NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsTRP CHANNELS: DIVERSITY OF FORM AND FUNCTION
The Drosophila trp mutation is responsible for the phenotype called transient receptor potential, an alteration of the fly’s electrorentinogram in which its sustained phase is missing [1,2]. The responsible gene was cloned in 1989 [3]. Its amino acid sequence predicted a protein with eight hydrophobic segments that could potentially form transmembrane segments. Purification and cloning of a calmodulin-binding protein from Drosophila heads showed it to be a homologue of trp. It received the name trp-like or trpl [4]. Its discoverers highlighted the existence of limited sequence similarities between trp/trpl and voltage-sensitive Na+ and Ca2+ channels. Expression of trpl in silkworm cells of Spodoptera frugiperda (Sf9 cells) did indeed lead to appearance of cation channels [5]. In keeping with both a role for trp and trpl in insect phototransduction, and the fact that insect phototransduction is biochemically akin to mammalian signal transduction based on the Gq-PLC pathway instead of a transducin-phosphodiesterase (Gt-PDE) pathway [6,7], the trpl channels expressed in Sf9 cells could be activated by a Gq-coupled GPCR [8].
Activation of the Gq-PLC signaling system results in hydrolysis of PIP2 with formation of the second messengers diacylglycerol (DAG) and inositol trisphosphate (IP3), followed by IP3-induced release of Ca2+ from intracellular stores and the activation of type-C protein kinases (PKCs) by the combined action of DAG and the released Ca2+. Further, the depletion of intracellular Ca2+ stores then activates Ca2+-permeable cation channels in the plasma membrane. The molecular basis by which store depletion activates plasma membrane Ca2+ entry channels is as yet incompletely defined. Two questions need to be answered: (1) which molecules make up the channels that mediate the store depletion–activated Ca2+ entry?; and (2) by what mechanism do the stores “inform” the plasma membrane channels of their state of replenishment? TRP channels have been postulated as the pore-forming molecules through which store depletion–activated Ca2+ entry takes place [9]. While there are data in support of this hypothesis, the final word is not in, and much is still to be elucidated (vide infra The ROCE SOCE Conundrum). On the other hand, recent studies that screened for a Ca2+ sensor have successfully identified a single pass membrane protein termed STIM1 as the store’s Ca2+ sensor [10,11]. Interestingly, store depletion promotes a translocation of STIM1 from endomembranes to the plasma membrane [12], where it presumably “talks” to the Ca2+ entry channels.
Ca2+ entering after activation of the PLC-IP3R store depletion pathway serves both as a substrate for the sarcoplasmic-endoplasmic reticulum Ca2+ pumps (SER-CAs), which replenish the depleted stores, and as a signaling molecule. We will refer to Ca2+ entry that follows activation of PLC by receptors as receptor-operated Ca2+ entry or ROCE. Store depletion can also be caused by inactivating SERCA pumps with an inhibitor such as thapsigargin [13,14] or by loading cells with Ca2+ chelator that acts as a sink for Ca2+ that leaks passively from the stores [15]. Both maneuvers activate Ca2+ entry that can be assessed either with fluorescent indicator dyes, such as fura2, in which case it is referred to as capacitative Ca2+ entry (CCE [16,17]), or as store-operated Ca2+ entry (SOCE) or by electrophysiologic means, where Ca2+ entry presents itself as an inward Ca2+ current, termed Ca2+ release-activated Ca2+ current (Icrac) [15,18]. Icrac and CCE are commonly accepted as being the measures of the same phenomenon.
In 1992, Hardie and Minke [19] showed that the missing sustained phase of the electroretinograms of trp mutant Drosophila eyes has as its underlying basis the absence of a Ca2+ conductance. In 1993, they formally raised the question whether the trp and trpl proteins might be functional homologues of capacitative Ca2+ entry channels, and by extension the pore-forming molecules of Icrac channels [20] in mammalian cells. The finding that a trpl/trp chimera could be activated by store depletion in Sf9 cells [21] lent strong support to Hardie and Minke’s hypothesis.
The mammalian homologues of Drosophila trp genes (TRPs) were cloned to test Hardie and Minke’s hypothesis. Six such homologues were identified in our initial 1995–96 screen [22–24], and a seventh was discovered three years later [25]. Initially called TRPs, they are now referred to as TRPCs [26]. Expression and assembly studies have shown that TRPCs can selectively form heteromeric complexes, such as 1:2, 1:3, 1:5, 4:5, 3:6:7 and 1:4:5 that co-immunoprecipitate [27–29]. Presumably in all cases active channels are tetrameric.
