M. et al. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25(15):3605–3617. [PMC free article: PMC1538563] [PubMed: 16888629]
- 39.
- Cullinan SB, Gordan JD, Jin J. et al. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24(19):8477–8486. [PMC free article: PMC516753] [PubMed: 15367669]
- 40.
- Kobayashi A, Kang MI, Okawa H. et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. [PMC free article: PMC479737] [PubMed: 15282312]
- 41.
- Zhang DD, Lo SC, Cross JV. et al. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–10953. [PMC free article: PMC533977] [PubMed: 15572695]
- 42.
- Petroski M, Deshaies R. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20. [PubMed: 15688063]
- 43.
- Patton E, Willems A, Tyers M. Combinatorial control in ubiquitin-dependent proteolysis: don’t Skp the F-box hypothesis. Trends Genet. 1998;14(6):236–243. [PubMed: 9635407]
- 44.
- Han L, Mason M, Risseeuw E. et al. Formation of an SCF(ZTL) complex is required for proper regulation of circadian timing. Plant J. 2004;40(2):291–301. [PubMed: 15447654]
- 45.
- Imaizumi T, Schultz T, Harmon F. et al. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309(5732):293–297. [PubMed: 16002617]
- 46.
- Sawa M, Nusinow D, Kay S. et al. FKF1 and GIGANTEA Complex Formation is Required for Day-Length Measurement in Arabidopsis. Science. 2007;318(5848):261–265. [PMC free article: PMC3709017] [PubMed: 17872410]
- 47.
- Stogios P, Downs G, Jauhal J. et al. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005;6(10):R82. [PMC free article: PMC1257465] [PubMed: 16207353]
- 48.
- Xu L, We i Y, Reboul J. et al. BTB proteins are substrate specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425(6955):316–321. [PubMed: 13679922]
- 49.
- Rybakin V, Clemen C. Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. Bioessays. 2005;27(6):625–632. [PubMed: 15892111]
- 50.
- Uetrecht A, Bear J. Coronins: the return of the crown. Trends Cell Biol. 2006;16(8):421–426. [PubMed: 16806932]
- 51.
- Ono S. Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments. Biochemistry. 2003;42(46):13363–13370. [PubMed: 14621980]
- 52.
- Xiang X. LIS1 at the microtubule plus end and its role in dynein-mediated nuclear migration. J Cell Biol. 2003;160(3):289–290. [PMC free article: PMC2172655] [PubMed: 12566423]
- 53.
- McNally KP, Bazirgan OA, McNally FJ. Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci. 2000;113(Pt 9):1623–1633. [PubMed: 10751153]
- 54.
- Kelso R, Hudson A, Cooley L. Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J Cell Biol. 2002;156(4):703–713. [PMC free article: PMC2174084] [PubMed: 11854310]
- 55.
- Sun S, Footer M, Matsudaira P. Modification of Cys-837 identifies an actin-binding site in the beta-propeller protein scruin. Mol Biol Cell. 1997;8(3):421–430. [PMC free article: PMC276094] [PubMed: 9188095]
- 56.
- Soltysik-Espanola M, Rogers RA, Jiang S. et al. Characterization of Mayven, a novel actin-binding protein predominantly expressed in brain. Mol Biol Cell. 1999;10(7):2361–2375. [PMC free article: PMC25454] [PubMed: 10397770]
- 57.
- Kim IF, Mohammadi E, Huang RC. Isolation and characterization of IPP, a novel human gene encoding an actin-binding, kelch-like protein. Gene. 1999;228(1-2):73–83. [PubMed: 10072760]
- 58.
- Hernandez MC, Andres-Barquin PJ, Martinez S. et al. ENC-1: a novel mammalian kelch-related gene specifically expressed in the nervous system encodes an actin-binding protein. J Neurosci. 1997;17(9):3038–3051. [PMC free article: PMC6573641] [PubMed: 9096139]
- 59.
- Eichinger L, Bomblies L, Vandekerckhove J. et al. A novel type of protein kinase phosphorylates actin in the actin-fragmin complex. EMBO J. 1996;15(20):5547–5556. [PMC free article: PMC452299] [PubMed: 8896448]
- 60.
- Steinbacher S, Hof P, Eichinger L. et al. The crystal structure of the Physarum polycephalum actin-fragmin kinase: an atypical protein kinase with a specialized substrate-binding domain. EMBO J. 1999;18(11):2923–2929. [PMC free article: PMC1171374] [PubMed: 10357805]
- 61.
- Lecuyer C, Dacheux JL, Hermand E. et al. Actin-binding properties and colocalization with actin during spermiogenesis of mammalian sperm calicin. Biol Reprod. 2000;63(6):1801–1810. [PubMed: 11090452]
- 62.
- Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89(6):939–949. [PubMed: 9200612]
- 63.
- The PyMOL Molecular Graphics System (http://www
.pymol.org) [computer program]. Version 0.99: DeLano Scientific, Palo Alto, CA; 2002. - 64.
- Nolen B, Pollard T. Insights into the influence of nucleotides on actin family proteins from seven structures of Arp2/3 complex. Mol Cell. 2007;26(3):449–457. [PMC free article: PMC1997283] [PubMed: 17499050]
- 65.
- Wu G, Xu G, Schulman B. et al. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell. 2003;11(6):1445–1456. [PubMed: 12820959]
- 66.
- Appleton B, Wu P, Wiesmann C. The crystal structure of murine coronin-1: a regulator of actin cyto-skeletal dynamics in lymphocytes. Structure. 2006;14(1):87–96. [PubMed: 16407068]
- 67.
- Pickles L, Roe S, Hemingway E. et al. Crystal structure of the C-terminal WD40 repeat domain of the human Groucho/TLE1 transcriptional corepressor. Structure. 2002;10(6):751–761. [PubMed: 12057191]
- 68.
- Cerna D, Wilson D. The structure of Sif2p, a WD repeat protein functioning in the SET3 corepressor complex. J Mol Biol. 2005;351(4):923–935. [PubMed: 16051270]
- 69.
- Cheng Z, Liu Y, Wang C. et al. Crystal structure of Ski8p, a WD-repeat protein with dual roles in mRNA metabolism and meiotic recombination. Protein Sci. 2004;13(10):2673–2684. [PMC free article: PMC2001155] [PubMed: 15340168]
- 70.
- Sprague E, Redd M, Johnson A. et al. Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast. EMBO J. 2000;19(12):3016–3027. [PMC free article: PMC203344] [PubMed: 10856245]
- Phylogenetic, Structural and Functional Relationships between WD-and Kelch-Repea...Phylogenetic, Structural and Functional Relationships between WD-and Kelch-Repeat Proteins - Madame Curie Bioscience Database
- PROMOTER-ASSOCIATED LONG NONCODING RNAs REPRESS TRANSCRIPTION THROUGH AN RNA BIN...PROMOTER-ASSOCIATED LONG NONCODING RNAs REPRESS TRANSCRIPTION THROUGH AN RNA BINDING PROTEIN TLS - Madame Curie Bioscience Database
- The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exoc...The Role of Synaptotagmin and Synaptotagmin-Like Protein (Slp) in Regulated Exocytosis - Madame Curie Bioscience Database
- Circadian Organization in the Algal Flagellate Euglena - Madame Curie Bioscience...Circadian Organization in the Algal Flagellate Euglena - Madame Curie Bioscience Database
- Pro-Inflammatory Responses in Macrophages during Toxoplasma gondii Infection - M...Pro-Inflammatory Responses in Macrophages during Toxoplasma gondii Infection - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...