NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
All eukaryotic cells contain membrane-bounded compartments that interact with the cell's environment. Vesicles transport proteins and lipids between these compartments via two major pathways: the outwards, exocytic pathway, carries material synthesized in the cytoplasm to the cell milieu, and the inwards, endocytic pathway, internalizes material from the environment to the inside of the cell. This communication of the cell with its environment is crucial for all tissue and organ function. Here, we summarize progress made during the last two decades in our understanding of bi-directional transport pathways between intracellular compartments. The accumulated knowledge of intracellular compartments and pathways that connect them formed the basis for advancements made in our understanding of the molecular machinery components, mechanisms and regulation of intracellular trafficking. Whereas the major compartments and pathways are well defined, less is known about the dynamic nature and biogenesis of compartments.
Introduction
All cells are surrounded by a membrane that serves as a barrier between the inside of a cell and its environment. Moreover, different cellular processes occur on membranes, e.g., DNA replication and respiration. Most prokaryotic cells contain only one membrane, the plasma membrane (PM), which surrounds the cell, and all membrane-attached processes occur on it. In some prokaryotes, specific patches of the PM specialize in separate functions. This specialization is more advanced in eukaryotic cells, which contain membrane-bound intracellular compartments that carry out specific functions, e.g., nucleus for DNA replication and mitochondria for respiration. Membrane expansion and compartmentalization in eukaryotic cells enabled the development of larger cells (1000-10,000 fold increase in volume) and an efficient separation of cell functions. However, at the same time compartmentalization creates a new problem, namely the need for communication between the different cellular compartments.
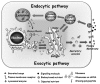
A major process of communication between the compartments that connect the cell with its environment is achieved by vesicular transport. In this process, cargo-loaded vesicles form at a donor compartment with the help of specific coat and adaptor proteins (e.g., COPI, COPII and clathrin). These vesicles are then targeted to the appropriate acceptor compartment, to which they attach with the help of tethers, and with which they fuse with the help of SNAREs.1 Vesicular transport enables proteins in membrane-bound vesicles to move between the cell compartments, including the outer-cell membrane, the PM. The first section of this book focuses on the different trafficking pathways and cellular compartments connected by vesicular transport (Fig. 1).2
Two major cellular pathways shuttle material outward and inward. In the exocytic pathway, proteins synthesized in the cytoplasm are translocated into the endoplasmic reticulum (ER). Rough ER is the site of synthesis of all secreted proteins, and resident proteins for all compartments connected by vesicular transport. The ER is also the site where synthesis of most of the lipids in the cells begins. From the ER, membranous vesicles shuttle cargo to the Golgi apparatus. ER-derived cargo enters the Golgi in its cis cisterna, and moves through the medial and trans cisternae. In the trans Golgi, proteins destined for secretion or to be presented on the PM are packed into secretory vesicles that subsequently fuse with the PM. This fusion occurs either constitutively or, as in the case of regulated secretion, in response to an external signal (summarized in Chapters 3 and 5, respectively refs. 3 and 4). The Golgi apparatus is the major sorting compartment of the cell because in the Golgi cargo is sorted not only to the PM for constitutive and regulated secretion, but also to endosomes and lysosomes, or back to the ER (see below).
In the endocytic pathway, proteins and membrane are internalized from the cell environment via a set of endosomes, early and late, to the lysosome (summarized in Chapter 4, ref. 5). The lysosome is a major degradation site for both internalized and cellular proteins. Thus, cellular proteins can get to lysosomes either from the PM via the endocytic pathway or from the cytoplasm via the autophagy and the cytoplasm-to-vacuole targeting (CVT) pathways.6
In addition, there is cross-talk between the exocytic and endocytic pathways. First, endosomal and lysosomal resident proteins and enzymes are shuttled from the ER via the Golgi to endosomes and lysosomes.7 Second, in polarized cells, proteins can be moved between two different environments, from one side of the cell to the other, via the transcytotic pathway.8 Lastly, macromolecules can be released from cells in small vesicles called exosomes by fusion of late endosomes, also known as multivesicular bodies (MVBs), with the PM.9
Transport of lipids and proteins between compartments creates another problem, which is how compartment identity is maintained in the context of the flow of material through the compartments. In addition, massive membrane flow needs to be balanced to maintain compartmental size. Therefore, for each step of forward transport, both in the exocytic and endocytic pathways, there is a retrograde transport step in which membrane and resident proteins are recycled back to their original compartment. This bi-directional trafficking requires sophisticated machinery and has to be regulated (summarized in the second and third sections of this book, respectively ref. 2).