Unexpectedly, independent research from several laboratories uncovered the existence of TRP-related cation channels that together constitute a “superfamily” whose members play differing and sometimes still unknown roles in cellular physiology.
One set of TRP-related channels, with a role in pain and thermosensing, was uncovered in David Julius’s laboratory. In 1997, Julius isolated the cDNA that encodes the capsaicin (also vanilloid) receptor (VR1) through expression cloning [30]. This was followed, shortly afterward, by the cloning of its close relative VRL1 [31]. Both turned out to be heat sensors and structural homologues of the fly trp channels. Independently, VRL1 was also cloned in 1999 as a growth factor–activated channel, which translocated from endomembranes to the plasma membrane [32]. Between 1999 and 2000, two groups, one in the Netherlands and one in Boston, identified two epithelial calcium transporters: renal ECaC and intestinal CaT. Amino acid sequence analysis revealed ECaC (also CaT2) and CaT (also ECaC2) to be related to VR1 and VRL1, and hence to TRPs. In 2001, the original mammalian TRPs were renamed to TRPCs (for classic or canonical) and VRs and ECaC/CaT were renamed to TRPVs, of which there are six.
Mlsn1 (melastatin 1), identified in 1998 as a gene product down-regulated in melanoma cells [33], is the founding member of the TRPMs, of which there are eight. As these families were being identified, it became evident that there existed other, more distant relatives that include the polycystins [34] and their relatives, TRPPs 1–4 (reviewed in reference [35]). Genes responsible for mucolipidosis also encode TRP-related proteins [36]. At present we recognize three TRPMLs. A mechanosensory trans-duction channel, TRPA1, is the latest addition to mammalian TRP genes [37,38]. TRPA1’s most outstanding structural characteristic is an unusually large number of ankyrin motifs in its N-terminus.
Most TRPs are calcium-permeable nonselective cation channels, with notable exceptions: ECaC/CaT channels are highly selective for Ca2+ [39–41], and TRPM4/5 are nonselective monovalent cation channels activated by Ca2+ without being permeant to Ca2+ (reviewed in reference [42]). TRPM2 and TRPM6/7 incorporate enzymatic functions into their C-termini. TRPM2 has a NUDIX domain able to bind ADP-ribose and to sense H2O2; TRPM6/7 carry an atypical (alpha) protein kinase domain; and while TRPV1, 2, and 3 sense and are activated by distinct temperatures, TRPM8 (also CMR, for cold and menthol receptor) is activated upon cooling [43]. TRPV4 and TRPM3 are osmo-sensitive. Physiologically, TRPV4 participates in mediating pain sensations, and TRPM5 is a taste transduction channel expressed in sensory neurons mediating bitter, sweet, and amino acid (unami) tastes. TRPC3 has been proposed to be the melanopsin-activated transduction channel of the intrinsically photosensitive retinal ganglion cells (ipRGCs) responsible for entrainment of the circadian clock of the suprachiasmatic nucleus [44,45]. TRPC3 and TRPC6 were recently implicated as essential components of the machinery guiding Ca2+-dependent growth cone turning of pontine neuron axon extensions [45], and Xenopus TRPC1 was identified by Wang and Poo [46] in a similar phenomenon whereby netrin guides axonal growth of Xenopus spinal neurons. In a parallel study, TRPC3 and TRPC6 were implicated in BDNF-directed axonal outgrowth from cerebellar granule cells [47]. In rodents, TRPC2 has been shown to be the transduction channel activated in vomeronasal sensory neurons in response to activation of vomeronasal pheromone-responsive GPCRs [48,49] (reviewed in references [50] and [51]). Recently, TRPA1, also ANKTM1, a channel with fourteen N-terminal ankyrin repeats, was characterized as a channel that transduces noxious cold sensation as well as the mechanical bending of stereocilia of inner ear hair cells [37,38].
