NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The conjugative transposon Tn916 was first discovered in the late 1970s and is, together with the related conjugative transposon Tn1545, the paradigm of a large family of related conjugative transposons known as the Tn916/Tn1545 family, which are found in an extremely diverse range of bacteria. With the huge increase in bacterial genomic sequence data available, due to the widespread use of next generation sequencing, more putative conjugative transposons belonging to the Tn916/Tn1545 family are being reported. Many of these are capable of excision, integration and conjugation. Nearly all of the Tn916/Tn1545-like elements discovered to date encode tetracycline resistance however, increasingly resistance to other antimicrobials is being found. Some of the members of the Tn916/Tn1545 family of elements are composite structures which contain smaller mobile genetic elements which are also capable of transposition. Tn916/Tn1545-like elements themselves are also found within larger and more complex elements. This review will give an overview of the current knowledge of the Tn916/Tn1545 family of conjugative transposons highlighting recently characterized composite elements carrying additional and novel resistance genes.
Introduction
The Conjugative Transposon Tn916
The existence of chromosomally, as opposed to plasmid encoded transferable resistance was first suspected in the late 1970s. When Enterococcus faecalis strain DS16 was mated with the plasmid-free E. faecalis strain JH2-2, some transconjugants resistant to tetracycline contained the Tn916 determinant linked to the co-resident plasmid pAD1 which had also transferred from DS16. In addition, derivatives of DS16 devoid of pAD1 were capable of transferring tetracycline resistance to recipient strains. Transconjugants (plasmid-free) from such matings could subsequently act as donors in the transfer of tetracycline resistance. Further work showed that tetracycline resistance was conferred by an integrative element which was called a conjugative transposon and was designated Tn916.1 This was the first conjugative transposon found to carry an antibiotic resistance gene and it was hypothesized that it may explain the widespread presence of tetracycline resistance among streptococci at the time.1
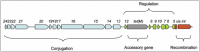
The genetic organization of Tn916, and many other mobile genetic elements (MGEs), is modular.2-4 The modules are involved in conjugation, excision and integration (recombination), regulation and accessory functions which are not involved in mobility or regulation (Fig. 1).4 Each of these functional modules will be considered separately and in detail below.
The Tn916 Family of Conjugative Transposons; An Ever Expanding Family of MGEs
In the past few decades, numerous mobile genetic elements with similarities to Tn916 have been characterized (Fig. 2). While the conjugation and the regulatory genes are generally conserved the genes encoding the excisionases, integrases or recombinases and the accessory genes vary (Fig. 2). A number of elements within the family also contain insertions of smaller MGEs encoding resistance to other antimicrobials e.g., macrolides5,6 and mercury.7 Group II introns and IS elements have also been found in some of the Tn916/Tn1545 family of MGEs (Fig. 2).8-10 Tn916-like elements are found in a wide range of bacterial species belonging to at least 36 genera spanning six phyla (Fig. 3), giving the Tn916/Tn1545 family an exceptionally broad host range.4
Here we present an overview of the current knowledge of the biological functions of Tn916 encoded proteins and explore their genetic diversity.
The Functions of the Transposon Encoded Proteins by Module
Recombination
All of the Tn916/Tn1545 family of MGEs possess a recombination module which is located at one end of the element with the direction of transcription leading out of that end of the element (Fig. 1).11-14 In Tn916, this consists of two genes: encoding a tyrosine integrase, IntTn, and an excisionase, XisTn. Excision begins with the introduction of staggered endonucleolytic cuts made at each end of the element generating single-stranded, non-complementary hexanucleotides at each end of the element termed the coupling sequences.13,15 The coupling sequences then join forming a covalent bond creating a circular intermediate molecule with a heteroduplex at the joint, while the target site from which the element has excised is also ligated. On integration into a target site, heteroduplex regions are produced on either side of the conjugative transposon which are then resolved by DNA repair or replication. One study has shown that Tn916 is also capable of inversion in its target site. PCRs performed on DNA extracted from a broth culture of Enterococcus faecium DPC3675 which carries one copy of Tn916 showed the ends of the transposon in both orientations within the target site,16 work in our lab has shown that this may be a general property of Tn916/Tn1545-like elements (Roberts et al., unpublished).
Tn916 and Tn1545 both encode a tyrosine recombinase which are highly related to each other.14,17 There is variation however between tyrosine recombinases associated with different Tn916-like elements e.g., Int600018 is more related to the tyrosine integrases of staphylococcal pathogenicity islands than it is to the integrases of Tn916 and Tn1545, probably reflecting different recombination events between different MGEs which have led to the formation of the different elements (Fig. 4). In some members of the Tn916/Tn1545 family integration and excision is mediated by large serine recombinase (these proteins do not require an excisonase in the recombination reactions) these include Tn5397 and Tn1116.8,19
Target Sites of Tn916
The Tn916 IntTn protein can use multiple target sites and these have been shown to be A:T-rich. A recent study has characterized 123 insertion sites in the genome of Butyrivibrio proteoclasticus strain B31620 and has shown that the consensus sequence TTTTT TATATA AAAAA is used (the hexanucleotide in italics is variable and forms the coupling sequences). In addition we have recently performed a similar study in Clostridium difficile and have shown a nearly identical consensus sequence based on almost 200 insertion sites in two different strains (Mullany et al., unpublished). Interestingly however Tn916 has a preferred insertion site in C. difficile strain CD37. Here the target insertion site also consists of an A:T-rich region but is preferentially used in this strain.21
Conjugation
Knowledge of the specific mechanism of conjugation among the Tn916 family members is somewhat limited, however early Tn5 mutagenesis indicated that ORFs 24 to 13 are involved in this process (Fig. 1).22 However none of the Tn5 insertions were complemented so polar effects cannot be ruled out. The specific functions of some of these proteins, or homologs of these proteins, have been experimentally proven and are shown in Table 1. Orf20 is a relaxase nicking at the origin of transfer (oriT), constituting the first step of the conjugation process.23 Tn916 IntTn is a specificity factor for this reaction and is responsible for both the strand and sequence selection of Orf20. OriT itself spans a 466 bp region containing a number of inverted repeats and is positioned between orf20 and orf21 of the transposon.24 Orf18 encodes a putative ArdA homologue which is responsible for the transposon's immunity to DNA restriction modification following conjugation by mimicking the DNA substrate for restriction enzymes. This likely contributes to the broad host range of Tn916.25 The putative product of orf14 shows some similarity to the NPL/p60 family of proteins which are associated with virulence in Listeria monocytogenes.26,27
Table 1.
