NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Chemokines are chemoattractant cytokines that play an important role in immunity. The role of chemokines against invading pathogens is emphasized by the expression of chemokine inhibitors by many pathogens. A mechanims employed by poxviruses and herpesviruses is the secretion of chemokine binding proteins unrelated to host receptors that bind chemokines with high affinity and block their activity. Soluble chemokine binding proteins have also been identified in the human parasite Schistosoma mansoniand in ticks. The binding specificity of these inhibitors of cell migration point at chemokines that contribute to host defense mechanisms against various pathogens. Chemokine binding proteins modulate the immune response and may lead to new therapeutic approaches to treat inflamatory diseases.
Modulation of the Chemokine System by Pathogens
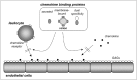
The infection with pathogens triggers signals that initiate the immune response and the recruitment of immune cells to sites of infection. The migration of leukocytes is largely controlled by chemokines, a family of chemoattractant cytokines that play a key role in inflammation and host defence against infectious agents.1-3 Members of the chemokine family share structural similarities and can be divided into four classes: CC, CXC, C and CX3C chemokines. The induction of particular chemokines together with the differential expression of specific seven-transmembrane-domain G-protein-coupled chemokine receptors by leukocyte subsets determines the immune cells that migrate towards sites of replication of pathogens within the animal host.
Chemokines interact with both their specific receptors and with cell surface glycosaminoglycans (GAGs) via distinct binding sites.4,5 Receptor binding is the means by which chemokines transduce their biological signals and trigger leukocyte migration. It is believed that under physiological conditions chemokines do not act in solution but are presented to chemokine receptors on leukocytes as ligands immobilized to a solid phase via interaction with GAGs. Disruption of either chemokine-receptor or chemokine-GAG complex formation might therefore inhibit chemokine biological activity.6,7
Infectious agents that replicate in an animal host must actively evade host immune defences that would otherwise eliminate them.8-10 The immune evasion strategies encoded by parasites, bacteria and viruses are diverse and illustrate an adaptation to their replication strategy either outside the cell or within cell compartments, ability to mutate, host cell and tissue tropism, transmission mechanism and genome coding capacity. The large DNA genome of poxviruses and herpesviruses allow these viruses, in contrast to the smaller RNA viruses, to encode many genes that control their interaction with the host cell and the immune system. One of the immune evasion strategies employed by poxviruses and herpesviruses is the molecular mimicry of cytokines and their receptors to intercept the cytokine networks that control the immune response to infection.11 Viral mechanisms that modulate the activity of chemokines include the expression of chemokine homologues, seven-transmembrane-domain chemokine receptor homologues or secreted chemokine binding protiens (CKBPs). More recently, some examples of CKBPs have been found in other pathogens different to viruses.
Here we review our current knowledge of pathogen-encoded CKBPs and describe their unique properties, mechanism of action and, if known, their contribution to pathogenesis.
The M-T7 Protein Encoded by Myxoma Virus (MYXV)
Myxomatosis is a severe disease of European rabbits that is caused by MYXV. Initial work identified the MYXV M-T7 gene encoding a 37-kDa glycoprotein that is abundantly secreted from infected cells.12 The M-T7 ORF showed significant similarity to the extracellular binding domain of human and mouse interferon-γ receptors (IFN-γRs) and was shown to bind to and inhibit the biological activity of rabbit IFN-γ in a species specific manner.12,13
M-T7 was also found to bind a broad range of C, CXC and CC chemokines and studies with CXCL8 mutants suggested that chemokine binding to M-T7 is via the conserved C-terminal GAG binding domain found in a variety of chemokines.14 This finding was unexpected and is a unique property of the IFN-γR encoded by MYXV since the IFN-γR orthologue encoded by VACV does not bind chemokines.14,15 The interaction of M-T7 with the chemokine GAG binding domains led to the suggestion that M-T7 might prevent the correct localization of chemokines and the formation of a chemokine gradient, rather than the blockade of chemokine binding to specific receptors.14
Table 1
CKBPs encoded by pathogens.
Infection of rabbits with a MYXV mutant with an inactivated M-T7gene demonstrated de contribution of M-T7 to MYXV pathogenesis in European rabbits.16 Marked differences were seen in the size and progression of skin lesions, the onset and severity of secondary bacterial infections and clearance of the virus. M-T7 was implicated in the control of migration of inflammatory cells to sites of infection. However, these results are difficult to interpret because M-T7 targets IFN-γ and chemokines and both have important roles in inflammatory responses.
The 35-kDa CKBP Encoded by Poxviruses
Poxvirus genomes encode a second class of CKBP that inhibits CC chemokines by interfering with the interaction of chemokines with their receptor.15,17,18 The major secreted protein of 35 kDa encoded by VACV strains Lister and rabbitpox was identified as a CKBP. This protein is not expressed by the most-commonly used VACV strains Western Reserve (WR) and Copenhagen. In the WR strain the protein is truncated and its promoter, known as the p7.5 early-late promoter, has been widely used in VACV vectors driving the expression of genes of interest. Parallel experiments with the variola virus (VARV) orthologue indicated that the virus which caused smallpox in humans also expressed this activity.18 The 35-kDa CKBP is also expressed by a wide variety of poxviruses including MYXV (M-T1 protein), cowpox virus (CPXV) and ectromelia virus (ECTV).18,19 Orf virus is a parapoxvirus that causes infections in sheep and cattle and sporadic infections in humans. It was shown that orf virus encodes a protein related to the 35-kDa CKBP but with a broader chemokine binding specificity that included the C chemokine lymphotactin in addition to CC chemokines.20 The binding of members of the 35-kDa CKBP family to numerous chemokines has demonstrated that it binds to almost all human and mouse CC chemokines with high affinity.21 Low-affinity binding to CXCL1 and CXCL8 has been detected, but it has not been possible to demonstrate binding to a range of other CXC, C or CX3C chemokines.15,18,21,22
The mechanism of action of the 35-kDa CKBP is competitive inhibition of CC-chemokine binding to cellular receptors, inhibiting as a consequence the induction of transient increases in calcium concentrations and the migration of cells along chemotactic gradients.15,18,22 The M-T1 protein of MYXV has the unique ability to interact with GAGs via a GAG binding domain at its C-terminus that is not present in other 35-kDa family members.23 This unique property of M-T1 would retain the protein in the vicinity of infected cells and may enhance its ability to protect the sites of infection from chemokine-mediated anti-viral responses.