The progress in our understanding of the pathways, machinery and regulation of vesicular transport was made possible by the development of novel techniques (summarized in Chapter 2, ref. 10). In particular, live-cell microscopy approaches provide dynamic views of intracellular trafficking. Recent live-cell studies have challenged the prevailing paradigm of compartments as static “bus stations.” The dynamic view envisions compartments as constantly changing entities in response to the cell needs. Here, we summarize our current understanding of the major intracellular compartments and trafficking pathways that connect them.
How We Study Intracellular Trafficking
The exocytic pathway and its compartments were defined in the 1960s by Palade and coworkers using pulse-chase analysis combined with electron microscopy.11 The endocytic pathway and its compartments were defined in the early 1970s by Brown and Goldstein, while studying human mutations that result in atherosclerosis due to defects in the recycling of low-density lipoprotein (LDL) receptors.12 The idea that all the steps of any biological pathway can be identified by mutations was further exploited during the early 1980s using yeast genetics to uncover all the steps of the exocytic pathway and define the genes whose products mediate these steps.13 At around the same time, reconstitution of protein transport steps in cell extracts combined with protein purification techniques allowed a complementary approach to identify transport machinery components.14
Progress in the intracellular trafficking field during the last two decades was made possible by further advances in available techniques (summarized in Chapter 2, ref. 10), and especially by combining these techniques. First, a powerful combination of genetic and biochemical strategies allowed the identification of vesicular trafficking machinery components and regulators. Genomics and proteomics studies carry the promise for the identification of the full inventory of these components in the near future. Various protein interaction studies placed these components into “molecular machines”. Second, combining fluorescence and electron microscopy with molecular genetics made it possible to localize these machinery components to their cellular compartments.
The most exciting recent development in cell biology, which will shape the future of this field, is the development of fluorescent tags and cutting edge fluorescence microscopy, which together allow following single molecules in live cells.15 Because it is clear that proteins function in complexes, the future of this field also belongs to techniques like fluorescence resonance energy transfer (FRET) and bi-molecular fluorescent complementation (BiFC),16 which allow identification of protein interactions in situ. Together, studies using these techniques should provide a detailed picture of the molecular machines that mediate intracellular trafficking in real time.
The Exocytic Pathway
The exocytic pathway moves cargo from the ER through the Golgi to the PM (Fig. 1). In the ER and the Golgi, proteins are modified by the addition of sugars and lipids. These modifications are highly ordered and occur successively in the ER and in the three cisternae of the Golgi, cis, medial and trans. Cargo-packed vesicles formed at the trans-Golgi fuse with the PM to deliver PM resident proteins such as receptors, channels and pumps and secreted proteins such as extracellular matrix components and signaling molecules. These vesicles also allow the expansion of the PM during cell growth.