Functions of orf2413 of the Tn916 conjugation module.
Regulation
Our knowledge of the regulation of Tn916/Tn1545-like elements is almost completely limited to Tn916. Su, et.al. (28), proposed that the regulatory system of Tn916 comprises of orf12, orf9, orf7 and orf8 (Fig. 1). This region is conserved in nearly all Tn916-like elements which suggest that it is extremely important for the function and / or maintenance of the elements.
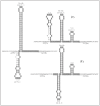
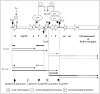
The presence of several inverted repeat sequences within orf12 are key to the proposed regulatory mechanism. It has been proposed that regulation of Tn916 involves transcriptional attenuation and is regulated by tetracycline.28,29 The proposed regulatory region consists of stem-loop structures, 5S:6S and 7:8 followed by a series of uracil residues in the RNA, predicted to be the transcriptional terminators ("T" on Fig. 5). In the presence of tetracycline most ribosomes are inactivated by the reversible binding of tetracycline, resulting in a build-up of charged t-RNA molecules due to a lower rate of protein synthesis. At this stage, a few ribosomes are thought to be protected by the low and basal level of Tet(M). Accumulation of charged t-RNA enables the more rapid translation of orf12 by the protected ribosomes. This event speeds up the translation by the protected ribosomes, which is normally slow due to the presence of rare codons in orf12, and is predicted to allow the ribosome to catch up to the RNA polymerase and therefore prevent the formation of, or destroy, the terminator structures 5S:6S and 7:8. This allows transcription from the promoter upstream of orf12 to extended into, and through, tet(M) and into the downstream genes (Fig. 6). However, in the absence of tetracycline, the ribosome pauses on the leader peptide of orf12 due to both the shortage of charged t-RNA molecules and the rare codons within orf12. This results in the ribosome lagging behind the RNA polymerase allowing the formation of the predicted strong 5S:6S terminator and/or the weaker 7:8 terminator which is predicted to terminate the majority of transcription.
Orf9 is proposed to repress the transcription from the orf7 promoter Porf7 (Fig. 6). Increased transcription through tet(M) and downstream regions will lead to the production of antisense orf9 RNA which leads to de-repression of Porf7 and increased transcription of Orf7 and Orf8 from the upstream promoter Porf7. Increased transcription from Porf7 will lead to an increase in the translation of Orf7 and Orf8. Orf7 and Orf8 are predicted to upregulate their own transcription from Porf7, thereby providing an amplification of the environmental signal (tetracycline) sensed upstream of tet(M). The increase in transcription from Porf7 will lead to an increase in the translation of downstream genes (xisTn and intTn) promoting excision of the element from the host replicon. In its circular form, transcription continues into the conjugation module (Figs. 1 and 6) presumably promoting transfer.
The regulatory model of Tn916 has never been experimentally proven although it is fundamentally important. When considering the regulation of Tn916; it is not actually dependent on the presence or absence of tetracycline but on the translation rate, where an increased pool of charged t-RNAs is likely to result in the upregulation of Tn916 genes. This means that any malfunction in the cell's translational apparatus will cause the translation rate to drop and therefore increase the tRNA concentration, which is expected to be deleterious to the cell. Tn916 should be able to sense this response to cellular distress and respond by activating its own transcription and movement.
Accessory Genes
Most of the Tn916/Tn1545 family of elements possess tet(M) which encodes a ribosomal protection protein (RPP) conferring resistance to tetracyclines.17,28 A number of other accessory genes have also been found among the Tn916/Tn1545 family of transposons (Fig. 2, Table 2). These range from other RPP genes such as tet(S) and the efflux gene tet(L), a number of macrolide resistance genes such as erm(B) and mef(E), as well as the mercury resistance gene mer(A), the kanamycin resistance gene aphA, and the quarternary ammonium compound (QAC) resistance gene qrg (Table 2).
Table 2.
Accessory genes found among the Tn916 family of transposons.
Tn916 Can Have Multiple Effects on the Host Genome
The insertion of Tn916 into a genome can affect the host in various ways. An insertion within a gene can lead to loss or alteration of gene function. Insertion near genes may lead to polar effects, e.g., in some E. faecalis strains Tn916 has inserted upstream of a hemolysin located on plasmid pAD1 which has resulted in its overexpression.30 Another mechanism of introducing heritable change in host cells is by transporting non Tn916 DNA (the coupling sequences) into the cell when the element transfers. In a study Tn916 was used to create insertional mutations in Desulfitobacterium dehalogenans, an anaerobic organism capable of halorespiration. The transposon mutagenesis generated a relatively high percentage of halorespiration deficient mutants in the strain.31 This study also revealed that the coupling sequences formed during recombination remained in some of the mutants in the empty target sites following excision of Tn916. Therefore, Tn916 was responsible for the introduction of short fragments of foreign DNA which remained after excision of the element causing deficiency in halorespiration. Another study has shown that Tn916 had replaced six nucleotides of its target site in Erysipelothrix rhusiopathiae with six alternative nucleotides, most likely the coupling sequence.32 In this case the transposon was responsible for the replacement of six chromosomal nucleotides (AAACAA) by a six new nucleotides (GTATTA) as a result of its insertion and subsequent excision.
