NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The WD repeat containing family of coronin proteins is generally referred to as F-actin-interacting proteins. While in lower eukaryotes such as Dictyostelium discoideum, the single short coronin protein regulates several F-actin dependent processes such as motility, phagocytosis and macropinocytosis, the function of any of the seven coronin isoforms in mammals is far less understood. This chapter describes the current knowledge on mammalian coronin 1 (coronin 1A), the closest homologue to Dictyostelium short coronin that is exclusively expressed in leukocytes. Recent work based on biochemical, molecular biological and genetic analysis suggest that coronin 1 has evolved a function that is quite different from the F-actin regulatory function of Dictyostelium short coronin. Rather, mammalian coronin 1 is involved in the regulation of leukocyte specific signaling events.
Introduction
Almost twenty years ago, a protein was isolated from the social amoeba Dictyostelium discoideum that was purified from precipitated actin/myosin complexes. Antibodies against this protein decorated the crown-shaped surface projections of growth phase Dictyostelium cells and hence the protein was termed ‘coronin’.1 The finding that coronin cosediments with F-actin in vitro and that a Dictyostelium mutant lacking coronin shows reduced motility and phagocytosis, both of which are dependent on F-actin, prompted the designation of coronin as an actin binding protein. The most striking feature of Dictyostelium coronin is the presence of a large, central WD (Tryp-Asp) repeat domain linked to a C-terminal coiled coil region. As a consequence, when mammalian proteins were identified harboring a similar central WD repeat followed by a coiled coil domain, they were referred to as members of the actin-binding protein family of coronins.
This chapter presents a different view for coronin 1 (coronin 1A), which is a leukocyte-specific, WD repeat containing protein with ~35% homology to Dictyostelium short coronin.2,3 Based on experimental evidence that will be discussed below, it will be argued that coronin 1 has a function unrelated to the regulation of F-actin dynamics, instead functioning in the regulation of leukocyte signaling processes.
Coronins From Unicellular Organisms
The identification of coronin in Dictyostelium was followed by the characterization of a coronin-related molecule in yeast.4 Unlike Dictyostelium that harbors three coronins, the single short coronin as well as a coronin 7 homologue (corA) and villidin (see Chapter 7), yeast contains a single coronin gene, termed crn1. Although, yeast coronin interacts with Arp2/3 in vitro,5 in contrast to the deletion of the short coronin in Dictyostelium, crn1-null mutants do not have any obvious phenotype and display normal F-actin dynamics.4
Coronins in Multicellular Invertebrate Organisms
Not much is known on the biology of coronins in the multicellular organisms Caenorhabditis elegans and Drosophila melanogaster. In C. elegans, a molecule named POD-1 that contains two stretches of WD repeats but no coiled coils, may be involved in the establishment of polarity in the developing embryo.6 In Drosophila, a Pod-1 homologue plays a role in axonal growth cone targeting.7 Besides Pod-1, both C. elegans as well as D. melanogaster contain a gene that encodes a protein (coro) that is far closer to Dictyostelium short coronin than Pod-1. However, while several Drosophila deletion mutants lacking coro show a rather pleiotropic phenotype, no information is available on a function for C. elegans coronin.
Mammalian Coronin 1
Coronin 1 (coronin 1A) is exclusively expressed in mammalian leukocytes, with no detectable expression in any other cell type (3 and data not shown). Several other coronin isoforms are expressed as well in leukocytes and therefore coronin 1 may have evolved to perform a highly specialized function in leukocytes. Interestingly, of all mammalian coronin isoforms, coronin 1 is the closest homologue to Dictyostelium short coronin. Whether or not the other coronin isoforms have evolved from coronin 1 or have arisen through a different evolutionary path is discussed to in Chapter 4.
