NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Earthworms belonging to oligochaete annelids became a model for comparative immunologists in the early sixties with the publication of results from transplantation experiments that proved the existence of self/nonself recognition in earthworms. This initiated extensive studies on the earthworm immune mechanisms that evolved to prevent the invasion of pathogens. In the last four decades important cellular and humoral pathways were described and numerous biologically active compounds were characterized and often cloned.
Introduction
Considering the fact that the majority of the immunologists all over the world are focused on the mammalian or particularly human immunology, it may sound surprising that invertebrates—and among others earthworms—have been an important experimental model since the very beginning of immunology. For example, phagocytosis, an important and evolutionarily conserved defense mechanism of innate immunity, was discovered in the late 1800s by a Nobel Prize winner Elie Mechnikoff while studying the origin of the digestive organs in the floating larvae of starfish.1
From the total number of extant animal species, certainly surpassing 2 millions, 95% are included in the invertebrate taxa. Invertebrates have evolved for hundreds of millions of years, often surviving in very hostile environments. Their successful survival strategies are likely based on short life span combined with numerous offspring. More importantly, all invertebrate species have developed a variety of defense mechanisms efficiently recognizing and responding to nonself substances.2,3
In contrast to adaptive immunity, which is a highly sophisticated system based on antigen-specific T and B cells and antibodies and which is observed in vertebrates only, many innate immunity mechanisms are conserved from invertebrates to vertebrates. Cellular mechanisms of invertebrate innate immunity include wound repair, clotting and coagulation responses, phagocytosis of invading microorganisms and encapsulation reactions. Apart from these cellular mechanisms, invertebrates possess a broad range of antimicrobial factors such as lysozyme-like proteins, proteases, cytolytic proteins, antimicrobial peptides and enzyme activation-base cascades; humoral defense also includes lectin-like and pattern recognition molecules that are designed to recognize a few highly conserved structures present in many different microorganisms. The majority of the above immune responses of invertebrates are non-adaptive with no or very limited ability either to "remember" or to respond more vigorously and effectively to repeated exposures to the same pathogens.4
Basic Information on Earthworm Anatomy
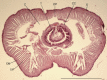
The earthworms are protostomian animals possessing true coelom of mesenchymal origin. The coelomic cavity is filled with coelomic fluid containing free wandering cells, named coelomocytes, originating in the mesenchymal lining of the cavity. The coelomic cavity is metameric and the segments are separated by transversal septa. Transport of the coelomic fluid and coelomocytes between the segments is regulated by channels. Each segment of the coelomic cavity is opened to the outer environment by a pair of nephridia and by a dorsal pore (Fig. 1).
In terms of systematic biology, the earthworm family—Lumbricidae—is the largest member of the class Oligochaeta, phylum Annelida. The earthworms are found in leaf litter, manure, under stones and logs as well as some arid areas, but most species prefer wetter, more heavily vegetated regions. Earthworms range in size from two centimeters to over one meter. There are over 3000 described earthworm species known worldwide and they have adapted to a wide range of soil habitats as well as freshwater lakes and streams (for a review see ref. 5). Immunological research is performed mainly in two genera—Lumbricus and Eisenia.
Cellular Defense Mechanisms
The first nonspecific barrier of earthworms is the skin covering their entire body. The skin consists of the epidermis and a thin cuticle, which contains mucopolysaccharides acting as an antimicrobial barrier.6,7 The epidermis is formed by a single layer epithelium of supporting cells, basal cells and secretory cells. The basal cells play an important role in wound healing and graft rejection, often exerting phagocytic activity.8,9 Thus, these basal cells are sometimes considered not to be of epidermal origin, but rather homologous to coelomocytes.10-12
Each segment of the coelomic cavity communicates with outer environment by a dorsal pore; the skin therefore cannot sufficiently prevent the microorganisms from entering the coelomic cavity. Consequently, the coelomic cavity is not aseptic and always contains bacteria, protozoans and fungi from the outer environment. Nevertheless, there are efficient mechanisms that keep the growth of microorganisms under control.13 It was reported that coelomic fluid contains 6 × 105/ml naturally occurring bacteria while the number of potentially phagocytic cells is more than ten times higher. These abundant phagocytes combined with the presence of various humoral factors can easily prevent the microorganisms from outgrowth.
The invading microorganisms can be eliminated by a number of ways. First, they can be excreted by nephridia14 or engulfed by the cells of nephrostome or middle tube.15 Second, as mentioned above, the microorganisms can be phagocytosed by certain coelomocytes and phagocytic cells that, when they become exhausted, are expelled through dorsal pores. The pores are equipped with muscular sphincters controlling intracoelomic pressure and the exchange of material between the outer and inner environments.14 Third, large foreign bodies, e.g., agglutinated bacteria or parasites are eliminated by encapsulation.16,17 This process begins, similarly to phagocytosis, by the recognition of foreign material which, however, cannot be engulfed due to its size. Within the first day, the foreign body is surrounded by free coelomocytes and after several days a dense capsule (often called a brown body because of its melanin content as a consequence of the prophenoloxidase cascade) composed of flattened cells is formed. When the capsule is about 1-2 mm in diameter, its external cells lose their adhesiveness so that the capsule can migrate towards the posterior segments of the coelomic cavity where it is eliminated by autonomy followed by wound.18-21 It was documented in Eisenia fetida earthworms that most brown bodies contain tissue wastes, agglutinated bacteria, gregarines or nematodes.17
As mentioned above, coelomic fluid contains different types of coelomocytes. Their nomenclature is based mainly on morphological and cytochemical criteria (for a review see refs. 22,23) though more recent studies attempt to determine superficial and functional markers for cell classification.24 In general, there are three main coelomocyte types—eleocytes, free chloragogen cells with nutritive and accessory functions and either hyaline or granular amoebocytes, both representing effectory immunocytes involved in a broad range of defense functions including phagocytosis.