Interactions with Other Mobile Genetic Elements
When investigating the structure and functions of the Tn916/Tn1545 family of transposons, it quickly becomes apparent that one is not looking at a static picture. These elements are constantly evolving and interacting with other MGEs including transposons, plasmids, insertion sequences and introns.
Group II introns have been found inserted into Tn916/Tn1545-like elements e.g., Tn5397,33 Tn5386,34 Tn6000,9 and Tn6084.10 All of the above are inserted into various orfs of the conjugation module of the host elements.
There are multiple insertions in Tn916/Tn1545-like elements which contain the erm(B) gene conferring resistance to macrolide, lincosamide and streptogramin (MLS) antibiotics. Tn91735,36 is found upstream of the recombination module of the elements disrupting orf9 in SPnRi3erm, Tn3872 and Tn2017. In fact the transposition of Tn917 has been found to be inducible by the presence of erythromycin,37 much like Tn916 transfer is thought to be induced by the presence of tetracycline although the molecular mechanisms of induction are different. The macrolide, aminoglycoside, streptothricin (MAS) element38 has been found in SPnRi3erm, Tn6003 and Tn1545 within the conjugation module within orf20. Macrolide efflux genetic assembly (MEGA) elements5,39 are also found among the Tn916/Tn1545 family. MEGA, which includes the mef(E) efflux gene, has been found in the regulatory region between orf6 and orf9 of Tn2009, Tn2010 and Tn2017 (Fig. 2).
Multiple copies of Tn916/Tn1545-like elements have been found in various genomes and mobilisation of other Tn916/Tn1545-like elements has been shown to occur.40 A recent study describes the presence of three highly similar elements (Tn6085a, Tn6085b and Tn6084) which are all found in one strain, E. faecium C68.10 Interestingly the presence of three transposons does not significantly increase the organism's resistance to tetracycline. In a previous study by the same group, the presence of two related elements in the same strain (Tn916 and Tn5386) resulted in the deletion of a large 178 kb genomic fragment suggesting interaction between the elements.41 Further investigations indicated that excision of Tn5386 was catalyzed by the Tn916 integrase, IntTn, resulting in the simultaneous excision of both elements and the region between them. Another study investigating the target site of Tn5397 demonstrated that introduction of Tn916 to strains already containing Tn5397, resulted in its loss in > 95% of cases, presumably due to trans acting factors from the other element.42
Variations on the Tn916 Theme
Tn5397 from Clostridium difficile
Tn5397 was originally identified in Clostridium difficile and has subsequently been found or transferred into E. faecalis,43,44 B. subtilis,45 and an oral Streptococcus sp.46 A Tn5397-like element; Tn1116, has also been discovered in Streptococcus pyogenes.19
Instead of the tyrosine integrase and the excisionase genes Tn5397 encodes a large serine recombinase; TndX which catalyzes recombination (Fig. 2).42 This protein is related to the TnpX resolvase found in the chloramphenicol resistance encoding Clostridium perfringens and Clostridium difficile transposons, Tn4451 and Tn4453 respectively.42,47 Copies of Tn5397 are always flanked by a direct repeat of a GA dinucleotide, during excision endonucleolytic staggered cuts, mediated by TndX, occur at the GA leading to G/C and A/T Crick and Watson base pairing at the joint of the circular form.42,43 Upon excision the target sequence is also regenerated.
The regulation of Tn5397 is subtly different to that hypothesized for Tn916. While the ORFs orf7 and orf8 of Tn5397 and Tn916 are homologous and the promoters upstream of orf7 (Porf7) and tet(M) are almost identical, an 88-bp deletion in Tn5397 effectively removes orf12 replacing this region with two alternative ORFs; orf25 and orf26.48 This deletion results in the disruption of the 5S:6S terminator (Fig. 5) which is predicted to be crucial for the regulation of tet(M) in Tn916.28 Despite these differences, we have shown, both at the phenotypic and genotypic level, that the expression of tetracycline resistance is still inducible (Roberts et al., unpublished). An alternative hypothesis for the regulation of Tn5397 is currently being tested.
The other major difference between Tn5397 and Tn916 is that Tn5397 has a group II intron inserted in the 3' end of orf14. This intron has been shown to splice out of the pre-mRNA,33 however splicing is not a prerequisite for conjugal transfer as Tn5397 containing a mutant intron (with a kanamycin resistance gene inserted into the reverse transcriptase) incapable of splicing, could still transfer from B. subtilis to C. difficile. As the intron has inserted close to the 3' end of orf14 the interrupted gene can presumably still produce a functional protein.33
Tn6000 from Enterococcus casseliflavus
Tn6000 was originally isolated from a cynomolgus monkey in a study investigating the microbiological effects of amalgam fillings.49 Tn6000 encodes a tyrosine integrase; Int6000 which is homologous to Int (42% identical) and Sip (41% identical), the integrases from the bovine staphylococcal pathogenicity islands SaPIbov and SaPIbov2, respectively (Fig. 4).9,50 It has been shown that the element is flanked by perfect 18 bp direct repeats which are also found in the target site as well as the circular form.18 This is also the case in SaPIbov2, although the 18bp sequences are different.
