NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Recent experimental and epidemiologic evidence suggests that systemic physiologic stress-responsive pathways may help shape the tumor microenvironment to promote metastasis. These pathways act through the peripheral sympathetic nervous system to release catecholaminergic neurotransmitters that stimulate signaling through β-adrenergic receptors on tumor cells and tumor-associated macrophages. Experimental studies found that chronic stress accelerated breast cancer metastasis through β-adrenergic signaling pathways that recruit alternatively activated macrophages to primary mammary tumors. Consistent with β-adrenergic regulation of breast cancer, recent clinical studies found that inhibiting β-adrenergic signaling with β-blockers was associated with improved breast-cancer specific outcomes. These and other studies described here suggest that β-blockade of sympathetic nervous system signaling pathways may be a novel adjuvant therapeutic strategy to slow cancer progression and prevent metastasis.
Introduction
The study of metastasis developed rapidly from the successful identification and characterization of genes that promote or suppress metastasis.1,2 More recently this traditional tumor-centric focus has expanded to include an appreciation of the influence of the tumor microenvironment on metastasis.3,4 In addition to regulation by tumor-derived factors and microenvironment-derived factors, it is possible that systemic physiologic conditions might impact microenvironmental dynamics to affect tumor metastasis. The nervous system plays a key role in coordinating activity of multiple physiologic systems in response to organismic threat. Furthermore, epidemiologic studies have long suggested that psychological factors that lead to activation of physiologic stress-response pathways might impact metastasis and tumor progression. Consistent with these ideas, recent research suggests that the fight-or-flight response from the sympathetic nervous system (SNS) may modulate multiple physiologic processes in the tumor microenvironment that collectively affect the propensity of cancer cells to disseminate from the primary tumor and colonize distant tissue sites.5-7 Here we review recent findings that describe the molecular and cellular mechanisms of SNS-enhanced metastasis in the context of breast cancer and discuss implications for new therapeutic strategies that target the SNS to slow or prevent cancer progression.
Beta-Adrenergic Signaling Promotes Metastasis
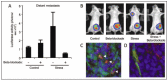
To investigate systemic physiologic regulation of metastasis our laboratory conducted a series of experimental studies to examine the impact of the peripheral SNS on breast cancer progression. These studies found that experimentally imposed chronic stress or pharmacologic activation of SNS signaling pathways accelerated breast cancer metastasis.5 Activation of SNS signaling by repeated restraint stress significantly increased metastasis from primary mammary tumors to distant target organs in an orthotopic syngenic model of breast cancer (Fig. 1A,B). Chronic stress increased metastatic colonization to lung by 10-fold, and to lymph node by 3-fold, which suggested that both vascular and lymphatic pathways of dissemination may be sensitive to SNS regulation. In response to activation, SNS nerve fibers release catecholaminergic neurotransmitters including norepinephrine which binds to adrenergic receptors on target cells. Pharmacologic activation of SNS signaling pathways with the β-adrenergic agonist isoproterenol also increased metastasis from primary mammary tumors. The kinetics and magnitude of target colonization that was induced by pharmacologic β-adrenergic stimulation was similar to that induced by physiologic SNS activation by chronic restraint. Conversely, when mice were treated with propranolol which blocks signaling through β-adrenergic receptors, stress-enhanced metastasis was completely suppressed. These findings suggest that β-adrenergic signaling is sufficient to enhance metastasis to distant tissues and that β-blockers—which have been used for many years to treat hypertension—may also have a protective role in slowing breast cancer progression.
In addition to these findings for breast cancer, progression of other cancer types may also be regulated by β-adrenergic signaling. Pharmacological SNS activation using isoproterenol increased both the frequency and magnitude of peritoneal colonization by disseminated ovarian cancer cells.6 Tumor growth was similarly enhanced by physiologic SNS activation using repeated restraint, and this effect was blocked by propranolol which demonstrated dependence on β-adrenergic signaling.6 SNS signaling increased both the number and size of metastatic foci in models of breast and ovarian cancer.5,6 Beta-blockade similarly inhibited norepinephrine-induced PC3 prostate cancer cell dissemination from thigh to lymph nodes8 and growth of breast cancer cells in lung after surgical stress.9 Collectively, these studies suggest that β blockade of β-adrenergic signaling pathways may be a novel and effective therapeutic strategy to slow progression in multiple solid tumor types.
SNS Accelerates Metastasis through Macrophage Recruitment
Signaling through β-adrenergic receptors modulates immune cell function, but little is known about neural-immune interactions in the context of cancer.10-13 To examine the communication pathways that transmit SNS signals into the tumor microenvironment, we investigated β-adrenergic receptor levels within primary tumors in an orthotopic mouse model of breast cancer. Low-level β-adrenergic receptors were detected on mammary tumor cells, but high-level β-adrenergic receptor expression was predominately confined to macrophages that infiltrated the tumor parenchyma.5 Beta-adrenergic receptors also were detected on human archival tumor tissue14,15 and on tumor-infiltrating macrophages in human breast cancer (Fig. 1C). Tumor-associated macrophages promote cancer progression through multiple pathways including accelerated angiogenesis, extracellular matrix remodeling, chemoattraction of immune and tumor cells, generation of a pro-inflammatory environment and evasion of anti-tumor immune responses.16-18 Myeloid progenitor cells and monocytes also express β-adrenergic receptors and may respond to β-adrenergic signaling while in the bone marrow or in circulation before being recruited to the primary tumor and transitioning to a macrophage phenotype.19,20 These observations suggest that tumor-associated macrophages may be sensitive to SNS signaling and raise the possibility that activation of a patients physiological stress-response pathways might influence macrophage recruitment to the primary tumor to enhance metastasis.