Although both types of amoebocytes have phagocytic properties, their activity differs. In contrast to granular amoebocytes, the cytoplasm of hyaline amoebocytes is occasionally full of engulfed material.22 It should be mentioned that amoebocytes engulf all kinds of material including inert particles, microbial cell wall components as well as foreign cells. However, the phagocytosis of eukaryotic cells depends on the source of the cells. Unlike allologous cells, xenologous cells (both from different earthworm species and from non-invertebrate species) are rapidly phagocytosed.14,25
Phagocytosis by coelomocytes, similarly to that of vertebrates, can be modulated by humoral components, opsonins, which coat the engulfed particle and thus promote its phagocytosis. It was proven that preincubation of both yeast and synthetic copolymer particles with the coelomic fluid significantly increased their phagocytosis.26,27 It is noteworthy that also mammalian opsonins, IgG immunoglobulin and C3b complement fragment, were described to enhance coelomocyte phagocytic activity, in contrast to IgM and C3d fragment, which did not affect phagocytosis.28
The earthworms are regarded as an important model organism of comparative immunology since 1960s when transplantation experiments were performed (for a review see ref. 29) and cell-mediated short-term memory was observed.30 All these experiments proved the existence of self and nonself recognition in earthworms and initiated extensive studies of earthworm immune mechanisms. The ability to recognize and respond to allografts as well as xenografts and, on the other hand, the ability to accept or not to destroy autografts was observed in many annelid species.29 This process begins like the reaction to injury. The first major change, which occurs after the healing of wounds is, regardless of the graft origin, the accumulation of coelomocytes near the graft sites and their infiltration into the matrix. The response to the xenografts results in complete walling off of the graft and its destruction by encapsulation reaction.31 The number of invading coelomocytes during the autograft transplantation is markedly lower32,33 but the reaction seems to be more rapid. The maximum number of coelomocytes surrounding the graft was detected within 24 hours, returning to the normal level by 72 hours. In contrast, the peak response to xenografts is on day 3 or 4 and normal levels are not reached before day 7. The destruction of xenografts is completed approximately by day 17 after the transplantation. If a second graft is transplanted at this time, an accelerated rejection within 6 or 7 days occurs. Moreover, the number of the invading coelomocytes is 20-30 % higher. The increased number of coelomocytes during the retransplantation is probably caused by an increased proliferating activity of mesenchymal lining of the coelomic cavity and the septa. These data suggest the existence of short-term and very limited memory, which is based solely on cells as the transfer of either the coelomic fluid or other substances does not induce any accelerated reaction.30,33
Allo- and xenorecognition mediated by earthworm coelomocytes was evidenced in experiments showing cell-mediated cytotoxicity to allogeneic coelomocytes34 and, more interestingly, capability of earthworm non/phagocytic coelomocytes to kill efficiently tumor cell targets such as K562.35,36
Humoral Defense Mechanisms
The coelomic fluid of annelids exerts numerous biological activities that are involved in effective defense mechanisms against invaders. It was documented that it contains various antimicrobial factors like lysozyme37,38 and antimicrobial peptides.39-41 Moreover, coelomic fluid was found to cause the lysis of vertebrate erythrocytes and, subsequently, several hemolytic factors were isolated and described. The majority of proteins with hemolytic properties have hemagglutination activity as well and, more interestingly, a spectrum of antibacterial and bacteriostatic activities against pathogenic soil bacteria.42-44 Furthermore, it was observed that coelomic fluid lyses eukaryotic cells other than erythrocytes, namely fibroblasts and insect hemocytes45 and various tumor cell lines.46,47 Hereinafter, each activity of the coelomic fluid is thoroughly described.
Lysozyme is a bacteriolytic enzyme which catalyzes the hydrolysis of 1,4-β-D-links between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in the peptidoglycan of bacterial cell walls and thus efficiently protects against infections caused particularly by Gram-positive bacteria. Lysozyme activity was observed in coelomocyte extracts as well as in the coelomic fluid.37 Later, the active protein was isolated and partially sequenced.48 Based on the N-terminal sequence, a novel class of lysozymes including those of molluscs, echinoderms, nematodes and earthworms was proposed.48 Recently, cDNA coding for lysozyme-like molecule of E. andrei earthworms was characterized and cloned.38 Earthworm lysozyme exhibited both lysozyme and isopeptidase activity and shared homology with other invertebrate lysozymes, with the highest similarity (72 % identity) to destabilase I from medicinal leech. Moreover, lysozyme expression can be up-regulated after a challenge with Gram-positive as well as Gram-negative bacteria.
Antimicrobial peptides are an abundant and diverse group of molecules that are produced by many cell types in invertebrates, vertebrates and plants. To date, only a limited number of bioactive peptides have been described in annelids. An antimicrobial peptide named Lumbricin I was identified in Lumbricus rubellus.39 Lumbricin I is a proline-rich antimicrobial peptide which is constitutively expressed in adult animals and is not induced by bacterial infection. A Lumbricin I analog named PP-1 was found in the Asian earthworm Pheretima tschiliensis, is synthesized in the body wall only and its localization in the mucus of the epidermis suggests its role in the mucosal defense.40 Furthermore, an antimicrobial short peptide OEP3121 of only 5 amino acids was found in Eisenia fetida earthworms.41
The coelomic fluid of Eisenia earthworms was described to exhibit strong hemolytic activity that is tightly connected with bacteriostatic and antibacterial properties against pathogenic soil bacteria. The first hemolytic proteins were described by Du Pasquier and Duprat49 and later on they were named EFAF (Eisenia fetida andrei factors) and characterized as two glycoproteins secreted by chloragocytes and eleocytes.42,50,51 The 45-kDa protein is encoded by a single nonpolymorphic gene and has a pI of 6.0, while the 40-kDa protein is encoded by a gene having four alleles, each representing one of isoforms with pI of 6.3, 6.2, 5.95 and 5.9. Each individual earthworm possesses the 45-kDa protein and 1 or 2 isoforms of the 40-kDa protein.42,52 In addition to EFAFs hemolytic activity, these proteins were found to agglutinate red blood cells53 and to participate in the cytotoxic activity of the coelomic fluid.45 Moreover, they exhibit antibacterial activity against both Gram-positive and Gram-negative bacteria,43,54,55 particularly against strains that are pathogenic for earthworms.44,52,56 In addition to their bacteriolytic activity, they may also mediate opsonization57 and participate in the clotting of the coelomic fluid.58
It was documented, that upon binding to sphingomyelin, a major lipid constituent of plasma membranes of most mammalian cells, these proteins polymerize and form 10-nm channels through the lipid bilayer.50,59
Later, EFAFs were characterized at the molecular level and were named fetidins. A gene for the 40-kDa protein was cloned60,61 and it was found that its putative amino acid sequence comprises an N-glycosylation site and a peroxidase motif. This is in accordance with the finding that both fetidins have peroxidase activity.
