NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Sensory hair cells (HCs) and their associated nonsensory supporting cells (SCs) exhibit a typical mosaic pattern in each of the sensory patches in the inner ear. Notch signaling has been considered to conduct the formation of this mosaic pattern through one of its famous functions, known as 'lateral inhibition'. The two Notch ligands Delta-like1 and Jagged2 are believed to act synergistically at the stage of cell diversification in mammals. In addition, many current studies suggest that Notch signaling has another inductive, but not inhibiting, role in the determination of the prosensory region, which precedes the cell diversification of HCs and SCs and Jagged1 is thought to be an essential ligand in this process. Earlier in ear development, the first cell fate determination begins with the delamination of the neuroblasts from the otic epithelium. The delaminated neuroblasts migrate and coalesce to form cochleovestibular ganglion. Notch signaling pathway is thought to function during the delamination through its lateral inhibitory mechanism. Recently, many experiments examining Notch-related gene expression patterns and direct functional analyses of genes have revealed multiple important functions of Notch in inner ear development. Here, we survey a series of studies and discuss the issues that remain to be elucidated in the future.
INTRODUCTION: STRUCTURE AND DEVELOPMENT OF MAMMALIAN INNER EAR
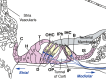
The mammalian inner ear is a finely structured organ, which consists of a turned cochlea, a central vestibule including saccule and utricle, three semicircular canals that are positioned at right angles to each other and a dorsally protruding endolymphatic duct and sac. In the inner ear, there are three different kinds of sensory regions: the organ of Corti, lining the cochlear duct; the maculae, contained within the saccule and utricle; the cristae, located at the base of each semicircular canal. The macula sacculi, macula utriculi, and the cristae of semicircular canals are responsible for detecting gravity and linear and angular acceleration, respectively. These five organs are crucial for balance; only one sensory organ, the Organ of Corti, is required for hearing (Fig. 1). All six sensory organs are populated by mechanosensory hair cells (HCs) and their associated supporting cells (SCs). Unfortunately, the production of HCs in the cochlea is completed before birth in mammals. Any subsequent loss of auditory HCs is not corrected, resulting in permanent hearing loss. In the vestibular sensory epithelia of adult mammals, HC regeneration in response to amynoglycoside ototoxicity does occur, although it is extremely limited.1,2
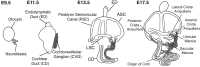
The inner ear is derived entirely from the otic placode, which can be initially recognized as a bilateral thickening of the surface ectoderm near the developing hindbrain at embryonic day (E) 8.5 in mice.3 Once established, the otic placode invaginates to form an enclosed otocyst by E10.5. As the otic vesicle closes, the neuroblasts that will give rise to the cochleovestibular ganglion (CVG) start to delaminate from the ventral region of the otocyst around E9.5 (Fig. 2).4-6 Almost simultaneously, a narrow extension originates in the dorsomedial region of the otocyst, extending toward the brain to form the endolymphatic duct and sac. Similarly, the cochlear duct forms as an outpocketing from the ventral region of the otocyst and continues to grow until E18. Individual regions in the inner ear under go specialization and develop as the prosensory regions that will contain HCs and SCs (for review see ref. 7). During the growth of the cochlear duct, the cells in the primordial organ of Corti exit the cell cycle between E13.5 and E14.5 to establish a distinct zone of nonproliferating cells delimited by the expression of the cyclin kinase inhibitors p27Kip1 and p19Ink4d in the cochlea.8-11 Subsequently, HCs and SCs differentiate within the sensory primordium to form a precise mosaic cell pattern. Initially, HC progenitors expressing Math1 appear in the mid-basal region of the cochlea at developmental stages co-incident with or just after terminal mitosis.12,13 Math1, a close homologue of the Drosophila proneural gene atonal, encodes a member of the bHLH (basic helix-loop-helix) family of transcriptional factors. Math1 is absolutely required for the generation of HC progenitors as well as their survival.13-15 As the development continues, additional HC progenitors start to appear in a stereotypical wave of differentiation, that is, from the mid-basal region to both apical and basal directions.12 SC progenitors develop in the area surrounding the HC progenitors, although with a small delay and the robust activation of Notch1 was detected in these cells.16
MULTIPLE ROLES OF NOTCH SIGNALING PATHWAY DURING INNER EAR DEVELOPMENT
Hair Cell and Supporting Cell Differentiation
Classic Notch signaling is associated with inhibitory interactions during which the activation of Notch via the ligands expressed on the membranes of a cell that is about to undergo differentiation prevents the adjacent cells from assuming the same cell fate. Thus, this inhibiting interaction between the neighboring cells creates a mosaic cell pattern from initially equivalent cells. The mechanism was originally studied in Drosophila melanogaster and Caenorhabditis elegans and is usually referred to Notch mediated 'lateral inhibition'.17-19 As each HC is surrounded by SCs in the sensory epithelium of the inner ear, it is not surprising that the Notch signaling pathway is thought to play a crucial role in the formation of the HC and SC mosaic through lateral inhibition. In addition, retroviral lineage tracing in the chicken auditory system has identified the existence of two cell clones in which one cell developed as an HC, while the other had developed as an SC, suggesting that the same progenitors give rise to both HCs and SCs.20 This finding also consistent with a lateral inhibitory mechanism in which different cell types arise from an initially equivalent epithelium.