Proteins enter the ER during their translation via the translocon pore. This entry requires a tag, the “signal sequence”, on the entering protein and signal recognition machinery on the ER membrane. Once in the ER, proteins stay either on or inside membranes. To exit the ER, proteins must get through a quality-control surveillance that ensures proper folding and assembly.17 From regions on the ER called ER exit sites, vesicles form and move to the cis Golgi. The area between the ER and the cis Golgi, termed intermediate compartment (IC), is filled with vesicles and tubules; the IC is not well defined functionally.18
The three Golgi cisternae are well-defined biochemically.3 Different protein-modifying enzymes are enriched in each cisterna. Currently, the way in which cargo or Golgi enzymes move between the three Golgi cisternae is still controversial. The vesicular transport model suggests that vesicles move cargo forward and resident proteins backward between the Golgi cisternae. The cisternal maturation model suggests that cargo stays enclosed inside a Golgi cisterna, which matures by fusing with retrograde vesicles carrying Golgi enzymes from a more mature cisterna and by giving rise to retrograde vesicles that return Golgi enzymes to younger cisternae. The rapid partitioning model suggests that Golgi cisternae within a stack are continuous, with cargo proteins equilibrating rapidly between the cisternae. In this model, the partitioning of enzymes into the different Golgi cisternae is a result of differential distribution of lipids within the continuous cisternae.19 Future experiments should help resolve this controversy.
In the last step of the exocytic pathway, exocytosis, secretory vesicles form at the trans-Golgi and fuse with the PM to deliver their protein and lipid cargo. Therefore, there are two major steps in the exocytic pathway mediated by vesicles: ER-to-cis Golgi and trans Golgi-to-PM. Vesicles mediating these two steps differ in size and coat composition.20,21
The forward exocytic pathway delivers more membrane than needed for PM expansion. In addition, resident proteins that move to the next compartment have to be retrieved back to the original compartment for maintenance of compartment identity. Therefore, for every step of forward transport, there is a corresponding retrograde transport step. The two major intersections of this bi-directional trafficking are the IC, which recycles proteins back to the ER, and recycling endosomes, which recycle proteins back to the PM or the Golgi.22
The Endocytic Pathway
In the endocytic pathway, cargo is internalized from the cell milieu (Fig. 1, summarized in Chapter 4, ref. 5). Cargo can be internalized at the PM by a number of routes. Membrane receptors are mainly internalized via clathrin-coated vesicles, whereas other proteins and viruses are internalized by caviolar- or raft-dependent routes. These three internalization routes depend on the GTPase dynamin for fission of the forming PM vesicle. However, fluid-phase cargo can also enter the cell via a dynamin-independent process. Each of these internalization routes delivers cargo to an internal compartment, endosomes, although the nature of the endosomal compartments may differ between routes.
The best characterized endocytic pathway proceeds from clathrin-coated vesicles through early and late endosomes to lysosomes. In the first set of endosomes, the sorting endosomes, cargo is sorted for recycling back to the PM (or the Golgi) via recycling endosomes, or to the lysosome via late endosomes. Patches of late endosomal membranes are internalized as vesicles to form multivesicular bodies (MVBs), which fuse with lysosomes. The lysosome is a major degradation site for internalized material and for cellular membrane proteins.
Like transport through the exocytic pathway, the first and last steps of the endocytic pathway are mediated by vesicular transport machinery: PM-to-early endosome and late endosome to lysosome. Using 3-dimensional time-lapse fluorescence microscopy (4D microscopy) and multiple fluorescent chromophores, it was shown that movement from early-to-late endosomes is achieved by endosome maturation, which is in turn mediated by Rab conversion.23
Future research in the endocytic pathway field will address the nature of the signals for the various internalization routes and the way in which cargo is sorted in sorting endosomes. This sorting is crucial for cell signaling because it determines the ratio between receptors that recycle back to the PM and continue to signal, and receptors that are shuttled to the lysosome for degradation. Cargo sorting is also of crucial importance for the function of neurons or neurosecretory cells as protein components of synaptic vesicles have to be retrieved efficiently to maintain PM identity.
Cross-Talk between the Exocytic and Endocytic Pathways
There are a few examples of cross-talk between the exocytic and endocytic pathways: bi-directional transport between the Golgi and endosomes, transport from one side of a polarized cell to the other and secretion of material from late endosomes.