The structure of the CPXV 35-kDa CKBP was determined by X-ray crystallography and it was shown to be a compact globular protein composed of two large parallel β-sheets, two short α-helices and several large connecting loops.24 The β sandwich topology of the protein is thought to be unique. By looking for exposed charged residues that have no apparent structural role and which are conserved among different members of the 35-kDa CKBP family, potential chemokine binding sites were suggested on the exposed face of β sheet II and at the edge of β sheet II. Further structural studies in solution by nuclear magnetic resonance demonstrated that the interface of interaction of the 35-kDa protein from VACV strain rabbitpox and human chemokine CCL2 involved the domains predicted from the initial crystalographic structure.25 The N-terminal residues of CCL2, as well as residues in the 20′s region and 40′s loop of the chemokine are involved in binding to the CKBP and established the structural basis for the ability of this vCKBP to promiscuously recognize CC chemokines. These results confirmed previous binding studies with human CCL2 mutants that identified the amino acid residues required for high affinity interaction with the VACV 35-kDa CKBP which were similar to those involved in CCL2-CCR2b chemokine receptor binding.26,27 The 35-kDa protein encoded by ECTV was also crystalized and shown to have a folding nearly identical to that of the CPXV and VACV proteins.28
The expression of an abundantly secreted CKBP would be expected to make a significant contribution to virus virulence in vivo by inhibiting CC-chemokine-mediated host inflammatory responses. Surprisingly, the experimental evidence in mouse and rabbit models of infection indicated that the 35-kDa protein encoded by VACV rabbitpox and MYXV inhibit the chemokine-mediated infiltration of immune cells into primary sites of infection but have little influence on the progression of disease.17,29,30 The expression of the 35-kDa protein from VACV WR, a strain that does not encode the CKBP, caused a slight attenuation of the virus associated with reduced inflammatory pathology in the lungs, suggesting that this CKBP may attenuate the immune-mediated pathology caused by VACV infection.31
The A41 Family of Poxvirus CKBPs
The VACV A41 protein has immunomodulatory activity and sequence similarity to the 35-kDa CKBP from VACV and other poxviruses. Deletion of the A41Lgene from VACV strain WR enhanced virulence slightly and showed an altered inflammatory response to infection in a dermal model.32 Clark et al33 showed that a VACV strain modified virus Ankara lacking the A41Lgene induced better protection than control virus. However, none of these studies identify the ligand(s) for A41. Two different groups carried out an extensive screening of chemokines by Surface Plasmon Resonance (SPR) for their potential binding to recombinant A41 from ECTV and VACV and recently reported the identification of a set of CC and CXC chemokines that interact with the A41 protein.34,35 In contrast to the poxvirus 35-kDa CKBP, the interaction of A41 with chemokines is inhibited in the presence of GAGs and the A41 protein does not block migration induced by chemokines. By using mutant forms of the chemokines CXCL10 and CXCL12α, which have reduced ability to interact with chemokine receptors or GAGs, Ruiz-Argüello et al35 showed that the A41 CKBP interacts with the GAG-binding domain of chemokines, suggesting that the property of this protein to inhibit leukocyte migration in vivo may be related to its ability to block the correct interaction of chemokines with GAGs, which is required for appropriate chemokine function in vivo.35
The crystal structure of the A41 protein from VACV was determined and found to be related to the 35-kDa CKBP, but has notable structural differences particularly in surface loops and electrostatic charge distribution.34 Although the A41-chemokine complex was not crystalized, structural modelling suggested that the interaction of A41 and 35-kDa proteins with chemokines involves the same domains. Based on the structural data, Bahar et al34 suggested a revised model in which the functional distinction between A41 and 35-kDa proteins arises from the differences in binding affinity. The binding site on chemokines for GAGs and chemokine receptors frequently overlap and the high affinity of the 35-kDa protein is sufficient to interfere with receptor binding while the lower affinity of A41 for chemokines can block GAG binding but not receptor binding.
The A41 and 35-kDa CKBPs from poxviruses are structurally related but block the chemokine system in different but complementary ways, probably being advantageous for the virus to control the host anti-viral responses.
A Family of Poxvirus Proteins Containing the Smallpox Virus-Encoded Chemokine Receptor (SECRET) Domain
The finding of the SECRET domain was related to the study of the VARV-encoded Cytokine response modifier B (CrmB). CrmB is one of the four tumor necrosis factor receptor (TNFR) homologues encoded by poxviruses, named CrmB, CrmC, CrmD and CrmE and the only one encoded by VARV, the causative agent of smallpox.36-39 A fifth homologue of the TNFR superfamily encoded by the poxviruses ECTV and CPXV is the viral homologue of CD30.40,41 Sequence comparison of the poxvirus TNFRs showed that the N-terminal region of each molecule shares amino acid sequence similarity with the cysteine-rich domain of the mammalian counterparts that interact with TNF. However, it was evident that CrmB and CrmD have an additional C-terminal extension that contributes to half of the molecule size and for which no similarity was found. The C-terminal domain is not required for TNF binding and no function was predicted.
To explore the immunomodulatory activity of VARV CrmB, Alejo et al42 expressed the protein in the baculovirus system to circumvent the direct manipulation of VARV, which is currently retricted to two high security laboratories.43 The purified recombinant CrmB was tested in a screening with different cytokines by SPR and it was found that CrmB not only bound TNF but also various chemokines. Independent expression of the N-terminal and C-terminal CrmB domains demonstrated that the C-terminal domain interacts with chemokines. An extensive screening of all human and mouse chemokines by SPR showed that CrmB interacted with a restricted set of chemokines with binding affinities similar to that of TNF.42 The human chemokines that best bound to VARV CrmB were CCL25, CCL28, CXCL12β, CXCL13 and CXCL14. Two additional chemokines (CCL27 and CXCL11) also interact with CrmB in the mouse system.
As the other CKBPs identified so far, the SECRET domain has no amino acid sequence similarity to host chemokine receptors or previously described CKBPs. However, the restricted chemokine binding specificity of the SECRET domain differs from the broad binding specificity of the previously identified CKBPs.