Tn6000 contains some insertions and additions likely derived from diverse sources. Upstream of the conjugation region are a group of five genes of which four are predicted to be involved in restriction/modification and anti-restriction (Fig. 2). These genes are in addition to the Tn916 anti-restriction gene orf18 which Tn6000 also possesses meaning that five of the 29 predicted ORFs are likely to be involved with protecting DNA against restriction enzymes. Next, there is an insertion of a fragment of DNA that shares nucleotide identity and gene order to a region of the virulence-related locus (vrl) from Dichelobacter nodosus, the causative agent of ovine foot rot.51 The vrl is a 27.1-kb genomic island associated with more virulent strains of D. nodosus. It has also been found in Desulfococcus multivorans, indicating that it has undergone horizontal gene transfer. The vrl is hypothesized to undergo horizontal gene transfer possibly mediated by a bacteriophage such as DinoHI.52 In Tn6000, the genes vap and hel (Fig. 2) are in the same order as vrlR and vrlS, a virulence-associated protein and a DEAD helicase of the Super-family 2 from vrl. The proteins Vap and Hel are 35% and 36% identical to VrlR and VrlS, respectively. The DEAD-DEAH helicases are involved in ATP-dependent unwinding of nucleic acids and may have a role in the conjugation process of Tn6000.
Finally, there is a group II intron present in exactly the same place as the one present in Tn5397. These two group II introns are > 99% identical to each other at the nucleotide level. It is therefore likely that the progenitors of one of these elements has previously inhabited the same cell as the other and acquired the intron, either by a retro-homing mechanism or by homologous recombination.
Tn6079 from Streptococcus gallolyticus
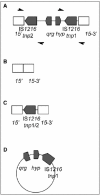
Tn6079 was recently isolated from a fecal metagenomic fosmid library of a one month old healthy infant boy.53 It is a composite transposon (28872 bp) carrying both tetracycline and erythromycin resistance genes (Fig. 2). The sequence and overall structure of Tn6079 is highly similar to putative Tn916-like transposons detected in S. gallolyticus-like strains, and flanking sequences from the fosmid insert of Tn6079 were used to assign the original host of the fragment to species level.53
Tn6079 is located at the 3′ end of a gene predicted to encode protein L33 from the ribosomal 50S subunit. The element contains complete Tn916-like conjugation and recombination modules, but in the regulation module only orf12 and orf5 is present (Fig. 1 and 2). Regarding accessory genes, in addition to tet(M), Tn6079 carries another tetracycline resistance gene, tet(L) (Table 2) predicted to encode an efflux protein and in addition it carries an erythromycin resistance gene, erm(T). The tet(L) gene is located just downstream of tet(M) and is closely linked to plasmid recombination/mobilization (pre/mob) and replication (rep) genes. Next to this, erm(T) is surrounded by IS1216 transposase sequences. Thus, apparently Tn6079 has evolved by the integration of different MGEs. Comparison of sequence and structure of Tn6079 and corresponding MGEs detected in other S. gallolyticus strains54,55 showed that the element with erm(T) and IS1216 genes most likely was introduced into Tn6079 by intraspecific genetic exchange.53 Another accessory gene predicted to encode a cell-surface protein is located just upstream of orf24. This gene is highly similar to genes present in the end of CTn1 from C. difficile 630 and Tn5386 from E. faecium.34,56 Finally, two hypothetical genes with unknown functions are present on both sides of orf21.
Tn6087 from Streptococcus oralis
Tn6087 was isolated from Streptococcus oralis cultured from pooled saliva collected from healthy volunteers.57 Its architecture is much like that of Tn916 with the same functional modules and identity in both the regulation and recombination genes. The Tn6087 tet(M) is more similar to that found in an E. faecalis Tn916-like conjugative transposon (DQ223248) than the one in Tn916 (U09422). The main differences are found in the conjugation region where a number of the ORFs are truncated and a 3 kb insertion is found within orf15 (Fig. 2).
The insertion within orf15 was found to be a composite transposon consisting of a QAC resistance gene (qrg) and a gene predicted to encode a hypothetical protein flanked by two nearly identical IS1216 sequences. The Qrg protein sequence showed some limited identity (46-57%) to known small multidrug resistance (SMR) proteins. SMR proteins are known to increase resistance to QACs, and the MIC to cetyltrimethyl ammonium bromide (CTAB) was found to be higher than expected (64 mg/ml) in the Streptococcus oralis isolate. To determine whether the increase in resistance to CTAB was due to the presence of qrg, the gene was mutated by allelic replacement with a chloramphenicol resistance gene. Resistance to CTAB was indeed found to be lower (16 mg/ml) in the qrg deficient mutant, and was restored when the mutant was complemented with qrg on a plasmid (32 mg/ml). To investigate how widespread the novel qrg was, the gene was amplified from eight metagenomic DNA samples. One sample extracted from pooled saliva and pooled faecal samples taken from volunteers from four European countries.58 All samples were found to be positive for the novel qrg gene and sequence analysis of the amplicons showed at least 97.81% identity to the Tn6087 qrg.
PCR analysis demonstrated that recombination occurred in this region resulting in a range of different molecules including the excision of the entire 3 kb composite transposon and a circular form of the qrg region and one of the IS elements was also detected (Fig. 7). As in the case of Tn6079, it seems likely that the IS1216 elements were able to mobilise genes foreign to Tn916 and insert them into this MGE thereby facilitating their spread.