Independently, a 41-kDa hemolytic protein, which is produced by coelomocytes and causes contraction of rat vascular smooth muscles, was characterized and named lysenin.62 Simultaneously, two 42-kDa lysenin-related proteins with weak contractive activity were identified.63 More recently, a new member of this lysenin-like multi-gene family has been cloned and provisionally called lysenin-related protein 3.64 Lysenin has a high amino acid sequence homology with fetidin (89% identity, 95% positivity), with lysenin-related protein 1 (76% identity, 89% positivity) and lysenin-related protein 3 (81% identity, 90% positivity). Amino acid sequence of lysenin-related protein 2 corresponds to that of fetidin. All these data suggest a close relationship between these lytic molecules. Individual sequence analyses have revealed that fetidin and lysenin are encoded by two distinct highly homologous genes but their expression level differs in individual earthworms.65
The hemolytic activity of lysenin is dependent on the presence of sphingolipids in the membrane.66 Moreover, the presence of cholesterol in the membrane facilitates hemolysis. Upon binding to the sphingomyelin, lysenin forms oligomers and subsequently pores 3 nm in diameter in the target membranes.66,67 Oligomerization does not occur on bacterial membranes since they are devoid of sphingomyelin. Therefore the mechanism of antibacterial activity must be different from its cytolytic activity.64
As sphingomyelin is crucial for the cytolytic activity of lysenin, it has been proposed to use lysenin as a valuable probe for sphingomyelin detection in sphingomyelin storage diseases, particularly in the cells of Niemann-Pick A patients,66 although the multiplicity of hemolysins in the natural source and the cytolytic activity appeared to be a major obstacle.
Independently on fetidin and lysenin, eiseniapore and hemolysins H1, H2, H3, CL39 and CL41 were described.68-71 Nevertheless, their more detailed analyses revealed close relationships with fetidin, lysenin or lysenin-related proteins.
Coelomic Cytolytic Factor as a Pattern Recognition Molecule
The coelomic fluid does not cause either the lysis of the coelomocytes of other earthworm species or of the hemocytes of mollusks, nematodes and protozoans. However, it was documented that coelomic fluid of E. fetida lyses a broad spectrum of various cell types including chicken fibroblasts, guinea-pig polymorphonuclear leukocytes and insect hemocytes.45 A proteinase-independent cytolytic effect of the coelomic fluid was observed in experiments with TNF-sensitive tumor L929 cell line. Subsequent isolation of lytic proteins led to the identification of a 42-kDa protein, which was named coelomic cytolytic factor—CCF.46
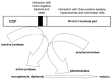
CCF acts in earthworm defense as a pattern-recognition molecule. Upon binding microbial pathogen-associated molecular patterns, namely O-antigen of LPS of Gram-negative bacteria, muramyl dipeptide and muramic acid of peptidoglycan from the cell walls of Gram-positive bacteria and β-1,3-glucans and N, N'-diacetylchitobiose of yeast, CCF triggers the activation of the prophenoloxidase cascade, which results in the formation of cytotoxic and antimicrobial compounds and thus represents an important invertebrate defense mechanism.72-75 The broad specificity of CCF for pathogen-associated molecular patterns results from the presence of two spatially distinct pattern recognition lectin-like domains. One domain, which shows homology with the polysaccharide and glucanase motifs of β-1,3-glucanases and invertebrate defense molecules, is located in the central part of CCF molecule and interacts with LPS and β-1,3-glucans. The C-terminal tryptophan-rich domain mediates interactions of CCF with N, N'-diacetylchitobiose, muramyl dipeptide and muramic acid (Fig. 2).74
The binding activity of C-terminal domain of CCF is rather unique for Eisenia fetida earthworms. Comparative analysis of CCF-like pattern-recognition proteins in seven other lumbricid species (Aporrectodea caliginosa, A. icterica, A. longa, A. rosea, Dendrobaena veneta, Lumbricus rubellus and L. terrestris) revealed high homology in polysaccharide-binding and glucanase motifs while C-terminal part was more heterogeneous.76 This is in a good agreement with the absence of cytolytic activity and binding capacity for N, N'-diacetylchitobiose and peptidoglycan components. E. fetida is an epigeic earthworm living in decaying organic matter, in compost and mold, where the diversity as well as quantity of microorganisms is substantially higher as compared to other soil layers. It is therefore obvious that E. fetida appears to be best equipped to resist microbial load as reflected by the broader CCF pattern-recognition repertoire.
As indicated above, CCF displays amino acid sequence homology with bacterial and animal β-1,3-glucanases but it does not exhibit their enzymatic activity.77-79 Moreover, CCF shows homology with the α subunit of the β-1,3-glucan sensitive factor G from the horseshoe crab Tachypleus tridentatus,80 with the Gram-negative bacteria-binding proteins of various insects81-84 and β-1,3-glucan recognition protein of arthropods.85,86 All these invertebrate homologs have been suggested to play a role in invertebrate innate immunity by acting as pattern recognition molecules.