Given the sequence similarity between VARV CrmB and ECTV CrmD, this molecule was also tested for chemokine binding activity and shown to interact with the same limited set of human and mouse chemokines.42 The number of molecules belonging to the SECRET family increased following the analysis of poxviral genomes and identification of other gene products encoding SECRET domain-containing proteins (SCPs), which were predicted to be secreted and of previously unknown function. The CPXV strain Brighton Red protein V218 and the ECTV strain Naval proteins E12 and E184 were shown to bind chemokines and named SCP-1, 2 and 3, respectively.42 The fact that all members of the SECRET family bind the same set of chemokines, despite their relatively low sequence similarity, suggests that the SECRET domain has a specific folding, allowing it to bind chemokines with high affinity, either independently or fused to TNFRs.
The identification of the SECRET domain in five different poxvirus proteins is intriguing. This distribution may explain, in part, the variety of genes encoding TNFR homologues in poxvirus genomes, some of which (CrmB and CrmD) encode this additional chemokine-inhibitory activity. It may also provide the virus the ability to differentially block chemokines involved in controlling distinct antiviral responses, inhibit chemokines at different stages of infection in the animal host or simultaneously inhibit chemokines and TNF. It is likely that as poxviruses with narrow host species specificity adapted to particular hosts (i.e., VARV to humans or ECTV to mice), genes were selected to facilitate viral replication and transmission in each host.
The ability of VARV CrmB, CPXV CrmB, ECTV CrmD and CPXV SCP-1 to inhibity chemokine-induced migration in vitro has been demonstrated,42 but the immunomodulatory activity of the SECRET domain has yet to be defined in vivo. Deletion of the SCP B7 encoded by VACV strain WR had no major effects on virulence.44 It is interesting that the SECRET domain binds chemokines that are likely to be relevant in anti-viral defense: (i) chemokines mediating T-and B-cell recruitment that are expressed by epithelial cells in mucosal surfaces (CCL25 and CCL28) or the skin (CCL27),45-47 which constitute the sites of virus entry; (ii) CCL25 and CCL28 recruit IgA-producing B-cells to mucosal sites;47,48 (iii) CXCL14 is involved in dendritic cell migration to epidermal tissues;49 and (iv) CXCL13 attracts B-cells to the spleen and lymph nodes.46
The CPXV CrmB protein, which blocks both TNF and chemokines in vitro,42 has anti-inflammatory potential in vivo and increased approximately 50-fold the LD50 in infected mice.50 However, the CPXV strain used still contained other active vTNFRs and the model of intracranial infection used can hardly mimick the natural route of infection. The MYXV M-T2 protein, a secreted vTNFR sharing the same domain organization as CrmB and CrmD, acts as a virulence factor in european rabbits causing a slight reduction of mortality.51 However, none of these studies have defined the contribution of the anti-TNF vs anti-chemokine domains present in CrmB and CrmD to immune regulation and virulence.
The M3 Protein Encoded by Murine Gammaherpesvirus 68 (MHV-68)
MHV-68 is a pathogen of wild rodents that is related to the primate gammaherpesviruses Epstein-Barr virus, Kaposi's Sarcoma associated herpesvirus and Herpesvirus Saimiri. MHV-68 provides a useful mouse model of gammaherpesvirus pathogenesis. The M3 protein, unique to MHV-68 and not encoded by primate gamaherpesviruses, is a major secreted protein unrelated to host chemokine receptors that was identified as a CKBP.52,53 M3 was identified as a CKBP in chemokine binding assays but, unlike the poxvirus 35-kDa CKBP that is specific for CC chemokines, M3 is able to bind to CC, CXC, C and CX3C chemokines.52,53 Although M3 binds to chemokines of all four classes in the human and mouse systems, it is not likely to be an effective inhibitor of some CXC chemokines such as murine CXCL1 and CXCL5 or human CXCL12α.
The mechanism by which M3 inhibits chemokines is similar to that of the poxvirus 35-kDa protein. Both proteins bind free chemokine with high affinity in a manner which prevents their interaction with chemokine receptors and the induction of intracellular signalling events leading to cell migration and activation.52,53 M3 has been shown to display a distinct property of inhibiting the interaction of chemokines with GAGs.54 Moreover, it is able to disrupt preformed chemokine-GAG interactions in vitro, suggesting that M3 has a dual anti-chemokine function: the inhibition of chemokine-receptor and chemokin-GAG interactions.
The crystal structure of M3 complexed to a P8A variant of CCL2 showed that the dimerization of M3 brings in close proximity the N-terminal domain of a monomeric M3 molecule to the C-terminal domain of the second monomer to generate a binding site for chemokines.55 The crystal structure was in accordance with binding studies of mutant chemokines showing that M3 interacts with chemokine residues involved in receptor binding, providing the structural basis for the ability of M3 to inhibit chemokine binding to cellular receptors.55,56 Recent crystalographic studies have determined the structure of M3 complexed with wild type CCL2 or XCL1, showing that M3 engages the different chemokine classes with the same overall binding geometry.57 The M3 C-terminal domain engages conserved residues involved in receptor binding whereas the acidic N-terminal domain exhibits electrostatic complementarity contacting chemokine basic clusters involved in GAG association, providing the structural basis for the interference of chemokine-GAG interactions by M3.54
Studies on the role of the M3 protein in MHV-68 pathogenesis have provided conflicting results. After intranasal infection, MHV-68 replicates transiently in respiratory epithelial cells and spreads to lymphoid tissue where latency is established in B-lymphocytes, macrophages and dendritic cells. An initial report showed that targeted disruption of the M3gene had surprisingly little effect on lytic virus replication in the respiratory tract or the initial spread of virus to lymphoid tissues after intranasal inoculation.58 However, the mutant virus failed to establish normal levels of latency in splenic B-cells. Interestingly, in vivo CD8+ T-cell depletion largely reversed the phenotype, suggesting that chemokine neutralization by M3 may function to block CD8+ T-cell recruitment into lymphoid tissue and to enable the establishment of MHV-68 latency. A second report found no effect of the deletion of the M3 gene in the intranasal model, but demonstrated a role for M3 in controling brain inflammation in an intracranial model of infection.59
The Glycoprotein G (gG) Encoded by Alphaherpesviruses
The screening of supernatants from cultures infected with various alphaherpesviruses identified chemokine binding activity encoded by equine herpesvirus-1 (EHV-1), bovine herpesvirus 1 (BHV-1) and other alphaherpesviruses.60 The activity was mapped to gG, which is expressed as a membrane-anchored protein at the surface of the enveloped virus particles of alphaherpesviruses. The chemokine binding activity identified in the supernatants was a secreted version of gG generated after proteolytic cleavage of the membrane form. Chemokine binding activity was also observed at the surface of insect cells infected with a recombinant baculovirus expressing the full-length gG. This is the first CKBP known to be expressed both as a membrane-anchored protein and as a secreted polypeptide.
