NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The concept of organotropism is by no means novel; for over a century it has been observed that certain primary tumor types have a tendency to metastasize to specific secondary sites. Yet it is only since the dawn of the 21st century that genomics has emerged as a valuable tool for probing the mysteries behind what drives organotropism. Metastasis is a complicated process involving many steps: intravasation, survival in circulation, extravasation, and colonization and growth at a distant site. The multi-step nature of metastasis and the inherent complications associated with cancer cells make finding and studying the genes associated with organotropism difficult. However, in recent years, great strides have been made in the field of organotropism and metastasis. Here we examine some specific examples of genes associated with organotropism, along with the complicated processes involved with the regulation of metastatic tumor growth.
Introduction
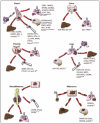
The hypothesis that metastasis is organotropic has influenced medical research since the publication of Dr. Pagets "Seed and Soil" paper.1 Throughout the first half of the 20th century, researchers attempted to find a rationale for the seemingly random process of metastasis. Dr. Pagets idea was simple: if a primary tumor sends out "seeds" (i.e., cells) throughout the body, they can only grow in good "soil" (i.e., a new organ site). Indeed, as techniques for locating metastatic tumors were refined, it became clearer that there is a distinct patterns of metastasis to distant organs: breast cancer tends to metastasise to the bone, liver, lymph nodes, and lung, and later to the brain; gastrointestinal, ovarian, and colorectal cancers to the liver and lung; prostate cancer to the bones, liver, and lung; lung cancer to the liver, brain, bone, and lymph nodes; neuroblastoma to the lung, liver, bone, and skin; and melanoma cells to lung, liver, brain and bone2-6 (Fig. 1). Although correlations could be found between primary tumors and their preferred metastatic destination, there was no clear rationale for the organ preference.
Logic would dictate that primary tumors in certain organs would spread spatially through the body, affecting the nearest neighboring organs.7 In the early 1950s, it was shown that intravascular injection of tumor cells into rabbits led to visible metastases in many different visceral organs, e.g., the adrenal glands, diaphragm, iris, kidneys, masseter muscle, pituitary gland, spleen, testis, and thyroid.8 In the organs where metastases were found, tumor cells were lodged in the capillaries. These results illustrate the importance of the circulatory system and mechanical factors in the delivery of metastatic cancer "seeds" to new locations. Indeed, others have shown by videomicroscopy that circulating tumors cells get lodged in capillaries and begin to grow in the organs where they arrest.9,10 Additionally, autopsy studies provide evidence that many metastases are in secondary sites that would be predicted based on blood circulation pattern, e.g, liver metastases in colon cancer patients. At the same time, however, there is an absence of metastases in organs where metastases would be expected based on circulation patterns, e.g., no metastases to the thyroid in colon cancer patients.11,12 Established correlative studies have proved that metastases have a tendency to consistently form in known secondary locations, distant from the primary tumor.13
Technology has now advanced to allow for the examination of gene regulation in regards to organotropism. One of the earliest tools applied to genetic studies was the bacterialLacZ gene, which allowed researchers to stain and quantify micrometastases.14 More recently, the use of firefly andrenillaluciferase in mouse model systems has allowed for a more refined picture of metastatic progression. Studies show that human tumor cells injected via different routes into immunocompromised mice lead to high metastases burdens in different locations.4 The tumor cells can be tracked over a period of time using bioluminescent imaging to see what organ the cells preferentially colonize. For example, immediately after injection of breast cancer cells into the left ventricle of the mouse heart, the cells are spread across the circulatory system, but 412 weeks later the cells have high rate of colonization almost exclusively in the brain and bone.15 One would expect that since the cells make contact with many different organs via the circulatory system there would be metastases all over the body; yet instead there are an increased number of metastases in the specific organs seen in human patients, e.g., bone and brain. Circulation has an obvious role in metastasis, but it is not the key regulator of organtropism in cancer metastasis. A more thorough review of the concept of seed and soil is covered in the chapter entitled "Metastatic cascade: seed and soil revisited."
Understanding the mechanism of metastasis organotropism is of interest to cancer biologists and oncologists since oftentimes the metastasis proves fatal, rather than the primary malignancy. Metastasis is a complicated process, and most likely there is not a single gene in any type of cancer cell that determines the destination of its metastases. Over the past decade, more refined tools such as qPCR, fluorescent and bioluminescent imaging, and microarray analysis have allowed a more complete understanding of both the genetic profile and the phenotypic expression of specific cancer cell types. Gene signatures have been identified which indicate which genes play a role in the metastasis of the primary tumor to a specific organ.16-19 Understanding the link between the regulation of these genes and the destination of certain metastases will impact the treatment of patients and the prevention of cancer fatalities. Presently, breast cancer is the most well characterized in terms of its genetic profile in relation to organotropism, and therefore, much of this review will discuss genes that mediate organotropism of breast cancer. A few examples of other cancers will also be examined. This review is by no means exhaustive; many genes in a variety of cancers have been implicated in the tropism of metastases, and it is not feasible to address all of them.
Genes That Mediate Breast Cancer Organotropism
Metastasis occurring from primary breast tumors has been studied exhaustively for many years. Breast cancer is known to most often metastasize initially to the lymph nodes, bone, and lungs, and later to the brain and liver; how and why these organs are preferred is not fully elucidated.4,20 Studies regarding the genetic forces driving organotropism in breast cancer have advanced dramatically in the past decade as a result of more refined molecular probing. As these phenomena become better understood, they open up possibilities for new biomarkers, drug targets, or other molecular therapies to prevent potentially fatal metastases.
Early studies in organotropism sought to establish a correlation between key prognostic markers in the primary tumor and the site of relapse. Breast cancer has long been classified based on the positive or negative presence of the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2). The tropism of breast cancer to the bone and brain was first linked with the presence or absence of ER/PR in the primary tumor, respectively21; however, the presence or absence of ER, PR, or HER2 alone could not account for the phenotypic diversity seen in breast cancer. Five molecular subtypes have now been mapped to specific gene expression patterns describing this phenotypic diversity and predicting patient outcome.22-24 Follow up studies utilizing immunohistochemical (IHC) staining of key markers from each subtype found that frequencies of the site of relapse of breast cancer patients are related to the molecular subtype of the patients tumor, with brain relapses in patients with basal tumors and HER2+ tumors, bone relapses in patients with luminal type tumors, and lung relapses associated with patients with basal tumors.25 Though there are some clinicopathologic analyses that suggest these subtypes have little correlation with any specific tropisms,26 many other studies utilizing both IHC staining and gene array analysis suggest the contrary.27,28 Harrell et al.27 recently analyzed 1,319 genes in an expression microarray from patients primary tumors, metastases, and cancer cell lines. They found that patients with breast tumors that were ER/PR negative subtypes generally presented with higher metastases, except those found in the bones, which had both ER+ and ER- tumors; HER2+ tumors were associated with tropism to the liver; and the basal-like and claudin-low subtypes were associated with brain and lung tropisms.27 These findings were similar to those of earlier studies.25,28 Further analysis showed that the brain-seeking metastases had basal-like, low claudin markers, with a gene signature similar to that of lung metastases and the primary tumors from which they originated. Overall there were very similar gene signatures shared between the primary tumor and metastatic tumor. These results suggest that the primary tumor molecular subtype can predict the potential metastatic site. Analysis with multiple organotropic-metastatic gene signatures (described below) further predict the site of relapse and indicate the importance of identifying genes and gene signatures that mediate organotropism.
