NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Nuclear receptors for steroid and non-steroid hormones act through both genomic and non-genomic mechanisms. Genomic events involve binding to cognate specific DNA sequences and subsequent recruitment of a battery of coregulators at the promoter. Non-genomic events involve the rapid activation of kinase cascades and steroid as well as non-steroid hormones trigger NR-mediated activation of the MAPK/MSK1 pathway. Here we review the recent insights concerning the crosstalk between the genomic and non-genomic actions of NRs, focusing on the mechanisms of MSK1 activation and influence on NR-target genes transcriptional regulation.
Introduction
Nuclear receptors (NRs) form a super family of ligand-regulated transcription factors, which regulate various physiological functions from development and reproduction to homeostasis and metabolism. This super family includes receptors for steroid hormones [Estrogen receptors (ER), Progesterone receptors (PR), Androgen receptors (AR) and Glucocorticoid receptors (GR)] and for non-steroid ligands [Vitamin D receptor (VDR), Retinoic Acid receptors (RAR), Retinoic X receptors (RXR) and the Peroxisome Proliferator Activated Receptors (PPAR)]. They also include a large number of so-called orphan receptors for which ligands do not exist or have not been identified yet.1-3
Classically NRs are known to act through genomic events, which involve binding of liganded homo or heterodimerized NRs to cognate specific DNA sequences followed by recruitment of coactivators and remodeling of chromatin at the promoter sequences of the activated genes.1,4
In addition to these so-called genomic effects, steroid and non-steroid hormones and their cognate NRs crosstalk with kinase cascades activated by signals impinging on membrane receptors. As an example, progestins and retinoic acid (RA) have been shown to activate the Mitogen-Activated Protein Kinase (MAPK) pathways and the downstream Mitogen- and Stress-activated protein Kinase 1 (MSK1).5,6 Traditionally, the non-genomic and genomic effects of steroid and non-steroid hormones have been considered as independent pathways. However it appeared that hormone-activated MSK1 is involved in the regulation of NR-target genes by phosphorylating NRs themselves and/or chromatin associated proteins. Here we will review the current knowledge on MSK1 activation by steroid and non-steroid hormones and on MSK1 molecular targets, focusing on recent insights into the role of MSK1 in transcriptional regulation of NR-target genes.
The Classical Picture of NRs: Ligand-Dependent Regulators of Transcription
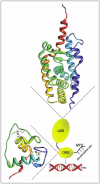
NRs have a well-defined domain organization and structure, consisting mainly of two highly conserved and structured domains: the central DNA-binding domain (DBD) linked to the C-terminal Ligand-Binding Domain (LBD). The structure of the DBDs and LBDs has been determined by nuclear magnetic resonance and crystallographic studies1,2,7 (Fig. 1). Briefly, the DBD contains two typical cysteine-rich zinc-binding motifs and two α-helices, which cross at right angles and fold into a globular conformation to form the core of the DBD. In contrast, the LBD is more complex because it contains not only the ligand-binding pocket, but also the main dimerization domain and a hydrophobic cleft involved in coregulators binding. The LBD shows a common fold comprising 12 conserved α-helices and a short β-turn, separated by exposed and flexible loops and arranged in three layers to form an antiparallel alpha-helical sandwich.
Gene induction by NRs is based on a complex network of NRs conformational changes and dynamic interactions with coregulatory proteins.2,7,8 Ligand binding is the first and crucial molecular event that switches NRs from inactive to active state by inducing conformational changes in the LBD. These changes favor the dimerization of NRs and increase their DNA affinity. They also create a new surface for coactivators binding, which initiates an ordered and coordinated dissociation and/or recruitment of a series of coregulator complexes with different enzymatic activities including Histone Acetyl- and Methyl-Transferases, and DNA-dependent ATPases.9 In fine, these events alter the chromatin structure surrounding the promoter of target genes and pave the way for the recruitment of the transcription machinery including RNA Polymerase II and general transcription factors.
NRs Are Phosphoproteins
In addition to this scenario NRs are targets for phosphorylation processes, which modulate their transcriptional activity.8,10 A number of studies demonstrated that the majority of the phosphorylated residues lie within the N-terminal domain (NTD) (Fig. 1). The phosphorylation sites located in the NTD of NRs are serine residues surrounded by prolines and therefore correspond to consensus sites for cyclin-dependent protein kinases (cdks) and Mitogen-Activated Protein Kinases (MAPKs). Accordingly, the NTD of PR, ER, AR, GR, RARs and PPARs were reported to be substrates for cdks, p42/p44MAPK (also called Erk1/2), p38MAPK, and c-Jun N-terminal kinases ( JNKs).10-16 Note that VDR is an exception, probably due to its very short NTD.
It must be noted that, in contrast to the DBDs and LBDs, the NTDs of NRs are not conserved and there are still no high-resolution structures available. Several biochemical studies coupled to structure prediction algorithms suggested that the NTDs of any member of the NR family are naturally disordered,17,18 providing the flexibility that is needed for modifications by enzymes such as kinases.19 Such modifications would induce changes in the structural properties of the domain with profound impacts on its interactions with coregulators and/or on the dynamics of adjacent structural domains.5,20
Interestingly, loops between the α-helices of the LBDs are exposed and flexible and thus are accessible for phosphorylation processes. As an example, in the LBD of RARs, loop L910 contains a serine residue located within an arginine/lysine rich motif, which corresponds to a consensus phosphorylation site for several kinases including MSK15,21 (Fig. 1).