gG encoded by EHV-1, BHV-1 and BHV-5 were characterized in more detail and found to bind a variety of CC and CXC chemokines.60 These proteins block chemokine activity by interfering with chemokine interaction with cellular receptors and the subsequent activation of cell migration. EHV-1 gG was shown to inhibit chemotaxis of equine neutrophils by equine CXCL8.61 As described for the MHV-68 M3 protein, it also prevents the interaction of chemokines with GAGs, suggesting that gG blocks chemokine activity at two different levels.60
Felid herpesvirus 1 (FeHV-1) gG was also found to bind a variety of chemokines with high affinity and to inhibit chemokine activity.62 Studies on FeHV-1 demonstrated that the gG present at the surface of the virion binds chemokines.63
The contribution of gG to virus virulence has been addressed in several models of alphaherpesvirus infection. The infection of mice with low doses of an EHV-1 gG mutant led to an exacerbation of respiratory disease, with higher virus titers and a more pronounced inflammatory response in the lungs, compared to wild type infections.61 EHV-1 gG was also found to reduce the infiltration of mouse neutrophils and macrophages into the lungs of infected mice and the chemotactic function of CCL3 in mice.64Infectious laryngotracheitis virus (ILTV) is an alphaherpesvirus that causes acute respiratory disease in chicken. A gG-deficient mutant of ILTV was attenuated compared to wild type virus and caused an increased tracheal mucosal thikness that reflects increased inflammatory cell infiltration at the site of infection.65 These results suggest immunomodulatory activity of gG in this virus system, but the interaction of ILTV gG with chemokines has not beed formally demonstrated yet.
Chemokine binding activity has not been reported in the gG encoded by the human pathogens herpes simplex virus 1 (HSV-1) and HSV-2.60 Interestingly, in contrast to gG encoded by HSV-2, HSV-1 gG is not secreted and suggests different functions of these proteins. A mutant HSV-1 lacking gG expression was tested in a mouse ear model but marginal attenuation and no effect on the ability of the virus to establish latency in neurons was observed.66 Varicella zoster virus is an important human pathogen that causes a systemic disease but the gene encoding gG is not present in the varicella zoster virus genome.67 The ability of gG encoded by HSV-1 and HSV-2 to bind chemokines and to contribute to virus-mediated pathology remains to be elucidated.
The Secreted CKBP from Human Cytomegalovirus (HCMV)
The pUL21.5 protein encoded by HCMV is a small secreted glycoprotein that has been demonstrated to bind CCL5 with high affinity and to block the interaction of CCL5 with specific cellular receptors.68 This CKBP was reported to be highly specific for one chemokine, in contrast to the broad binding specificity of previously described virus-encoded CKBPs, but only three chemokines were tested in this study and the possibility that pUL21.5 binds some other chemokines of this complex family of chemoattractant cytokines cannot be ruled out. The mRNA encoding the pUL21.5 protein is packaged into virions and it was proposed that pUL12.5 may be expressed and secreted to modulate the host anti-viral response even before the newly infecting viral genome becomes transcriptionally active.68
A Schistosoma Mansoni-Encoded Secreted Chemokine Inhibitor
S. mansoniis a trematode parasite that infects humans, causing schistisomiasis, a disease that is common in the developing world and that cause severe disease in 10% of the infected individuals.69 In most cases, however, schistosomes are able to achieve chronic infections that cause high morbility. Schistosomes are particularly adept at manipulating the host's immune system to the benefit of the parasite. For example, the granulomatous inflammation around parasite eggs trapped in various organs, which, though a major cause of pathology, is evoked by the parasite to facilitate the expulsion of its eggs from the host. In situations where the formation of the granuloma is compromised, such as in immuno-supressed individuals, the excretion of eggs is diminished.70,71
A CKBP secreted by S. mansoniwas identified in a cross-linking assay with radiolabelled chemokines and named smCKBP.72 This protein was found to be expressed only in schistosome egg secretions but not in the other life cycle stages (cercariae, schistosomular, worms) of S. mansoni. Moreover, the smCKBP was also produced by eggs from the two other major schistosome species that infect man, S. haematobiumand S. japonicum. smCKBP was the first CKBP identified in a human pathogen and is the only one identified in a parasite to date.
The gene encoding smCKBP was identified following a proteomic approach.72 Despite having chemokine binding activity, smCKBP shares no amino acid sequence similarity to known viral CKBPs or mammalian proteins. Characterization of the binding properties showed that smCKBP interacts with several chemokines including CCL2, CCL3, CCL5, CXCL8 and CX3CL1. Furthermore, smCKBP was shown to prevent the interaction of chemokines with their specific cellular receptors and therefore chemokine-mediated cell activation and migration.
The study of the in vivo role of smCKBP might prove important to the understanding of the schistosomiasis pathology. It will be of interest to determine, in the murine model, the course of infection of a S. mansonimutant lacking smCKBP. This, however, awaits the development of methodologies for the construction of deletion mutants in this parasite. It has been shown that, in an experimental granulomatous inflammation model, secretion of smCKBP by live eggs profoundly modulated the differential recruitment of cells and the size of the egg granuloma,72 suggesting that smCKBP may ultimately facilitate granuloma formation and the propagation of the S. mansoni eggs.