Expression studies have also demonstrated consistent results; Notch1 is broadly expressed within the cochlear duct, including the prosensory region.21 Cells destined to develop as HCs up-regulate expression of two Notch ligands, Jagged2 (Jag2) and Delta-like1 (Dll1), with the first signs of ligand expression occurring around E14 in the basal region of the cochlea and subsequently extending towards the apex.21,22 Within 24 hours of ligand expression, activated Notch1 is observed in adjacent cells16 as well as expression of at least two Notch target basic helix-loop-helix genes, Hes1 and Hes5 (Hes: Mammalian homologues of Drosophila hairy and Enhancer of split); these cells ultimately develop into SCs.23,24 Hes1 and Hes5 are supposed to repress the transcription of Math1, a crucial gene for HC formation.25
A large amount of direct functional evidence exists supporting lateral inhibition in HC and SC differentiation during the development of the mouse inner ear. The deletion of the Jag2 gene resulted in extra rows of both inner and outer HCs in the cochlea.21 Similarly, mice lacking one copy of the Notch1 gene and mice deleted for Hes1 and Hes5 deletions exhibited increased number of HCs.23,26 More recent studies using Dll1/ Jag2 double mutant embryos27 or Dll1 conditional knockout (cko)mouse embryos28 have revealed that the Notch ligands Dll1 and Jag2 act synergistically to regulate the HC and SC differentiation in a manner consistent with the lateral inhibition theory. Interestingly, the authors suggested that the Notch signaling pathway, via the ligands Dll1 and Jag2, may regulate adequate cellular differentiation through the prevention of excessive cellular proliferation in addition to inducing lateral inhibition.
Determination of Prosensory Region
Jagged1 and Prosensory Determination
Jagged1 (Jag1) and Lunatic Fringe (Lfng) are both components of the Notch signaling pathway, with Jag1 acting as a ligand for Notch and Lfng modulating the activity of some Notch ligands.29,30 The results of in situ hybridization analyses have shown that Jag1 mRNA5,22,31 and Lfng mRNA32 are both expressed in patterns compatible with a role in prosensory formation during inner ear development. Functional analyses also revealed that mice heterozygous for two different N-ethyl-N-nitrosourea-induced point mutations in Jag1 gene (slalom33 and head-turner)34 showed mild defects in the sensory organs, especially the reduction of outer HCs in the cochlea. Because embryos homozygous for mutant alleles of the Jag1 gene do not survive past E11.5, Jag1 cko mutant mice were created using the Foxg1-Cre mouse line to express Cre-recombinase in early otocysts.28,35 In Jag1 cko mice, no HCs or SCs were found at the basal turn of the cochlea, while the number of HCs and SCs was reduced at the apical turn. In the vestibular region, the cristae of semicircular canals were completely lacking and the utricular macula was extremely small. As the saccule and its macula were also mildly affected, the formations of all the six sensory regions of the inner ear were affected to various degrees in Jag1 cko inner ears. Moreover, markers of the prosensory domain, such as Sox2 and the cyclin-dependent kinase inhibitor p27Kip1, are down regulated in Jag1 cko inner ears. These results suggested that Jag1 is likely to be essential for the determination of the prosensory region in the inner ear.