A New Picture of NRs: NRs Are Associated to the Cell Membrane and Activate MAPK Signaling Pathways
It is becoming increasingly evident that NRs induce rapid non-genomic responses in addition to their classical genomic effects. These non-genomic effects involve the rapid and transient activation of several kinase cascades mediated by a subpopulation of NRs anchored at the cytoplasmic side of the cell membrane.
Indeed, most of the classical steroid receptors (ER, PR, GR, AR) have been found in specialized plasma membrane structures such as caveolae and lipid rafts22-24 that contain lipids, structural proteins like flotillin and caveolin, and several proteins involved in signal transduction including heterodimeric G proteins, c-Src, Rho and RAC GTPases and Phosphoinositide 3-kinase (PI3K).25,26 The membrane localization of steroid NRs depends on post-translational modifications and it has been shown that palmitoylation of a highly conserved nine-amino acids motif in the LBD is critical for membrane localization of ER and PR via caveolin-1 association.27 In addition, steroid NRs are part of membrane molecular complexes, which differ depending on the cell type and context and contain c-Src, the regulatory subunit of PI3K (p85α) and heterodimeric Gα proteins.25,28,29 In response to the hormone, ERα rapidly activates the Src/ p21ras/Erk pathway via direct interaction with the SH2 domain of c-Src (Fig. 2). Progestins and androgens also activate this signaling cascade via direct interaction of the cognate receptor with the SH3 domain of c-Src or with ERα which itself activates c-Src30-33 (Fig. 2). Note however that, in response to glucocorticoids, GR does not activate Erk but p38MAPK and JNKs11,34 (Fig. 2).
Non-steroid receptors such as VDR and RARs have been also found associated to cell membrane fractions, in association with caveolin-1,35 PI3K,36 c-Src37 or G alpha Q proteins.38 However, vitamin D39 and RA5,40-42 rather activate p38MAPK (Fig. 2). Activation of this pathway occurs very rapidly through the transient activation of small GTPases such as RhoA and its immediate effector ROCK43 or RAC-137,40 suggesting a non-genomic activation event similar to that described for steroid receptors. Of note is that this process appears to be cell specific as RA has been shown to activate Erks in neuronal and Sertoli cells.36,44-46
NRs Activate MSK1, Downstream of Erk and p38MAPK
Among the targets of NRs-activated Erk and p38MAPK, there is MSK1 (Mitogen- and stress-activated protein kinase), which presents a large structural analogy with the N-terminal ribosomal S6 kinase (RSK) domain.47-49 MSK1 is predominantly localized in the nucleus and is composed of two kinase domains connected with a linker region. A C-terminal docking domain assures binding of activating Erk or p38MAPK, which then phosphorylates a threonine residue located in the C-terminal kinase domain. Then this activated C-terminal kinase domain phosphorylates a serine in the N-terminal kinase domain, which at the end is responsible for the phosphorylation of MSK1 substrates.
After activation by the steroid hormone progestin, phosphorylated Erk translocates to the nucleus and forms a complex with nuclear PR homodimers. Then Erk binds and phosphorylates MSK150 leading to its activation6 (Fig. 2 and Fig. 3, left). Non-steroid hormones such as RA and Vitamin D also activate MSK1 downstream of p38MAPK 5,39,43 (Fig. 2), in line with the fact that MSK1 can be activated through both Erks and p38MAPK. Whether VDR and RAR form with p38MAPK and MSK-1 trimeric complexes has not been elucidated yet and will require further investigations.
In conclusion, it appears that steroid and non-steroid NRs are able to activate the MAPK/MSK1 pathway in response to their cognate hormone. However whether this mechanism is general has not been demonstrated yet. Indeed, ER and estrogens do not activate51 or rather inactivate52 MSK1. It is interesting to note that p90 ribosomal S6 kinase (RSK), which is another member of the subfamily of MAPK-activated protein kinases downstream of Erk47 and which is overexpressed in several cancers including breast cancers,53 has been shown to bind and phosphorylate ER.12,54 Whether ER and estrogens activate RSK instead of MSK1, will require further investigations (Fig. 2).
Concerning AR and androgens, there are still no data concerning MSK1. However, as for ER, RSK would be an interesting candidate53,55,56 (Fig. 2). Similarly, whether GR and glucocorticoids activate MSKs or not has not been reported yet. Note however that an activation of the Erk/ MSK1 pathway has been observed in neurons upon concomitant activation of GR and the N-methyl-D-aspartate receptor (NMDA-R) in stress processes57,58 (Fig. 2).