Further, it was shown that CCF agglutinates both Gram-positive and Gram-negative bacteria72 and contributes to the opsonizing properties of the coelomic fluid, thereby providing an efficient mechanism for phagocytosis in earthworm defense reactions.46 CCF is also involved in the cell-mediated cytotoxic reactions and potentiates the lytic activity of coelomic fluid against red blood cells from various species.34
More interestingly, CCF shares functional analogies with mammalian tumor necrosis factor. The ability of the coelomic fluid to lyse TNF-sensitive tumor cell line L929 is caused by CCF. This activity is not inhibited by anti-TNF neutralizing monoclonal antibodies, suggesting that the structure of TNF and CCF as well as the mechanism of TNF and CCF mediated lysis differ. In addition to this TNF-like lytic activity, CCF exhibits other similarities with this cytokine. CCF is secreted by phagocytic coelomocytes upon LPS stimulation (Fig. 3), while TNF is produced by LPS-activated macrophages.34,87 TNF and CCF have opsonizing properties46,88 bind β-1,3-glucans and N, N'-diacetylchitobiose via lectin-like interactions.72,89 In addition, monoclonal antibodies elicited against the lectin-like TIP domain of TNF cross-react with CCF and, conversely, monoclonal antibody against CCF reacts with TNF without impairing the interaction of TNF with its specific receptor.90,91 However, the activity of CCF is not inhibited by anti-TNF antibody suggesting different mechanisms of TNF- and CCF-mediated lysis.
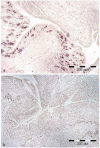
Figure 3
Up-regulated tissue expression of CCF in chloragogen tissue after LPS challenge (a) as compared to nonstimulated controls (b) followed by in situ hybridization. Reproduced by courtesy of Dr. Ellen Kauschke, Giessen.
The lectin-like domain of TNF was shown to be involved in the killing of African and American trypanosomes.90-92 Hence, in view of the similar lectin-like activity of CCF and TNF, the possible trypanolytic activity of CCF was investigated.73 The coelomic fluid of E. fetida as well as purified CCF were described to have a potent trypanolytic activity that can be inhibited not only by anti-CCF monoclonal antibodies but also by N, N'-diacetylchitobiose and anti-TNF antibodies. The possible target for both CCF and TNF on the trypanosome surface is the N-linked N, N'-diacetylchitobiose core of the variant-specific glycoprotein (VSG) that acts as a protective coat. This idea is strongly supported by the fact that CCF and TNF are able to lyse only bloodstream forms of parasites expressing VSG but not insect-stage procyclic forms expressing procyclin as a surface protein.
It was documented that TNF increases the membrane conductance in mammalian cells, interacting with ion-channels or ion-channel-coupled molecules through a lectin-like domain.93,94 Similarly, when endothelial cells or macrophages were activated with CCF, an increase in membrane conductance occurred.95 As observed with TNF, the ion-gating effect of CCF appeared when cells from TNF-receptor I and TNF-receptor II knockout mice were used. Moreover, this effect is blocked by N, N'-diacetylchitobiose and amiloride—an epithelial sodium channel inhibitor—suggesting that the effect is mediated by the lectin-like domain of CCF. In macrophages, CCF-induced depolarization results in the release of TNF, IL-6 and nitric oxide via NF-κB signaling. This pathway based on an interaction of lectin domain with saccharide moiety of ion channel may represent an evolutionary ancient mechanism of cell activation.96
Surprisingly, despite the functional analogies of CCF and TNF and cross-reactivity of anti-CCF and anti-TNF antibodies, these molecules do not show any gene or amino acid sequence homology, indicating a lack of common evolutionary origin.73
Conclusion
Earthworms rely on innate defense mechanisms that are sufficient for survival in often hostile environment. The choice of earthworms for comparative immunology studies was pertinent since they represent an inexpensive, appropriate and noncontroversial model for experimentation. Described defense mechanisms and molecules help to better understand more sophisticated immunity in vertebrates. Moreover, earthworms were found useful in monitoring environmental pollution. The Organization for Economic Cooperation and Development (OECD Guidelines for testing of Chemicals 1984) and the American Environmental Protection Agency accepted official protocols involving screening of earthworm immunological parameters as markers/indicators of impaired environmental conditions.97,98 Furthermore, earthworms might be considered as a source of biologically active compounds with potential industrial or medical use. Actually, earthworm powder has been used as a traditional medicine in some South Asia countries for years to treat various diseases. Currently, the therapeutic effect of earthworm active factors is being evaluated by a modern scientific approach. Some therapeutics containing fibrinolytic enzymes from Lumbricus rubellus and Eisenia fetida earthworms99-102 are already commercially available to support coagulation and fibrinolysis balance in the body and thus prevent or treat cardiac and cerebrovascular diseases (Boluoke® (lumbrokinase), Canada RNA Biochemical Inc.).
Acknowledgements
The authors acknowledge the support by the Czech Science Foundation (206/07/0378), Grant Agency of the Academy of Sciences of the Czech Republic (A600200704), the Ministry of Education, Youth and Sports (2B06155) and an Institutional Research Concept (AV0Z50200510).
References
- 1.
- Mechnikoff EE. Sur la lutte des cellules de l'organisme contre de l'invasion des microbes. Ann Inst Pasteur. 1887;1:322–340.
- 2.
- Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. [PubMed: 2700931]
- 3.
- Little TJ, Hultmark D, Read AF. Invertebrate immunity and the limits of mechanistic immunology. Nat Immunol. 2005;6:651–654. [PubMed: 15970937]
- 4.
- Schulenburg H, Boehnisch C, Michiels NK. How do invertebrates generate a highly specific innate immune response? Mol Immunol. 2007;44:3338–3344. [PubMed: 17391764]
- 5.
- Mill PJ. Physiology of Annelids. New York: Academic Press. 1978:1–683.
- 6.
- Rahemtulla F, Lovtrup S. The comparative biochemistry of invertebrate mucopolysaccharides II. Nematoda; Annelida. Comp Biochem Physiol B. 1974;49:639–646. [PubMed: 4279797]
- 7.