Bone Tropism
Many genes have been associated with metastasis. There are a wide range of genetic profiles associated with certain cancers; determining the particular genes that regulate the metastatic destination of primary tumors is a daunting task. Once it was clear that cancer metastasis did have organ preferences, methods were developed to find out what drove specific metastatic events. As early as 1973, Fidler29 showed that by successively extracting and re-suspending melanoma cells from pulmonary metastases and re-injecting them into mice, the metastatic tendency (based on number of tumors present after sacrifice) increased with each subsequent selection and injection. Though these early experiments indicated that organotropic cells existed within the heterogeneous parental cell population, there was no progress made toward understanding the driving force of organotropism for some time. This method for creating cells lines with strong metastatic proclivity to certain organs has changed little over time. Today, in vivo selection of many cancer cell lines has allowed researchers to examine their role in discrete metastatic events, e.g., MDA-231 breast cancer cells with very specific organotropisms to brain, lung, or bone15-18 or metastatic colon carcinomas that metastasize to the liver.30
Since many genes regulate the overall process of metastasis, the ability to develop these specific-destination cell lines is valuable in creating the genetic profiles associated with certain kinds of organotropism. Gene array analysis has been an indispensable tool for creating these profiles. Researchers have compared the gene profile of parental tumor cell lines, which metastasize to many organs, to the gene signatures associated with organ-specific metastatic cell lines developed by the above methods. One of the first tropisms studied in breast cancer was bone. Kang, et al. examined MDA-231 breast cancer cell lines that preferentially metastasized to the bones, comparing them against both the parental MDA-231 cell line and the normal breast epithelial line MCF10A as controls.17 The deferential genes they found were then compared with 70 genes associated with poor outcome prognosis in human patients.31 They found a bone metastatic gene signature (BoMS) of up- or down- regulated genes. These genes often had a role in the cell membrane or encoded secretory proteins, e.g., chemokine receptor CXCR4; growth factors FGF5 and CTGF; interleukin 11 (IL-11), a known regulator in the recruitment of osteoclasts; osteopontin (OPN), and the collagenase matrix metalloprotease-1 (MMP1), a promoter of osteolysis.17 These results suggest that the metastatic cells are interacting with and influencing their microenvironment.
Once a genetic profile for bone-destined metastatic cancer cells was determined, the role specific genes played in mediating the bone tropism was examined. For years researchers have been studying the growth of cancer cells in the bone.32 Cancer growth in the bone can cause osteoblastic or osteolytic lesions. Breast cancer patients can present with either one or both types of lesions; however, the most common type is osteolytic.32 In the bones, there is a tightly regulated homeostasis maintained between the production of osteoblasts and osteoclasts, two distinct bone marrow cell types that produce or readsorb the bone scaffolding, respectively.33 The chemokine IL-11 is a known upregulator of the formation of osteoclasts, which enhance bone reabsorption and promote apoptosis of bone marrow cells promoting osteolytic lesions.34 However, the overexpression of IL-11 alone is not enough to increase metastases to the bone; only when coupled with overexpression of OPN do the tumor cells display an increased bone tropism.17 OPN is a secretory protein that plays a complex role in stimulating new bone formation, and is found to be overexpressed in multiple kinds of highly metastatic cancers.35 OPN acts as an anchor for the osteoclasts, and when expression of OPN is upregulated, there is more recruitment of osteoclasts to the affected site. The osteoclasts can be activated by the tumor cells to degrade the bone and release more tumorgenic factors in the metastatic site.36 Furthermore, the recruitment of osteoclasts results in the increased expression of genes known to play a role in metastasis, such as transforming growth factor-b (TGFb), bone morphogenic proteins (BMPs), and platelet-derived growth factor (PDGF), which in turn induce more bone-specific metastatic genes, such as IL-11, MMP1, and CXCR4.37 The breast cancer cells also secrete parathyroid hormone-related protein (PTHrP), which stimulates osteoblasts to secrete receptor activator of nuclear factor kappa-B ligand (RANKL), which in turn can determine the activity of osteoclasts. Breast, as well as lung and prostate cancer cells, express RANK, the receptor for RANKL, suggesting that RANKL may be an attractive factor for breast, lung, and prostate cancer tropism to bone.38 This feedback loop constitutes the "vicious cycle" associated with bone metastasis and suggests that the interaction between the tumor cell and its microenvironment in the metastatic site plays an important role in organotropsim.
Some factors important in this "vicious cycle" are genes expressed in the normal breast during development or lactation. For example, during lactation, mammary epithelial cells release PTHrP to increase the amount of calcium in milk via induction of osteoclast bone resorption and calcium release.39 Another important gene, Runx2, plays a role in cellular events during lactation, but is overexpressed in breast-to-bone metastases, where it activates MMP9, OPN, BSP and RANKL expression, genes all associated with bone metastasis. Breast cancer cells transfected with dominant negative Runx2 have a significantly reduced incidence of bone metastases.40 These examples illustrate that the intrinsic properties of healthy breast cells may influence their preferred tropism to bone upon their mutation to the cancerous phenotype. Only a few genes that play a role in the vicious cycle have been described here; a more complete examination of bone-tropic breast cancer metastasis is presented in the chapter entitled "Breast cancer metastasis to bone."
Additional genes found in the BoMS were evaluated for their role in bone-specific metastasis. CXCR4 was found to be upregulated in breast cancer cells compared with normal breast epithelia using quantitative real-time PCR methods.41 This chemokine receptor is found on breast cancer cells and its ligand, CXCL12, is found in lymph nodes, lung, and bone marrow, suggesting that CXCL12 is an attractive factor driving the tropism of the breast cancer cells to these organs.41 Kang, et al. found that CXCR4 overexpression alone is enough to cause a small increase in bone metastases, however, this increased metastasis to bone is much more pronounced in concert with IL-11 and OPN overexpression.17 On the other hand, CTGF, a similar chemokine, does not increase bone metastases when overexpressed alone, though it does when combined with IL-11 and OPN overexpression. These results illustrate the coordinated efforts of multiple genes in driving organotropic metastasis, and the function of the microenvironment as part of metastatic growth. Interestingly, Harrell, et al. did not find the BoMS to be strongly associated in any breast cancer subtype,27 suggesting that this gene signature associated with osteoclast recruitment may be more important than the subtype in predicting the ability of cancer cells to metastasize to the bone.