Structure of Coronin 1
Coronin 1 Has a Three-Domain Structure
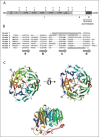
A detailed sequence analysis revealed that coronin 1 (coronin 1A) is made up of three distinct domains (see Fig. 1A): The first, N terminal domain that also contains the 5 WD repeats is rich in β-sheet and is referred to as β–propeller (residues 1-355). The second domain is comprised of a region with little regular secondary structure and is referred to as linker domain (residues 356-429). Finally, the third domain is a coiled coil containing segment which is rich in α-helices (residues 430-461).8
The Coronin 1 N-Terminal Domain Contains a 7-Bladed Propeller
One of the characteristic structure of all coronins are their central WD40 domains (see ref. 9 and Chapters 2 and 5). These repeats are characterized by a ~30-40 amino acid residue segment that are bordered by Gly-His (GH) and Trp-Asp (WD) peptide residues.10,11 The WD repeat unit was first recognized in the beta subunit of the GTP binding protein transducin.12 This domain seems to have evolved with the eukaryotic kingdom and may be involved in protein: protein interactions, although the precise function of the WD repeat domains in many proteins remains unknown.13,14
Coronin-1 possesses 5 WD repeats and based on the homology with the G protein beta subunits it has been proposed that the WD repeat folds into a 5-bladed beta propeller.9 However, an extensive sequence analysis of coronin 1 revealed the presence of two additional sequence stretches of 46 and 44 residues, respectively, that flank the WD repeat-containing core sequence and are predicted to form four short β-strands and align with the corresponding β-strands of the five WD repeats.8 Since WD repeats are not strictly necessary to assert a propeller fold,15-17 the prediction suggests that the coronin 1 propeller domain is, in fact, made up of at least seven blades instead of the previously proposed five blades.8 Consistent with this analysis, the crystal structure of coronin 1 indeed revealed the presence of a 7-bladed propeller18 (see Fig. 1B,C). Furthermore, the presence of a 7-bladed propeller in coronin 1 is consistent with the predicted similarity between the coronin 1 N-terminal domain and the yeast transcriptional repressor Tup119 as well as the G protein β-subunit,20 both WD repeat containing seven-bladed β-propeller proteins.
Coronin 1 Assembles into Coiled Coil Mediated Trimers
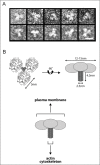
The C-terminal coiled coil in coronin 1 had been generally assumed to be involved in dimerization.9 However, observation of purified coronin 7 molecules isolated from macrophages by transmission electron microscopy revealed uniformly distributed particles with an apparent three-fold symmetry (Fig. 2). Further biochemical analysis suggested that the observed lobes apparent in Figure 2 correspond to the WD-repeat containing domains, that are assembled into trimers by virtue of the presence of the C-terminal coiled coil domain.8
The assembly of the coiled coil domain into trimers, rather than the previously assumed dimer was further confirmed by X-ray crystallography.21 Interestingly, it turned out that the coronin 1 coiled coil contains a distinct structural motif, encompassing specific networks of surface salt bridges that is conserved among a variety of trimeric coiled coils.21 In this light it will be interesting to analyze the biophysical and biochemical characteristics of the coiled coil domains from other coronin isoforms.
Function of Coronin 1 in Leukocytes
Coronin 1 (coronin 1A), in contrast to several ubiquitously expressed coronin isoforms, is exclusively expressed in leukocytes.3 Thus far, coronin 1 expression has been demonstrated in thymocytes, T-cells, macrophages and neutrophils.2,3,22-24 Morphological examination of coronin 1 in thymocytes and T-cells shows its accumulation at sites of membrane activity and actin rearrangement.22,23 It has recently been suggested that in T-cells, coronin 1 prevents F-actin induced apoptosis.23 However, in coronin 1 deficient mice all other leukocyte populations are present in normal numbers, arguing against a general role for coronin 1 in preventing apoptosis.