Tn6087 also differs from Tn916 in that it has not been shown to have the ability to transfer by conjugation. Single nucleotide polymorphisms in the Tn6087 sequence within the conjugation module resulted in a number of truncations within these ORFs: specifically in orf24, orf20, orf16, and orf15. Furthermore, the presence of the composite transposon within orf15 was also likely to have an effect on conjugation ability. However, Tn6087 could be transformed into another Streptococcus sp.57
Conclusion
The Tn916/Tn1545 family of transposons is diverse and ubiquitous among many bacterial genera. Nearly all members of the family encode tetracycline resistance, but some also have resistance to other antimicrobial agents, such as macrolide antibiotics and some antiseptics. These traits give the host an advantage in certain environments, and importantly in the healthcare setting. Some of the Tn916/Tn1545 family of elements have a composite structure which includes smaller mobile elements within a larger Tn916/Tn1545-like structure which are also mobilisable. Furthermore, the presence of these elements can have a number of effects on the host genome, from interruptions to genes to the deletions of large parts of the genome. All of the above traits make the Tn916/Tn1545 family of elements important players in bacterial genome evolution. Their ability to modify their host's genotype and phenotype makes further study of these MGEs of high importance.
Questions for the Future
The major gaps in our understanding of Tn916 and the members of its family lie within the regulation and conjugation modules of the elements. We have only minimal knowledge of the genes involved in conjugation which is one of the most important characteristics of these elements. There is also evidence that some of the genes within the regulation module are affected by the presence of tetracycline in the cell's environment which trigger the transfer of the element. It would also be interesting to investigate how the cells detect the presence of the antibiotic. We have described a high level of plasticity among the members of the Tn916/Tn1545 family, with more being discovered all the time.
Acknowledgments
Research in the authors (PM and APR) laboratories is performed with financial support from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 241446 (ANTIRESDEV) and under grant agreement no. 223585 (HYPERDIFF) and the Medical Research Council (grant no. G0601176). AJ is funded by the Malaysian Government.
References
- 1.
- Franke AE, Clewell DB. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococ-cus-faecalis that is capable of conjugal transfer in the absence of a conjugative plasmid. J Bacteriol. 1981;145:494–502. PMID:6257641. [PMC free article: PMC217299] [PubMed: 6257641]
- 2.
- Toussaint A, Merlin C. Mobile elements as a combination of functional modules. Plasmid. 2002;47:26–35. PMID:11798283 doi:10.1006/plas.2001.1552. [PubMed: 11798283]
- 3.
- Osborn AM, Boltner D. When phage, plasmids, and transposons collide: genomic islands, and conjugative- and mobilizable-transposons as a mosaic continuum. Plasmid. 2002;48:202–12. PMID:12460536 doi:10.1016/S0147-619X(02)00117-8. [PubMed: 12460536]
- 4.
- Roberts AP, Mullany P. A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol. 2009;17:251–8. PMID:19464182 doi:10.1016/j.tim.2009.03.002. [PubMed: 19464182]
- 5.
- Del Grosso M, d'Abusco AS, Iannelli F, et al. Tn2009, a Tn916-like element containing mef(E) in Streptococcus pneumoniae. Antimicrob Agents Ch. Jun. 2004;48(6):2037–2042. [PMC free article: PMC415626] [PubMed: 15155196]
- 6.
- Warburton PJ, Palmer RM, Munson MA, Wade WG. Demonstration of in vivo transfer of doxycycline resistance mediated by a novel transposon. J Antimicrob Chemother. 2007;60:973–80. PMID:17855723 doi:10.1093/jac/dkm331. [PubMed: 17855723]
- 7.
- Soge OO, Beck NK, White TM, et al. A novel transposon, Tn6009, composed of a Tn916 element linked with a Staphylococcus aureus mer operon. J Antimicrob Chemother. 2008;62:674–80. PMID:18583328 doi:10.1093/jac/dkn255. [PMC free article: PMC2536709] [PubMed: 18583328]
- 8.
- Mullany P, Pallen M, Wilks M, et al. A Group II intron in a conjugative transposon from the Gram-positive bacterium, Clostridium difficile. Gene. 1996;174:145–50. PMID:8863741 doi:10.1016/0378-1119(96)00511-2. [PubMed: 8863741]
- 9.
- Brouwer MSM, Mullany P, Roberts AP. Characterization of the conjugative transposon Tn6000 from Enterococcus casseliflavus 664.1H1 (formerly Enterococcus faecium 664.1H1) . FEMS Microbiol Lett. 2010;309:71–6. PMID:20528943. [PubMed: 20528943]
- 10.
- Rice LB, Carias LL, Rudin S, et al. Multiple copies of functional, Tet(M)-encoding Tn916-like elements in a clinical Enterococcus faecium isolate. Plasmid. 2010;64:150–5. PMID:20600284 doi:10.1016/j.plasmid.2010.06.003. [PMC free article: PMC2943565] [PubMed: 20600284]
- 11.
- Caparon MG, Scott JR. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989;59:1027–34. PMID:2557157 doi:10.1016/0092-8674(89)90759-9. [PubMed: 2557157]
- 12.
- Salyers AA, Shoemaker NB, Stevens AM, Li LY. Conjugative transposons - an unusual and diverse set of integrated gene-transfer elements. Microbiol Rev. 1995;59:579. PMID:8531886. [PMC free article: PMC239388] [PubMed: 8531886]
- 13.
- Scott JR, Churchward GG. Conjugative transposition. Annu Rev Microbiol. 1995;49:367–97. PMID:8561465 doi:10.1146/annurev.mi.49.100195.002055. [PubMed: 8561465]
- 14.
- Jaworski DD, Flannagan SE, Clewell DB. Analyses of traA, int-Tn, and xis-Tn mutations in the conjugative transposon Tn916 in Enterococcus faecalis. Plasmid. 1996;36:201–8. PMID:9007015 doi:10.1006/plas.1996.0047. [PubMed: 9007015]
- 15.
- Rudy CK, Scott JR. Length of the coupling sequence of Tn916. J Bacteriol. 1994;176:3386–8. PMID:8195096. [PMC free article: PMC205512] [PubMed: 8195096]
- 16.