To investigate the role of the peripheral nervous system in regulating tumor-associated macrophage dynamics, we examined the effect of chronic stress on macrophage recruitment to primary tumors. Physiologic activation of stress-response pathways increased recruitment of macrophages to primary mammary tumors by 50%. The effect of chronic stress on macrophage recruitment was mimicked by pharmacologic β-adrenergic activation with isoproterenol and was suppressed by β-blockade, confirming dependence on β-adrenergic signaling pathways. The macrophage chemoattractant CSF-1 is overexpressed in many solid tumors, including breast tumors, and is associated with poor prognosis.21-23 To examine the role of macrophage recruitment in stress-enhanced metastatic dissemination we treated mice with a CSF-1 receptor small molecule antagonist, GW2580, to suppress macrophage infiltration of primary mammary tumors.5 GW2580 had no impact on the kinetics of primary tumor growth, but reduced macrophage recruitment to primary tumors and blocked stress-enhanced metastasis to distant tissues. These studies identified the SNS as a novel physiologic regulator of tumor-associated macrophages and suggest that β-blockade modulates macrophage dynamics to slow or prevent metastasis.
Macrophages Induce a Switch to Pro-Metastatic Gene Expression
Studies of the tumor microenvironment found that SNS-regulated macrophage recruitment induced a pro-metastatic gene expression signature in primary mammary tumors. SNS signaling to macrophages increased expression of Tg fb and Arg1 genes,5 suggesting a shift toward an immunosuppressive M2-like myeloid phenotype that is characterized by local transforming growth factor-β (TGFβ) activity and a shift in arginine metabolism toward proliferative polyamine production.16 SNS activity increased expression of prometastatic factors including extracellular matrix-degrading proteases (Mmp2 and Mmp9), inflammatory mediators (Ptgs2/ Cox2), and pro-angiogenic factors (Veg f), but downregulated expression of anti-metastatic type I interferon β (Ifnb). Beta-adrenergic stimulation of bone marrow-derived macrophages with the SNS neurotransmitter norepinephrine induced similar patterns of pro-metastatic gene expression as those found in the tumor parenchyma of stressed mice, suggesting that the SNS may directly induce tumor-associated macrophages to produce pro-metastatic molecules in vivo.5 SNS regulation of tumor-associated macrophages may also be relevant for patients with cancer. Ovarian cancer patients who reported greater number and severity of stressful life events had significantly higher levels of MMP-9 in ovarian tumor-associated macrophages, consistent with the proposed model that SNS regulates macrophage-derived pro-metastatic factors in the tumor microenvironment.24
SNS control of tumor-associated macrophage gene expression may be a specific example of a more general physiologic immune regulatory mechanism as whole genome transcriptional profiling found that myeloid lineage cells are particularly sensitive to SNS-mediated social regulation.25 Consistent with findings of stress-responsive genes in mouse models of cancer,5 chronically lonely individuals showed changes in gene expression in antigen presenting cells, including upregulation of genes involved in inflammation and suppression of genes involved in type I interferon responses.25 SNS regulation of tumor-associated immune cells has also been found in experimental models of other tumor types, including UV-induced squamous cell carcinoma-like skin lesions, metastatic breast cancer, and leukemia.12,26-28
To explore the functional impact of SNS-regulated gene expression changes on the tumor microenvironment, we investigated the effect of SNS activation on angiogenesis. Chronic stress increased Veg f gene expression and increased blood vessel density by 3-fold in primary mammary tumors. Despite increased angiogenesis, stress did not increase primary tumor size. Beta-blockade reversed stress-enhanced angiogenesis, showing that β-adrenergic signaling is necessary for stress-induced changes in blood vessel density. Stress also increased Veg f expression and vascular density in ovarian tumor metastases in a β-adrenergic dependent manner.6 Pharmacologic inhibitor studies demonstrated that stress-enhanced angiogenesis was dependent on macrophage recruitment to the tumor.5 Tumor-infiltrating macrophages have also been linked to increased angiogenesis and reduced survival in invasive breast cancer in humans,29 although the role of β-adrenergic regulation of macrophage dynamics in patients has yet to be explored.