Evasins, a Family of CKBPs in Ticks
A novel family of CKBPs, termed Evasins, has recently been described in ticks.73,74 The Evasin family comprises four members and are small proteins of 7-12 kDa that are produced in the tick saliva. Ticks are blood-sucking parasites that feed on their hosts for several days but cause no inflammatory response. It has been suggested that Evasins may help ticks to inhibit chemokine-mediated innate responses that protect from parasites. In contrast to the broad binding specificity of the first CKBPs identified in viruses (M-T7, 35-kDa or M3 proteins), evasins show a restricted chemokine binding specificity. Evasin-1 binds CCL3, CCL4 and CCL18, Evasin-3 binds CXCL8 and CXCL1 and Evasin-4 binds CCL5 and CCL11.73,74 Interestingly, it appears that ticks have evolved a family of CKBPs with narrow binding specificity as an alternative to a single CKBP with broad binding activity. Evasins block the interaction of chemokines with their cellular receptors and inhibit in this way chemokine-induced recruitment of leukocytes in vitro and in vivo.
The crystal structure of Evasin-1 and Evasin-3 has been determined and reveal novel protein folds.73 Both proteins are unrelated in amino acid sequence and in their secondary and terciary structure and interact with host chemokines presumably in different ways. The determination of the structure of Evasins complexed with chemokines will define the structural basis for the Evasin selectivity of chemokines.
The Evolutionary Origin of CKBPs and Their Potential Therapeutic Applications
The production of secreted versions of cytokine receptors to control the activity of cytokines is a mechanism employed by large DNA viruses and other pathogens. The same strategy is used by the immune system to limit the activity of cytokines in order to avoid immune pathology. For example, it is well documented the proteolytic cleavage of TNFRs to release the ectodomain that retains TNF binding activity, the secretion of type II IL-1 receptors to neutralize the activity of IL-1 or the production of secreted IL-18 binding proteins of structure different to that of the membrane-bound IL-18 receptor.75,76
Due to the structural nature of the seven-transmembrane-domain chemokine receptors, the production of secreted versions of these receptors is not feasable and alternative mechanisms have evolved to limit chemokine activity. The host immune system uses decoy receptors, such as the Duffy antigen receptor for chemokines (DARC), D6 or CCX-CKR, that bind chemokines but do not transduce signals and function as chemokine scavengers.77,78 Alternatively, the anti-inflammatory cytokine IL-10 decouples chemokine receptors from intracellular signaling processes, thereby allowing them to sequester chemokines without inducing biological responses.78 In contrast, viruses and other pathogens encode secreted proteins of unique structure not found in host proteins that are able to bind chemokines with high affinity and to block their activity.9,11
The chemokine family is complex, with more than 40 chemokine ligands3 and the binding properties of the CKBPs reflects the targeting of specific immune functions mediated by particular sets of chemokines. Some CKBPs, such as the gammaherpesvirus M3 or the poxvirus 35-kDa proteins,21,52,53 bind a broad range of chemokines, whereas a narrow binding specificity has been recently described for the poxvirus SECRET domain and the Evasins encoded by ticks.42,73 Future in vivo studies in animal models of infection will be highly relevant to understand the immune modulatory activity of the different CKBPs and will shed light into the physiological role of the target chemokines in immunity.
Another interesting property of some virus-encoded CKBPs is that they may inhibit the interaction of chemokines with specific receptors, blocking chemokine-induced signalling, or may interfere with chemokine-GAG interactions. It is likely that the ability of gammaherpesvirus M3 and alphaherpesvirus gG to inhibit the binding of chemokines to both receptors and GAGs may enhance the anti-chemokine activity of these CKBPs in vivo.54,57,60
The chemokine network is a major target for the development of drugs useful in the control of inflammatory diseases.6,7,79 The identification of CKBPs with no amino acid sequence similarity among themselves or to host receptors is providing us with novel protein structures capable of neutralizing chemokines and have great potential as new medicaments.80,81 Many studies have demonstrated the therapeutic value of CKBPs in different animal models of human diseases. For example, treatment with the poxvirus 35-kDa CKBP significantly reduced inflammation of the airway and lung parenchyma and improved the physiological function of the lungs during airway hyperreactivity in a mouse model of allergen-induced asthma.82 In another study, a single injection of MYXV M-T7 given to rats or rabbits caused a significant attenuation of restenosis, a response to vascular injury that leads to recurrent atherosclerotic plaque growth.83 The generation of transgenic mice expressing the gammaherpesvirus M3 protein has been an important tool to demonstrate the efficient blockade of chemokine-mediated cell migration in vivo by M3 and its ability to block intimal hyperplasia in response to arterial injury and the development of diabetes.84-87 The anti-inflammatory activity of the CKBPs encoded by S. mansoniand ticks has been shown in models of contact hypersensitivity model, pulmonary inflammation or arthritis.72,73 Future studies will no doubt further assess the utility of pathogen-encoded chemokine inhibitors in other models of inflammation. It is interesting that products derived from pathogenic organisms hold substantial promise for the treatment of human inflammatory diseases.81
The vCKBPs identified in the genome of pathogens were not predicted from sequence analysis to bind chemokines and it is possible that the secretion of CKBPs of unrelated structure is a strategy used by other pathogens. Further studies on chemokine inhibitors from pathogens will provide interesting information on the contribution of these immune evasion proteins to pathogenesis and the function of chemokines in immunity.
Acknowledgments
We acknowledge the support from the Wellcome Trust, Spanish Ministry of Science and Innovation, Comunidad de Madrid and European Union.
References
- 1.
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392(6676):565–568. [PubMed: 9560152]
- 2.
- Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001;2(2):95–101. [PubMed: 11175800]
- 3.
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7(12):243. [PMC free article: PMC1794421] [PubMed: 17201934]
- 4.
- Johnson Z, Proudfoot AE, Handel TM. Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005;16(6):625–636. [PubMed: 15990353]
- 5.
- Handel TM, Johnson Z, Crown SE. et al. Regulation of protein function by glycosaminoglycans-as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. [PubMed: 15952892]
- 6.
- Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2(2):106–115. [PMC free article: PMC7097668] [PubMed: 11910892]
- 7.
- Wells TN, Power CA, Shaw JP. et al. Chemokine blockers—therapeutics in the making? Trends Pharmacol Sci. 2006;27(1):41–47. [PubMed: 16310864]
- 8.
- Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767–782. [PubMed: 16497587]
- 9.
- Seet BT, Johnston JB, Brunetti CR. et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. [PubMed: 12543935]
- 10.
- Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunol Today. 2000;21(9):447–455. [PMC free article: PMC7141567] [PubMed: 10953097]
- 11.
- Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol. 2003;3(1):36–50. [PubMed: 12511874]
- 12.
- Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258(5086):1369–1372. [PubMed: 1455233]
- 13.
- Mossman K, Upton C, McFadden G. The myxoma virus-soluble interferon-gamma receptor homolog, M-T7, inhibits interferon-gamma in a species-specific manner. J Biol Chem. 1995;270(7):3031–3038. [PubMed: 7852384]
- 14.
- Lalani AS, Graham K, Mossman K. et al. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J Virol. 1997;71(6):4356–4363. [PMC free article: PMC191652] [PubMed: 9151824]
- 15.
- Alcami A, Symons JA, Collins PD. et al. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160(2):624–633. [PubMed: 9551896]
- 16.
- Mossman K, Nation P, Macen J. et al. Myxoma virus M-T7, a secreted homolog of the interferon-gamma receptor, is a critical virulence factor for the development of myxomatosis in euroapean rabbits. Virology. 1996;215(1):17–30. [PubMed: 8553583]
- 17.
- Graham KA, Lalani AS, Macen JL. et al. The T1/35 kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology. 1997;229(1):12–24. [PubMed: 9123853]
- 18.
- Smith CA, Smith TD, Smolak PJ. et al. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta chemokine activity yet lacks sequence homology to known chemokine receptors. Virology. 1997;236(2):316–327. [PubMed: 9325239]
- 19.
- Smith VP, Alcami A. Expression of secreted cytokine and chemokine inhibitors by ectromelia virus. J Virol. 2000;74(18):8460–8471. [PMC free article: PMC116357] [PubMed: 10954546]
- 20.
- Seet BT, McCaughan CA, Handel TM. et al. Analysis of an orf virus chemokine-binding protein: shifting ligand specificities among a family of poxvirus viroceptors. Proc Natl Acad Sci USA. 2003;100(25):15137–15142. [PMC free article: PMC299921] [PubMed: 14657392]
- 21.
- Burns JM, Dairaghi DJ, Deitz M. et al. Comprehensive mapping of poxvirus vCCI chemokine-binding protein. Expanded range of ligand interactions and unusual dissociation kinetics. J Biol Chem. 2002;277(4):2785–2789. [PubMed: 11696549]
- 22.
- Lalani AS, Ness TL, Singh R. et al. Functional comparisons among members of the poxvirus T1/35 kDa family of soluble CC-chemokine inhibitor glycoproteins. Virology. 1998;250(1):173–184. [PubMed: 9770431]
- 23.
- Seet BT, Barrett J, Robichaud J. et al. Glycosaminoglycan binding properties of the myxoma virus CC-chemokine inhibitor, M-T 1. J Biol Chem. 2001;276(32):30504–30513. [PubMed: 11369757]
- 24.
- Carfi A, Smith CA, Smolak PJ. et al. Structure of a soluble secreted chemokine inhibitor vCCI (p35) from cowpox virus. Proc Natl Acad Sci USA. 1999;96(22):12379–12383. [PMC free article: PMC22925] [PubMed: 10535930]
- 25.
- Zhang L, Derider M, McCornack MA. et al. Solution structure of the complex between poxvirus-encoded CC chemokine inhibitor vCCI and human MIP-1beta. Proc Natl Acad Sci USA. 2006;103(38):13985–13990. [PMC free article: PMC1599900] [PubMed: 16963564]
- 26.
- Beck CG, Studer C, Zuber JF. et al. The viral CC chemokine-binding protein vCCI inhibits monocyte chemoattractant protein-1 activity by masking its CCR2B-binding site. J Biol Chem. 2001;276(46):43270–43276. [PubMed: 11551937]
- 27.
- Seet BT, Singh R, Paavola C. et al. Molecular determinants for CC-chemokine recognition by a poxvirus CC-chemokine inhibitor. Proc Natl Acad Sci USA. 2001;98(16):9008–9013. [PMC free article: PMC55364] [PubMed: 11470923]
- 28.
- Arnold PL, Fremont DH. Structural determinants of chemokine binding by an ectromelia virus-encoded decoy receptor. J Virol. 2006;80(15):7439–7449. [PMC free article: PMC1563704] [PubMed: 16840324]
- 29.
- Lalani AS, Masters J, Graham K. et al. Role of the myxoma virus soluble CC-chemokine inhibitor glycoprotein, M-T1, during myxoma virus pathogenesis. Virology. 1999;256(2):233–245. [PubMed: 10191189]
- 30.
- Martinez-Pomares L, Thompson JP, Moyer RW. Mapping and investigation of the role in pathogenesis of the major unique secreted 35-kDa protein of rabbitpox virus. Virology. 1995;206(1):591–600. [PubMed: 7831815]
- 31.
- Reading PC, Symons JA, Smith GL. A soluble chemokine-binding protein from vaccinia virus reduces virus virulence and the inflammatory response to infection. J Immunol. 2003;170(3):1435–1442. [PubMed: 12538705]
- 32.
- Ng A, Tscharke DC, Reading PC. et al. The vaccinia virus A41L protein is a soluble 30 kDa glycoprotein that affects virus virulence. J Gen Virol. 2001;82(Pt 9):2095–2105. [PubMed: 11514718]
- 33.
- Clark RH, Kenyon JC, Bartlett NW. et al. Deletion of gene A41L enhances vaccinia virus immunogenicity and vaccine efficacy. J Gen Virol. 2006;87(Pt 1):29–38. [PubMed: 16361415]
- 34.
- Bahar MW, Kenyon JC, Putz MM. et al. Structure and function of A41, a vaccinia virus chemokine binding protein. PLoS Pathog. 2008;4(1):e5. [PMC free article: PMC2211551] [PubMed: 18208323]
- 35.
- Ruiz-Arguello MB, Smith VP, Campanella GS. et al. An ectromelia virus protein that interacts with chemokines through their glycosaminoglycan binding domain. J Virol. 2008;82(2):917–926. [PMC free article: PMC2224573] [PubMed: 18003726]
- 36.
- Hu FQ, Smith CA, Pickup DJ. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204(1):343–356. [PubMed: 8091665]
- 37.
- Loparev VN, Parsons JM, Knight JC. et al. A third distinct tumor necrosis factor receptor of orthopoxviruses. Proc Natl Acad Sci USA. 1998;95(7):3786–3791. [PMC free article: PMC19915] [PubMed: 9520445]
- 38.