Lung Tropism
As pathways mediating bone-specific metastasis were elucidated, research was also being conducted on lung-destined metastases. MDA-231 cells were used again to develop lung-specific metastatic cancer cell lines, including lines that were either weakly or strongly lung tropic. Microarray analysis was performed to compare these lung tropic lines to the parental lines, and the results generated "lung metastasis signature" (LMS) genes. Comparing the difference between weakly lung-seeking metastases and strong lung-preferring metastases, a slight difference in the profile was evident; this perhaps indicates a baseline "lung metastagenicity" in the weakly metastatic line, while the strong metastatic line had a handful of exclusive genes classified as "lung-metastatic virulent"18. Overexpression of any single gene did not markedly increase lung metastasis, again illustrating that a multi-gene coordinated effort is needed to regulate lung-specific tropism. The list of up- or down- regulated genes discovered was extensive.18 Many of these genes are secreted or have a receptor function, e.g., MMP2, a secreted protease; osteonectin/SPARC, a cell adhesion molecule; IL13Ra2, a decoy receptor for the chemokine IL-13; and vascular cell adhesion molecule-1 (VCAM1), a cell adhesion receptor.18 One of these genes, tenascin C (TNC), an ECM protein, secreted from the LM cells has been found to be vital for tumor survival and outgrowth in the lung.42 TNC was found to be suppressed by miR-335, and loss of miR-335 is found in breast cancer patients who relapse.43 As with the BoMS, these genes found in the LMS may be involved in the interaction of breast cancer cells with the microenvironment at the metastatic site.
Additional genes were upregulated that were not lung-specific, indicating that promoters of organ-specific metastasis are mediated by more general oncogenes in concert with their specific genes. When primary breast tumors of patients were examined in a clinical setting, the markers that were highest in the most aggressive tumors were for more general metastatic genes: MMP1, chemokine CXCL1, prostagladin-endoperoxide synthase (PTGS2), inhibitor of cell differentiation and senescence (ID1), and epiregulin (EREG), an epidermal-growth factor family member.18 Some of the LMS genes (ID1, CXCL1, PTGS2/COX2, TNC and MMP1) promoted growth in the mammary gland and lung, while others (SPARC, VCAM, and IL13Ra2) only promoted growth in the lung.18,44 These results suggest that some lung metastatic (LM) genes promote tumor growth and lung tropism while others only give growth advantage in the lung microenvironment.18 Interestingly, the isolated LM cell lines appeared to have an accelerated growth rate in the mammary fat pad compared with their parental cell type. These LM subtype tumors grew larger more quickly than non-LM tumors; their quick expansion led them to overtake the circulatory system more swiftly, resulting in a population of highly invasive cells that move through the bloodstream and to the lung aveoli.45 The LMS genes COX2, EREG, and MMP1 facilitate angiogenesis, intravasation, and extravasation of the breast cancer cells to the lung,46 suggesting that large tumors with a higher number of cells expressing these genes will have greater ability to move out of the primary tumor and into the lung. Patients with LMS+ tumors and large tumor size were more likely to have lung metastases than bone or other visceral metastases. When determining if a patient is likely to have lung metastases, the predictive accuracy increases when researchers examine the LMS+ and tumor size in concert.45 Furthermore, the LMS genes identified in these studies are associated with the primary tumor intrinsic subtype (most commonly basal-like and claudin-low).27 Thus, the LMS when combined with other prognostic markers (tumor size and intrinsic subtype) help to further predict the tropism of the primary tumor.
Landemaine, et al. identified a six gene signature (6GS) predicting breast cancer metastasis to lung (DSC2, TFCP2L1, UGT8, ITGB8, ANP32E, and FERMT1).47,48 These genes were all associated with the basal-like molecular subtype, but were still an independent predictor of lung-tropism.48,49 Desmosomal protein (DSC2), focal adhesion molecule (FERMT1), and integrin b8 (ITGB8) are all cell adhesion molecules,47 suggesting changes in cancer cell adhesion properties play a key role in organotropism. Other adhesion molecules have been shown to play a role in breast cancer tropism to the lung. Metadherin was identified, through a phage expression library, as a protein on tumor cells that mediates lung tropism to a yet to be identified receptor expressed only in the lung endothelium.50 In addition, expression of integrin b4 in breast cancer cells mediates cancer cell adhesion to the lung endothelium through hCLCA2, a Ca2+ sensitive chloride channel protein, suggesting that this interaction can mediate the arrival of tumor cells to the lung.51 Other investigators have found receptors on breast cancer cells that bind to factors in the lung, indicating that there is an attractive force driving the breast cancer cells to metastasize to the lung. As mentioned above, the chemokine receptors CXCR4 and CCR7 are expressed on breast cancer cells and their respective ligands CXCL12 and CCL21 are expressed in the lung.41
These chemokines are also found in the bone and lymph nodes, and thus, these receptors are not exclusively lung-tropic genes, suggesting they confer a preference to multiple metastatic sites. Additionally, integrin β4 is expressed in many benign breast tumors, indicating that interactions between integrin β4 and hCLCA2 are not sufficient for metastasis of the cells.51 These results suggest, as found in other studies, that a coordinate expression of genes is necessary for lung-tropism.
Recent studies have examined the role of cell fusion in metastasis. Once the genetic profiles associated with breast cancer metastasis to certain locations were established, researchers were interested in how these profiles interacted with each other in the heterogeneous primary tumor environment. Though cellular fusion occurs infrequently in normal cells, cancer cells can undergo this process at a relatively high rate of about 1%.52 Using MDA-231 breast cancer cell lines that preferred either the lung (LM2) or bone (SCP2) metastatic sites, it was established that the cells underwent fusion to become bi-nucleated cells with 4n chromosomes. These synkaryonic cells, when selected into a single population, had a tendency to metastasize to both destinations and retained the genetic profile associated with the original singly organotopic sublines. Furthermore, they were chromosomally and phenotypically stable. These findings demonstrate that cell fusion may have a greater impact on tropism and metastatic aggressiveness than previously realized; for example, if a virulent lung metastatic line fuses with a weakly metastatic bone line, the result could be high rates of metastasis to both the lung and the bone, which would affect treatments for the patient.