In addition to regulating metastasis via effects on tumor-associated macrophages, it is possible that SNS signaling acts directly on tumor cells to promote metastatic behavior. In addition to expression on breast tumor cells, β-adrenergic receptors have been documented on tumor cells in archived human patient samples and on multiple tumor cell lines, including hematopoietic malignancies, melanoma and carcinomas including, prostate, ovarian, oral squamous cell, salivary and lung.8,14,15,30-35 Tumor cell stimulation with the SNS neurotransmitter norepinephrine increased tumor cell migration and invasion in in vitro assays through β-adrenergic-dependent pathways.36-39 Consistent with SNS-induced invasion, norepinephrine induced expression of proteases (MMP2, MMP9) and pro-angiogenic cytokines (IL6, IL8, VEGF).15,31,38,40-42 SNS regulation of tumor cell-derived cytokines has also been found in vivo. In an orthotopic model of ovarian cancer metastasis, suppression of tumor-derived interleukin-8 blocked stress-enhanced metastasis by modulating protease expression and decreasing angiogenesis.31 In ovarian cancer patients, low levels of social support have been linked to high interleukin-6 levels in peripheral blood and ascites.43 These studies suggest that SNS-stimulated tumor cells may be additional sources of proteases and cytokines that complement macrophage-derived protease production to promote tumor cell dissemination, and raise the possibility that β-blockers may offer protection through direct effects on tumor cells as well as through modulation of the tumor microenvironment.
Neural Mechanisms
In response to chronic stress or threat, cognitive and emotional information is integrated by the central nervous system and transmitted by the peripheral autonomic nervous system to the body. This fight-or-flight response results in release of neurotransmitters including norepinephrine from SNS nerve fibers located within organs, as well as release of cortisol and neurotransmitters including epinephrine from the adrenal gland into systemic circulation.6,11,44 Ligation of these neurotransmitters to β-adrenergic receptors triggers a signaling cascade that induces G-protein-coupled cyclic 3′,5′-adenosine monophosphate (cAMP) synthesis, phosphorylation of protein kinase A (PKA), and transcription factor activation.45,46 The resulting changes in gene expression may affect the tumor microenvironment or directly modulate tumor cell behavior.
SNS nerve fibers innervate organs that are preferentially targeted by breast cancer metastasis, including lungs, lymph nodes and bone marrow.47-49 This anatomic structure suggests that neural signaling may also regulate the metastatic microenvironment to influence tumor cell colonization of these organs. Furthermore, the experience of chronic stress may increase neural signaling to these organs. In studies to investigate the impact of chronic stress on peripheral neural architecture, we found that experimental social stress increased the density of SNS nerve fibers in axillary lymph nodes of adult primates.50 Chronic stress increased transcription of the sympathetic neurotrophin nerve growth factor (NGF), which is essential for development and maintenance of SNS neurons.51 The impact of stress on neural density occurred specifically in the lymph node paracortex where macrophages interact with other immune cells to shape an immune response. These findings showed that the peripheral nervous system is surprisingly plastic, even in fully developed adult primates. Stress-regulation of neural density suggests an anatomic mechanism for increased SNS signaling in lymph nodes and possibly other metastatic targets in chronically stressed individuals.
In addition to innervation of metastatic target organs, it is possible that primary tumors receive direct sympathetic innervation. Nerve fibers positive for tyrosine hydroxylase—the rate limiting enzyme required for biosynthesis of SNS neurotransmitters—were detected in peripheral parenchyma of human breast and ovarian tumor archival samples and in mammary tumors from a syngenic mouse model (Fig. 1D).52 However, functionality of these tyrosine hydroxylase-positive nerve fibers in tumors has yet to be demonstrated, for example through microdialysis studies.53 SNS neurotransmitters have been found to accumulate in primary ovarian tumors and in associated ascites.54 Patients with lower social support or more advanced tumors were found to have higher SNS neurotransmitter levels in tumors, consistent with stress-induced neural regulation of cancer progression.55 The source of these catecholamines is unclear but may be SNS fibers that innervate the ovary parenchyma or vasculature.56
Very little is known about central nervous system regulation of tumor progression. Hippocampal brain derived neurotrophic factor (BDNF) levels have been linked to adrenergic regulation of the tumor microenvironment57 and further studies are needed to investigate central mechanisms that control SNS-enhanced metastasis.