- Saraiva M, Alcami A. CrmE, a novel soluble tumor necrosis factor receptor encoded by poxviruses. J Virol. 2001;75(1):226–233. [PMC free article: PMC113916] [PubMed: 11119592]
- 39.
- Smith CA, Hu FQ, Smith TD. et al. Cowpox virus genome encodes a second soluble homologue of cellular TNF receptors, distinct from CrmB, that binds TNF but not LT alpha. Virology. 1996;223(1):132–147. [PubMed: 8806547]
- 40.
- Panus JF, Smith CA, Ray CA. et al. Cowpox virus encodes a fifth member of the tumor necrosis factor receptor family: a soluble, secreted CD30 homologue. Proc Natl Acad Sci USA. 2002;99(12):8348–8353. [PMC free article: PMC123070] [PubMed: 12034885]
- 41.
- Saraiva M, Smith P, Fallon PG. et al. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J Exp Med. 2002;196(6):829–839. [PMC free article: PMC2194064] [PubMed: 12235215]
- 42.
- Alejo A, Ruiz-Arguello MB, Ho Y. et al. A chemokine-binding domain in the tumor necrosis factor receptor from variola (smallpox) virus. Proc Natl Acad Sci USA. 2006;103(15):5995–6000. [PMC free article: PMC1458686] [PubMed: 16581912]
- 43.
- Smith GL, McFadden G. Smallpox: anything to declare? Nat Rev Immunol. 2002;2(7):521–527. [PubMed: 12094226]
- 44.
- Price N, Tscharke DC, Hollinshead M. et al. Vaccinia virus gene B7R encodes an 18-kDa protein that is resident in the endoplasmic reticulum and affects virus virulence. Virology. 2000;267(1):65–79. [PubMed: 10648184]
- 45.
- Homey B, Alenius H, Muller A. et al. CCL27-CCR10 interactions regulate T-cell-mediated skin inflammation. Nat Med. 2002;8(2):157–165. [PubMed: 11821900]
- 46.
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16(1):1–4. [PubMed: 11825560]
- 47.
- Lazarus NH, Kunkel EJ, Johnston B. et al. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170(7):3799–3805. [PubMed: 12646646]
- 48.
- Bowman EP, Kuklin NA, Youngman KR. et al. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med. 2002;195(2):269–275. [PMC free article: PMC2193602] [PubMed: 11805153]
- 49.
- Schaerli P, Willimann K, Ebert LM. et al. Cutaneous CXCL14 targets blood precursors to epidermal niches for langerhans cell differentiation. Immunity. 2005;23(3):331–342. [PubMed: 16169505]
- 50.
- Palumbo GJ, Buller RM, Glasgow WC. Multigenic evasion of inflammation by poxviruses. J Virol. 1994;68(3):1737–1749. [PMC free article: PMC236634] [PubMed: 8107235]
- 51.
- Upton C, Macen JL, Schreiber M. et al. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184(1):370–382. [PubMed: 1651597]
- 52.
- Parry CM, Simas JP, Smith VP. et al. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J Exp Med. 2000;191(3):573–578. [PMC free article: PMC2195820] [PubMed: 10662803]
- 53.
- van Berkel V, Barrett J, Tiffany HL. et al. Identification of a gammaherpesvirus selective chemokine binding protein that inhibits chemokine action. J Virol. 2000;74(15):6741–6747. [PMC free article: PMC112190] [PubMed: 10888612]
- 54.
- Webb LM, Smith VP, Alcami A. The gammaherpesvirus chemokine binding protein can inhibit the interaction of chemokines with glycosaminoglycans. Faseb J. 2004;18(3):571–573. [PubMed: 14734646]
- 55.
- Alexander JM, Nelson CA, van Berkel V. et al. Structural basis of chemokine sequestration by a herpesvirus decoy receptor. Cell. 2002;111(3):343–356. [PubMed: 12419245]
- 56.
- Webb LM, Clark-Lewis I, Alcami A. The gammaherpesvirus chemokine binding protein binds to the N terminus of CXCL8. J Virol. 2003;77(15):8588–8592. [PMC free article: PMC165246] [PubMed: 12857930]
- 57.
- Alexander-Brett JM, Fremont DH. Dual GPCR and GAG mimicry by the M3 chemokine decoy receptor. J Exp Med. 2007;204(13):3157–3172. [PMC free article: PMC2150966] [PubMed: 18070938]
- 58.
- Bridgeman A, Stevenson PG, Simas JP. et al. A secreted chemokine binding protein encoded by murine gammaherpesvirus-68 is necessary for the establishment of a normal latent load. J Exp Med. 2001;194(3):301–312. [PMC free article: PMC2193474] [PubMed: 11489949]
- 59.
- van Berkel V, Levine B, Kapadia SB. Critical role for a high-affinity chemokine-binding protein in gamma-herpesvirus-induced lethal meningitis. J Clin Invest. 2002;109(7):905–914. [PMC free article: PMC150927] [PubMed: 11927617]
- 60.
- Bryant NA, Davis-Poynter N, Vanderplasschen A. et al. Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. Embo J. 2003;22(4):833–846. [PMC free article: PMC145452] [PubMed: 12574120]
- 61.
- Van de Walle GR, May ML, Sukhumavasi W. et al. Herpesvirus chemokine-binding glycoprotein G (gG) efficiently inhibits neutrophil chemotaxis in vitro and in vivo. J Immunol. 2007;179(6):4161–4169. [PubMed: 17785855]
- 62.
- Costes B, Ruiz-Arguello MB, Bryant NA. et al. Both soluble and membrane-anchored forms of felid herpesvirus 1 glycoprotein G function as a broad-spectrum chemokine-binding protein. J Gen Virol. 2005;86(Pt 12):3209–3214. [PubMed: 16298965]
- 63.
- Costes B, Thirion M, Dewals B. et al. Felid herpesvirus 1 glycoprotein G is a structural protein that mediates the binding of chemokines on the viral envelope. Microbes Infect. 2006;8(11):2657–2667. [PubMed: 16962359]
- 64.
- Van de Walle GR, Sakamoto K, Osterrieder N. CCL3 and viral chemokine-binding protein gg modulate pulmonary inflammation and virus replication during equine herpesvirus 1 infection. J Virol. 2008;82(4):1714–1722. [PMC free article: PMC2258710] [PubMed: 18077722]
- 65.