TRP SUPERFAMILY GENES AND THEIR GENE PRODUCTS
TRP Subfamilies
As is evident from the above discussion, a variety of different approaches involving many laboratories—some working in parallel—were involved in identifying the various TRP channels and hence naming the cloned genes in a somewhat unruly manner. The nomenclature of the C, M, and V channels has been agreed upon [26]. The use of TRPP for the PKD family of channels is still tentative. Table 1.1 tabulates the TRP genes we analyzed and provides their mRNA accession numbers; the lengths, in amino acids, predicted by their coding sequences (CDS), restricted for the most part to the longest if splice variants exist; location of the TRP channel domain (ion channel domain) within the cDNAs; and the genomic accession numbers, chromosomal loci, and number of exons, as predicted by GenBank’s annotated genomic contigs. Using the ion channel domains identified in Table 1.1 as a basis, we constructed a phylogenetic tree that in turn organized members of the C-, V-, M-, P-, ML-, and A-type TRP channels into subgroups (Figure 1.1). The years in which their founding members were identified are highlighted.
TABLE 1.1
TRP Channel Superfamily GenBank Accession Numbers and Numerical Parameters of Their mRNAs and Genes
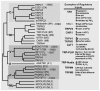
FIGURE 1.1
(Left) Phylogenetic tree illustrating the successive discovery of TRP and TRP-related channel. Years denote when the founding member of each subfamily was identified. (Right) Functional diversity among the major subfamilies of TRP channels. The figure (more...)
Figure 1.2 presents an amino acid alignment in which we compare the ion channel domains of a representative member of each of the six families (C, V, M, P, ML, A), highlighting in white on black background positions at which three or more amino acid identities occur among the six sequences. The result documents the “relatedness” of the six subfamilies. The segregation of TRP channels into the C, M, V, P, ML, and A subfamilies emerges very clearly from these alignments.

FIGURE 1.2
Amino acid sequence alignment of the ion channel domain of representative members of the TRP superfamily of cation channels: TRPC3, TRPM2, TRPP2, TRPA1, TRPML1, and TRPV1. Amino acids shown in white on a black background denote three identities among (more...)
Kyte and Doolittle analysis and subsequent glycosylation scanning mutagenesis and limited epitope mapping led to the definition of six transmembrane domains in TRPC3 preceded by a cytosolic hydrophobic domain (h or h1) [52]. Based on both the individual hydrophobicity plots and TRPC3 as a template, we defined the putative transmembrane domains of all the TRPC channels listed in Table 1.1.
We used the annotations that accompany the genomic sequence files to reconstruct the open reading frames (ORFs) of the C-, V-, M-, P-, ML-, and A-type TRP mRNAs (panels A through F of Figure 1.3) and located the positions of the exon boundaries. The figure panels show the deduced location of the putative transmembrane domains and channel pore-forming segment, and highlight exons coding for specific domains such as calmodulin binding, ankyrin repeats (absent in TRPM channels), IP3 receptor interacting sites, etc.

FIGURE 1.3
Comparison of exon boundary locations along the open reading frames of TRP channels. A, TRPCs; B, TRPMs; C, TRPVs; D, TRPAs; E, TRPP; F, TRPML. Black boxes, 5′ and 3′ untranslated sequences; open boxes, coding exons; diagonally hatched (more...)
The sequence alignments also revealed that C, M, and V TRP channels but not the TRPP, TRPML, or TRPA channels contain a typical six amino acid motif about fifteen amino acids after the predicted sixth transmembrane segment of the TRP channel domain (Table 1.2), and that h1, the intracellular hydrophobic region that precedes the ion channel domain [52], is a feature found in all the TRP subfamilies with the exception of the TRPV subfamily. The cytosolic h domain also appears to be absent from some TRPs of the M, ML, and A1 classes. Domains and binding sites found in the various TRP channels are illustrated in Figure 1.4.
TABLE 1.2
TRP Motifs

FIGURE 1.4
Overview of domains found in TRP channels. CaMBD, calmodulin-binding domains, are according to Zhang et al. [87], Tang et al. [88], Trost et al. [89], and Yildirim et al. [90] IP3R Bdg Sites, inositol-trisphosphate receptor binding sites, are according (more...)
Genomic Structures
Figure 1.5 illustrates the organization of the TRPC channels in the murine and human genomes, and that of the M-, V-, and P-type TRPs. The number of exons of each gene is given in Table 1.1. At this level of analysis, TRP genes show no special features. They extend from as little as 23.5 kb (mouse TRPC2) to as much as 304 kb (TRPC5). Large introns are spliced out both from within 5′ untranslated segments of the transcripts (e.g., intron A of TRPC5 = 164 kb) and from within the coding region of the transcripts (e.g., intron B of TRPC6 = 65.9 kb). The number of exons also varies from as many as over 40 (TRPP1) to as few as 2 (one coding and one 3′ noncoding: PKD-REJ [TRPP4]).