Trafficking between the Golgi and Endosomes
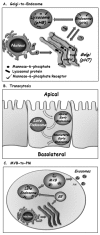
Because almost all proteins and lipids destined to reside and function in any of the compartments connected by vesicular transport are translocated first into the ER, there should be a pathway to transport newly synthesized endosomal and lysosomal proteins and lipids to endocytic compartments. Indeed, cargo can be shuttled from the trans Golgi not only to the PM via exocytosis, but also to endosomes and lysosomes (Fig. 2A). In mammalian cells, most endosomal and lysosomal proteins are labeled with mannose-6-phosphate (M6P) in the Golgi. In the trans Golgi, M6P-labeled cargo is sorted by M6P receptors (M6PR) into vesicles that are targeted to the endocytic compartments. Lower pH in endosomes causes dissociation of the cargo from the M6PR for its further delivery to the right endosomal compartment. Retrograde transport recycles M6PRs back from endosomes to the Golgi for further functioning.7 Thus, bi-directional trafficking between the Golgi, endosomes and lysosomes connects the two major intracellular trafficking pathways.
Transcytosis
Polarized cells, such as epithelial cells and neurons, contain distinct functional PM domains: apical and basolateral or somatodendritic and axonal, respectively. The mechanisms by which this cell polarity is established and maintained are still not clear.24 Regardless, polarized cells use the endocytic pathway to shuttle cargo between their distinct PM domains. Here, cargo, soluble or membranous, is internalized from the PM on one side of the cell, e.g., the apical side of epithelial cells, which faces the lumen of organs. In this case, cargo delivered first to apical early endosomes can be shuttled via a common set of late endosomes, and then through basolateral early endosomes, to the PM of the basolateral side of the cell (Fig. 2B). Thus, transcytosis can selectively move material through cells across tissue barriers; for example, from the luminal (apical) side to the underlying interstitium (basolateral) side of endothelium that lines blood vessels or epithelium that lines the intestines.8 It seems that even though this transport is mediated by endosomes, exocytic machinery components, like the tethering complex exocyst and SNAREs, are required for this process.25
Late Endosome-to-Plasma Membrane
This is the newest addition to the connection between the endocytic and exocytic pathways. Here, transport of macromolecules from a late endocytic compartment is redirected to the PM and secreted inside small vesicles, termed exosomes, to the cell's surroundings. MVBs are late endosomes that contain internal membrane-surrounded cargo. Usually, MVBs fuse with lysosomes and send their cargo for degradation. However, under certain conditions MVBs can fuse with the PM, thus secreting exosomes to the cell milieu (Fig. 2C).9 This process is important for communication between cells and has been implicated in secretion of components to the blood stream and as a signaling device. On the other hand, exosomes might play a role in spreading infectious agents; for example, viruses like HIV can hijack this route to be released from cells.26 Currently, the regulation and function of this process is still unknown.
Regulated Trafficking
Trafficking through the exocytic and endocytic pathways is coordinated by internal regulators that ensure fidelity and uninterrupted flow.27 In addition, some trafficking steps can be regulated by external signals. For example, transport of membranes and proteins to and from the PM can be regulated by extracellular signaling molecules, while the autophagy pathway can be induced under stress conditions.