- O'Keeffe T, Hill C, Ross RP. In situ inversion of the conjugative transposon Tn916 in Enterococcus faecium DPC3675. FEMS Microbiol Lett. 1999;173:265–71. PMID:10220904 doi:10.1111/j.1574-6968.1999.tb13511.x. [PubMed: 10220904]
- 17.
- Flannagan SE, Zitzow LA, Su YA, Clewell DB. Nucleotide-sequence of the 18-Kb conjugative transposon Tn916 from Enterococcus-faecalis. Plasmid. 1994;32:350–4. PMID:7899523 doi:10.1006/plas.1994.1077. [PubMed: 7899523]
- 18.
- Roberts AP, Davis IJ, Seville L, et al. Characterization of the ends and target site of a novel tetracycline resistance-encoding conjugative transposon from Enterococcus faecium 664.1H1 . J Bacteriol. 2006;188:4356–61. PMID:16740942 doi:10.1128/JB.00129-06. [PMC free article: PMC1482970] [PubMed: 16740942]
- 19.
- Brenciani A, Bacciaglia A, Vecchi M, et al. Genetic elements carrying erm(B) in Streptococcus pyogenes and association with tet(M) tetracycline resistance gene. Antimicrob Agents Ch. Apr. 2007;51(4):1209–1216. [PMC free article: PMC1855496] [PubMed: 17261630]
- 20.
- Cookson AL, Noel S, Hussein H, et al. Transposition of Tn916 in the four replicons of the Butyrivibrio proteoclasticus B316(T) genome. FEMS Microbiol Lett. 2011;316:144–51. PMID:21204937 doi:10.1111/j.1574-6968.2010.02204.x. [PubMed: 21204937]
- 21.
- Wang H, Roberts AP, Mullany P. DNA sequence of the insertional hot spot of Tn916 in the Clostridium difficile genome and discovery of a Tn916-like element in an environmental isolate integrated in the same hot spot. FEMS Microbiol Lett. 2000;192:15–20. PMID:11040422 doi:10.1111/j.1574-6968.2000.tb09352.x. [PubMed: 11040422]
- 22.
- Senghas E, Jones JM, Yamamoto M, et al. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988;170:245–9. PMID:2826394. [PMC free article: PMC210634] [PubMed: 2826394]
- 23.
- Rocco JM, Churchward G. The integrase of the conjugative transposon Tn916 directs strand- and sequence-specific cleavage of the origin of conjugal transfer, oriT, by the endonuclease Orf20. J Bacteriol. 2006;188:2207–13. PMID:16513750 doi:10.1128/JB.188.6.2207-2213.2006. [PMC free article: PMC1428151] [PubMed: 16513750]
- 24.
- Jaworski DD, Clewell DB. A functional origin of transfer (oriT) on the conjugative transposon Tn916. J Bacteriol. 1995;177:6644–51. PMID:7592445. [PMC free article: PMC177520] [PubMed: 7592445]
- 25.
- Serfiotis-Mitsa D, Roberts GA, Cooper LP, et al. The orf18 Gene Product from conjugative transposon Tn916 is an ArdA antirestriction protein that inhibits Type I DNA restriction-modification systems. J Mol Biol. 2008;383:970–81. PMID:18838147 doi:10.1016/j.jmb.2008.06.005. [PubMed: 18838147]
- 26.
- Rigden DJ, Jedrzejas MJ, Galperin MY. Amidase domains from bacterial and phage autolysins define a family of gamma-D,L-glutamate-specific amidohydrolases. Trends Biochem Sci. 2003;28:230–4. PMID:12765833 doi:10.1016/S0968-0004(03)00062-8. [PubMed: 12765833]
- 27.
- Clewell DB, Flannagan SE, Jaworski DD. Unconstrained bacterial promiscuity - the Tn916-Tn1545 family of conjugative transposons. Trends Microbiol. 1995;3:229–36. PMID:7648031 doi:10.1016/S0966-842X(00)88930-1. [PubMed: 7648031]
- 28.
- Su YA, Ping H, Clewell DB. Characterization of the tet(M) determinant of Tn916 - evidence for regulation by transcription attenuation. Antimicrob Agents Chemother. 1992;36:769–78. PMID:1323953. [PMC free article: PMC189400] [PubMed: 1323953]
- 29.
- Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–17. PMID:9593300 doi:10.1046/j.1365-2958.1998.00778.x. [PubMed: 9593300]
- 30.
- Ike Y, Flannagan SE, Clewell DB. Hyperhemolytic phenomena associated with insertions of Tn916 into the hemolysin determinant of Enterococcus faecalis Plasmid Pad1. J Bacteriol. 1992;174:1801–9. PMID:1312528. [PMC free article: PMC205781] [PubMed: 1312528]
- 31.
- Smidt H, Song DL, van der Oost J, de Vos WM. Random transposition by Tn916 in Desulfitobacterium dehalogenans allows for isolation and characterization of halorespiration-deficient mutants. J Bacteriol. 1999;181:6882–8. PMID:10559152. [PMC free article: PMC94161] [PubMed: 10559152]
- 32.
- Shimoji Y, Mori Y, Sekizaki T, et al. Construction and vaccine potential of acapsular mutants of Erysipelothrix rhusiopathiae: Use of excision of Tn916 to inactivate a target gene. Infect Immun. 1998;66:3250–4. PMID:9632592. [PMC free article: PMC108339] [PubMed: 9632592]
- 33.