RARα Is Phosphorylated by MSK1 While the Other NRs Are Targets for the Upstream MAPK
MSK1 was originally shown to phosphorylate several transcription factors including CREB, ATF1 and Nuclear Factor-κB p65 (NF-κB).48,49 Similarly, MSK1 phosphorylates rapidly RARα at a serine residue (S369) located in loop L910 within the LBD5,7 (Fig. 1). This serine is an exposed residue located in a flexible loop and belongs to an Arginine-Lysine-rich motif that corresponds to a consensus phosphorylation motif for several kinases including MSK1. The interesting point is that phosphorylation of this serine initiates a coordinated phophorylation cascade (Fig. 3, right). Indeed, our laboratory recently demonstrated that phosphorylation of S369 by MSK1 increases the dynamics/flexibility of the nearby loop L89,59 which corresponds to the docking site of cyclin H60 (Fig. 1) that forms with cdk7 and MAT1, the CAK subcomplex of the general transcription factor TFIIH. Consequently, the binding efficiency of cyclin H is increased, allowing the right positioning of the cdk7 kinase and the phopshorylation of serine 77 located in the NTD by this kinase.21 To our knowledge, it was the first example of cooperation between the N-and C-terminal domains of RARs through a kinase complex. The serine in L910 and the docking site of cyclin H are conserved between RARs but not in other NRs indicating that this kinase cascade would be RAR specific.59
Note that in contrast to RAR, PR within the trimeric PR/Erk/MSK1 complex is phosphorylated by Erk and not by MSK1.6 However, concerning VDR, there are no data showing whether it is phosphorylated by MSK1 or by the upstream p38MAPK. Finally, concerning the other NRs such as ER, AR and GR, which were not found to activate MSK1 in response to the hormone, they are phosphorylated by MAPKs.11,12,61
MSK1 Participates in the Activation of NR-Target Genes and Is Recruited at the Promoters of NR-Target Genes
Steroid and non-steroid hormones are well known to activate the expression of several target genes.1,3,4,62,63 Traditionally, this genomic action has been considered as independent of the non-genomic effects. However, it now emerges that the two pathways converge in the modification of structural components of the chromatin. Indeed MSKs are well known to facilitate gene relaxation47,49 and a number of studies indicated that inhibition of MSK1 abolished the induction of progestin-, RA- or Vitamin D-target genes.5,6,43
One of the well characterized example of transcriptional control by progestins is the promoter of the mouse mammary tumor virus (MMTV), which is organized into positioned nucleosomes with one nucleosome (nucleosome B) covering the hormone response element (HRE) and which is induced upon hormone treatment.50,64,65 Though progestin activation of the MMTV promoter is sensitive to Erk and MSK1 inhibition, this behavior does not represent an exception, as about 25% of the hormonally-regulated genes are also sensitive to Erk inhibition in microarrays analysis.65 Series of chromatin immunoprecipitation experiments indicated that phosphorylated PR complexed with Erk and MSK1 is rapidly recruited to the MMTV promoter nucleosome containing the HRE6 (Fig. 3, left).
A similar strategy has been followed for RA-target genes and it has been demonstrated that phosphorylated RARα and MSK1 are also rapidly recruited to RA response elements5 (Fig. 3, right). However, MSK1 was not associated with RARα, in contrast to what was reported for PR. In fact, TFIIH, the second kinase complex of the MSK-1 initiated kinase cascade, was found associated with RARα at the promoters.5 Unfortunately, there is no data indicating whether MSK1 is also recruited to the promoters of VDR-target genes.
MSK 1 Regulates the Chromatin Environment of NR-Target Promoters
Once recruited to target promoters, liganded NRs are known to induce an ordered and cyclical recruitment of coactivator complexes with enzymatic activities, which modify histones and remodel chromatin in an ATP-dependent manner.66,67 Given that MSK1 is also recruited to NRs-target promoters, the question was whether the kinase also contributes to histone phosphorylation and chromatin remodeling at these promoters, as previously described for genes implicated in cell transformation.49,68
It has been found that, concomitantly to the recruitment of the ternary PR/Erk/MSK1 complex to nucleosome B of the MMTV promoter, histone H3 becomes phosphorylated at serine 10 by MSK1 (Fig. 3, left), an event coupled to acetylation of lysine 14 by pCAF and displacement of the repressive complex containing HP1g.6,64,69 BAF complexes (SWI/SNF ATP-dependent chromatin remodeling complexes) are also recruited to the promoter (Fig. 3, left) through a direct interaction with the activated PR and H3K14 acetylation participate in anchoring the complex to the promoter.69 Unfortunately no precise order of the different events can be proposed up to now.69 Nevertheless, BAF uses the energy of ATP hydrolysis to initiate nucleosome remodeling and to remove H2A/H2B dimers from nucleosome B, allowing binding of further PR molecules, coactivators and the transcriptional machinery including RNA polymerase II.64
Most interestingly, there are similarities between PR and RARα. Indeed the recruitment of RARα to target promoters was also concomitant with histone phosphorylation and acetylation and the subsequent recruitment of the transcriptional machinery5 (Fig. 3, right). However, whether MSK1 also controls the recruitment/dissociation of other complexes will require further investigations. Of note is that histone H3 phosphorylation-acetylation was also observed in response to concomitant activation of GR and NMDA-R.57
MSK1: a Novel Regulator of NR-Target Genes?