Role of Notch Signaling in Induction for Prosensory Region
As Jag1 is a Notch ligand, the above-described results imply that Jag1-mediated Notch signaling is essential for establishing the prosensory regions in both of the cochlea and the vestibule. Consistent with this hypothesis, in vitro inhibition of the Notch signaling in cochlear explants with the γ-secretase inhibitor DAPT during early developmental stages reduced the number of HCs and SCs that develop.36 In gain-of-function studies using the constitutively active, intracellular domain of Notch1 (NICD), NICD induced the expression of prosensory markers when over-expressed in the embryonic mouse cochlea,37 and caused the formation of ectopic sensory patches in embryonic chickens.38 These studies support an inductive role for Notch signaling in the formation of prosensory patches within the inner ear, in addition to its subsequent role in lateral inhibitory signaling that determines the HC versus SC fates later during development. Lewis and coworkers have suggested that a Notch-mediated lateral induction mechanism is involved in the prosensory formation: The activation of Notch1 positively regulates the expression of Jag1 autonomously, gradually strengthening the Notch1 activation and the expression of Jag1 in the prosensory cells.18,38,39 However, this hypothesis has not been tested in long term with direct gain of function in mammals.
In 2010, a Cre-/loxP approach was used to conditionally activate the Notch pathway in nonsensory regions of mouse inner ear epithelia during different stages of otic vesicle morphogenesis.40 The RosaNotch transgenic mouse yielded a heritable, constitutive co-expression of NICD and nuclear GFP in the presence of Cre-recombinase. The authors selected a FoxG1Cre transgenic line that expresses Cre-recombinase in early otic vesicles and several other regions of embryos and found that the broad ectopic activation of Notch at very early stages caused induction of prosensory markers, such as Sox2 and Hey1 throughout the entire otic epithelium. Unfortunately, as Foxg1Cre; RosaNotch embryos did not survive past E13.5, they had to use an hGFAPCCre transgenic line that provides a more restricted pattern of recombination in the nonsensory region of the developing inner ear during later stages. They observed that at intermediate stages of otic morphogenesis, the activation of Notch1 in a nonsensory region led to the induction of ectopic sensory patches containing HCs and SCs. Moreover, they demonstrated that the activation of Notch1 in isolated nonsensory cells induced the lateral induction of Jag1 in adjacent cells. Another group of the researchers also showed that NICD expression resulted in ectopic HCs and SCs in nonsensory regions of the cochlea and vestibule using a combined Tet-On (tetracycline-on)/Cre induction system in mice.41 These recent reports substantiate the hypothesis that Jag1-Notch-mediated lateral induction may propagate and maintain the prosensory character during inner ear development in both mice and chicks.
Possible Effectors of the Notch Signaling Pathway in the Context of Early Prosensory Formation
Though Jag1-dependent Notch activation has been revealed to be important for prosensory determination during the earlier stage of cochlear development, little is known about the effectors of the Notch pathway in this context. The Notch effectors Hey1 and Hey2 (sometimes referred to as Hesr1 and Hesr2) have been identified as likely mediators of Notch signaling at this stage, mainly based on the expression patterns.36 However, the presumed functional redundancy and early embryonic lethality made a definitive loss-of-function analysis impossible. Another Notch signaling mediator, Hes1, is broadly expressed during the early otocyst stages and may also be involved in defining the prosensory domain.24,42 A cyclin-dependent kinase inhibitor, p27Kip1, is also known to demarcate the prosensory region in the cochlear primordium, which consists of the sensory progenitors that have completed their terminal mitoses.9,10 Hes1 reportedly promotes precursor cell proliferation through the transcriptional down-regulation of p27Kip1 in the thymus, liver and brain.43 We showed that Hes1, not Hes5 was weakly expressed at the time of onset of p27Kip1 expression and the expression pattern of Hes1 prior to cell differentiation was similar to that of activated Notch1. In addition, p27Kip1 was up-regulated and the number of BrdU-positive S-phase cells was reduced in the developing cochlear epithelium of Hes1-null mice.24 The results suggest that Hes1, as one of the effectors of the Notch pathway, may contribute to the adequate proliferation of sensory precursor cells via the potential transcriptional down-regulation of p27Kip1 expression and may play a pivotal role in the correct prosensory determination.
The HMG-box transcription factor Sox244, 45 has also been thought to play an important role in prosensory formation based on its expression pattern during inner ear development and the absent or severely disturbed prosensory patches in two mouse Sox2 mutant lines, LCC and Ysb.46 As the expression of Sox2 is markedly reduced in Jag1 cko mutant mice,35 it is supposed that Sox2 may function downstream of the Jag1-Notch1 signaling pathway during prosensory formation. However, the mechanism of the contribution of Sox2 in this context still remains to be elucidated.