Coronin 1 in Macrophages
One of the first reports on a role for coronin 1 in leukocytes implicated coronin 1 in the intracellular survival of pathogenic mycobacteria.3 While most other bacteria upon internalization in macrophages are rapidly transferred from phagosomes to lysosomes followed by their destruction, mycobacteria, once internalized, actively block the fusion of phagosomes with lysosomes.25-27
A search for host molecules possibly involved in mediating the block in phagosome-lysosome fusion, identified coronin 1 (then known as TACO, for Tryptophan Aspartate containing Coat protein) as the sole detectable protein exclusively retained on mycobacterial phagosomes.3 Coronin 1, which in non-infected macrophages distributes equally between the cytosol and the membranes, is exclusively retained on phagosomes of macrophages infected with live mycobacteria, whereas in macrophages infected with dead mycobacteria, coronin 1 is initially co-internalized but rapidly dissociates from phagosomes. Following dissociation, the noncoated phagosomes fuse with or mature into lysosomes, resulting in the subsequent degradation of the internalized mycobacteria. This suggested an essential role for coronin 1 in preventing the fusion of mycobacterial phagosomes with lysosomes.3,28 Moreover, mycobacteria are effectively destroyed within Kupffer cells, the resident macrophages of the liver that do not express coronin 1.3 Interestingly, virulent strains of the human pathogen H. pylori are equally capable to actively retain coronin 1 at the phagosomal membrane upon internalization, suggesting that the coronin 1-mediated block in lysosomal fusion might be utilized also by other pathogens.29 However, whether or not pathogen specific molecules are involved in the active phagosomal retention of coronin 1 remains is as yet unclear.
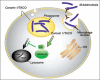
How coronin 1 mediates the survival of pathogenic mycobacteria has remained obscure. While a direct contribution of coronin 1 to the internalization of mycobacteria could be excluded early on, its molecular role remained enigmatic. The analysis of a genetic model for coronin 1, however, has recently shed some light on the molecular activities of coronin 1 in macrophages. It turned out that, while being fully dispensible for all F-actin-mediated functions analyzed (phagocytosis, macropinocytosis, motility), coronin 1 is required for the activation of the Ca2+ dependent phosphatase calcineurin.30 In wild type macrophages, upon internalization of mycobacteria this phosphatase becomes activated, thereby blocking phagosome-lysosome fusion by an as yet unknown mechanism and allowing mycobacterial survival (see also Fig. 3). In the absence of coronin 1, calcineurin activation does not occur resulting in phagosome-lysosome fusion and intracellular killing of the internalized mycobacteria. Strikingly, the genetic depletion of coronin 1 can be phenocopied by the addition of the calcineurin inhibitors cyclosporin A and FK506. Thus, it appears that coronin 1 has evolved to activate Ca2+ dependent signaling reactions in macrophages thereby promoting the survival of pathogenic mycobacteria.30 How, exactly, coronin 1 mediates the activation of calcineurin remains to be analyzed and may be related to its activity in modulating the association of membranes with the cytoskeleton.8
A role for coronin 1 in the regulation of signaling reactions in leukocytes is also consistent with its localization within cholesterol-enriched domains31,32 as well as to a possible role for protein kinase C in the modulation of its localization.33,34 Cholesterol-enriched domains are known to harbor a subset of molecules involved in signaling.35-37 Since in the absence of cholesterol mycobacteria are not even phagocytosed,31,38 it is well possible that mycobacteria have evolved mechanisms that ensure their uptake in a cholesterol dependent manner such that once they are internalized, they can reside within coronin 1-coated phagosomes. Consequently, the recruited coronin 1 then activates calcineurin resulting in blocking phagolysosome fusion and preventing degradation of the bacilli, thereby allowing the mycobacteria to survive intracellularly (Fig. 3).
Importantly, corroborating the original observation that coronin 1 was not involved in mycobacterial uptake, macrophages devoid of coronin 1 were perfectly capable to internalize mycobacteria as well as a range of other phagocytic cargo.30 Thus, in contrast to the situation in Dictyostelium, coronin 1 is dispensable for phagocytosis in macrophages. Also, several other actin-dependent processes, such as macropinocytosis, cell motility, cell spreading and membrane ruffling were unaffected in macrophages lacking coronin. These results strongly suggest that in macrophages, coronin 1 is dispensable for F-actin mediated processes.
Conclusions and Perspectives
Coronin 1 (coronin 1A) is probably one of the best-characterized members of the coronin protein family expressed in vertebrates. Emerging evidence suggests that, at least in macrophages, rather than being involved in F-actin mediated processes, coronin 1 modulates the activation of signaling reactions. In doing so, the presence or absence of coronin 1 regulates the intracellular trafficking as well as the survival of pathogenic mycobacteria. Since macrophages lacking coronin 1 are fully functional, the role for coronin 1 in uninfected cells remains enigmatic. The availability of different model systems is however likely to allow the dissection of the true role of this abundant molecule not only in macrophages but also in other leukocyte subpopulations.