MSK1 is well known to be activated by many physiological and pathological stimuli and to regulate gene transcription at multiple levels.49 Indeed, MSK1 targets directly several transcription factors such as CREB, ATF-1 and NF-κB and induces histone phosphorylation, chromatin relaxation and facilitated recruitment of other coregulators at the cognate target promoters.70
The present review points out that MSK1 is also activated by several steroid and non-steroid hormones and regulates the expression of the cognate NR-target genes by regulating the transcriptional activity of NRs and by interfering with chromatin environment. Indeed MSK1-mediated phosphorylation of H3 contributes to several NR-target genes induction, very probably as a chromatin mark accounting in cooperation with other histones modifications for the dissociation of repressive complexes and/or the recruitment of chromatin-remodeling complexes.5,6,69 Thus one can propose that MSK1-mediated phosphorylation events might act as a transcriptional clock fine-tuning the dynamics of chromatin so that at the end the correct proteins are present with the right activity, at the right place and at the right time.
Then the question is whether all NR-target genes promoters are modified and remodeled through H3 phosphorylation by MSK1. Another question is whether MSK1 is also involved in the phosphorylation of NRs coregulators, given that most coactivators and corepressors are known to be also targets for phosphorylation processes.7
Future Applications
NRs and Cancer: a Versatile Role of MSK1
Steroid hormone receptors are well established in the etiology of many cancers including classical hormone-dependent cancers like breast and prostate cancer. MSKs are also well known to play a role in cell proliferation and malignant transformation through transcription regulation of the immediate early genes c-fos and junB,68,70 ER8171 and the Nur77, Nurr1 and Nor1 orphan nuclear receptors,72 coupled with histone H3 phosphorylation at specific loci and AP-1 activation.
In line with this, the non-genomic progestin signaling to MSK1 has been correlated with tumor proliferation.6 In addition, given that MAPK signaling is hyperactivated in several cancers such as breast and prostate cancer,47,73 one can speculate that signaling to MSK is also increased, potentiating the proliferative effects of ER, PR and AR. Knowing this, MSK-specific drugs would have therapeutic effect in such cancers where MSK deregulation is clearly involved. Note however, that in other cancer types such as colon cancer, the p38MAPK/MSK1 pathway rather cooperates with vitamin D and VDR for the expression of genes involved in tumor suppression.39 Thus, depending on the type of cancer and on the NR, the MAPK/MSK1 pathway can have opposite effects on cell growth, restricting the use of MSK specific drugs in cancer therapy.
NRs and MSK1 Inhibition
Evidence has accumulated over the past few years that the action of NRs is not restricted to the regulation of cognate target gene expression, but also concerns several other gene programs by interfering with other transcription factors. A well-known example is the anti-inflammatory action of GR and glucocorticoids of which the action mechanism is mainly based on interference with the activity of the transcription factor NF-κB. Indeed, in response to inflammatory cytokines, the classical NF-κB activation pathway determines the expression of various pro-inflammatory genes. The transactivation of NF-κB is fine-tuned by MSK1, which phosphorylates NF-κB p65 and histone H3 S10 at inflammatory genes promoters.49,74 All these events participate in the establishment of a transcription-prone chromatin environment. Recent research by Beck et al.75 demonstrated a completely new aspect of the anti-inflammatory action of glucocorticoids. Indeed, they found that glucocorticoids counteract MSK1 recruitment at inflammatory gene promoters, through the formation of a complex between activated MSK1 and GR, followed by a subcellular relocalization of activated MSK1 to the cytoplasm. The subsequent absence of MSK1 at inflammatory gene promoters leads to impaired phosphorylation of histone and transcription factor components, resulting in a lack of activation of MSK1-dependent NF-κB-driven promoters.
Another well known example is the inhibition of AP-1 by certain NRs such as GR, ER and RARs.1,7 AP-1 complexes (heterodimers of the proto-oncogene products c-Fos and c-Jun) regulate the expression of several genes involved in oncogenesis and cell transformation. Most interestingly, a network of phosphorylation processes involving MSK1 controls the activity of AP-1 complexes.68 Though the molecular basis of the anti-AP-1 activity of GR and RARs has remained elusive,1,7 one cannot exclude a nucleocytoplasmic shuttling of MSK1 as above. Nevertheless, nucleocytoplasmic shuttling of MSK appears to be another new action mechanism for certain NRs, opening perspectives for novel therapeutic strategies.76
Conclusion
It is clear that MSK-mediated chromatin remodeling plays a role in many physiological and pathological processes and the list of the agents that can activate the MAPK/MSK signaling pathway is still growing. In this context, hormones and their cognate NRs can activate this pathway, which then contributes to the activation of target genes. However, depending on the NR, MSK1 activation has been related to cell growth6 or differentiation.39 Moreover, under certain conditions, NRs do not activate but inhibit MSK1 and thereby the activity of several genes regulated by this kinase. Therefore it does not appear to be a general rule for the cross talk between NRs and MSKs and MSK-specific drugs should have therapeutic benefits only in specific NR-related cancers or diseases.