- Rahemtulla F, Lovtrup S. The comparative biochemistry of invertebrate mucopolysaccharides III. Oligocheta and Hirudinea. Comp Biochem Physiol B. 1975;50:627–629. [PubMed: 123493]
- 8.
- Chapron C. Etude histologique, infrastructurale et experimentale de la regeneration cephalique chez les lombriciens, Eisenia foetida. Ann Embr Morphol. 1970;3:235–239. [PubMed: 5450205]
- 9.
- Valembois P. Degenerescence et regeneration de l'epiderme a la suite d'une xenogreffe de paroi du corps entre lombriciens. C R Acad Sci Paris. 1971;96:59–64.
- 10.
- Burke JM. Wound healing in Eisenia foetida (Oligochaeta). I. Histology and 3H-thymidine radioautography of the epidermis. J Exp Zool. 1974;188:49–63. [PubMed: 4822549]
- 11.
- Burke JM. Wound healing in Eisenia foetida (Oligochaeta). II. A fine structural study of the role of the epidermis. Cell Tissue Res. 1974;154:61–82. [PubMed: 4442103]
- 12.
- Burke JM. Wound healing of Eisenia foetida (Oligochaeta). III. A fine structural study of the role of non-epidermal tissues. Cell Tissue Res. 1974;154:83–112. [PubMed: 4442104]
- 13.
- Dales RP, Kalac Y. Phagocytic defence by the earthworm Eisenia foetida against certain pathogenic bacteria. Comp Biochem Physiol. 1992;101A:487–490.
- 14.
- Cameron GR. Inflammation in earthworms. J Pathol. 1932;35:933–972.
- 15.
- Villaro AC, Sesma P, Alegría D, et al. Relationship of symbiotic microorganisms to metanephridium: phagocytic activity in the metanephridial epithelium of two species of Oligochaeta. J Morphol. 1985;186:307–314. [PubMed: 29996572]
- 16.
- Ratcliffe NA, Rowley AF, Fitzgerald SW, et al. Invertebrate immunity: basic concepts and recent advances. Internat Rev Cytol. 1985;97:183–349.
- 17.
- Valembois P, Lassegues M, Roch P. Formation of brown bodies in the coelomic cavity of the earthworm Eisenia fetida andrei and attendant changes in shape and adhesive capacity of constitutive cells. Dev Comp Immunol. 1992;16:95–101. [PubMed: 1499843]
- 18.
- Keilin ND. Parasitic autotomy of the host as a mode of liberation of coelomic parasites from the body of the earthworm. Parasitology. 1925;17:170–172.
- 19.
- Herlan-Meewis H. Regeneration in annelids. Advances in Morphogenesis, Vol. 4. M. Abercrombie and J. Brachet. New York:Academic Press. 1965:155–215. [PubMed: 5331921]
- 20.
- Alonso-Bedate M, Sequeros E. Neorosecretory phenomena in the cerebral ganglia of clitellated Allolobophora caliginosa. Acta Embryol Morphol Exp. 1983;4:93–103.
- 21.
- Alonso-Bedate M, Sequeros E. Suggested regulatory mechanisms for caudal regeneration in Allolobophora molleri (Annelida: Oligochaeta). Comp Biochem Physiol. 1985;81A:225–228.
- 22.
- Stein E, Avtalion RR, Cooper EL. The coelomocytes of the earthworm Lumbricus terrestris: morphology and phagocytic properties. J Morphol. 1977;153:467–477. [PubMed: 71353]
- 23.
- Šíma P. Annelid coelomocytes and haemocytes: roles in cellular immune reactions.In: Větvička V, Šíma P, Cooper EL, Bilej M, Roch P, eds.Immunology of Annelids. Boca Raton:CRC Press. 1994:115–165.
- 24.
- Engelmann P, Pal J, Berki T, et al. Earthworm leukocytes react with different mammalian antigen-specific monoclonal antibodies. Zoology. 2002;105:257–265. [PubMed: 16351874]
- 25.
- Stein E, Cooper EL. The role of opsonins in phagocytosis by coelomocytes of the earthworm, Lumbricus terrestris. Dev Comp Immunol. 1981;5:415–425. [PubMed: 7024010]
- 26.
- Bilej M, Scheerlinck JP, Van Den Driessche T, et al. The flow cytometric analysis of in vitro phagocytic activity of earthworm coelomocytes (Eisenia foetida, Annelida). Cell Biol Internat Rep. 1990;14:831–837.
- 27.
- Bilej M, Vetvicka V, Tuckova L, et al. Phagocytosis of synthetic particles in earthworms. Effect of antigenic stimulation and opsonization. Folia Biol (Praha). 1990;36:273–280. [PubMed: 2279582]
- 28.
- Laulan A, Lestage J, Bouc AM, et al. The phagocytic activity of Lumbricus terrestris leukocytes is enhanced by the vertebrate opsonins: IgG and complement C3b fragment. Dev Comp Immunol. 1988;12:269–277. [PubMed: 3384156]
- 29.
- Cooper EL, Roch P. Immunological profile of annelids: transplantation immunity In: Větvička V, Šíma P, Cooper EL, Bilej M, Roch P, eds. Immunology of Annelids. Boca Raton: CRC Press. 1994:201–243.
- 30.
- Bailey S, Miller BJ, Cooper EL. Transplantation immunity in annelids. II. Adoptive transfer of the xenograft reaction. Immunology. 1971;21:81–86. [PMC free article: PMC1408112] [PubMed: 5558033]
- 31.
- Parry MJ. Survival of body wall autografts, allografts and xenografts in the earthworm Eisenia foetida. J Invert Pathol. 1978;31:383–388. [PubMed: 355560]
- 32.
- Cooper EL. Transplantation immunity in helminths and annelids. Transplantation Proc. 1970;2:216–221. [PubMed: 5521747]
- 33.
- Hostetter R, Cooper EL. Cellular anamnesis in earthworms. Cell Immunol. 1973;9:384–392. [PubMed: 4758875]
- 34.