Brain Tropism
In more recent years, studies have been performed to examine the processes involved in breast-to-brain metastases. Though brain metastases are not as prevalent as lung or bone metastases, occurring in approximately 20% of metastatic breast cancer patients, they are almost always debilitating due to the grave neurological impact even a small metastasis can have. In addition, brain metastases are difficult to treat due to the inability for many cancer drugs to cross the blood-brain barrier (BBB). The BBB not only serves as a hurdle for drug delivery, but also as an impediment to disseminated cancer cells. The BBB has a unique vascular structure comprised of brain endothelial cells that form tight junctions and are surrounded by a basement membrane, pericytes, and astrocytes.53 The brain astrocytes in particular can secrete many biological factors, such as certain cytokines or interleukin factors (IL)-1, -3, and -6 that may play a role in the metastasis of cancer to the brain. Indeed, Sierra et al.54 found that astrocytes secrete IL-6, which rescues the MDA-435 brain-tropic metastatic variant cell line (MDA-435Br1) from apoptosis in serum deprived cultures, but had no effect on parental line or the lung-tropic variant of MDA-435. The MDA-435Br1 cells have increased expression levels of the IL-6 receptor (IL-6R). IL-6 secretion from astrocytes and its subsequent binding to tumor cells expressing IL-6R may play a key role in determining if frank metastases occur in the brain by allowing the outgrowth of these IL-6R expressing tumor cells in the brain.54 Direct contact of the tumor cells with the astrocytes is also important in the survival of the tumor cells. Survival genes GSTA5, BCL2L1, and TWIST, are all upregulated in breast cancer cells that are co-cultured with astroctyes.55 The upregulation of these genes mediates chemotherapeutic resistance, and implies that the microenvironment of the metastatic site has a large influence on therapeutic success. Other genes expressed in breast cancer cells that promote survival of breast cancer cells in the brain microenvironment have been identified. Genes involved in glucose metabolism, e.g., hexokinase 2, are upregulated in breast cancer cells that are highly metastatic to the brain and in brain metastases of breast cancer patients,56,57 suggesting that breast cancer brain metastatic cells derive energy from glucose oxidation and that these are important genes in the growth of breast cancer cells in the brain. Additionally, Her2+ expression and αvβ3 integrin activation are important in metastatic tumor growth in the brain.58,59 The activation of αvβ3 integrin increases VEGF production in areas of hypoxia, leading to increased angiogenesis of these brain metastases.58 Interestingly, αvβ3 integrin activation does not affect angiogenesis when these cells are grown in the mammary fat pad, showing an important interaction of these cancer cells with the brain microenvironment. Additionally, the surrounding glial cells have been shown to have much more than a simple scaffolding role in the brain. Although the effect of glial cells on the metastatic tumor niche is not fully elucidated, they have been implicated in the inflammatory responses associated with tumor invasion.60,61 These results show that, as in the bone, genes expressed in the brain microenvironment are important in survival/growth of tumor cells in the brain.
Most likely due to the unique properties of the brain, brain-destined metastases express a number of distinct genes.16 As early as 2001, brain-seeking MDA-231 cells were cultivated through successive in vivo selection.62 However, it was still some time before the genes regulating brain tropism were examined. As with earlier bone and lung studies, the gene expression of two breast cancer cell lines, CN34 and MDA-231, were in vivo selected for brain tropism as described above, and these brain-tropic cell lines were compared with their parental lines. Over 240 genes with altered expression levels were identified, 17 of which were found to be altered in clinical cases of brain relapse but not bone, liver, or lymph. Six genes overlapped with the previously established LMS genes,18 e.g., COX2 and epidermal growth factor receptor (EGFR). As these genes are not found in bone-preferring metastasis, this may indicate that these genes play a role in non-fenestrated capillary adhesion.16 Interestingly, it was shown that COX2 RNAi knockdown or treatment with EGFR inhibitor cetuximab lead to a decrease in brain metastases,16 with no effect on the CN34-BrM2 cells' growth in culture or their basal lung seeding ability, nor any effect on the ability of the MDA-231-LMS-4175 to colonize the lung, suggesting a unique function these genes play in breast cancer metastasis to the brain. The inhibition of COX2 and EGFR decreased the ability of breast cancer cells to cross the BBB in vitro, suggesting that these genes play a role in the extravasation of breast cancer cells into the brain.
Of these uniquely brain-metastatic genes, 2,6 sialyltransferase (ST6GALNAC5) was of particular interest to researchers, as it is expressed almost exclusively in the brain of both humans and mice.63 ST6GALNAC5 binds to a lectin called sambucus nigra agglutinin (SNA), which was found to stain highly in breast tumor and related brain lesions.16 The regulation of cell-cell surface interactions has been linked to sialyltransferases, and it is believed that such interactions mediate the ability of a tumor cell to dock to the brain endothelium.16 Inhibiting ST6GALNAC5 affected the ability of the tumor cells to pass through an in vitro BBB, while expression of this gene in lung-tropic tumor cells induces brain, as well as lung metastases to form in vivo. These results together indicate that ST6GALNAC5 plays a crucial role in the unique events associated with metastatic tropisms to the brain. As with the LMS, the brain metastatic signature (BrMS) identified in these studies is correlated with basal-like and claudin-low subtypes, and this BrMS along with the subtype signature helps to further predict the proclivity of breast cancer cells to metastasize to the brain.27 Though brain metastases had some genetic similarities to lung metastases derived from the same primary tumor, there are unique gene signatures associated solely with brain metastases, which can be used to develop therapies to treat the fatal metastases associated with tumor colonization of the brain.
Role of Cancer Stem Cells in Breast Cancer Organotropism
Cancer stem cells (CSC) have become a growing focus for cancer researchers, and many have hypothesized that CSCs are responsible for the generation of distant metastases.64 The Differentiation Score (DS) is a system for scoring how far along the differentiation axis a tumor cell has progressed, ranging from mammary stem cells to mature breast luminal cells.23 The DSs were determined for the MDA-231 and CN34 brain-tropic cells. A lower DS is associated with markers of stem-like cells. The brain-tropic cells had a lower DS, i.e., were less differentiated than their parental subtype, suggesting these cells have stem cell properties.27 In addition, the claudin-low subtype was the least differentiated based on the DS. Furthermore, a lower DS was correlated with higher incidence of brain metastasis, which dropped off dramatically as the tumor cells became more differentiated.27 The DS identifies a subset of tumors within the ER-, claudin-low, basal-like tumors that are more likely to metastasize to the brain and/or lung.27 This study indicates that CSC characteristics may predict tropism of breast cancer cells to the lung and brain but not to the bone. The signatures strongest in the brain- and lung-tropic tumors were weakly expressed in bone-tropic tumors.27 Further characterization of specific genes involved in CSC that drive organotropism is needed.