Acknowledgments
Funds from CNRS, INSERM, the Agence Nationale pour la Recherche (ANR-05BLAN-039002 and and ANR-09-BLAN-0297-01), the Foundation pour la Recherche Medicale (FRM, DEQ20090515423) and the Institut National du Cancer (INCa-PL09194) supported this work. FRM and the Lady TATA Memorial Trust supported AP.
References
- 1.
- Germain P, Altucci L, Bourguet W, Rochette-Egly C, Gronemeyer H. Nuclear receptor superfamily: principles of signaling. Pure Appl Chem. 2003;75:1619–64. http://dx.doi.org/10.1351/pac200375111619.
- 2.
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. http://dx.doi.org/10.1124/pr.58.4.2. [PubMed: 17132848]
- 3.
- Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–64. http://dx.doi.org/10.1038/nrd1551. [PubMed: 15520817]
- 4.
- Laudet V, Gronemeyer H. London: Academic Press. 2001. Nuclear Receptor Factsbook.
- 5.
- Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de Thé H, et al. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters . EMBO J. 2009;28:34–47. http://dx.doi.org/10.1038/emboj.2008.256. [PMC free article: PMC2633082] [PubMed: 19078967]
- 6.
- Vicent GP, Ballare C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, et al. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–81. http://dx.doi.org/10.1016/j.molcel.2006.10.011. [PubMed: 17081988]
- 7.
- Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors. Nucl Recept Signal. 2009;7:e005. [PMC free article: PMC2686084] [PubMed: 19471584]
- 8.
- Rochette-Egly C. Dynamic combinatorial networks in nuclear receptor-mediated transcription. J Biol Chem. 2005;280:32565–8. http://dx.doi.org/10.1074/jbc.R500008200. [PubMed: 16076839]
- 9.
- Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B. Transcriptional activities of retinoic acid receptors. Vitam Horm. 2005;70:199–264. http://dx.doi.org/10.1016/ S0083-6729(05)70007-8. [PubMed: 15727806]
- 10.
- Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–66. http://dx.doi.org/10.1016/S0898-6568(02)00115-8. [PubMed: 12618210]
- 11.
- Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, et al. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol. 2008;22:1754–66. http://dx.doi.org/10.1210/me.2007-0219. [PMC free article: PMC2725771] [PubMed: 18483179]
- 12.
- Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. http://dx.doi.org/10.1016/S0039-128X(02)00110-1. [PubMed: 12475718]
- 13.
- Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–34. http://dx.doi.org/10.1210/me.2005-0338. [PubMed: 17185395]
- 14.
- Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007;21:2311–9. http://dx.doi.org/10.1210/me.2007-0101. [PubMed: 17536004]
- 15.
- Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nucl Recept Signal. 2007;5:e005. [PMC free article: PMC1876600] [PubMed: 17525795]
- 16.
- Weigel NL, Moore NL. Cyclins, cyclin dependent kinases, and regulation of steroid receptor action. Mol Cell Endocrinol. 2007;265-266:157–61. http://dx.doi.org/10.1016/j.mce.2006.12.013. [PMC free article: PMC1940111] [PubMed: 17207919]
- 17.
- Lavery DN, McEwan IJ. Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J. 2005;391:449–64. http://dx.doi. org/10.1042/BJ20050872. [PMC free article: PMC1276946] [PubMed: 16238547]
- 18.
- Wärnmark A, Treuter E, Wright AP, Gustafsson JA. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol. 2003;17:1901–9. http://dx.doi.org/10.1210/me.2002-0384. [PubMed: 12893880]
- 19.
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. http://dx.doi.org/10.1038/nrm1589. [PubMed: 15738986]
- 20.
- Lalevée S, Bour G, Quinternet M, Samarut E, Kessler P, Vitorino M, et al. Vinexin{beta}, an atypical sensor of retinoic acid receptor g signaling: union and sequestration, separation, and phosphorylation. FASEB J. 2010;24:4523–34. http://dx.doi.org/10.1096/fj.10-160572. [PubMed: 20634350]
- 21.
- Gaillard E, Bruck N, Brelivet Y, Bour G, Lalevée S, Bauer A, et al. Phosphorylation by PKA potentiates retinoic acid repeptor a activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc Natl Acad Sci USA. 2006;103:9548–53. http://dx.doi.org/10.1073/ pnas.0509717103. [PMC free article: PMC1480444] [PubMed: 16769902]
- 22.
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. http://dx.doi. org/10.1038/nrd1283. [PubMed: 14708019]
- 23.
- Márquez DC, Chen HW, Curran EM, Welshons WV, Pietras RJ. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246:91–100. http://dx.doi.org/10.1016/j.mce.2005.11.020. [PubMed: 16388889]
- 24.
- Matthews L, Berry A, Ohanian V, Ohanian J, Garside H, Ray D. Caveolin mediates rapid glucocorticoid effects and couples glucocorticoid action to the antiproliferative program. Mol Endocrinol. 2008;22:1320–30. http://dx.doi.org/10.1210/me.2007-0154. [PMC free article: PMC5419537] [PubMed: 18308897]
- 25.
- Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290:8–13. http://dx.doi.org/10.1016/j.mce.2008.04.005. [PMC free article: PMC2565274] [PubMed: 18502030]
- 26.