Regulated Exocytosis
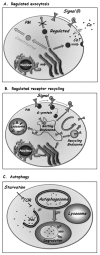
At the trans Golgi, specific proteins can be sorted into special secretory vesicles that accumulate and fuse with the PM only when triggered by an extracellular signal (Fig. 3A). In these systems, the level of the signal controls the rate of exocytosis. The best-studied examples of regulated exocytosis are secretion of neurotransmitters in synaptic vesicles by neurons and secretion of hormones in secretory granules by endocrine cells.4 However, even in yeast there are examples of regulated exocytosis, such as the regulated sorting of a general amino-acid permease to the PM in response to external nitrogen availability.28
The basic machinery of regulated exocytosis, in both endocrine and neuronal cells, is adapted from the core vesicular transport machinery. In the case of secretory granules, regulated exocytosis starts with the sorting step that occurs at the trans-Golgi. In this step, appropriate cargo proteins often form aggregates, which are then packaged into immature secretory granules. These vesicles undergo maturation by the recycling of membrane and Golgi-resident proteins back to the Golgi. As a result, cargo in mature vesicles becomes condensed to form dense-core granules.29 In addition, some polypeptides are proteolytically processed in the maturing vesicles to generate active hormones or neuropeptides. Mechanisms of synaptic vesicle biogenesis remain unresolved, with potential sorting steps at the TGN and at different stages of the endocytic pathway.30 In the cases of both secretory granules and synaptic vesicles, a fraction of the mature granules, called “primed” vesicles, attach to the PM and are ready to fuse in response to a signal. Signals, like hormones or neurotransmitters, interact with PM surface receptors to cause calcium influx through membrane channels, which results in a transient increase in cytoplasmic calcium near the prospective vesicle fusion site. The machinery components that mediate secretory granule and synaptic vesicle attachment and fusion are modified to function only upon stimulation by specific regulators. These specific regulators are calcium sensors that ensure vesicle attachment at the right place and fusion only upon elevation of local calcium levels.
In addition, a specific feature of secretion in neuronal synapses is that synaptic vesicles can undergo multiple rounds of fusion. This is achieved by two mechanisms unique to synapses. First, vesicles can be refilled with neurotransmitters from the cytoplasm by transporters present in the vesicle membrane. In addition, fast release of neurotransmitters in the synapse can be facilitated by a transient link of vesicles with a fusion pore on the PM, in a mechanism called “kiss and run”.
Because regulated exocytosis is crucial for proper functioning of two major body systems, endocrine and neuronal, uncovering the details of this process is important for understanding and treating neural and endocrine dysfunctions. Future studies should help to identify calcium sensors that ensure vesicle fusion only upon excitation and determine the way by which these sensors regulate the precise rate of vesicle fusion.
Regulated Receptor Endocytosis
Endocytosis of signaling receptors and plasma membrane transporters also can be regulated by extracellular signals. One well-characterized example involves G-protein coupled receptors (GPCR), the largest family of signaling receptors (∼900 in mammalian cells). Internalization of some GPCR can be induced by the addition of their cognate signal (Fig. 3B). This induction is mediated by phosphorylation of activated receptors, which elicits arrestin binding and uncoupling of the receptor from the G-protein. Phosphorylated receptor/arrestin complexes then interact with specific clathrin coat adaptors that mediate their concentration in clathrin-coated pits. Subsequently, activated receptors are internalized via clathrin-coated vesicles to early endosomes, where they can be sorted to recycling endosomes for recycling back to the PM, or to late endosomes for degradation in the lysosome. This regulated internalization and sorting of activated receptors determines the length and amplitude of multiple cell-signaling processes. The specific internalization mechanisms for many GPCRs that regulate important cell functions are still unknown, and future studies should elucidate these mechanisms.31
Autophagy
Under nutrient deprivation conditions, cells can induce the autophagy pathway, which allows them to engulf areas of their cytoplasm, including membrane-bounded organelles, and deliver the material for degradation in the lysosome to generate nutrients (Fig. 3C). In mammalian cells, autophagy is crucial for multiple processes such as programmed cell death and cellular defense against pathogens. Improper regulation of autophagy can result in cancer and in muscular and neurodegenerative disorders.32
The machinery components of the autophagy pathway, first defined in yeast, are conserved. This pathway is regulated by the target-of-rapamycin (TOR) kinase, which inhibits autophagy under normal growth conditions. Once TOR inhibition is removed, a new organelle, the autophagosome, is generated de novo. In this process, a membrane “sac” engulfs portions of the cytoplasm and closure of this sac results in the formation of the double-membrane autophagosome. Fusion of the outer membrane of the autophagosome with the lysosome results in the exposure of the inner membrane and its content to lysosomal hydrolases, leading to their degradation.33,34 Much is known about the steps of the autophagy pathway and its machinery components. However, little is currently known about the beginning of the process, especially how the “sac” is generated.