Biphasic Regulation of Cell Proliferation by the Notch Signaling Pathway during the Inner Ear Development
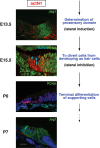
We investigated the spatio-temporal expression pattern of activated Notch1 (actN1) during the mouse cochlear development.24 The results showed that actN1 was diffusely observed in the prosensory region and greater epithelial ridge (GER) during the early developmental stage and Notch1 was activated robustly in SC progenitors at a later stage of cell diversification after E14.5 (Fig. 3). The level of Notch1 signaling switched from a diffuse state to a robust state at the time of cell cycle exit, when the Notch pathway started to promote cell diversification through lateral inhibition.24 On the other hand, the diffuse pattern of Notch1 activation before E13.5 was closely related to the expression of Hes1 as well as Jag1. Thus, as discussed in the former chapter, the diffuse activation of Notch1 should be critical for the prosensory formation by continuing the adequate proliferation of sensory precursor cells.24 Interestingly, the higher level of Notch1 activation during the later stage of cochlear development was supposed to inhibit excessive cell division in several studies. A huge increase in HCs was observed in Dll1+/- Jag2-/- cochlea and the authors suspected that their observation resulted from the inhibition of excessive cell division via the Notch pathway during cell diversification.27 A similar conclusion was drawn in a study examining in vitro culture of mouse fetal cochleae and the reversible inhibition of the Notch-signaling pathway using inhibitors of γ-secretase and TNF-α-converting enzyme.47 Previous reports have revealed that Notch signaling has similar biphasic roles during the development of the central nervous system (CNS).48, 49 We propose that the deletion of the Hes1 allele may suppress cell proliferation during the period of prosensory formation and that excessive cell division might occur during the next cell diversification stage, resulting in the relatively mild increase in HCs observed in Hes1-/- mice at E17.5 in previous studies.23,24,26
Delamination of Neural Progenitors
Early during ear development, a subset of cells in the anteroventral region of the otocyst becomes determined as neuroblasts and starts to delaminate from the epithelium at about E9.5 in mice. They migrate and co-alesce to form the primary neurons of the auditory and vestibular systems in cochleovestibular ganglion (CVG).4-6 Neurogenic basic helix-loop-helix (bHLH) transcriptional factors, such as Neurogenin1 (Ngn1), NeuroD1 (NeuD1) and Math1, are crucial for in the development of various nervous systems. In the inner ear, Ngn150, 51 and NeuD152 are required for the generation or delamination and survival of CVG neurons respectively. Lateral inhibition has been suppered to be involved in this process as well as in diversification of HCs and SCs at the later stage during the inner ear development. That is, Dll1-expressing cells delaminate as neuroblasts from the early otocyst and Notch1 is activated in other cells surrounding the Dll1-positive cells. The differentiation of these surrounding cells into neuroblasts are inhibited and they instead become sensory or nonsensory epithelial cells. The volume of the CVG reportedly increased, with the saccular and utricular maculae being lost or severely reduced in Dll1 cko mice.28 The phenomenon can be interpreted as indication that when the lateral inhibition failed via the deletion of Dll1, an excessive proportion of cells was diversified towards a neuronal fate, resulting in the depletion of the prosensory population in the anteroventral otic epithelium, which is supposed to develop as maculae.
In contrast, a recent study demonstrated that the CVG was smaller in Jag1 cko mice, with significantly fewer neuroblasts (Ngn1-positive cells) within the otic vesicle.54 The authors supposed that Jag1 might be important for the specification and maintenance of 'neurosensory progenitors' given the decrease in both sensory and neural progenitors in Jag1 cko inner ears. Lineage experiments indicate that sensory cells in the maculae and neuronal cells can share a common progenitor in chik.56 In addition, fate-mapping experiments have demonstrated that not all Ngn1-positive cells become neuroblasts, with some becoming sensory and nonsensory cells in the macular regions of mice.54 These data support the existence of a neurosensory progenitor and may reinforce the hypothesis that Notch-dependent lateral inhibition among the neurosensory progenitors plays a crucial role in neuroblast delamination.