- de Laurentiis A, Donovan L, Arcaro A. Lipid rafts and caveolae in signaling by growth factor receptors. Open Biochem J. 2007;1:12–32. [PMC free article: PMC2570545] [PubMed: 18949068]
- 27.
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–88. http://dx.doi.org/10.1074/jbc.M611877200. [PubMed: 17535799]
- 28.
- Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol. 2009;308:3–8. http://dx.doi.org/10.1016/j.mce.2009.03.025. [PMC free article: PMC2701909] [PubMed: 19549586]
- 29.
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–57. http://dx.doi.org/10.1016/j. yfrne.2007.08.003. [PubMed: 18083219]
- 30.
- Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, Bottero D, et al. Androgen-stimulated DNA synthesis and cytoskeletal changes in fibroblasts by a nontranscriptional receptor action. J Cell Biol. 2003;161:547–56. http://dx.doi.org/10.1083/jcb.200211099. [PMC free article: PMC2172930] [PubMed: 12743104]
- 31.
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–17. http://dx.doi.org/10.1093/emboj/19.20.5406. [PMC free article: PMC314017] [PubMed: 11032808]
- 32.
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–18. http://dx.doi.org/10.1093/emboj/17.7.2008. [PMC free article: PMC1170546] [PubMed: 9524123]
- 33.
- Hagan CR, Faivre EJ, Lange CA. Scaffolding actions of membrane-associated progesterone receptors. Steroids. 2009;74:568–72. http://dx.doi.org/10.1016/j.steroids.2008.12.004. [PMC free article: PMC3969614] [PubMed: 19135465]
- 34.
- Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–83. http://dx.doi.org/10.1210/ me.2004-0528. [PubMed: 15817653]
- 35.
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–71. http://dx.doi.org/10.1210/me.2004-0116. [PubMed: 15272054]
- 36.
- Masiá S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic Acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic Acid receptor. Mol Endocrinol. 2007;21:2391–402. http://dx.doi.org/10.1210/me.2007-0062. [PubMed: 17595318]
- 37.
- Dey N, De PK, Wang M, Zhang H, Dobrota EA, Robertson KA, et al. CSK controls retinoic acid receptor (RAR) signaling: a RAR-c-SRC signaling axis is required for neuritogenic differentiation. Mol Cell Biol. 2007;27:4179–97. http://dx.doi.org/10.1128/MCB.01352-06. [PMC free article: PMC1900023] [PubMed: 17325034]
- 38.
- Piskunov A, Rochette-Egly C. A retinoic acid receptor RARα pool present in membrane lipid rafts forms complexes with G protein αQ to activate p38MAPK. Oncogene. 2011. [Epub ahead of print] http://dx.doi.org/10.1038/onc.2011.499. [PubMed: 22056876]
- 39.
- Ordoñez-Morán P, Alvarez-Diaz S, Valle N, Larriba MJ, Bonilla F, Munoz A. The effects of 1,25-dihydrovitaminD3 on colon cancer cells depend on RhoA-ROCK-p38MAPK-MSK signaling. J Steroid Biochem Mol Biol. 2010;121:355–61. http://dx.doi.org/10.1016/j. jsbmb.2010.02.031. [PubMed: 20223287]
- 40.
- Alsayed Y, Uddin S, Mahmud N, Lekmine F, Kalvakolanu DV, Minucci S, et al. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to all-trans-retinoic acid. J Biol Chem. 2001;276:4012–9. http://dx.doi.org/10.1074/jbc.M007431200. [PubMed: 11060298]
- 41.
- Giannì M, Bauer A, Garattini E, Chambon P, Rochette-Egly C. Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-indced RARg degradation and transactivation. EMBO J. 2002;21:3760–9. http://dx.doi.org/10.1093/emboj/cdf374. [PMC free article: PMC126119] [PubMed: 12110588]
- 42.
- Giannì M, Parrella E, Raska I, Gaillard E, Nigro EA, Gaudon C, et al. P38MAPK-dependent phosphorylation and degradation of SRC-3/AIB1 and RARalpha-mediated transcription. EMBO J. 2006;25:739–51. http://dx.doi.org/10.1038/sj.emboj.7600981. [PMC free article: PMC1383562] [PubMed: 16456540]
- 43.
- Ordóñez-Morán P, Larriba MJ, Palmer HG, Valero RA, Barbáchano A, Duñach M, et al. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697–710. http://dx.doi.org/10.1083/ jcb.200803020. [PMC free article: PMC2582889] [PubMed: 19015318]
- 44.
- Chen N, Napoli JL. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J. 2008;22:236–45. http://dx.doi.org/10.1096/fj.07-8739com. [PubMed: 17712061]
- 45.
- Gupta P, Ho P, Huq MD, Ha SG, Park SW, Khan AA, et al. Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci USA. 2008;105:11424–9. http://dx.doi.org/10.1073/ pnas.0710561105. [PMC free article: PMC2516243] [PubMed: 18682553]
- 46.
- Pan J, Kao YL, Joshi S, Jeetendran S, Dipette D, Singh US. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93:571–83. http://dx.doi.org/10.1111/j.1471-4159.2005.03106.x. [PubMed: 15836616]
- 47.
- Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. http://dx.doi.org/10.1139/ o04-121. [PubMed: 15746962]
- 48.
- Lazou A, Markou T. MSK1. UCSD Nature Molecule Pages. 2010. In press. http://dx.doi. org/10.1038/mp.a001562.01.
- 49.
- Vermeulen L, Berghe WV, Beck IM, De Bosscher K, Haegeman G. The versatile role of MSKs in transcriptional regulation. Trends Biochem Sci. 2009;34:311–8. http://dx.doi.org/10.1016/j.tibs.2009.02.007. [PubMed: 19464896]
- 50.
- Vicent GP, Ballare C, Zaurin R, Saragueta P, Beato M. Chromatin remodeling and control of cell proliferation by progestins via cross talk of progesterone receptor with the estrogen receptors and kinase signaling pathways. Ann N Y Acad Sci. 2006;1089:59–72. http://dx.doi.org/10.1196/ annals.1386.025. [PubMed: 17261755]
- 51.
- Espino PS, Li L, He S, Yu J, Davie JR. Chromatin modification of the trefoil factor 1 gene in human breast cancer cells by the Ras/mitogen-activated protein kinase pathway. Cancer Res. 2006;66:4610–6. http://dx.doi.org/10.1158/0008-5472.CAN-05-4251. [PubMed: 16651411]
- 52.
- Titolo D, Mayer CM, Dhillon SS, Cai F, Belsham DD. Estrogen facilitates both phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J Neurosci. 2008;28:6473–82. http://dx.doi.org/10.1523/JNEUROSCI.0514-08.2008. [PMC free article: PMC6670897] [PubMed: 18562618]
- 53.
- Eisinger-Mathason TS, Andrade J, Lannigan DA. RSK in tumorigenesis: connections to steroid signaling. Steroids. 2010;75:191–202. http://dx.doi.org/10.1016/j.steroids.2009.12.010. [PMC free article: PMC2823981] [PubMed: 20045011]
- 54.
- Clark DE, Poteet-Smith CE, Smith JA, Lannigan DA. Rsk2 allosterically activates estrogen receptor alpha by docking to the hormone-binding domain. EMBO J. 2001;20:3484–94. http://dx.doi.org/10.1093/emboj/20.13.3484. [PMC free article: PMC125527] [PubMed: 11432835]
- 55.
- Clark DE, Errington TM, Smith JA, Frierson HF Jr, Weber MJ, Lannigan DA. The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 2005;65:3108–16. [PubMed: 15833840]
- 56.
- Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–51. http://dx.doi.org/10.1111/ j.1471-4159.2005.03318.x. [PubMed: 16011741]
- 57.
- Reul JM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32:S21–5. http://dx.doi.org/10.1016/j. psyneuen.2007.03.016. [PubMed: 17644269]
- 58.
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–28. http://dx.doi.org/10.1111/j.1471-4159.2006.04396.x. [PubMed: 17250652]
- 59.
- Samarut E, Amal I, Markov GV, Stote R, Dejaegere A, Laudet V, Rochette-Egly C. Evolution of nuclear retinoic acid receptor alpha (RARα) phosphorylation sites. Serine gain provides fine-tuned regulation. Mol Biol Evol. 2011;28:2125–37. [PubMed: 21297158]
- 60.
- Bour G, Gaillard E, Bruck N, Lalevée S, Plassat JL, Busso D, et al. Cyclin H binding to the RAR{alpha} activation function (AF)-2 domain directs phosphorylation of the AF-1 domain by cyclin-dependent kinase 7. Proc Natl Acad Sci USA. 2005;102:16608–13. http://dx.doi.org/10.1073/ pnas.0505556102. [PMC free article: PMC1283805] [PubMed: 16275922]
- 61.
- Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–8. http://dx.doi.org/10.1016/j.biopha.2006.07.082. [PubMed: 16949786]
- 62.
- Su D, Gudas LJ. Gene expression profiling elucidates a specific role for RARgamma in the retinoic acid-induced differentiation of F9 teratocarcinoma stem cells. Biochem Pharmacol. 2008;75:1129–60. http://dx.doi.org/10.1016/j.bcp.2007.11.006. [PMC free article: PMC2988767] [PubMed: 18164278]
- 63.
- Bour G, Taneja R, Rochette-Egly C. Mouse embryocarcinoma F9 cells and retinoic acid. A model to study the molecular mechanisms of endodermal differentiation. In: Taneja R, ed. Nuclear Receptors in Development, Vol. 16. San Diego: Elsevier Press. 2006:211–253.
- 64.
- Vicent GP, Nacht AS, Zaurin R, Ballare C, Clausell J, Beato M. Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol Endocrinol. 2010;24:2088–98. http://dx.doi.org/10.1210/me.2010-0027. [PMC free article: PMC5417384] [PubMed: 20484412]
- 65.
- Vicent GP, Zaurin R, Ballare C, Nacht AS, Beato M. Erk signaling and chromatin remodeling in MMTV promoter activation by progestins. Nucl Recept Signal. 2009;7:e008. [PMC free article: PMC2807634] [PubMed: 20087429]
- 66.