- Bilej M, Rossmann P, Sinkora M, et al. Cellular expression of the cytolytic factor in earthworms Eisenia foetida. Immunol Lett. 1998;60:23–29. [PubMed: 9541459]
- 35.
- Cotuk A, Dales RP. Lysozyme activity in the coelomic fluid and coelomocytes of the earthworm Eisenia foetida SAV in relation to bacterial infection. Comp Biochem Physiol. 1984;78A:469–474.
- 36.
- Cossarizza A, Cooper EL, Suzuki MM, et al. Earthworm leukocytes that are not phagocytic and cross-react with several human epitopes can kill human tumor cell lines. Exp Cell Res. 1996;224:174–182. [PubMed: 8612683]
- 37.
- Quaglino D, Cooper EL, Salvioli S, et al. Earthworm coelomocytes in vitro: cellular features and "granuloma" formation during cytotoxic activity against the mammalian tumor cell target K562. Eur J Cell Biol. 1996;70:278–288. [PubMed: 8832212]
- 38.
- Josková R, Šilerová M, Procházková P, et al. Identification and cloning of an invertebrate-type lysozyme from Eisenia andrei. Dev Comp Immunol. 2009;33:932–938. [PubMed: 19454335]
- 39.
- Cho JH, Park CB, Yoon YG, et al. Lumbricin I, a novel proline-rich antimicrobial peptide from the earthworm: purification, cDNA cloning and molecular characterization. Biochim Biophys Acta. 1998;1408:67–76. [PubMed: 9784609]
- 40.
- Wang X, Wang X, Zhang Y, et al. An antimicrobial peptide of the earthworm Pheretima tschiliensis: cDNA cloning, expression and immunolocalization. Biotechnol Lett. 2003;25:1317–1323. [PubMed: 14514059]
- 41.
- Liu YQ, Sun ZJ, Wang C, et al. Purification of a novel antibacterial short peptide in earthworm Eisenia foetida. Acta Biochim Biophys Sin (Shanghai). 2004;36:297–302. [PubMed: 15253156]
- 42.
- Roch P. Protein analysis of earthworm coelomic fluid: 1) polymorphic system of the natural hemolysin of Eisenia fetida andrei. Dev Comp Immunol. 1979;3:599–608. [PubMed: 43268]
- 43.
- Valembois P, Roch P, Lassegues M, et al. Antibacterial activity of the haemolytic system from the earthworm Eisenia fetida andrei. J Invert Pathol. 1982;40:21–27.
- 44.
- Roch P, Lassegues M, Valembois P. Antibacterial activity of Eisenia fetida andrei coelomic fluid: III. Relationship within the polymorphic hemolysins. Dev Comp Immunol. 1991;15:27–32. [PubMed: 2050244]
- 45.
- Kauschke E, Mohrig W. Cytotoxic activity in the coelomic fluid of the annelid Eisenia foetida Sav. J Comp Physiol [B] 1987;157:77–83. [PubMed: 3571568]
- 46.
- Bilej M, Brys L, Beschin A, et al. Identification of a cytolytic protein in the coelomic fluid of Eisenia foetida earthworms. Immunol Lett. 1995;45:123–128. [PubMed: 7622179]
- 47.
- Cooper EL, Cossarizza A, Suzuki MM, et al. Autogeneic but not allogeneic earthworm effector coelomocytes kill the mammalian tumor cell target K562. Cell Immunol. 1995;166:113–122. [PubMed: 7585971]
- 48.
- Ito Y, Yoshikawa A, Hotani T, et al. Amino acid sequences of lysozymes newly purified from invertebrates imply wide distribution of a novel class in the lysozyme family. Eur J Biochem. 1999;259:456–461. [PubMed: 9914527]
- 49.
- Du Pasquier L, Duprat P. Humoral and cellular aspects of a nonspecific natural immunity in the oligochetee Eisenia foetida Sav. (Lumbricinae), C R Acad Sci Hebd Seances Acad Sci D. 1968;266:538–541. [PubMed: 4969240]
- 50.
- Roch P, Valembois P, Davant N, et al. Protein analysis of earthworm coelomic fluid. II. Isolation and biochemical characterization of the Eisenia foetida andrei factor (EFAF). Comp Biochem Physiol. 1981;69B:829–836.
- 51.
- Roch P. Isolation of agglutinins and lysins from earthworm coelomic fluid by gel filtration followed by chromatofocusing. J Chromat. 1984;290:231–235.
- 52.
- Roch P, Valembois P, Lassegues M. Genetic and biochemical polymorphism of earthworm humoral defenses. Prog Clin Biol Res. 1987;233:91–102. [PubMed: 3295895]
- 53.
- Valembois P, Roch P, Lassegues M. Simultaneous existence of haemolysins and hemagglutinins in the coelomic fluid and in the cocoon albumen of the earthworm Eisenia fetida andrei. Comp Biochem Physiol. 1984;78A:141–145.
- 54.
- Lassegues M, Roch P, Valembois P. Antibacterial activity of Eisenia fetida andrei coelomic fluid: evidence, induction and animal protection. J Invert Pathol. 1989;53:1–6.
- 55.
- Hirigoyenberry F, Lassegues M, Roch P. Antibacterial activity of Eisenia foetida andrei coelomic fluid: immunological study of the two major antibacterial proteins. J Invert Pathol. 1992;59:69–74.
- 56.
- Valembois P, Roch P, Lassegues M. Antibacterial molecules in annelids. In: Brehelin M, ed.Immunity in Invertebrates. Berlin:Springer-Verlag. 1986:74–93.
- 57.
- Sinkora M, Bilej M, Tuckova L, et al. Hemolytic function of opsonizing proteins of earthworms coelomic fluid. Cell Biol Int. 1993;17:935–939. [PubMed: 8287023]
- 58.
- Valembois P, Roch P, Lassegues M. Evidence of plasma clotting system in earthworms. J Invertebr Pathol. 1988;51:221–228.
- 59.