- Roberts AP, Braun V, von Eichel-Streiber C, Mullany P. Demonstration that the group II intron from the clostridial conjugative transposon Tn5397 undergoes splicing in vivo. J Bacteriol. 2001;183:1296–9. PMID:11157942 doi:10.1128/JB.183.4.1296-1299.2001. [PMC free article: PMC95003] [PubMed: 11157942]
- 34.
- Rice LB, Carias LL, Marshall SH, et al. Characterization of Tn5386, a Tn916-related mobile element. Plasmid. 2007;58:61–7. PMID:17408741 doi:10.1016/j.plasmid.2007.01.002. [PubMed: 17408741]
- 35.
- Tomich PK, An FY, Clewell DB. Transposon (Tn917) in Streptococcus faecalis that exhibits enhanced transposition during induction of drug-resistance. Cold Spring Harb Sym. 1978;43:1217–1221. [PubMed: 114353]
- 36.
- Shaw JH, Clewell DB. Complete nucleotide-sequence of "macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985;164:782–96. PMID:2997130. [PMC free article: PMC214320] [PubMed: 2997130]
- 37.
- Tomich PK, An FY, Clewell DB. Properties of Erythromycin-inducible transposon-Tn917 in Streptococcus faecalis. J Bacteriol. 1980;141:1366–74. PMID:6245068. [PMC free article: PMC293835] [PubMed: 6245068]
- 38.
- Cochetti I, Tili E, Vecchi M, et al. New Tn916-related elements causing erm(B)-mediated erythromycin resistance in tetracycline-susceptible pneumococci. J Antimicrob Chemother. 2007;60:127–31. PMID:17483548 doi:10.1093/jac/dkm120. [PubMed: 17483548]
- 39.
- Maria-Marimon J, Valiente A, Ercibengoa M, et al. Erythromycin resistance and genetic elements carrying macrolide efflux genes in Streptococcus agalactiae. Antimicrob Agents Chemother. 2005;49:5069–74. PMID:16304174 doi:10.1128/AAC.49.12.5069-5074.2005. [PMC free article: PMC1315971] [PubMed: 16304174]
- 40.
- Flannagan SE, Clewell DB. Conjugative transfer of Tn916 in Enterococcus faecalis - Transactivation of homologous transposons. J Bacteriol. 1991;173:7136–41. PMID:1657880. [PMC free article: PMC209219] [PubMed: 1657880]
- 41.
- Rice LB, Carias LL, Marshall S, et al. Tn5386, a novel Tn916-like mobile element in Enterococcus faecium D344R that interacts with Tn916 to yield a large genomic deletion. J Bacteriol. 2005;187:6668–77. PMID:16166528 doi:10.1128/JB.187.19.6668-6677.2005. [PMC free article: PMC1251567] [PubMed: 16166528]
- 42.
- Wang H, Roberts AP, Lyras D, et al. Characterization of the ends and target sites of the novel conjugative transposon Tn5397 from Clostridium difficile: Excision and circularization is mediated by the large resolvase, TndX. J Bacteriol. 2000;182:3775–83. PMID:10850994 doi:10.1128/JB.182.13.3775-3783.2000. [PMC free article: PMC94550] [PubMed: 10850994]
- 43.
- Jasni AS, Mullany P, Hussain H, Roberts AP. Demonstration of conjugative transposon (Tn5397)-mediated horizontal gene transfer between Clostridium difficile and Enterococcus faecalis. Antimicrob Agents Chemother. 2010;54:4924–6. PMID:20713671 doi:10.1128/AAC.00496-10. [PMC free article: PMC2976158] [PubMed: 20713671]
- 44.
- Agersø Y, Pedersen AG, Aarestrup FM. Identification of Tn5397-like and Tn916-like transposons and diversity of the tetracycline resistance gene tet(M) in enterococci from humans, pigs and poultry. J Antimicrob Chemother. 2006;57:832–9. PMID:16565159 doi:10.1093/jac/dkl069. [PubMed: 16565159]
- 45.
- Mullany P, Wilks M, Puckey L, Tabaqchali S. Gene cloning in Clostridium difficile using Tn916 as a shuttle conjugative transposon. Plasmid. 1994;31:320–3. PMID:8058827 doi:10.1006/plas.1994.1036. [PubMed: 8058827]
- 46.
- Roberts AP, Pratten J, Wilson M, Mullany P. Transfer of a conjugative transposon, Tn5397 in a model oral biofilm. FEMS Microbiol Lett. 1999;177:63–6. PMID:10436923 doi:10.1111/j.1574-6968.1999.tb13714.x. [PubMed: 10436923]
- 47.
- Lyras D, Adams V, Lucet I, Rood JI. The large resolvase TnpX is the only transposon-encoded protein required for transposition of the Tn4451/3 family of integrative mobilizable elements. Mol Microbiol. 2004;51:1787–800. PMID:15009902 doi:10.1111/j.1365-2958.2003.03950.x. [PubMed: 15009902]
- 48.
- Roberts AP, Johanesen PA, Lyras D, Mullany P, Rood JI. Comparison of Tn5397 from Clostridium difficile, Tn916 from Enterococcus faecalis and the CW459tet(M) element from Clostridium perfringens shows that they have similar conjugation regions but different insertion and excision modules. Microbiology. 2001;147:1243–51. PMID:11320127. [PubMed: 11320127]
- 49.
- Summers AO, Wireman J, Vimy MJ, et al. Mercury released from dental "silver" fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother. 1993;37:825–34. PMID:8280208. [PMC free article: PMC187773] [PubMed: 8280208]
- 50.
- Ubeda C, Tormo MA, Cucarella C, et al. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol. 2003;49:193–210. PMID:12823821 doi:10.1046/j.1365-2958.2003.03577.x. [PubMed: 12823821]
- 51.