- Devlin JM, Browning GF, Hartley CA. et al. Glycoprotein G is a virulence factor in infectious laryngotracheitis virus. J Gen Virol. 2006;87(Pt 10):2839–2847. [PubMed: 16963741]
- 66.
- Balan P, Davis-Poynter N, Bell S. et al. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75(Pt 6):1245–1258. [PubMed: 8207391]
- 67.
- Gomi Y, Sunamachi H, Mori Y. et al. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J Virol. 2002;76(22):11447–11459. [PMC free article: PMC136748] [PubMed: 12388706]
- 68.
- Wang D, Bresnahan W, Shenk T. Human cytomegalovirus encodes a highly specific RANTES decoy receptor. Proc Natl Acad Sci USA. 2004;101(47):16642–16647. [PMC free article: PMC534536] [PubMed: 15536129]
- 69.
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2(7):499–511. [PubMed: 12094224]
- 70.
- Fallon PG, Richardson EJ, Smith P. et al. Elevated type 1, diminished type 2 cytokines and impaired antibody response are associated with hepatotoxicity and mortalities during Schistosoma mansoni infection of CD4-depleted mice. Eur J Immunol. 2000;30(2):470–480. [PubMed: 10671202]
- 71.
- Karanja DM, Colley DG, Nahlen BL. et al. Studies on schistosomiasis in Western Kenya: I. evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56(5):515–521. [PubMed: 9180601]
- 72.
- Smith P, Fallon RE, Mangan NE. et al. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med. 2005;202(10):1319–1325. [PMC free article: PMC2212990] [PubMed: 16301741]
- 73.
- Deruaz M, Frauenschuh A, Alessandri AL. et al. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med. 2008;205(9):2019–2031. [PMC free article: PMC2526197] [PubMed: 18678732]
- 74.
- Frauenschuh A, Power CA, Deruaz M. et al. Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus. J Biol Chem. 2007;282(37):27250–27258. [PubMed: 17640866]
- 75.
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S1–13. [PubMed: 14989423]
- 76.
- Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–371. [PubMed: 12033742]
- 77.
- Graham GJ, McKimmie CS. Chemokine scavenging by D6: a movable feast? Trends Immunol. 2006;27(8):381–386. [PubMed: 16814608]
- 78.
- Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6(12):907–918. [PubMed: 17124512]
- 79.
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190. [PubMed: 16369557]
- 80.
- Lucas A, McFadden G. Secreted immunomodulatory viral proteins as novel biotherapeutics. J Immunol. 2004;173(8):4765–4774. [PubMed: 15470015]
- 81.
- Fallon PG, Alcami A. Pathogen-derived immunomodulatory molecules: future immunotherapeutics? Trends Immunol. 2006;27(10):470–476. [PubMed: 16920025]
- 82.
- Dabbagh K, Xiao Y, Smith C. et al. Local blockade of allergic airway hyperreactivity and inflammation by the poxvirus-derived pan-CC-chemokine inhibitor vCCI. J Immunol. 2000;165(6):3418–3422. [PubMed: 10975861]
- 83.
- Liu L, Lalani A, Dai E. et al. The viral anti-inflammatory chemokine-binding protein M-T7 reduces intimal hyperplasia after vascular injury. J Clin Invest. 2000;105(11):1613–1621. [PMC free article: PMC300852] [PubMed: 10841520]
- 84.
- Jensen KK, Chen SC, Hipkin RW. et al. Disruption of CCL21-induced chemotaxis in vitro and in vivo by M3, a chemokine-binding protein encoded by murine gammaherpesvirus 68. J Virol. 2003;77(1):624–630. [PMC free article: PMC140591] [PubMed: 12477865]
- 85.
- Martin AP, Alexander-Brett JM, Canasto-Chibuque C. et al. The chemokine binding protein M3 prevents diabetes induced by multiple low doses of streptozotocin. J Immunol. 2007;178(7):4623–4631. [PubMed: 17372021]
- 86.
- Martin AP, Canasto-Chibuque C, Shang L. et al. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol. 2006;177(10):7296–7302. [PubMed: 17082648]
- 87.
- Pyo R, Jensen KK, Wiekowski MT. et al. Inhibition of intimal hyperplasia in transgenic mice conditionally expressing the chemokine-binding protein M3. Am J Pathol. 2004;164(6):2289–2297. [PMC free article: PMC1615775] [PubMed: 15161661]
- Modulation of the Chemokine System by Pathogens
- The M-T7 Protein Encoded by Myxoma Virus (MYXV)
- The 35-kDa CKBP Encoded by Poxviruses
- The A41 Family of Poxvirus CKBPs
- A Family of Poxvirus Proteins Containing the Smallpox Virus-Encoded Chemokine Receptor (SECRET) Domain
- The M3 Protein Encoded by Murine Gammaherpesvirus 68 (MHV-68)
- The Glycoprotein G (gG) Encoded by Alphaherpesviruses
- The Secreted CKBP from Human Cytomegalovirus (HCMV)
- A Schistosoma Mansoni-Encoded Secreted Chemokine Inhibitor
- Evasins, a Family of CKBPs in Ticks
- The Evolutionary Origin of CKBPs and Their Potential Therapeutic Applications
- Acknowledgments
- References
- Chemokine Binding Proteins Encoded by Pathogens - Madame Curie Bioscience Databa...Chemokine Binding Proteins Encoded by Pathogens - Madame Curie Bioscience Database
- Cytokines, Receptors and Signalling Pathways Involved in Macrophage and Dendriti...Cytokines, Receptors and Signalling Pathways Involved in Macrophage and Dendritic Cell Development - Madame Curie Bioscience Database
- Physiological Roles and Mechanisms of Signaling by TRAF2 and TRAF5 - Madame Curi...Physiological Roles and Mechanisms of Signaling by TRAF2 and TRAF5 - Madame Curie Bioscience Database
- Sympathetic Nervous System Regulation of Metastasis - Madame Curie Bioscience Da...Sympathetic Nervous System Regulation of Metastasis - Madame Curie Bioscience Database
- VEGF and Endothelial Guidance in Angiogenic Sprouting - Madame Curie Bioscience ...VEGF and Endothelial Guidance in Angiogenic Sprouting - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...