Additional Examples of Organotropic Genes in Other Primary Tumor Types
While breast cancer remains the best categorized in terms of organotropic metastases, a few other genes have been found to regulate specific tropisms of other cancers. In primary lung cancer bone-, liver-, and kidney-tropic genes have been identified. Lung cancer is often diagnosed late in progression, after metastases have already been established in different secondary locations. The preferred destinations of lung metastases are the liver, lymph nodes, brain, and bone.65 SBC-5 cells have been established as a lung cancer cell line with a proclivity to metastasise to the bone, liver, kidney and lung. For this experiment, researchers used gene array analysis to examine the genetic profiles of the SBC-5 cells after they had reached their metastatic terminus. Genes specific to each metastatic location were identified from each subpopulation. Liver-tropic genes include IGFBP7, CDH2, CTSL2, ADAM17 and P4HA1; bone tropic genes include FGFR, FST, PTHrP, COL3A1, MST1, FAP and POV1; kidney tropic genes include LGALS9, COL1A1, BMP6, INHBA, and C1S.65 Notably, the bone metastases from the primary lung tumor-derived cells had a similar genetic profile to bone-tropic metastases derived from breast cancer; upregulated genes included RANKL, PTHrP, and CXCR4. RANKL and CXCR4 are also involved in neuroblastoma and prostate cancer tropism to bone.66-69 These results indicate that the same genes present in different primary tumor types can guide metastases to the bone. Similarly, genes correlated with breast cancer metastasis to the brain are linked to lung and melanoma tropism to the brain. In lung cancer, 10 miRNAs were found to correlate with metastasis to the brain, including miR-450b-3p, miR-29c, miR-145, miR-148a, miR-1, miR-30d, miR-187, miR-218, miR-708, and miR-375.70 miR-145 has been shown to suppress invasion and metastasis through inhibition of mucin-1 expression.71 Interestingly, expression of mucin-1 has also been correlated with breast cancer metastasis to the brain.72 In melanoma cells, which were selected for their ability to metastasize to the brain, expression of ANGPTL4, PTGS2/ COX2, and MMP1, were upregulated.73 These genes are also upregulated in the breast cancer BrMS.16 Additionally, another 35 genes were up or downregulated in the brain metastasis and micrometastasis cells. Higher levels of preferentially expressed antigen in melanoma (PRAME) and lower levels of claudin-1 and cysteine-rich protein 61 were found in the brain metastasizing cells versus their corresponding parental cell lines.73 Claudin-1 functions in tight junction formation suggesting that loss of cell-cell adhesion is important in tropism of melanoma cells to the brain. The molecular subtype claudin-low also correlates with breast cancer metastasis to the brain.27 These results suggest that gene clusters or similar gene families may play a role in organotropism of primary tumors from different organs to the same destination.
The current understanding the mechanism of colon cancer growth in the liver and lung is very limited, and there is very little known about which genes or gene signatures regulate the tropism of colon cancer cells to the liver or lung. Genes that are believed to increase colon cancer cell survival in the lungs include E2F and thymidylate synthase. High levels of E2F expression are associated with lung metastases of colon cancer patients. This transcription factor upregulates thymidylate synthase and is believed to increase the cells' survival and chemo resistance of colon cancer lung metastases.74 Recently, expression of miR-493 was shown to inhibit colon cancer metastasis to the liver.75 IGF1R is a direct target of miR-493 and inhibition of IGF1R leads to decreased metastasis to the liver and induced cancer cell death in the liver after the cells arrive. High levels of miR-493 in colon cancer tumors were associated with decreased liver metastases in these patients.75 Further understanding of miR-493 function in colon cancer metastasis to the liver will help elucidate additional genes involved in liver tropism.
In neuorblastoma, the suppression of caspase-8 and integrin α4 may confer tropism to the bone. Caspase-8 is a known tumor suppressor gene.76 When caspase-8 deficient neuroblastomas were injected into chick embryos, they showed increased metastatic capabilities compared with their wild-type counterparts.77 In addition, caspase-8 is not found in murine neuroblastomas that spontaneously metastasize. If integrins on a neuroblastoma cell are not activated through interactions with another cells surface receptor or extracellular matrix, they trigger integrin mediated death (IMD) via caspase-8, which recruits other apoptotic factors leading to cell death. Thus, downregulation of caspase-8 expression allows the neuroblastoma cells to survive better after they enter circulation. Loss of caspase-8 would confer a general increase in metastasis. Interestingly, some metastatic tumor cells still retain caspase-8 expression. In order for these cells to metastasize, IMD must be bypassed. Integrin α4 is expressed in neuroblastomas, and bone marrow contains the ligands for this integrin77; thus, the presence of ligated integrin-α4 on neuroblastoma cells in the bone could allow for the bypass of IMD and the survival of neuroblastoma cells in the bone. These results suggest that, as with breast cancer, coordinated gene expression is necessary for bone-tropism of neuroblastomas.
This is by no means a comprehensive list of all primary tumors and genetic profiles and their metastatic propensities. While breast, lung, bone, lymph, and brain cancers, as well as neuroblastomas and melanomas, exhibit an overlap in their metastases, the liver, gallbladder, bile ducts, and intestines have completely different primary profiles and metastatic tendencies. The genes which regulate specific organotropisms of these tumors remain poorly defined.78 Furthermore, much of this genetic profiling has been done only in the past ten years; while many genes have been identified, they have not all been closely examined for their regulatory roles in organ-specific metastasis. Additionally, as the technology for genetic profiling and in vivo studies is refined, there is no doubt that more genes, in both the cancer cells and their microenvironment, will be elucidated.
Genes that Mediate the Pre-Metastatic Niche in Organotropism
Many of the studies mentioned above demonstrate that the "soil," or the metastatic microenviroment, is important for organotropism. Studies have examined the role a pre-metastatic niche plays in attracting certain tropisms. Haematopoietic progenitor cells (HPCs) in the bone marrow that are positive for vascular endothelial growth factor receptor 1 (VEGFR1) are stimulated by conditioned media from either B16 melanoma or Lewis Lung carcinoma cells to increase fibronectin in the nearby fibroblasts, which primes the local environment into a niche for incoming metastases.79 Bone marrow-derived cells (BMDCs) tagged with GFP or b-galactosidase were found to inhabit the lungs several days before the arrival of either the B16 melanoma or Lewis Lung carcinoma cells (LLCs). Intravenously injecting B16 melanoma cell conditioned media into mice was enough to stimulate the mobilization of BMDCs into the pre-metastatic niche. Additionally, LLCs are known to colonize the lungs and liver preferentially, and it was shown that when intradermally injected into mice, a BMDC pre-metastatic niche in the lung and liver was established prior to actual tumor metastasis. Likewise, the B16 melanoma cells, which have a broader metastatic tropism potential, stimulated BMDC cluster formation in the lung, liver, testis, spleen, and kidney. Functionally, it is believed the VEGFR1 receptor is the key regulator of this clustering of BMDCs and the driving force behind the pre-metastatic niche; when the receptor was silenced using monoclonal antibodies, incidence of lung metastases from the LLCs dropped to nearly zero. It is speculated that VEGFR1+ cells express the integrin VLA-4, which allows for the interaction of VEGFR1+ cells with VCAM1 on endothelial cells in the pre-metastatic niche in the new location.79 These results suggest that soluble factors secreted from the primary tumor mediate the formation of the pre-metastatic niche in certain organs and thus organotropism. The chemokines VEGF and PIGF are believed to be two of these soluble factors.80 Further discovery of genes that mediate the interaction of the tumor cells with the BMDCs and how this interaction generates the pre-metastatic niche in an organ-specific manner will provide a better understanding of what mediates organotropism. The factors regulating the pre-metastatic niche are described in more detail in the chapter entitled "Pre-metastatic hematopoietic niche."