Clinical β-Blockade of Metastasis
The experimental studies of SNS regulation of metastasis described above provide an important mechanistic framework for understanding recent epidemiologic findings that β-blockers may provide protective benefits for women with breast cancer.58-61 These retrospective studies investigated the relationship between breast cancer patients use of β-blockers in the year prior to diagnosis—in the majority of cases to treat concurrent hypertension—with breast cancer recurrence and survival. A retrospective cohort study of hypertensive breast cancer patients who were prescribed β-blockers prior to diagnosis found reduced tumor recurrence in these women compared with non-hypertensive breast cancer patients or hypertensive breast cancer patients who received non-β-blocker anti-hypertensive medication.58 After controlling for other prognostic factors including tumor size, stage and grade, β-blocker usage was associated with significantly reduced risk of developing distant metastasis or breast-cancer specific mortality. A second retrospective study of breast cancer patients treated with neo-adjuvant chemotherapy, found that concurrent β-blocker usage was associated with better relapse-free survival.59 This effect was found in patients with tumors that were negative for the estrogen receptor, progesterone receptor and human epidermal growth factor receptor (HER-2)(triple negative breast cancer, TNBC). Patients with estrogen receptor-positive (ER+) breast cancer showed no improvement in cancer-specific outcomes with β-blockade. These findings may reflect hormone receptor modulation of the effects of β-blockade on cancer progression.59 However, it is also possible that the relatively short length of follow-up (55 mo) was insufficient to see an effect of β-blockade on ER+ breast cancer which typically has a longer time to relapse than TNBC.60 Finally, a third recent study found that use of the non-selective β-blocker propranolol in the year before diagnosis was associated with lower tumor grade and stage at diagnosis.61 Propranolol usage was linked to reduced local or distant metastasis and reduced incidence of breast cancer specific mortality. In contrast, the β1-adrenergic receptor inhibitor atenolol was not associated with improved outcome, raising the possibility that blockade of β2-adrenergic receptors may be required for the protective effects of β-blockade on cancer progression. Extending these findings in breast cancer patients, a prospective longitudinal study of cardiovascular patients found that β-blocker usage reduced risk of various cancer types by 49%, and case-control studies found that β-blocker usage was associated with reduced risk of prostate cancer and reduced deaths from melanoma progression.62-64 Collectively, these studies suggest that β-blockers may provide protection against progression of multiple solid tumor types, although it is not clear if protective effects extend to all cancer types.65
Before β-blockers may be used in the cancer clinic, it will be important to identify the steps in the metastatic cascade that are most sensitive to β-adrenergic regulation and therefore sensitive to β-blocker intervention. Two studies of women with advanced metastatic breast cancer at study entry found no evidence that β-blockers improved outcome which suggests that the protective window for the effects of β-blockers occurs earlier in cancer progression, e.g., during development of the primary tumor or during early stages of tumor cell dissemination.66,67 Consistent with these clinical findings, studies using experimental models of breast cancer found much greater effects of stress on metastatic burden when examining metastatic escape from a primary tumor than in models focused on later stages of metastasis (e.g., metastatic colonization by tumor cells injected into the bloodstream).5,6,68 These findings suggest that events early in the metastatic cascade—including degradation of the basement membrane and tumor cell intravasation - may be particularly sensitive to β-adrenergic signaling. In addition, it remains important to determine the impact of local SNS innervation on colonization of metastatic targets.
To further characterize opportunities for therapeutic intervention, we investigated the impact of stress after surgical resection of the primary tumor to model situational stress associated with cancer diagnosis. In a mouse model of breast cancer metastasis, physiologic activation of stress response pathways only after surgical removal of the primary mammary tumor induced ~3-fold increase in metastasis. While even 3-fold increased metastasis may be of clinical importance, this effect was modest compared with the effect of stress preceding tumor onset which increased metastasis by ~30-fold. These findings suggest that chronic lifetime stress that might precede diagnosis—rather than the stress associated with a single event—may more significantly impact cancer progression. This may be due, in part, to the capacity of long-term activation of stress response pathways to maximally remodel the microenvironment of a developing tumor in a way that promotes metastatic dissemination.5
Duration of SNS exposure may affect its capacity to modulate tumor progression. Some evidence suggests that short-term or intermittent activation of the stress response may delay cancer progression, in contrast to the effects of sustained neural activation.69 However, even acute stress may have the capacity to promote key steps in the metastatic cascade. A single stressful episode (swim stress) was sufficient to increase lung retention of circulating tumor cells by 2-fold, an effect that was blocked by either β1- or β2-selective inhibitors.70 The effect was not seen when tumor cells were injected 24 h after stress, suggesting that effects of acute stress on the microenvironment are fleeting and chronic repeated stress is needed for lasting impact on the tumor microenvironment. These temporal insights from mechanistic studies may help guide future clinical trials of β-blockers by identifying a window of therapeutic opportunity for the anti-metastatic effects of β-blockade.
Discussion
Neural regulation of metastasis highlights the importance of examining tumor metastatic dynamics in their broader physiologic context. Neurotransmitters released from SNS nerve fibers during times of chronic stress help shape the tumor microenvironment in ways that promote metastatic escape and dissemination of tumor cells. Experimental studies using animal models show that stress-enhanced metastasis depends on signaling through β-adrenergic receptors, suggesting that β-blockade may be a novel adjuvant therapeutic strategy to block metastasis when used in conjunction with conventional cancer treatments. Targeting peripheral neural networks is a radical departure from current clinical paradigms for treatment of metastasis. This approach may be particularly valuable when tumors are refractory to existing targeted therapies, e.g., in the context of TNBC. Beta-blockers are widely available, inexpensive and their effects are well-characterized suggesting rapid translation to the cancer clinic may be possible once responsive patient populations are identified and mechanistic pathways are delineated.