Compartment Dynamics and Biogenesis
Until recently, compartments were viewed as stable entities, like “bus stations”, with “bus-like carriers” moving cargo between them. This view was challenged especially when live-cell microscopy allowed observation of compartment dynamics. It became clear that compartments can disappear and reappear depending on the cell cycle, environmental cues and cargo waves.
One of the best-studied examples of compartment dynamics is the Golgi complex. In most eukaryotic cells, the Golgi apparatus disintegrates during mitosis. Golgi disintegration can also be induced by drugs like Brefeldin A (BFA). At the end of mitosis, or upon removal of the drug, the Golgi apparatus reassembles. Mechanistic questions addressed in the field are: what happens to Golgi resident proteins during disintegration and how does the Golgi reassemble. Currently these questions are under active investigation with one model suggesting that the Golgi contents completely recycle through the ER and another model proposing that Golgi fragments form the stage for its reassembly.35,36
Recent findings suggest that compartments change continuously, depending on cargo passing through them. For example, an extension of the cisternal maturation model suggests that the entire Golgi apparatus assembles and disassembles continuously. In this model, the cis Golgi cisterna is generated by fusion of ER-derived COPII vesicles that contain cargo, with retrograde COPI vesicles that contain cis-Golgi enzymes. On the other end of the Golgi, the trans cisterna is consumed as anterogade vesicles form to carry cargo to the PM or endosomes, and retrograde vesicles are generated to carry trans-Golgi enzymes to the medial compartment. This latter event is required for the maturation of the medial- to trans-Golgi cisterna. Thus, this model proposes the Golgi to be a dynamic compartment that changes not only during cell cycle, but also in the context of cargo transport.37 Therefore, intracellular compartments may be more like “bus stations” comprised of a collection of “buses” without a static structure.
Another important question is how compartments are inherited into newly divided cells. Do compartments self assemble de novo, with or without template, or do they grow and divide? Studies in yeast suggest that the Golgi is formed de novo without a template whereas the perinuclear ER, together with the nucleus, is partitioned between the two newly formed cells. In mammalian cells and some protozoa, the suggested mechanism for Golgi biogenesis is self-assembly that requires a template.38 The autophagosome is a non-essential compartment formed de novo under deprivation conditions.34 However, it is not clear whether phagosomes need a template for assembly. For example, yeast cells that grow under normal conditions have the cytoplasm-to-vacuole targeting, CVT, pathway to transport special proteins from the cytoplasm directly to the lysosome, called vacuole in yeast. Many components are shared between the CVT and autophagy pathways.6 Therefore, here again it is possible that under deprivation conditions, phagosomes use preexisting CVT structures as a template for their assembly.
Summary and Future Perspectives
Major advances in technology have made substantial progress in the intracellular trafficking field possible. During the past two decades, the field gained detailed understanding of the nature of cellular compartments and the connecting pathways. Each compartment is defined by its lipid and protein composition. Maintenance of compartment identity during massive internal flow of proteins and membrane is probably achieved by active recycling of proteins and lipids to their original compartment. However, there are still unanswered questions and areas of controversy.
The intracellular membrane-surrounded compartments can be clearly visualized by electron microscopy and the inventory of compartment components is almost complete (see Section II of this book, ref. 2). Does this mean that we know what compartments look like? It would be like trying to imagine how a car looks based on the inventory of its parts without actually seeing the car. Currently, very little is known about the architecture of intracellular compartments. The first glimpse into compartment architecture was recently provided for synaptic vesicles (SVs). A quantitative study of purified SVs was used for modeling an average SV. This model suggests that the outside of the SV is densely covered with proteins, that the proteins are highly divergent and include more than one percent of our proteome, and that abundant proteins are present in multiple copies per vesicle.39 Major questions are still open as to whether the protein divergence reflects averaging of sub-populations of SV, whether multiple copies of abundant proteins are distributed randomly over the surface of the SV or found concentrated in patches, and the nature of the architecture of larger, more complex compartments.