- Perissi V, Rosenfeld MG. Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol. 2005;6:542–54. http://dx.doi.org/10.1038/nrm1680. [PubMed: 15957004]
- 67.
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–28. http://dx.doi.org/10.1101/gad.1424806. [PubMed: 16751179]
- 68.
- Kim HG, Lee KW, Cho YY, Kang NJ, Oh SM, Bode AM, et al. Mitogen- and stress-activated kinase 1-mediated histone H3 phosphorylation is crucial for cell transformation. Cancer Res. 2008;68:2538–47. http://dx.doi.org/10.1158/0008-5472.CAN-07-6597. [PMC free article: PMC2288657] [PubMed: 18381464]
- 69.
- Vicent GP, Zaurin R, Nacht AS, Li A, Font-Mateu J, LeDily F, et al. Two chromatin remodeling activities cooperate during activation of hormone responsive promoters. PLoS Genet. 2009;5:e1000567. http://dx.doi.org/10.1371/journal.pgen.1000567. [PMC free article: PMC2704372] [PubMed: 19609353]
- 70.
- Drobic B, Perez-Cadahia B, Yu J, Kung SK, Davie JR. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic Acids Res. 2010;38:3196–208. http://dx.doi. org/10.1093/nar/gkq030. [PMC free article: PMC2879512] [PubMed: 20129940]
- 71.
- Janknecht R. Regulation of the ER81 transcription factor and its coactivators by mitogen- and stress-activated protein kinase 1 (MSK1). Oncogene. 2003;22:746–55. http://dx.doi. org/10.1038/sj.onc.1206185. [PubMed: 12569367]
- 72.
- Darragh J, Soloaga A, Beardmore VA, Wingate AD, Wiggin GR, Peggie M, et al. MSKs are required for the transcription of the nuclear orphan receptors Nur77, Nurr1 and Nor1 downstream of MAPK signalling. Biochem J. 2005;390:749–59. http://dx.doi.org/10.1042/BJ20050196. [PMC free article: PMC1199668] [PubMed: 15910281]
- 73.
- Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–8. http://dx.doi. org/10.1016/j.febslet.2009.11.041. [PMC free article: PMC8117181] [PubMed: 19925796]
- 74.
- Vermeulen L, DeWilde G, VanDamme P, Van denBerghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003;22:1313–24. http://dx.doi.org/10.1093/emboj/cdg139. [PMC free article: PMC151081] [PubMed: 12628924]
- 75.
- Beck IM, Van den Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, et al. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. EMBO J. 2008;27:1682–93. http://dx.doi.org/10.1038/emboj.2008.95. [PMC free article: PMC2435130] [PubMed: 18511904]
- 76.
- Beck IM, Berghe WV, Gerlo S, Bougarne N, Vermeulen L, De Bosscher K, et al. Glucocorticoids and mitogen- and stress-activated protein kinase 1 inhibitors: possible partners in the combat against inflammation. Biochem Pharmacol. 2009;77:1194–205. http://dx.doi.org/10.1016/j. bcp.2008.12.008. [PubMed: 19150610]
- 77.
- Rastinejad F, Wagner T, Zhao Q, Khorasanizadeh S. Structure of the RXR-RAR DNA-binding complex on the retinoic acid response element DR1. EMBO J. 2000;19:1045–54. http://dx.doi.org/10.1093/emboj/19.5.1045. [PMC free article: PMC305643] [PubMed: 10698945]
- 78.
- Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature. 1995;375:377–82. http://dx.doi.org/10.1038/375377a0. [PubMed: 7760929]
- Introduction
- The Classical Picture of NRs: Ligand-Dependent Regulators of Transcription
- NRs Are Phosphoproteins
- A New Picture of NRs: NRs Are Associated to the Cell Membrane and Activate MAPK Signaling Pathways
- NRs Activate MSK1, Downstream of Erk and p38MAPK
- RARα Is Phosphorylated by MSK1 While the Other NRs Are Targets for the Upstream MAPK
- MSK1 Participates in the Activation of NR-Target Genes and Is Recruited at the Promoters of NR-Target Genes
- MSK 1 Regulates the Chromatin Environment of NR-Target Promoters
- MSK1: a Novel Regulator of NR-Target Genes?
- Conclusion
- Acknowledgments
- References
- MSK1 and Nuclear Receptors Signaling - Madame Curie Bioscience DatabaseMSK1 and Nuclear Receptors Signaling - Madame Curie Bioscience Database
- Sonic Hedgehog Signaling in the Developing and Regenerating Fins of Zebrafish - ...Sonic Hedgehog Signaling in the Developing and Regenerating Fins of Zebrafish - Madame Curie Bioscience Database
- Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion - Madame Curie Bi...Muscle Morphogenesis: The Process of Embryonic Myoblast Fusion - Madame Curie Bioscience Database
- Development of the Larval Somatic Musculature - Madame Curie Bioscience DatabaseDevelopment of the Larval Somatic Musculature - Madame Curie Bioscience Database
- Tissue Engineering Constructs and Commercialization - Madame Curie Bioscience Da...Tissue Engineering Constructs and Commercialization - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...