Conclusion
Metastasis is an elaborate process in the progression of cancer, and understanding the mechanism of how it is regulated is crucial for the prevention and treatment of patients with advanced disease. The tendency of primary tumors to have preferred orthotopic destinations for their metastases is known as organotropism, and understanding the genes that control a specific tropism can help researchers develop more targeted treatments for the disease. With new technologies, the concept of a "seed" and "soil" has advanced since over a century ago. Though the molecular basis for organotropism is still largely unclear, mounting evidence shows there are unique genes and gene signatures that modulate specific tropisms of a primary tumor. To date, researchers have found genes that mediate: (a) the attraction of cancer cells to specific organs, e.g., CXCR4 in tumor cells binding the chemokine CXCL12 in bone, brain and lung,41 (b) adhesion/"homing" of cells to the endothelium in specific organs, e.g., expression of integrin β4 on breast cancer cells and its ligand, hCLCA2,51 on lung endothelium, (c) growth or survival in the metastatic site, e.g., the expression of PTHrP in prostate, lung, and breast cancer cells32 mediates tumor growth in the bone, (d) angiogenesis at the metastatic site, e.g., integrin αvβ3 activations leads to increased VEGF production and angiogenesis in brain metastases but not the primary tumor,58 (e) the premetastatic niche, e.g., VEGF and PIGF mediate formation of the premetastatic niche80 and, (f) the ability of cells to extravasate into a specific organ, e.g., expression of ST6GALNAC5 in breast cancer cells increases extravasation through the BBB.16 The evidence that genes involved in the cyclical interaction of primary tumors, their metastases, and the cellular microenvironment involved in organotropism indicates this process requires a coordinated effort. Indeed many of the genes identified functioned in coordination to increase metastasis to a specific organ.16-18 When multiple gene signatures and/or clinical variables are combined, the ability to predict the organ of metastasis is greatly increased.27 Individual genes found to play a role in organotropism can allow for the development of targeted therapy for specific metastases; however, since a coordinated effort is required for metastasis to a specific organ, a multi-therapeutic approach is needed to prevent and inhibit metastatic growth. Several recent studies indicate that loss of specific miRNAs play a role in organotropism and provide a selection advantage for cells in specific organs,43,70,75 suggesting that expression of certain miRNAs may be responsible for some of the coordinated gene expression required for organotropism. We are just now beginning to understand the mechanisms involved in the arrival and growth of cancer cells to specific organs. Future research will work toward defining the organotropic genes more extensively in cancers, determining when or if these genes are activated in the primary tumor cells or in the tumor cells at the metastatic site, and discovering new therapies and targets to prevent the occurrence of potentially fatal metastases.
References
- 1.
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:99–101. [PubMed: 2673568]
- 2.
- DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–9. http://dx.doi.org/10.1097/00043426-199905000-00005 . [PubMed: 10363850]
- 3.
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–33. http://dx.doi.org/10.1002/cncr.21778 . [PubMed: 16518827]
- 4.
- Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153–62. http://dx.doi.org/10.1007/s10911-007-9047-3 . [PubMed: 17566854]
- 5.
- Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. http:// dx.doi.org/10.1053/hp.2000.6698 . [PubMed: 10836297]
- 6.
- Meyers MLBC. Diagnosis and Treatment of Metastatic Melanoma. In: Balch CM HA, Sober AJ, Soong S-J, ed. Cutaneous Melanoma St Louis: Quality Medical Publishing, Inc. 1998
- 7.
- Ewing J. Neoplastic Diseases. A treatise on tumors. Philadelphia & London: W.B. Saunders Co. 1928
- 8.
- Coman DR, deLONG RP, MccUTCHEON M. Studies on the mechanisms of metastasis; the distribution of tumors in various organs in relation to the distribution of arterial emboli. Cancer Res. 1951;11:648–51. [PubMed: 14859232]
- 9.
- Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. [vii.] Surg Oncol Clin N Am. 2001;10:243–55, vii. [PubMed: 11382585]
- 10.
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–73. http://dx.doi.org/10.1016/S0002-9440(10)65628-3 . [PMC free article: PMC1853000] [PubMed: 9736035]
- 11.
- Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis. 1992;10:191–9. http://dx.doi.org/10.1007/BF00132751 . [PubMed: 1582089]
- 12.
- Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. http://dx.doi.org/10.1002/path.1711500308 . [PubMed: 3806280]
- 13.
- Fidler IJ. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45:773–82. [PubMed: 5513503]
- 14.
- Krüger A, Schirrmacher V, Khokha R. The bacterial lacZ gene: an important tool for metastasis research and evaluation of new cancer therapies. Cancer Metastasis Rev. 1998-1999;17:285–94. http://dx.doi.org/10.1023/A:1006066706040 . [PubMed: 10352882]
- 15.
- Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. [PMC free article: PMC539194] [PubMed: 15630443]
- 16.
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. http://dx.doi.org/10.1038/ nature08021 . [PMC free article: PMC2698953] [PubMed: 19421193]
- 17.
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. http:// dx.doi.org/10.1016/S1535-6108(03)00132-6 . [PubMed: 12842083]
- 18.
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. http://dx.doi.org/10.1038/nature03799 . [PMC free article: PMC1283098] [PubMed: 16049480]
- 19.
- Welch DR. Microarrays bring new insights into understanding of breast cancer metastasis to bone. Breast Cancer Res. 2004;6:61–4. http://dx.doi.org/10.1186/bcr736 . [PMC free article: PMC400646] [PubMed: 14979907]
- 20.
- Paget S. The distribution of secondary growths in cancer of the breast Lancet 1889. 133 571 3;http:// dx.doi.org/10.1016/S0140-6736(00)49915-0 . [PubMed: 2673568]
- 21.