To identify patients who will most benefit from β-adrenergic intervention, it will be important to identify tumor types that are responsive to β-adrenergic signaling. In the context of breast cancer, it is not clear if β-blockade may be equally protective against progression in all breast cancer subtypes.15,59 While one study found that β-blockers conferred protection only in women with TNBC,59 the generalizability of these findings is unclear as other studies have not separated patient populations by receptor status.58,61 In addition to those pharmacological studies, a meta-analysis of prospective studies that investigated the association of stressful life events with cancer progression identified other tumor types that may be sensitive to stress biology, and therefore potentially responsive to SNS interventions.71 In particular, progression of hematopoietic cancers and lung cancer showed sensitivity to stress biology across multiple studies, indicating that investigation of β-blockade as a novel therapeutic intervention to slow progression of these tumor types may be warranted.
In order to select the most appropriate β-blocker for randomized clinical trials in breast cancer patients, it will be important to define the β-adrenergic receptor subtype (or subtypes) that mediates effects on metastasis. Both β1 and β2 adrenergic receptors have been detected on tumor cells and on tumor-associated stromal cells including macrophages.5,14,15,42,72 However, findings that non-selective β-blockade but not β1-selective blockade conferred a survival advantage in breast cancer patients, suggested that signaling through β2-adrenergic (or non-β1 adrenergic) mechanisms are important in neural regulation of cancer.61 Other recent epidemiologic studies have not directly addressed this distinction.58,59 However, β-blockers that were selective for β2-but not β1-adrenergic receptors blocked stress-enhanced proliferation of metastatic tumor cells in an experimental model of ovarian cancer.6 With an increasing trend to prescribe β1-selective antagonists rather than non-selective β-blockers,73 it will be critically important to clarify the roles of specific β-adrenergic receptors leading into future intervention trials.
The experimental studies described here have begun to clarify cellular and molecular mechanisms that may underlie epidemiologic observations linking chronic stress to breast cancer progression in humans.7,71,74 By activating peripheral neural pathways, chronic stress may re-model the tumor microenvironment in a manner that favors tumor cell dissemination and colonization of distant sites. The effect of these changes on cancer initiation is unclear as few consistent relationships have been found between stress and incidence of breast cancer.75-78 However, several epidemiological studies and a large meta-analysis of prospective clinical studies have linked experience of significant negative life events with increased progression of established breast cancers.71,74,79-82 In contrast, positive social interactions including high levels of social support have been linked to improved cancer-specific outcomes.74 These findings suggest that β-blockers may be a novel strategy to protect breast cancer patients from the adverse effects of stress biology on tumor progression and metastasis.
Conclusion
Neural regulation of metastasis suggests that cancer progression is subject to regulation by the broader tumor macroenvironment of the whole patient. The nervous system regulates multiple physiologic pathways that converge through β-adrenergic signaling pathways to shape the tumor microenvironment. These processes coordinately influence the rate of tumor cell dissemination from the primary tumor and colonization of distant tissues. Systemic physiologic re-shaping of the tumor microenvironment raises the possibility that systemic interventions may provide new adjunctive approaches to complement existing anti-cancer therapeutics. These studies highlight the importance of the patients overall physiology in development of new therapeutic strategies to slow or reverse cancer metastasis.
Acknowledgments
Congressionally Directed Medical Research Programs (CDMRP) Breast Cancer Research Program grant W81XWH-081-0629, NIH grants CA138687, CA116778, National Health and Medical Research Council (Australia) grant 1008865, National Breast Cancer Foundation (Australia) grant ECR-11-11 and the UCLA Cousins Center for PNI.
References
- 1.
- Welch DR, Steeg PS, Rinker-Schaeffer CW. Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res. 2000;2:408–16. http://dx.doi. org/10.1186/bcr87 . [PMC free article: PMC138663] [PubMed: 11250734]
- 2.
- Steeg PS, Bevilacqua G, Sobel ME, Liotta LA. Identification and characterization of differentially expressed genes in tumor metastasis: the nm23 gene. Basic Life Sci. 1991;57:355–60. discussion 360-1. [PubMed: 1667573]
- 3.
- Cichon MA, Degnim AC, Visscher DW, Radisky DC. Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:389–97. http://dx.doi.org/10.1007/s10911-010-9195-8 . [PMC free article: PMC3011086] [PubMed: 21161341]
- 4.
- Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2010;37(Suppl 2):S2–14. http://dx.doi.org/10.1053/j.seminoncol.2010.10.007 . [PubMed: 21111245]
- 5.
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52. http://dx.doi.org/10.1158/0008-5472.CAN-10-0522 . [PMC free article: PMC2940980] [PubMed: 20823155]
- 6.
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. http://dx.doi.org/10.1038/nm1447 . [PubMed: 16862152]
- 7.
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. http://dx.doi.org/10.1038/nrc1820 . [PMC free article: PMC3146042] [PubMed: 16498446]
- 8.
- Palm D, Lang K, Niggemann B, Drell TL 4th, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–9. http://dx.doi.org/10.1002/ijc.21723 . [PubMed: 16381019]
- 9.
- Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–26. http://dx.doi.org/10.1016/j.bbi.2004.07.004 . [PubMed: 15664784]
- 10.
- Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, et al. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987;100:225–60. http://dx.doi.org/10.1111/j.1600-065X.1987.tb00534.x . [PubMed: 3326822]
- 11.
- Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–48. http://dx.doi.org/10.1146/ annurev.pa.35.040195.002221 . [PubMed: 7598501]
- 12.
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–8. [PubMed: 9531281]
- 13.
- Levi B, Benish M, Goldfarb Y, Sorski L, Melamed R, Rosenne E, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behav Immun. 2011;25:727–35. http://dx.doi.org/10.1016/j. bbi.2011.01.014 . [PMC free article: PMC3081380] [PubMed: 21277367]
- 14.
- Powe DG, Voss MJ, Habashy HO, Zänker KS, Green AR, Ellis IO, et al. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat. 2011;130:457–63. http://dx.doi.org/10.1007/ s10549-011-1371-z . [PubMed: 21298476]
- 15.
- Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, et al. The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351–62. http://dx.doi.org/10.1007/s10549-010-0822-2 . [PubMed: 20237834]
- 16.
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. http://dx.doi.org/10.1038/nrc1256 . [PubMed: 14708027]
- 17.
- DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. http://dx.doi.org/10.1186/bcr1746 . [PMC free article: PMC2206719] [PubMed: 17705880]
- 18.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. http://dx.doi.org/10.1038/nature07205 . [PubMed: 18650914]
- 19.
- Valledor AF, Borràs FE, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/ macrophage differentiation. J Leukoc Biol. 1998;63:405–17. [PubMed: 9544570]
- 20.
- Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. http://dx.doi.org/10.1016/j. jneuroim.2007.02.007 . [PMC free article: PMC2020805] [PubMed: 17428548]
- 21.
- Lin EY, Nguyen AV, Russell RG, Pollard JW, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. http://dx.doi.org/10.1084/ jem.193.6.727 . [PMC free article: PMC2193412] [PubMed: 11257139]
- 22.
- Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995;27:7985. http://dx.doi.org/10.3109/07853899509031941 . [PubMed: 7742005]
- 23.
- Beck AH, Espinosa I, Edris B, Li R, Montgomery K, Zhu S, et al. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res. 2009;15:778–87. http://dx.doi. org/10.1158/1078-0432.CCR-08-1283 . [PMC free article: PMC2987696] [PubMed: 19188147]
- 24.
- Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:683946. http://dx.doi.org/10.1158/1078-0432.CCR-08-0230 . [PMC free article: PMC2716059] [PubMed: 18980978]
- 25.
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT, et al. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci USA. 2011;108:3080–5. http://dx.doi.org/10.1073/pnas.1014218108 . [PMC free article: PMC3041107] [PubMed: 21300872]
- 26.
- Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–7. http://dx.doi.org/10.1093/jnci/dji401 . [PMC free article: PMC3422720] [PubMed: 16333031]
- 27.
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–8. http://dx.doi.org/10.1002/(SICI)1097-0215(19990315)80:6<880::AID-IJC14>3.0.CO;2-Y. [PubMed: 10074922]
- 28.
- Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS ONE. 2011;6:e19246. http://dx.doi.org/10.1371/journal.pone.0019246 . [PMC free article: PMC3084788] [PubMed: 21559428]
- 29.
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed: 8840975]
- 30.
- Yang EV, Donovan EL, Benson DM, Glaser R, et al. VEGF is differentially regulated in multiple myeloma-derived cell lines by norepinephrine. Brain Behav Immun. 2008;22:318–23. http://dx.doi. org/10.1016/j.bbi.2007.09.010 . [PMC free article: PMC2259392] [PubMed: 17981009]
- 31.
- Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–70. http://dx.doi.org/10.1074/jbc.M110.109579 . [PMC free article: PMC2975170] [PubMed: 20826776]
- 32.
- Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–21. [PubMed: 14555525]
- 33.
- Bernabé DG, Tamae AC, Biasoli R, Oliveira SH. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun. 2011;25:574–83. http://dx.doi.org/10.1016/j.bbi.2010.12.012 . [PubMed: 21187140]
- 34.
- Schuller HM, Tithof PK, Williams M, Plummer H 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510–5. [PubMed: 10493497]
- 35.
- Tsuji M, Kuno T, Tanaka C, Ichihashi M, Mishima Y. Beta-adrenergic receptors of B16 melanoma cell. Arch Dermatol Res. 1983;275:415–6. http://dx.doi.org/10.1007/BF00417345 . [PubMed: 6318672]
- 36.
- Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9. [PubMed: 11306460]
- 37.
- Lang K, Drell TL 4th, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer. 2004;112:231–8. http://dx.doi.org/10.1002/ijc.20410 . [PubMed: 15352035]
- 38.
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. http://dx.doi.org/10.1158/10780432.CCR-05-1698 . [PMC free article: PMC3141061] [PubMed: 16428474]
- 39.
- Drell TL 4th, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63–70. http://dx.doi.org/10.1023/A:1024491219366 . [PubMed: 12889599]
- 40.
- Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–26. http://dx.doi.org/10.1074/jbc.M611539200 . [PubMed: 17716980]
- 41.
- Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–75. http://dx.doi. org/10.1016/j.bbi.2008.10.005 . [PMC free article: PMC2652747] [PubMed: 18996182]
- 42.
- Madden KS, Szpunar MJ, Brown EB. beta-Adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130:747–58. http://dx.doi.org/10.1007/s10549-011-1348-y . [PMC free article: PMC3126869] [PubMed: 21234673]
- 43.
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–13. http://dx.doi. org/10.1002/cncr.21147 . [PubMed: 15954082]
- 44.
- Guérineau NC, Desarmenien MG. Developmental and stress-induced remodeling of cell-cell communication in the adrenal medullary tissue. Cell Mol Neurobiol. 2010;30:1425–31. http://dx.doi. org/10.1007/s10571-010-9583-z . [PMC free article: PMC3627125] [PubMed: 21061165]
- 45.
- Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. http://dx.doi.org/10.1146/annurev.ne.15.030192.000511 . [PubMed: 1575451]
- 46.
- Landen CN Jr, Lin YG, Armaiz Pena GN, Das PD, Arevalo JM, Kamat AA, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 2007;67:10389–96. http://dx.doi.org/10.1158/0008-5472.CAN-07-0858 . [PubMed: 17974982]
- 47.
- Felten DL, Livnat S, Felten SY, Carlson SL, Bellinger DL, Yeh P. Sympathetic innervation of lymph nodes in mice. Brain Res Bull. 1984;13:693–9. http://dx.doi.org/10.1016/0361-9230(84)90230-2 . [PubMed: 6532515]
- 48.
- Taylor EW, Jordan D, Coote JH. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev. 1999;79:855–916. [PubMed: 10390519]
- 49.
- Tang Y, Shankar R, Gamelli R, Jones S. Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. J Neuroimmunol. 1999;96:182–9. http://dx.doi.org/10.1016/S01655728(99)00032-6 . [PubMed: 10337916]
- 50.
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–65. http://dx.doi.org/10.1523/JNEUROSCI.1247-07.2007 . [PMC free article: PMC6672171] [PubMed: 17699667]
- 51.
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. http://dx.doi.org/10.1126/science.3306916 . [PubMed: 3306916]
- 52.
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci USA. 2010;107:5681–6. [PMC free article: PMC2851818] [PubMed: 20176930]
- 53.
- Shimizu N, Hori T, Nakane H. An interleukin-1 beta-induced noradrenaline release in the spleen is mediated by brain corticotropin-releasing factor: an in vivo microdialysis study in conscious rats. Brain Behav Immun. 1994;8:14–23. http://dx.doi.org/10.1006/brbi.1994.1002 . [PubMed: 8003768]
- 54.
- Lutgendorf SK, DeGeest K, Sung CY, Arevalo JM, Penedo F, Lucci J 3rd, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–83. http://dx.doi.org/10.1016/j.bbi.2008.04.155 . [PMC free article: PMC2677379] [PubMed: 18550328]
- 55.
- Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–5. http://dx.doi.org/10.1016/j.bbi.2010.10.012 . [PMC free article: PMC3103818] [PubMed: 20955777]
- 56.
- Lara HE, Dorfman M, Venegas M, Luza SM, Luna SL, Mayerhofer A, et al. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: Studies on norepinephrine release. Microsc Res Tech. 2002;59:495–502. http://dx.doi.org/10.1002/ jemt.10229 . [PubMed: 12467025]
- 57.
- Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. http://dx.doi.org/10.1016/j.cell.2010.05.029 . [PMC free article: PMC3784009] [PubMed: 20603014]
- 58.
- Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–38. [PMC free article: PMC3248123] [PubMed: 21317458]
- 59.
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–52. http://dx.doi.org/10.1200/JCO.2010.33.4441 . [PMC free article: PMC3139371] [PubMed: 21632501]
- 60.
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. [PubMed: 17671126]
- 61.
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–44. http://dx.doi.org/10.1200/JCO.2010.33.5422 . [PubMed: 21632503]
- 62.
- Algazi M, Plu-Bureau G, Flahault A, Dondon MG, L MG. Could treatments with beta-blockers be associated with a reduction in cancer risk? Rev Epidemiol Sante Publique. 2004;52:53–65. http://dx.doi. org/10.1016/S0398-7620(04)99022-0 . [PubMed: 15107693]
- 63.
- Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control. 2004;15:535–41. http://dx.doi.org/10.1023/B:CACO.0000036152.58271.5e . [PubMed: 15280632]
- 64.
- De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, et al. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med. 2011;171:779–81. http://dx.doi.org/10.1001/archinternmed.2011.131 . [PubMed: 21518948]
- 65.
- Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–61. http://dx.doi.org/10.1111/j.1365-2125.2011.03980.x . [PMC free article: PMC3141198] [PubMed: 21453301]
- 66.