- Roch P, Canicatti C, Valembois P. Interactions between earthworm hemolysins and sheep red blood cell membranes. Biochim Biophys Acta. 1989;983:193–198. [PubMed: 2758056]
- 60.
- Lassegues M, Milochau A, Doignon F, et al. Sequence and expression of an Eisenia-fetida-derived cDNA clone that encodes the 40-kDa fetidin antibacterial protein. Eur J Biochem. 1997;246:756–762. [PubMed: 9219536]
- 61.
- Milochau A, Lassegues M, Valembois P. Purification, characterization and activities of two hemolytic and antibacterial proteins from coelomic fluid of the annelid Eisenia fetida andrei. Biochim Biophys Acta. 1997;1337:123–132. [PubMed: 9003444]
- 62.
- Sekizawa Y, Hagiwara K, Nakajima T, et al. A novel protein, lysenin, that causes contraction of the isolated rat aorta: its purification from the coelomic fluid of the earthworm, Eisenia foetida. Biomed Res. 1996;17:197–203.
- 63.
- Sekizawa Y, Kubo T, Kobayashi H, et al. Molecular cloning of cDNA for lysenin, a novel protein in the earthworm Eisenia foetida that causes contraction of rat vascular smooth muscle. Gene. 1997;191:97–102. [PubMed: 9210594]
- 64.
- Bruhn H, Winkelmann J, Andersen C, et al. Dissection of the mechanisms of cytolytic and antibacterial activity of lysenin, a defence protein of the annelid Eisenia fetida. Dev Comp Immunol. 2006;30:597–606. [PubMed: 16386304]
- 65.
- Procházková P, Σilerová M, Felsberg J, et al. Relationship between hemolytic molecules in Eisenia fetida earthworms. Dev Comp Immunol. 2006;30:381–392. [PubMed: 16051356]
- 66.
- Yamaji A, Sekizawa Y, Emoto K, et al. Lysenin, a novel sphingomyelin-specific binding protein. J Biol Chem. 1998;273:5300–5306. [PubMed: 9478988]
- 67.
- Yamaji-Hasegawa A, Makino A, Baba T, et al. Oligomerization and pore formation of a sphingomyelin-specific toxin, lysenin. J Biol Chem. 2003;278:22762–22770. [PubMed: 12676961]
- 68.
- Lange S, Nussler F, Kauschke E, et al. Interaction of earthworm hemolysin with lipid membranes requires sphingolipids. J Biol Chem. 1997;272:20884–20892. [PubMed: 9252415]
- 69.
- Lange S, Kauschke E, Mohrig W, et al. Biochemical characteristics of Eiseniapore, a pore-forming protein in the coelomic fluid of earthworms. Eur J Biochem. 1999;262:547–556. [PubMed: 10336641]
- 70.
- Eue I, Kauschke E, Mohrig W, et al. Isolation and characterization of earthworm hemolysins and agglutinins. Dev Comp Immunol. 1998;22:13–25. [PubMed: 9617580]
- 71.
- Koenig S, Wagner F, Kauschke E, et al. Mass spectrometric analyses of CL(39), CL(41) and H(1), H(2), H(3) confirm identity with fetidin and lysenin produced by earthworm leukocytes. Dev Comp Immunol. 2003;27:513–520. [PubMed: 12697308]
- 72.
- Beschin A, Bilej M, Hanssens F, et al. Identification and cloning of a glucan- and lipopolysaccharide-binding protein from Eisenia foetida earthworm involved in the activation of prophenoloxidase cascade. J Biol Chem. 1998;273:24948–24954. [PubMed: 9733802]
- 73.
- Beschin A, Bilej M, Brys L, et al. Convergent evolution of cytokines. Nature. 1999;400:627–628. [PubMed: 10458158]
- 74.
- Bilej M, De Baetselier P, Van Dijck E, et al. Distinct carbohydrate recognition domains of an invertebrate defense molecule recognize Gram-negative and Gram-positive bacteria. J Biol Chem. 2001;276:45840–45847. [PubMed: 11585829]
- 75.
- Procházková P, Σilerová M, Stijlemans B, et al. Evidence for proteins involved in prophenoloxidase cascade in Eisenia fetida earthworms. J Comp Physiol B. 2006;176:581–587. [PubMed: 16636833]
- 76.
- Šilerová M, Procházková P, Josková R, et al. Comparative study of the CCF-like pattern recognition protein in different lumbricid species. Dev Comp Immunol. 2006;30:765–771. [PubMed: 16386303]
- 77.
- Yamamoto M, Aono R, Horikoshi K. Structure of the 87-kDa beta-1,3-glucanase gene of Bacillus circulans IAM1165 and properties of the enzyme accumulated in the periplasm of Escherichia coli carrying the gene. Biosci Biotechnol Biochem. 1993;57:1518–1525. [PubMed: 7764221]
- 78.
- Bachman ES, McClay DR. Molecular cloning of the first metazoan beta-1,3-glucanase from eggs of the sea urchin Strongylocentrotus purpuratus. Proc Natl Acad Sci USA. 1996;93:6808–6813. [PMC free article: PMC39109] [PubMed: 8692900]
- 79.
- Kozhemyako VB, Rebrikov DV, Lukyanov SA, et al. Molecular cloning and characterization of an endo-1,3-beta-D-glucanase from the mollusk Spisula sachalinensis. Comp Biochem Physiol B Biochem Mol Biol. 2004;137:169–178. [PubMed: 14990213]
- 80.
- Seki N, Muta T, Oda T, et al. Horseshoe crab (1,3)-beta-D-glucan-sensitive coagulation factor G.A serine protease zymogen heterodimer with similarities to beta-glucan-binding proteins. J Biol Chem. 1994;269:1370–1374. [PubMed: 8288603]
- 81.
- Lee WJ, Lee JD, Kravchenko VV, et al. Purification and molecular cloning of an inducible gram-negative bacteria-binding protein from the silkworm, Bombyx mori. Proc Natl Acad Sci USA. 1996;93:7888–7893. [PMC free article: PMC38844] [PubMed: 8755572]
- 82.