Acknowledgement
I thank the members of my laboratory for many stimulating discussions, Benoit Combulazier, Rajesh Jayachandran, Philipp Mueller and Varadha Sundaramurthy for critical reading of the manuscript and Annette Roulier for assistance in the artwork. Research in my laboratory is supported by the Swiss National Science Foundation, the Roche Research Foundation, the Olga Mayenfisch Foundations as well as the Swiss Lung Liga and the Swiss Life Jubileum Stiftung.
References
- 1.
- de Hostos EL, Bradtke B, Lottspeich F. et al. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991;10(13):4097–4104. [PMC free article: PMC453159] [PubMed: 1661669]
- 2.
- Suzuki K, Nishihata J, Arai Y. et al. Molecular cloning of a novel actin-binding protein, p57, with a WD repeat and a leucine zipper motif. FEBS Lett. 1995;364(3):283–288. [PubMed: 7758584]
- 3.
- Ferrari G, Langen H, Naito M. et al. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97(4):435–447. [PubMed: 10338208]
- 4.
- Heil-Chapdelaine RA, Tran NK, Cooper JA. The role of Saccharomyces cerevisiae coronin in the actin and microtubule cytoskeletons. Curr Biol. 1998;8(23):1281–1284. [PubMed: 9822583]
- 5.
- Humphries CL, Balcer HI, D'Agostino JL. et al. Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J Cell Biol. 2002;159(6):993–1004. [PMC free article: PMC2173993] [PubMed: 12499356]
- 6.
- Rappleye CA, Paredez AR, Smith CW. et al. The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode caenorhabditis elegans. Genes Dev. 1999;13(21):2838–2851. [PMC free article: PMC317117] [PubMed: 10557211]
- 7.
- Rothenberg ME, Rogers SL, Vale RD. et al. Drosophila pod-1 crosslinks both actin and microtubules and controls the targeting of axons. Neuron. 2003;39(5):779–791. [PubMed: 12948445]
- 8.
- Gatfield J, Albrecht I, Zanolari B. et al. Association of the Leukocyte Plasma Membrane with the Actin Cytoskeleton through Coiled Coil-mediated Trimeric Coronin 1 Molecules. Mol Biol Cell. 2005;16(6):2786–2798. [PMC free article: PMC1142424] [PubMed: 15800061]
- 9.
- de Hostos EL. The coronin family of actin-associated proteins. Trends Cell Biol. 1999;9(9):345–350. [PubMed: 10461187]
- 10.
- van der Voorn L, Ploegh HL. The WD-40 repeat. FEBS Lett. 1992;307(2):131–134. [PubMed: 1644165]
- 11.
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252(5007):802–808. [PubMed: 1902986]
- 12.
- Neer EJ, Schmidt CJ, Nambudripad R. et al. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371(6495):297–300. [PubMed: 8090199]
- 13.
- Li D, Roberts R. WD-repeat proteins: structure characteristics, biological function and their involvement in human diseases. Cell Mol Life Sci. 2001;58(14):2085–2097. [PubMed: 11814058]
- 14.
- Yu L, Gaitatzes C, Neer E. et al. Thirty-plus functional families from a single motif. Protein Sci. 2000;9(12):2470–2476. [PMC free article: PMC2144505] [PubMed: 11206068]
- 15.
- Fulop V, Jones DT. Beta propellers: structural rigidity and functional diversity. Curr Opin Struct Biol. 1999;9(6):715–721. [PubMed: 10607670]
- 16.
- Smith TF, Gaitatzes C, Saxena K. et al. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24(5):181–185. [PubMed: 10322433]
- 17.
- Jawad Z, Paoli M. Novel sequences propel familiar folds. Structure (Camb). 2002;10(4):447–454. [PubMed: 11937049]
- 18.
- Appleton BA, Wu P, Wiesmann C. The crystal structure of murine coronin-1: a regulator of actin cytoskeletal dynamics in lymphocytes. Structure. 2006;14(1):87–96. [PubMed: 16407068]
- 19.
- Sprague ER, Redd MJ, Johnson AD. et al. Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast. EMBO J. 2000;19(12):3016–3027. [PMC free article: PMC203344] [PubMed: 10856245]
- 20.