- Li CI, Malone KE, Weiss NS, Boudreau DM, Cushing-Haugen KL, Daling JR. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65-79 years. Cancer. 2003;98:1504–13. http://dx.doi.org/10.1002/cncr.11663 . [PubMed: 14508839]
- 67.
- Meier CR, Derby LE, Jick SS, Jick H. Angiotensin-converting enzyme inhibitors, calcium channel blockers, and breast cancer. Arch Intern Med. 2000;160:349–53. http://dx.doi.org/10.1001/ archinte.160.3.349 . [PubMed: 10668837]
- 68.
- Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain Behav Immun. 1991;5:193–205. http://dx.doi.org/10.1016/0889-1591(91)90016-4 . [PubMed: 1654166]
- 69.
- Dhabhar FS, Saul AN, Daugherty C, Holmes TH, Bouley DM, Oberyszyn TM. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 2010;24:127–37. http://dx.doi.org/10.1016/j.bbi.2009.09.004 . [PMC free article: PMC2788066] [PubMed: 19765644]
- 70.
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154–64. http://dx.doi.org/10.1159/000054276 . [PubMed: 11124582]
- 71.
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. http://dx.doi.org/10.1038/ncponc1134 . [PubMed: 18493231]
- 72.
- Simantov R, Sachs L. Differential desensitization of functional adrenergic receptors in normal and malignant myeloid cells: relationship to receptor-mediated hormone cytotoxicity. Proc Natl Acad Sci USA. 1978;75:1805–9. http://dx.doi.org/10.1073/pnas.75.4.1805 . [PMC free article: PMC392429] [PubMed: 25440]
- 73.
- Shah SM, Carey IM, DeWilde S, Richards N, Cook DG. Trends and inequities in beta-blocker prescribing for heart failure. Br J Gen Pract. 2008;58:862–9. http://dx.doi.org/10.3399/bjgp08X376195 . [PMC free article: PMC2593536] [PubMed: 19068160]
- 74.
- Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–11. http://dx.doi. org/10.1200/JCO.2005.04.2846 . [PubMed: 16505430]
- 75.
- Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415–23. http://dx.doi. org/10.1093/aje/kwg002 . [PubMed: 12615606]
- 76.
- Li J, Johansen C, Hansen D, Olsen J. Cancer incidence in parents who lost a child: a nationwide study in Denmark. Cancer. 2002;95:2237–42. http://dx.doi.org/10.1002/cncr.10943 . [PubMed: 12412179]
- 77.
- Levav I, Kohn R, Iscovich J, Abramson JH, Tsai WY, Vigdorovich D. Cancer incidence and survival following bereavement. Am J Public Health. 2000;90:1601–7. http://dx.doi.org/10.2105/ AJPH.90.10.1601 . [PMC free article: PMC1446385] [PubMed: 11029995]
- 78.
- Kvikstad A, Vatten LJ, Tretli S, Kvinnsland S. Widowhood and divorce related to cancer risk in middle-aged women. A nested case-control study among Norwegian women born between 1935 and 1954. Int J Cancer. 1994;58:512–6. http://dx.doi.org/10.1002/ijc.2910580410 . [PubMed: 8056447]
- 79.
- Ell K, Nishimoto R, Mediansky L, Mantell J, Hamovitch M. Social relations, social support and survival among patients with cancer. J Psychosom Res. 1992;36:531–41. http://dx.doi.org/10.1016/00223999(92)90038-4 . [PubMed: 1640391]
- 80.
- Soler-Vila H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African-American and white breast cancer patients: a population-based study. Cancer. 2003;98:1299–308. http://dx.doi. org/10.1002/cncr.11670 . [PubMed: 12973855]
- 81.
- Barraclough J, Pinder P, Cruddas M, Osmond C, Taylor I, Perry M. Life events and breast cancer prognosis. BMJ. 1992;304:1078–81. http://dx.doi.org/10.1136/bmj.304.6834.1078 . [PMC free article: PMC1881923] [PubMed: 1586819]
- 82.
- Graham J, Ramirez A, Love S, Richards M, Burgess C. Stressful life experiences and risk of relapse of breast cancer: observational cohort study. BMJ. 2002;324:1420. http://dx.doi.org/10.1136/ bmj.324.7351.1420 . [PMC free article: PMC115851] [PubMed: 12065263]
- Sympathetic Nervous System Regulation of Metastasis - Madame Curie Bioscience Da...Sympathetic Nervous System Regulation of Metastasis - Madame Curie Bioscience Database
- VEGF and Endothelial Guidance in Angiogenic Sprouting - Madame Curie Bioscience ...VEGF and Endothelial Guidance in Angiogenic Sprouting - Madame Curie Bioscience Database
- Physical Background and Technical Realizations of Hyperthermia - Madame Curie Bi...Physical Background and Technical Realizations of Hyperthermia - Madame Curie Bioscience Database
- The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway - Madame C...The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway - Madame Curie Bioscience Database
- RNA NETWORKS IN PROKARYOTES II: tRNA Processing and Small RNAs - Madame Curie Bi...RNA NETWORKS IN PROKARYOTES II: tRNA Processing and Small RNAs - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...