NOTCH SIGNALING PATHWAY AND THE INNER EAR REGENERATION
The production of HCs in the cochlea is finished before birth in mammals. Any subsequent loss of auditory HCs is not compensated, resulting in irreversible hearing loss. In contrast, many nonmammalian vertebrates readily regenerate HCs into adulthood and HC regeneration has been most thoroughly studied in birds (see review ref. 57). In birds, when acoustic trauma or ototoxic drugs destroy HCs, SCs give rise to new HCs in two different ways. First, SCs are directly converted into new HCs through a method described as transdifferentation.57, 58 A few days later, SCs adjacent to the dying HCs re-enter the cell cycle, dividing asymmetrically to generate new HCs and SCs.60, 61 In the normally quiescent bird auditory epithelium, HC regeneration seems to re-activate developmental programs and the Notch signaling pathway is probably one of the best candidates for investigation. During development, Notch receptor activation suppresses HC diversification through the up-regulation of the Hes genes, which are potent inhibitors of Math1. As a result, the control of the Notch pathway, such as through the deletion of Notch ligands, leads to supernumerary HCs. Correspondingly, during zebrafish lateral line62 and chick basilar papilla (BP; auditory epithelium) HC regeneration,63 the inhibition of the Notch signaling pathway increases the production of new HCs. Daudet and co-researchers showed that the blockade of Notch signaling by DAPT had no direct effect on SC division after HC damage in organ cultures of chick BP.63 However, DAPT caused the excessive regeneration of HCs at the expense of SCs, through both mitotic and nonmitotic mechanisms with the up-regulation of Delta1 and Hes5 genes. They also confirmed that the inhibition of γ-secretase in undamaged BP does not trigger HC production. Conversely, the over expression of NICD in SCs after damage caused them to maintain their phenotype and inhibited HC regeneration. These data imply that Notch signaling controls the switches of the cell diversification, but may not directly regulate the cell division in the regeneration of BP. The authors supposed that another signal (Q signal; signal for quiescence), which is independent of Notch signaling (N signal), functions during quiescence and regeneration and inhibits SCs from direct transdifferentiation into HCs and from cell division to product HC and SC progenitors.
In mammals, the applications of DAPT to explants of embryonic and neonatal organ of Corti resulted in the robust production of supernumerary HCs in the case of mice.47,64 Continuous inhibition through the application of DAPT also seems to induce the mitogenic proliferation of SCs in the cultures of embyronic organ of corti.47 However, experiments on damaged adult guinea pig organs of Corti are disappointing; local application of a γ-secretase inhibitor, MDL28170, resulted in the formation of very limited ectopic HCs in the inner sulcus region.65 The results might imply that when HCs are injured in adult mammals, the Q-signal that has been described in the case of chicks cannot be adequately weakened by some unknown factors. We might be able to elucidate these factors by investigating other signaling pathways that are act reciprocally with the Notch signaling pathway during inner ear development.
CONCLUSION
Auditory and vestibular sensory organs in the inner ear are composed of mechanosensory hair cells (HCs) and associated supporting cells (SCs). HCs are not neighboring each other and are separated by SCs. As a result, they form a typical mosaic cell pattern, which have intrigued the researchers to think that Notch mediated inhibitory mechanism called 'lateral inhibition' should play a pivotal role in HC and SC differentiation during inner ear development. Recent about 15 years' progress of molecular and developmental biology have revealed that the Notch signaling pathway have multiple important roles in developing inner ear, including the determination of prosensory region, the regulation of cell proliferation as well as the cell diversifications. Unfortunately, mammalian auditory epithelium do not have regenerative capacity when they have been injured by noise or modern drugs, contrasting to that of non-mammalian vertebrates including birds, in which HC regeneration seems to reactivate the developmental programs. Some researchers tried to induce HC regeneration in damaged adult mammalian cochlea via manipulating the Notch signaling pathway, for example, by applying γ-secretase inhibitors. However, they have not succeeded in the HC restoration yet. By investigating other factors and signaling pathways that act reciprocally with the Notch signaling pathway during inner ear development, we might be able to make progress in the regenerative medicine for hearing deficient.
REFERENCES
- 1.
- Forge A, Li L, Corwin JT, et al. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259(5101):1616–1619. [PubMed: 8456284]
- 2.
- Warchol ME, Lambert PR, Goldstein BJ, et al. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259(5101):1619–1622. [PubMed: 8456285]
- 3.
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131(17):4119–4130. [PubMed: 15319325]
- 4.