- Lambright DG, Sondek J, Bohm A. et al. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379(6563):311–319. [PubMed: 8552184]
- 21.
- Kammerer RA, Kostrewa D, Progias P. et al. A conserved trimerization motif controls the topology of short coiled coils. Proc Natl Acad Sci USA. 2005;102(39):13891–13896. [PMC free article: PMC1236532] [PubMed: 16172398]
- 22.
- Nal B, Carroll P, Mohr E. et al. Coronin-1 expression in T-lymphocytes: insights into protein function during T-cell development and activation. Int Immunol. 2004;16(2):231–240. [PubMed: 14734608]
- 23.
- Foger N, Rangell L, Danilenko DM. et al. Requirement for coronin 1 in T-lymphocyte trafficking and cellular homeostasis. Science. 2006;313(5788):839–842. [PubMed: 16902139]
- 24.
- Grogan A, Reeves E, Keep N. et al. Cytosolic phox proteins interact with and regulate the assembly of coronin in neutrophils. J Cell Sci. 1997;110(Pt 24):3071–3081. [PubMed: 9365277]
- 25.
- Armstrong JA, Hart DPA. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. [PMC free article: PMC2139093] [PubMed: 15776571]
- 26.
- Russell DG. Mycobacterium tuberculosis: here today and here tomorrow. Nat Rev Mol Cell Biol. 2001;2(8):569–577. [PubMed: 11483990]
- 27.
- Pieters J. Manipulation of the Macrophage Response by Pathogenic Mycobacteria. In: Kaufmann Sea, Rubin E eds. Handbook of Tuberculosis: Molecular Biology and Biochemistry: Wiley-VCH. 2007
- 28.
- Tailleux L, Neyrolles O, Honore-Bouakline S. et al. Constrained intracellular survival of Mycobacterium tuberculosis in human dendritic cells. J Immunol. 2003;170(4):1939–1948. [PubMed: 12574362]
- 29.
- Zheng PY, Jones NL. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol. 2003;5(1):25–40. [PubMed: 12542468]
- 30.
- Jayachandran R, Sundaramurthy V, Combaluzier B. et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130(1):37–50. [PubMed: 17632055]
- 31.
- Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288(5471):1647–1650. [PubMed: 10834844]
- 32.
- Pieters J, Gatfield J. Hijacking the host: survival of pathogenic mycobacteria inside macrophages. Trends Microbiol. 2002;10(3):142–146. [PubMed: 11864824]
- 33.
- Itoh S, Suzuki K, Nishihata J. et al. The role of protein kinase C in the transient association of p57, a coronin family actin-binding protein, with phagosomes. Biol Pharm Bull. 2002;25(7):837–844. [PubMed: 12132654]
- 34.
- Reeves EP, Dekker LV, Forbes LV. et al. Direct interaction between p47phox and protein kinase C: evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem J. 1999;344(Pt 3):859–866. [PMC free article: PMC1220709] [PubMed: 10585874]
- 35.
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275(23):17221–17224. [PubMed: 10770957]
- 36.
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. [PubMed: 9177342]
- 37.
- Kaul D, Anand PK, Verma I. Cholesterol-sensor initiates M. tuberculosis entry into human macrophages. Mol Cell Biochem. 2004;258(1-2):219–222. [PubMed: 15030187]
- 38.
- Peyron P, Bordier C, N'Diaye EN. et al. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J Immunol. 2000;165(9):5186–5191. [PubMed: 11046051]
- Coronin 1 in Innate Immunity - Madame Curie Bioscience DatabaseCoronin 1 in Innate Immunity - Madame Curie Bioscience Database
- CTLA-4 in Type 1 Diabetes Mellitus - Madame Curie Bioscience DatabaseCTLA-4 in Type 1 Diabetes Mellitus - Madame Curie Bioscience Database
- Cell Adhesion Molecules - Madame Curie Bioscience DatabaseCell Adhesion Molecules - Madame Curie Bioscience Database
- Coronin Structure and Implications - Madame Curie Bioscience DatabaseCoronin Structure and Implications - Madame Curie Bioscience Database
- Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications f...Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications for Carcinoma Metastasis - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...