- Billington SJ, Huggins AS, Johanesen PA, et al. Complete nucleotide sequence of the 27-kilobase virulence related locus (vrl) of Dichelobacter nodosus: Evidence for extrachromosomal origin. Infect Immun. 1999;67:1277–86. PMID:10024571. [PMC free article: PMC96457] [PubMed: 10024571]
- 52.
- Cheetham BF, Parker D, Bloomfield GA, et al. Isolation of the bacteriophage DinoHI from Dichelobacter nodosus and its interactions with other integrated genetic elements. Open Microbiol J. 2008;2:1–9. PMID:19088904 doi:10.2174/1874285800802010001. [PMC free article: PMC2593044] [PubMed: 19088904]
- 53.
- de Vries LE, Valles Y, Agerso Y, et al. The gut as reservoir of antibiotic resistance: microbial diversity of tetracycline resistance in mother and infant. PLoS ONE. 2011;6:e21644. doi:10.1371/journal.pone.0021644. [PMC free article: PMC3125294] [PubMed: 21738748]
- 54.
- Tsai JC, Hsueh PR, Chen HJ, et al. The erm(T) gene is flanked by IS1216V in inducible erythromycin-resistant Streptococcus gallolyticus subsp. pasteurianus. Antimicrob Agents Chemother. 2005;49:4347–50. PMID:16189118 doi:10.1128/AAC.49.10.4347-4350.2005. [PMC free article: PMC1251499] [PubMed: 16189118]
- 55.
- Rusniok C, Couve E, Da Cunha V, et al. Genome sequence of Streptococcus gallolyticus: Insights into its adaptation to the bovine rumen and its ability to cause Endocarditis. J Bacteriol. 2010;192:2266–76. PMID:20139183 doi:10.1128/JB.01659-09. [PMC free article: PMC2849448] [PubMed: 20139183]
- 56.
- Sebaihia M, Wren BW, Mullany P, et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–86. PMID:16804543 doi:10.1038/ng1830. [PubMed: 16804543]
- 57.
- Ciric L, Mullany P, Roberts AP. Antibiotic and antiseptic resistance genes are linked on a novel mobile genetic element: Tn6087. J Antimicrob Chemother. 2011. In press. [PMC free article: PMC3172042] [PubMed: 21816764]
- 58.
- Seville LA, Patterson AJ, Scott KP, et al. Distribution of tetracycline and erythromycin resistance genes among human oral and fecal metagenomic DNA. Microb Drug Resist. 2009;15:159–66. PMID:19728772 doi:10.1089/mdr.2009.0916. [PubMed: 19728772]
- 59.
- Ludwig W, Strunk O, Westram R, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–71. PMID:14985472 doi:10.1093/nar/gkh293. [PMC free article: PMC390282] [PubMed: 14985472]
- 60.
- Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–17. PMID:9593300 doi:10.1046/j.1365-2958.1998.00778.x. [PubMed: 9593300]
- 61.
- Wu LJ, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–5. PMID:8160014 doi:10.1126/science.8160014. [PubMed: 8160014]
- 62.
- Cochetti I, Tili E, Mingoia M, et al. erm(B)-carrying elements in tetracycline-resistant pneumococci and correspondence between Tn1545 and Tn6003. Antimicrob Agents Chemother. 2008;52:1285–90. PMID:18285489 doi:10.1128/AAC.01457-07. [PMC free article: PMC2292545] [PubMed: 18285489]
- 63.
- Del Grosso M, Camilli R, Iannelli F, et al. The mef(E)-carrying genetic element (MEGA) of Streptococcus pneumoniae: Insertion sites and association with other genetic elements . Antimicrob Agents Chemother. 2006;50:3361–6. PMID:17005818 doi:10.1128/AAC.00277-06. [PMC free article: PMC1610078] [PubMed: 17005818]
- 64.
- Del Grosso M, Camilli R, Libisch B, et al. New composite genetic element of the Tn916 family with dual macrolide resistance genes in a Streptococcus pneumoniae isolate belonging to clonal Complex 271. Antimicrob Agents Chemother. 2009;53:1293–4. PMID:19104015 doi:10.1128/AAC.01066-08. [PMC free article: PMC2650554] [PubMed: 19104015]
- 65.
- McDougal LK, Tenover FC, Lee LN, et al. Detection of Tn917-like sequences within a Tn916-like conjugative transposon (Tn3872) in erythromycin-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2312–8. PMID:9736555. [PMC free article: PMC105825] [PubMed: 9736555]
- 66.
- Lancaster H, Roberts AP, Bedi R, et al. Characterization of Tn916S, a Tn916-like element containing the tetracycline resistance determinant tet(S). J Bacteriol. 2004;186:4395–8. PMID:15205444 doi:10.1128/JB.186.13.4395-4398.2004. [PMC free article: PMC421593] [PubMed: 15205444]
- The Tn916/Tn1545 Family of Conjugative Transposons - Madame Curie Bioscience Dat...The Tn916/Tn1545 Family of Conjugative Transposons - Madame Curie Bioscience Database
- Functions of the Hsp90-Binding FKBP Immunophilins - Madame Curie Bioscience Data...Functions of the Hsp90-Binding FKBP Immunophilins - Madame Curie Bioscience Database
- Important Mammalian Respiratory Allergens Are Lipocalins - Madame Curie Bioscien...Important Mammalian Respiratory Allergens Are Lipocalins - Madame Curie Bioscience Database
- Biochemical Studies of Voltage-Gated Ca2+ Channels - Madame Curie Bioscience Dat...Biochemical Studies of Voltage-Gated Ca2+ Channels - Madame Curie Bioscience Database
- Tyrosyl-tRNA Synthetases - Madame Curie Bioscience DatabaseTyrosyl-tRNA Synthetases - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...