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215(4):359–369. [PubMed: 6863589]
- 5.
- Adam J, Myat A, Le Roux I, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125(23):4645–4654. [PubMed: 9806914]
- 6.
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. [PubMed: 12052904]
- 7.
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7(11):837–849. [PubMed: 17053809]
- 8.
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;Suppl 220:221–244. [PubMed: 6067797]
- 9.
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. [PubMed: 10079221]
- 10.
- Lowenheim H, Furness DN, Kil J, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci USA. 1999;96(7):4084–4088. [PMC free article: PMC22424] [PubMed: 10097167]
- 11.
- Chen P, Zindy F, Abdala C, et al. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5(5):422–426. [PubMed: 12717441]
- 12.
- Chen P, Johnson JE, Zoghbi HY, et al. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129(10):2495–2505. [PubMed: 11973280]
- 13.
- Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–1841. [PubMed: 10364557]
- 14.
- Jones JM, Montcouquiol M, Dabdoub A, et al. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26(2):550–558. [PMC free article: PMC6674393] [PubMed: 16407553]
- 15.
- Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. [PubMed: 15711559]
- 16.
- Murata J, Tokunaga A, Okano H, et al. Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497(3):502–518. [PubMed: 16736472]
- 17.
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Dev Biol. 1998;9(6):591–597. [PubMed: 10075488]
- 18.
- Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998;9(6):583–589. [PubMed: 9892564]
- 19.
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131(5):965–973. [PubMed: 14973298]
- 20.
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18(19):7811–7821. [PMC free article: PMC6793014] [PubMed: 9742150]
- 21.
- Lanford PJ, Lan Y, Jiang R, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21(3):289–292. [PubMed: 10080181]
- 22.
- Morrison A, Hodgetts C, Gossler A, et al. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84(1-2):169–172. [PubMed: 10473135]
- 23.
- Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127(15):3373–3383. [PubMed: 10887092]
- 24.
- Murata J, Ohtsuka T, Tokunaga A, et al. Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1. J Neurosci Res. 2009;87(16):3521–3534. [PubMed: 19598246]
- 25.
- Ohtsuka T, Ishibashi M, Gradwohl G, et al. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18(8):2196–2207. [PMC free article: PMC1171303] [PubMed: 10205173]
- 26.
- Zheng JL, Shou J, Guillemot F, et al. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127(21):4551–4560. [PubMed: 11023859]
- 27.
- Kiernan AE, Cordes R, Kopan R, et al. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132(19):4353–4362. [PubMed: 16141228]
- 28.
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133(7):1277–1286. [PubMed: 16495313]
- 29.
- Bruckner K, Perez L, Clausen H, et al. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406(6794):411–415. [PubMed: 10935637]
- 30.
- Moloney DJ, Panin VM, Johnston SH, et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406(6794):369–375. [PubMed: 10935626]
- 31.
- Lewis AK, Frantz GD, Carpenter DA, et al. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78(1-2):159–163. [PubMed: 9858718]
- 32.
- Cole LK, Le Roux I, Nunes F, et al. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1 and lunatic fringe. J Comp Neurol. 2000;424(3):509–520. [PubMed: 10906716]
- 33.
- Tsai H, Hardisty RE, Rhodes C, et al. The mouse slalom mutant demonstrates a role for Jagged1 in neuroepithelial patterning in the organ of Corti. Hum Mol Genet. 2001;10(5):507–512. [PubMed: 11181574]
- 34.
- Kiernan AE, Ahituv N, Fuchs H, et al. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci USA. 2001;98(7):3873–3878. [PMC free article: PMC31145] [PubMed: 11259677]
- 35.
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2(1):e4. [PMC free article: PMC1326221] [PubMed: 16410827]
- 36.
- Hayashi T, Kokubo H, Hartman BH, et al. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316(1):87–99. [PMC free article: PMC2362132] [PubMed: 18291358]
- 37.
- Dabdoub A, Puligilla C, Jones JM, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105(47):18396–18401. [PMC free article: PMC2587543] [PubMed: 19011097]
- 38.
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132(3):541–551. [PubMed: 15634704]
- 39.
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci USA. 2000;97(22):11692–11699. [PMC free article: PMC34337] [PubMed: 11050197]
- 40.
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15792–15797. [PMC free article: PMC2936601] [PubMed: 20798046]
- 41.