- Dimopoulos G, Richman A, Muller HM, et al. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA. 1997;94:11508–11513. [PMC free article: PMC23521] [PubMed: 9326640]
- 83.
- Shin SW, Park SS, Park DS, et al. Isolation and characterization of immune-related genes from the fall webworm, Hyphantria cunea, using PCR-based differential display and subtractive cloning. Insect Biochem Mol Biol. 1998;28:827–837. [PubMed: 9818384]
- 84.
- Kim YS, Han SJ, Ryu JH, et al. Lipopolysaccharide-activated kinase, an essential component for the induction of the antimicrobial peptide genes in Drosophila melanogaster cells. J Biol Chem. 2000;275:2071–2079. [PubMed: 10636911]
- 85.
- Ma C, Kanost MR. A beta1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J Biol Chem. 2000;275:7505–7514. [PubMed: 10713054]
- 86.
- Ochiai M, Ashida M. A pattern-recognition protein for beta-1,3-glucan. The binding domain and the cDNA cloning of beta-1,3-glucan recognition protein from the silkworm, Bombyx mori. J Biol Chem. 2000;275:4995–5002. [PubMed: 10671539]
- 87.
- Aggarwal BB, Kohr WJ, Hass PE, et al. Human tumor necrosis factor.Production, purification and characterization. J Biol Chem. 1985;260:2345–2354. [PubMed: 3871770]
- 88.
- Luo G, Niesel DW, Shaban RA, et al. Tumor necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect Immun. 1993;61:830–835. [PMC free article: PMC302808] [PubMed: 8381771]
- 89.
- Olson EJ, Standing JE, Griego-Harper N, et al. Fungal beta-glucan interacts with vitronectin and stimulates tumor necrosis factor alpha release from macrophages. Infect Immun. 1996;64:3548–3554. [PMC free article: PMC174262] [PubMed: 8751898]
- 90.
- Lucas R, Magez S, De Leys R, et al. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263:814–817. [PubMed: 8303299]
- 91.
- Magez S, Geuskens M, Beschin A, et al. Specific uptake of tumor necrosis factor-alpha is involved in growth control of Trypanosoma brucei. J Cell Biol. 1997;137:715–727. [PMC free article: PMC2139880] [PubMed: 9151676]
- 92.
- Olivares Fontt EO, De Baetselier P, Heirman C, et al. Effects of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha on Trypanosoma cruzi trypomastigotes. Infect Immun. 1998;66:2722–2727. [PMC free article: PMC108261] [PubMed: 9596739]
- 93.
- Hribar M, Bloc A, van der Goot FG, et al. The lectin-like domain of tumor necrosis factor-alpha increases membrane conductance in microvascular endothelial cells and peritoneal macrophages. Eur J Immunol. 1999;29:3105–3111. [PubMed: 10540321]
- 94.
- van der Goot FG, Pugin J, Hribar M, et al. Membrane interaction of TNF is not sufficient to trigger increase in membrane conductance in mammalian cells. FEBS Lett. 1999;460:107–111. [PubMed: 10571070]
- 95.
- Bloc A, Lucas R, Van Dijck E, et al. An invertebrate defense molecule activates membrane conductance in mammalian cells by means of its lectin-like domain. Dev Comp Immunol. 2002;26:35–43. [PubMed: 11687261]
- 96.
- Bilej M, Josková R, Van den Bergh R, et al. An invertebrate TNF functional analogue activates macrophages via lectin-saccharide interaction with ion channels. Int Immunol. 2006;18:1663–1670. [PubMed: 17035350]
- 97.
- Green JC, Bartels CL, Warren-Hicks WJ, et al. 1989. Protocols of Short-Term Toxicity Screening of Hazardous Waste Sites, US Environmental Protection Agency, EPA/600/3-88/029, ERLC, Corvallis (OR);
- 98.
- Goven AJ, Kennedy J. Environmental pollution and toxicity in invertebrates: an earthworm model for immunotoxicology. Adv Comp Environ Physiol. 1996;24:169–211.
- 99.
- Mihara H, Maruyama M, Sumi H. Novel thrombolytic therapy discovered from traditional oriental medicine using the earthworm. Southeast Asian J Trop Med Public Health. 1992;23(Suppl 2):131–140. [PubMed: 1298986]
- 100.
- Mihara H, Sumi H, Yoneta T, et al. A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn J Physiol. 41:461–472. [PubMed: 1960890]
- 101.
- Nakajima N, Mihara H, Sumi H. Characterization of potent fibrinolytic enzymes in earthworm, Lumbricus rubellus. Biosci Biotechnol Biochem. 1993;57:1726–1730. [PubMed: 7764268]
- 102.
- Nakajima N, Ishihara K, Sugimoto M, et al. Chemical modification of earthworm fibrinolytic enzyme with human serum albumin fragment and characterization of the protease as a therapeutic enzyme. Biosci Biotechnol Biochem. 1996;60:293–300. [PubMed: 9063978]
- Earthworm Immunity - Madame Curie Bioscience DatabaseEarthworm Immunity - Madame Curie Bioscience Database
- Overview of Intracellular Compartments and Trafficking Pathways - Madame Curie B...Overview of Intracellular Compartments and Trafficking Pathways - Madame Curie Bioscience Database
- Subcellular Compartmentalization of Insulin Signaling Processes and GLUT4 Traffi...Subcellular Compartmentalization of Insulin Signaling Processes and GLUT4 Trafficking Events - Madame Curie Bioscience Database
- Immunity and Autoimmunity Induced by Polyomaviruses: Clinical, Experimental and ...Immunity and Autoimmunity Induced by Polyomaviruses: Clinical, Experimental and Theoretical Aspects - Madame Curie Bioscience Database
- Methylenetetrahydrofolate Reductase Polymorphisms: Pharmacogenetic Effects - Mad...Methylenetetrahydrofolate Reductase Polymorphisms: Pharmacogenetic Effects - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...