The most controversial topic in the area of trafficking pathways has been how cargo moves through compartments, and especially through the Golgi cisternae. It seems that between compartments, e.g., ER and Golgi, or Golgi to the PM, cargo moves via vesicles. In contrast, between sub-compartments, e.g., cis-, medial- and trans-Golgi, or early-to-late endosomes, vesicles are probably not the carriers of cargo.19 The jury is still out as to whether intra-Golgi transport occurs by vesicular transport, cisternal maturation or gated transport through connecting tubules.
Another major open question concerns intracellular compartment biogenesis. The Golgi apparatus is the best-studied organelle for this question because it naturally disintegrates during mitosis. Here too, there are diverse results for Golgi biogenesis in different organisms and the question remains open as to which Golgi sub-structures or proteins, if any, form a template for assembly of the new Golgi after each mitotic division.38 Future studies will hopefully help solve these cell mysteries.
Acknowledgments
The authors thank Gregory Payne for critical reading of the manuscript, Eran Segev for text editing, and acknowledge support from the National Institutes of Health GM45-444 to N. S. and from the National Science Foundation to A. A. while working at the Foundation.
References
- 1.
- Costaguta G, Payne G. Overview of protein trafficking mechanisms. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 105–14. this volume.
- 2.
- Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin: Landes Bioscience and Springer Science+Business Media; 2009. pp. 103–438. this volume.
- 3.
- Hua Z, Graham T. The Golgi apparatus. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 42–66. this volume.
- 4.
- Nagarajan N, Custer K, Bajjalieh S. Regulated secretion. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 84–102. this volume.
- 5.
- Conibear E, Tam Y. The endocytic pathway. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 67–83. this volume.
- 6.
- Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9(3-4):65–76. [PMC free article: PMC1430730] [PubMed: 12865942]
- 7.
- Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4(3):202–12. [PubMed: 12612639]
- 8.
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83(3):871–932. [PubMed: 12843411]
- 9.
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–30. [PubMed: 11967126]
- 10.
- Pruess M, Weidman P, Nielsen E. How we study protein transport. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 15–41. this volume.
- 11.
- Palade G. Intracellular Aspects of the Process of Protein Secretion. Science. 1975;189(4206):867. [PubMed: 17812524]
- 12.
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. [PubMed: 3513311]
- 13.
- Schekman R. Genetic and biochemical analysis of vesicular traffic in yeast. Curr Opin Cell Biol. 1992;4(4):587–92. [PubMed: 1419039]
- 14.
- Rothman JE, Orci L. Molecular dissection of the secretory pathway. Nature. 1992;355(6359):409–15. [PubMed: 1734280]
- 15.
- Walter NG, Huang CY, Manzo AJ, et al. Do-it-yourself guide: how to use the modern single-molecule toolkit. Nat Methods. 2008;5(6):475–89. [PMC free article: PMC2574008] [PubMed: 18511916]
- 16.
- Ciruela F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Curr Opin Biotechnol. 2008;19(4):338–43. [PubMed: 18602005]
- 17.
- Fewell S, Brodsky J. Entry into the endoplasmic reticulum: protein translocation, folding and quality control. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 119–42. this volume.
- 18.
- Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119(Pt 11):2173–83. [PubMed: 16723730]
- 19.
- Simon SM. Golgi governance: the third way. Cell. 2008;133(6):951–3. [PMC free article: PMC2711685] [PubMed: 18555771]
- 20.
- McPherson P, Ritter B, Wendland B. Clathrin-mediated endocytosis. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 159–82. this volume.
- 21.
- Pagant S, Miller E. COP-Mediated vesicle transport. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 143–58. this volume.
- 22.
- Saraste J, Goud B. Functional symmetry of endomembranes. Mol Biol Cell. 2007;18(4):1430–6. [PMC free article: PMC1839002] [PubMed: 17267686]
- 23.