- Pan W, Jim Y, Stanger B, Kierman AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15798–15803. [PMC free article: PMC2936630] [PubMed: 20733081]
- 42.
- Li S, Mark S, Radde-Gallwitz K, et al. Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC Dev Biol. 2008;8:20. [PMC free article: PMC2277407] [PubMed: 18302773]
- 43.
- Murata K, Hattori M, Hirai N, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol Cell Biol. 2005;25(10):4262–4271. [PMC free article: PMC1087711] [PubMed: 15870295]
- 44.
- Uchikawa M, Kamachi Y, Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev. 1999;84(1-2):103–120. [PubMed: 10473124]
- 45.
- Lefebvre V, Dumitriu B, Penzo-Mendez A, et al. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39(12):2195–2214. [PMC free article: PMC2080623] [PubMed: 17625949]
- 46.
- Kiernan AE, Pelling AL, Leung KK, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434(7036):1031–1035. [PubMed: 15846349]
- 47.
- Takebayashi S, Yamamoto N, Yabe D, et al. Multiple roles of Notch signaling in cochlear development. Dev Biol. 2007;307(1):165–178. [PubMed: 17531970]
- 48.
- Guentchev M, Mc Kay RD. Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Eur J Neurosci. 2006;23(9):2289–2296. [PubMed: 16706837]
- 49.
- Tokunaga A, Kohyama J, Yoshida T, et al. Mapping spatio-temporal activation of Notch signaling during neurogenesis and gliogenesis in the developing mouse brain. J Neurochem. 2004;90(1):142–154. [PubMed: 15198674]
- 50.
- Ma Q, Chen Z, del Barco Barrantes I, et al. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20(3):469–482. [PubMed: 9539122]
- 51.
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1(2):129–143. [PMC free article: PMC2504536] [PubMed: 11545141]
- 52.
- Liu M, Pereira FA, Price SD, et al. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14(22):2839–2854. [PMC free article: PMC317056] [PubMed: 11090132]
- 53.
- Haddon C, Jiang YJ, Smithers L, et al. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125(23):4637–4644. [PubMed: 9806913]
- 54.
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15798–15803. [PMC free article: PMC2936630] [PubMed: 20733081]
- 55.
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132(7):1687–1697. [PubMed: 15743876]
- 56.
- Raft S, Koundakjian EJ, Quinones H, et al. Cross-regulation of Ngn1 and Math1 co-ordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134(24):4405–4415. [PubMed: 18039969]
- 57.
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51(6-7):633–647. [PubMed: 17891722]
- 58.
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205(1):17–20. [PubMed: 8867010]
- 59.
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78(4):461–471. [PubMed: 15372572]
- 60.
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. [PubMed: 3381100]
- 61.
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–1776. [PubMed: 3381101]
- 62.
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28(9):2261–2273. [PMC free article: PMC6671837] [PubMed: 18305259]
- 63.
- Daudet N, Gibson R, Shang J, et al. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326(1):86–100. [PMC free article: PMC2660390] [PubMed: 19013445]
- 64.
- Yamamoto N, Tanigaki K, Tsuji M, et al. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84(1):37–45. [PubMed: 16283144]
- 65.
- Hori R, Nakagawa T, Sakamoto T, et al. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18(18):1911–1914. [PubMed: 18007185]
- 66.
- Morsli H, Choo D, Ryan A, Johnson R, et al. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18(9):3327–3335. [PMC free article: PMC6792659] [PubMed: 9547240]
- NOTCH SIGNALING AND THE DEVELOPING INNER EAR - Madame Curie Bioscience DatabaseNOTCH SIGNALING AND THE DEVELOPING INNER EAR - Madame Curie Bioscience Database
- Restriction-Modification Systems as Mobile Epigenetic Elements - Madame Curie Bi...Restriction-Modification Systems as Mobile Epigenetic Elements - Madame Curie Bioscience Database
- Molecular Mechanisms of ErbB2-Mediated Breast Cancer Chemoresistance - Madame Cu...Molecular Mechanisms of ErbB2-Mediated Breast Cancer Chemoresistance - Madame Curie Bioscience Database
- The Role of Neuropilin in Vascular and Tumor Biology - Madame Curie Bioscience D...The Role of Neuropilin in Vascular and Tumor Biology - Madame Curie Bioscience Database
- DNA Vaccines against Bacterial Pathogens - Madame Curie Bioscience DatabaseDNA Vaccines against Bacterial Pathogens - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...