- Maki DD, Grossman RI. Patterns of disease spread in metastatic breast carcinoma: influence of estrogen and progesterone receptor status. AJNR Am J Neuroradiol. 2000;21:1064–6. [PMC free article: PMC7973900] [PubMed: 10871014]
- 22.
- Perou CM, Srlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. http://dx.doi.org/10.1038/35021093 . [PubMed: 10963602]
- 23.
- Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. Sep 2;12(5):R68. [PMC free article: PMC3096954] [PubMed: 20813035]
- 24.
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. http://dx.doi.org/10.1186/gb-2007-8-5-r76 . [PMC free article: PMC1929138] [PubMed: 17493263]
- 25.
- Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–14. http://dx.doi. org/10.1158/0008-5472.CAN-07-5644 . [PubMed: 18451135]
- 26.
- St Romain P, Madan R, Tawfik OW, Damjanov I, Fan F. Organotropism and prognostic marker discordance in distant metastases of breast carcinoma: fact or fiction- A clinicopathologic analysis. Hum Pathol. Mar;43(3):398–404. [PubMed: 21840040]
- 27.
- Harrell JC, Prat A, Parker JS, Fan C, He X, Carey L, et al. Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat. 2012;132:523–35. [PMC free article: PMC3303043] [PubMed: 21671017]
- 28.
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. Jul 10;28(20):3271–3277. [PubMed: 20498394]
- 29.
- Fidler IJ. Selection of successive tumour lines for metastasis. Nat New Biol. 1973;242:148–9. [PubMed: 4512654]
- 30.
- Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71. [PubMed: 2846163]
- 31.
- van tVeer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. http://dx.doi. org/10.1038/415530a . [PubMed: 11823860]
- 32.
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. http://dx.doi.org/10.1038/nrc867 . [PubMed: 12154351]
- 33.
- Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66:855–86. [PubMed: 3532144]
- 34.
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–11. http://dx.doi. org/10.1056/NEJM199502023320506 . [PubMed: 7816067]
- 35.
- Sharp JA, Sung V, Slavin J, Thompson EW, Henderson MA. Tumor cells are the source of osteopontin and bone sialoprotein expression in human breast cancer. Lab Invest. 1999;79:869–77. [PubMed: 10418827]
- 36.
- Roodman GD. Biology of osteoclast activation in cancer. J Clin Oncol. 2001;19:3562–71. [PubMed: 11481364]
- 37.
- Buijs JT, Stayrook KR, Guise TA. TGF-beta in the Bone Microenvironment: Role in Breast Cancer Metastases. Cancer Microenviron. Dec;4(3):261–281. [PMC free article: PMC3234330] [PubMed: 21748439]
- 38.
- Santini D, Perrone G, Roato I, Godio L, Pantano F, Grasso D, et al. Expression pattern of receptor activator of NFkB (RANK) in a series of primary solid tumors and related bone metastases. J Cell Physiol. 2011;226:780–4. http://dx.doi.org/10.1002/jcp.22402 . [PubMed: 20857484]
- 39.
- Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10:105–18. http://dx.doi.org/10.1007/s10911-005-5394-0 . [PubMed: 16025218]
- 40.
- Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, et al. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res. 2004;64:4506–13. http://dx.doi.org/10.1158/0008-5472.CAN-03-3851 . [PubMed: 15231660]
- 41.
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. http://dx.doi. org/10.1038/35065016 . [PubMed: 11242036]
- 42.
- Oskarsson T, Acharyya S, Zhang XH, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. Jul;17(7):867–874. [PMC free article: PMC4020577] [PubMed: 21706029]
- 43.
- Tavazoie SF, Alarcón C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–52. http://dx.doi. org/10.1038/nature06487 . [PMC free article: PMC2782491] [PubMed: 18185580]
- 44.
- Calvo A, Catena R, Noble MS, Carbott D, Gil-Bazo I, Gonzalez-Moreno O, et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene. 2008;27:5373–84. http:// dx.doi.org/10.1038/onc.2008.155 . [PMC free article: PMC2702869] [PubMed: 18504437]
- 45.
- Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–5. http://dx.doi.org/10.1073/pnas.0701138104 . [PMC free article: PMC1871856] [PubMed: 17420468]
- 46.
- Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. http:// dx.doi.org/10.1038/nature05760 . [PubMed: 17429393]
- 47.
- Landemaine T, Jackson A, Bellahcne A, Rucci N, Sin S, Abad BM, et al. A six-gene signature predicting breast cancer lung metastasis. Cancer Res. 2008;68:6092–9. http://dx.doi. org/10.1158/0008-5472.CAN-08-0436 . [PubMed: 18676831]
- 48.
- Driouch K, Bonin F, Sin S, Clairac G, Lidereau R. Confounding effects in "a six-gene signature predicting breast cancer lung metastasis": reply. [reply] Cancer Res. 2009;69:9507–11. http://dx.doi.org/10.1158/0008-5472.CAN-09-2688 . [PubMed: 19934332]
- 49.
- Culhane AC, Quackenbush J. Confounding effects in "A six-gene signature predicting breast cancer lung metastasis". Cancer Res. 2009;69:7480–5. http://dx.doi.org/10.1158/0008-5472. CAN-08-3350 . [PMC free article: PMC3128918] [PubMed: 19723662]
- 50.
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–74. http://dx.doi.org/10.1016/S1535-6108(04)00079-0 . [PubMed: 15093543]
- 51.
- Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. The breast cancer beta 4 integrin and endothelial human CLCA2 mediate lung metastasis. J Biol Chem. 2001;276:25438–46. http:// dx.doi.org/10.1074/jbc.M100478200 . [PubMed: 11320086]
- 52.
- Lu X, Kang Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proc Natl Acad Sci U S A. 2009;106:9385–90. http://dx.doi.org/10.1073/pnas.0900108106 . [PMC free article: PMC2695061] [PubMed: 19458257]
- 53.
- Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–20. http://dx.doi.org/10.1016/S00029440(10)61180-7 . [PMC free article: PMC1603675] [PubMed: 16192626]
- 54.
- Sierra A, Price JE, García-Ramirez M, Méndez O, López L, Fabra A. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest. 1997;77:357–68. [PubMed: 9354770]
- 55.
- Kim SJ, Kim JS, Park ES, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. Mar;13(3):286–298. [PMC free article: PMC3050871] [PubMed: 21390191]
- 56.
- Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–86. http://dx.doi. org/10.1158/0008-5472.CAN-06-3137 . [PubMed: 17308085]
- 57.
- Palmieri D, Fitzgerald D, Shreeve SM, Hua E, Bronder JL, Weil RJ, et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res. 2009;7:1438–45. http://dx.doi.org/10.1158/1541-7786. MCR-09-0234 . [PMC free article: PMC2746883] [PubMed: 19723875]
- 58.