- Rink J, Ghigo E, Kalaidzidis Y, et al. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–49. [PubMed: 16143105]
- 24.
- Prydz K, Dick G, Tveit H. How many ways through the Golgi maze? Traffic. 2008;9(3):299–304. [PubMed: 18088319]
- 25.
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12(4):483–90. [PubMed: 10873817]
- 26.
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–81. [PMC free article: PMC3636814] [PubMed: 18331451]
- 27.
- Donaldson J, Segev N. Regulation and coordination of intracellular trafficking: an overview. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 329–41. this volume.
- 28.
- Magasanik B, Kaiser CA. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290(1-2):1–18. [PubMed: 12062797]
- 29.
- Bowman G, Cowman A, Turkewitz A. Biogenesis of dense-core secretory granules. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Austin/New York: Landes Bioscience/Springer Science+Business Media; 2009. pp. 183–209. this volume.
- 30.
- Fei H, Grygoruk A, Brooks ES, et al. Trafficking of vesicular neurotransmitter transporters. Traffic. 2008;9(9):1425–36. [PMC free article: PMC2897747] [PubMed: 18507811]
- 31.
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8(5):462–70. [PubMed: 17376169]
- 32.
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–5. [PMC free article: PMC1705980] [PubMed: 15528435]
- 33.
- Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007;581(11):2156–61. [PubMed: 17382324]
- 34.
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–9. [PubMed: 17909521]
- 35.
- Colanzi A, Suetterlin C, Malhotra V. Cell-cycle-specific Golgi fragmentation: how and why? Curr Opin Cell Biol. 2003;15(4):462–7. [PubMed: 12892787]
- 36.
- Storrie B. Maintenance of Golgi apparatus structure in the face of continuous protein recycling to the endoplasmic reticulum: making ends meet. Int Rev Cytol. 2005;244:69–94. [PubMed: 16157178]
- 37.
- Mironov AA, Beznoussenko GV, Polishchuk RS, et al. Intra-Golgi transport: a way to a new paradigm? Biochim Biophys Acta. 2005;1744(3):340–50. [PubMed: 15979506]
- 38.
- Lowe M, Barr FA. Inheritance and biogenesis of organelles in the secretory pathway. Nat Rev Mol Cell Biol. 2007;8(6):429–39. [PubMed: 17505521]
- 39.
- Takamori S, Holt M, Stenius K, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–46. [PubMed: 17110340]
- Introduction
- How We Study Intracellular Trafficking
- The Exocytic Pathway
- The Endocytic Pathway
- Cross-Talk between the Exocytic and Endocytic Pathways
- Trafficking between the Golgi and Endosomes
- Transcytosis
- Late Endosome-to-Plasma Membrane
- Regulated Trafficking
- Regulated Exocytosis
- Regulated Receptor Endocytosis
- Autophagy
- Compartment Dynamics and Biogenesis
- Summary and Future Perspectives
- Acknowledgments
- References
- Overview of Intracellular Compartments and Trafficking Pathways - Madame Curie B...Overview of Intracellular Compartments and Trafficking Pathways - Madame Curie Bioscience Database
- Subcellular Compartmentalization of Insulin Signaling Processes and GLUT4 Traffi...Subcellular Compartmentalization of Insulin Signaling Processes and GLUT4 Trafficking Events - Madame Curie Bioscience Database
- Immunity and Autoimmunity Induced by Polyomaviruses: Clinical, Experimental and ...Immunity and Autoimmunity Induced by Polyomaviruses: Clinical, Experimental and Theoretical Aspects - Madame Curie Bioscience Database
- Methylenetetrahydrofolate Reductase Polymorphisms: Pharmacogenetic Effects - Mad...Methylenetetrahydrofolate Reductase Polymorphisms: Pharmacogenetic Effects - Madame Curie Bioscience Database
- NMD and Human Disease - Madame Curie Bioscience DatabaseNMD and Human Disease - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...