- Lorger M, Krueger JS, O'Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106:10666–71. http://dx.doi.org/10.1073/pnas.0903035106 . [PMC free article: PMC2697113] [PubMed: 19541645]
- 59.
- Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–8. http://dx.doi.org/10.1158/0008-5472.CAN-06-3316 . [PubMed: 17483330]
- 60.
- Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25:799–810. http://dx.doi.org/10.1007/s10585-008-9193-z . [PMC free article: PMC2679391] [PubMed: 18649117]
- 61.
- Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last). Trends Neurosci. 2003;26:520–2. http://dx.doi.org/10.1016/j.tins.2003.08.006 . [PubMed: 14522143]
- 62.
- Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–95. http://dx.doi. org/10.1359/jbmr.2001.16.8.1486 . [PubMed: 11499871]
- 63.
- Okajima T, Fukumoto S, Ito H, Kiso M, Hirabayashi Y, Urano T, et al. Molecular cloning of brain-specific GD1alpha synthase (ST6GalNAc V) containing CAG/Glutamine repeats. J Biol Chem. 1999;274:30557–62. http://dx.doi.org/10.1074/jbc.274.43.30557 . [PubMed: 10521438]
- 64.
- Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. http://dx.doi.org/10.1038/sj.cr.7310118 . [PubMed: 17179981]
- 65.
- Yano S, Kakiuchi S, Zhang H, Sone S, Meadows G. Organotropism of Lung Cancer Metastasis and its Molecular Targeted Therapy Integration/Interaction of Oncologic Growth. Vol 15: Springer Netherlands. 2005:387–405.
- 66.
- Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg. 2004;39:150611. http://dx.doi.org/10.1016/j.jpedsurg.2004.06.019 . [PubMed: 15486895]
- 67.
- Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. http://dx.doi.org/10.1002/pros.20318 . [PubMed: 16114056]
- 68.
- Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–96. http://dx.doi.org/10.1016/j.ccr.2005.04.013 . [PubMed: 15894268]
- 69.
- Granchi D, Amato I, Battistelli L, Avnet S, Capaccioli S, Papucci L, et al. In vitro blockade of receptor activator of nuclear factor-kappaB ligand prevents osteoclastogenesis induced by neuroblastoma cells. Int J Cancer. 2004;111:829–38. http://dx.doi.org/10.1002/ijc.20308 . [PubMed: 15300794]
- 70.
- Lu Y, Govindan R, Wang L, Liu PY, Goodgame B, Wen W, et al. MicroRNA profiling and prediction of recurrence/relapse-free survival in stage I lung cancer. Carcinogenesis. 2012;1 [PMC free article: PMC3334512] [PubMed: 22331473]
- 71.
- Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. Jan 1;70(1):378–387. [PMC free article: PMC2805032] [PubMed: 19996288]
- 72.
- Rye PD, Norum L, Olsen DR, Garman-Vik S, Kaul S, Fodstad O. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int J Cancer. 1996;68:682–7. http://dx.doi.org/10.1002/(SICI)1097-0215(19961127)68:5<682::AIDIJC20>3.0.CO;2-2 . [PubMed: 8938153]
- 73.
- Izraely S, Sagi-Assif O, Klein A, Meshel T, Tsarfaty G, Pasmanik-Chor M, et al. The metastatic microenvironment: Brain-residing melanoma metastasis and dormant micrometastasis. Int J Cancer. 2011;25 [PubMed: 22025079]
- 74.
- Banerjee D, Gorlick R, Liefshitz A, Danenberg K, Danenberg PC, Danenberg PV, et al. Levels of E2F-1 expression are higher in lung metastasis of colon cancer as compared with hepatic metastasis and correlate with levels of thymidylate synthase. Cancer Res. 2000;60:2365–7. [PubMed: 10811110]
- 75.
- Okamoto K, Ishiguro T, Midorikawa Y, Ohata H, Izumiya M, Tsuchiya N, et al. miR-493 induction during carcinogenesis blocks metastatic settlement of colon cancer cells in liver. EMBO J. 2012;28 [PMC free article: PMC3321205] [PubMed: 22373578]
- 76.
- Stafford LJ, Vaidya KS, Welch DR. Metastasis suppressors genes in cancer. Int J Biochem Cell Biol. 2008;40:874–91. http://dx.doi.org/10.1016/j.biocel.2007.12.016 . [PubMed: 18280770]
- 77.
- Lahti JM, Teitz T, Stupack DG. Does integrin-mediated cell death confer tissue tropism in metastasis. Cancer Res. 2006;66:5981–4. http://dx.doi.org/10.1158/0008-5472.CAN-06-0131 . [PubMed: 16778165]
- 78.
- Ballestero MR, Monte MJ, Briz O, Jimenez F, Gonzalez-San Martin F, Marin JJ. Expression of transporters potentially involved in the targeting of cytostatic bile acid derivatives to colon cancer and polyps. Biochem Pharmacol. 2006;72:729–38. http://dx.doi.org/10.1016/j.bcp.2006.06.007 . [PubMed: 16844096]
- 79.
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. http://dx.doi.org/10.1038/nature04186 . [PMC free article: PMC2945882] [PubMed: 16341007]
- 80.
- Kaplan RN, Rafii S, Lyden D. Preparing the "soil": the premetastatic niche. Cancer Res. 2006;66:1108993. http://dx.doi.org/10.1158/0008-5472.CAN-06-2407 . [PMC free article: PMC2952469] [PubMed: 17145848]
- 81.
- Erin N, Wang N, Xin P, Bui V, Weisz J, Barkan GA, et al. Altered gene expression in breast cancer liver metastases. Int J Cancer. 2009;124:1503–16. http://dx.doi.org/10.1002/ijc.24131 . [PubMed: 19117052]
- Genes That Mediate Metastasis Organotropism - Madame Curie Bioscience DatabaseGenes That Mediate Metastasis Organotropism - Madame Curie Bioscience Database
- The TRP Calcium Channel and Retinal Degeneration - Madame Curie Bioscience Datab...The TRP Calcium Channel and Retinal Degeneration - Madame Curie Bioscience Database
- PNA and Oligonucleotide Inhibitors of Human Telomerase - Madame Curie Bioscience...PNA and Oligonucleotide Inhibitors of Human Telomerase - Madame Curie Bioscience Database
- The Role of Serine/Threonine Protein Phosphatases in Ceramide Signaling - Madame...The Role of Serine/Threonine Protein Phosphatases in Ceramide Signaling - Madame Curie Bioscience Database
- Target Recognition of Guanylate Cyclase by Guanylate Cyclase-Activating Proteins...Target Recognition of Guanylate Cyclase by Guanylate Cyclase-Activating Proteins - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...