FIGURE 1.5
Chromosomal organization of exons in genes coding for TRP channels. (Left) Murine and human TRPC genes. (Right) TRPM, TRPV, and TRPP genes. Exon predictions were obtained from genomic contigs downloaded from the National Center for Biotechnology Information (more...)
Direct Chromosomal Repeats
There may be two instances in which TRPs are found as direct repeats on a chromosome: TRPV3 (VRL2) follows TRPV1 (VR1), and TRPV6 (CaT1, ECaC2) follows after TRPV5 (ECaC1, CaT2), separated from the Stop codon of one and the beginning of the ORF of the next by only 7.5 Kb and 22 Kb, respectively. Both of these duplications appear to have been recent because, when compared to other TRPVs, V1 is most similar to V3 as compared to being most similar to another TRPV. The same applies to TRPV5 (ECaC1) and TRPV6 (CaT1). By comparison, in the field of G protein α subunits, there are several pairs, such as Gi2α preceding Gtrα (locus 3p21), Gi3α preceding Gtcα (locus 1p13), Gi1α preceding Ggustα (locus 3p21), Gqα preceding G14α (locus 9p21), and G11α preceding G16α (locus 19p13). Giα’s are more similar to each other than to any of the transducins and gustducins, which in turn resemble each other much more than the Gi’s. Likewise Gqα is closer to G11α than to G14α, and G14α is closer to G16α than to G11α or Gqα, suggesting that gene duplications have happened very early in evolution, with duplicated genes duplicating further and allowing the leading and trailing member time to diverge.
MECHANISM(S) OF TRPC ACTIVATION
Receptor-Mediated Activation of TRPCs Requires Phospholipase C Activation: A Role for PDZ Scaffolds?
The major players involved in regulation of TRPC channel activity are illustrated in Figure 1.6. A description of their involvement follows. TRPC channels are activated when phospholipase C is activated either by a Gq-coupled GPCR pathway mediated by PLCβs or by a receptor tyrosine kinase signaling pathway mediated by the γ family of PLCs. Activation of TRPCs is lost by inhibition of PLCβ with the PLCβ inhibitor U73122 [53] or when activation is tested in systems lacking PLCs, such as the NorpA Drosophila mutant, which lacks PLCβ [54], and DT40 chicken B cells, in which PLCγ has been inactivated by gene disruption [55].
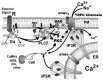
FIGURE 1.6
Overview of some of the mechanisms that regulate or are thought to regulate the Gq-PLCβ triggered activation of TRPCs. A ligand of a Gq-coupled G-protein-coupled receptor (GPCR) is shown to activate the receptor’s Gq-activating function (more...)
In Drosophila, the argument has been made for the formation of a “signalplex” with participation of INAD, a multi-PDZ domain–containing scaffold protein, as an intrinsic mechanism by which the trp/trpl-based phototransduction channel is activated. In agreement with this postulate, INAD binds to NorpA, PLC, trp, trpl, rhodopsin, and PKC [56–59] (reviewed in reference [60]).
In mammals, including humans, two of the TRPCs—TRPC4 and TRPC5—also interact with a PDZ scaffold protein, NHERF, the regulator factor of the Na-H exchanger [61]. NHERF is a two-PDZ domain protein. TRPC4, TRPC5, PLCβ1, and PLCβ2 interact with the first PDZ domain, while the other PDZ domain binds members of the Ezrin-Radixin-Moesin (ERM) family of proteins, known to interact with F-actin. Thus, it would appear that, rather than organizing a signalplex similar to INAD’s role in the Drosophila eye, NHERF’s role in vertebrates may be that of physically connecting members of the PLC-TRP signaling pathway to the cyto-skeleton. Yet this connection is unlikely to be part of the TRPC-activating process, because cortical actin, induced by calyculin A, blocks GPCR as well as store-depletion activated Ca2+ entry mediated by TRPC3 [62]. Calyculin A is a PP1 and PP2A phosphoprotein phosphatase inhibitor that causes accumulation of the C-terminally phosphorylated forms of ERM proteins. These in turn interact with F-actin, promoting its redistribution to the plasma membrane where the N-terminal portion of ERM proteins interact with membrane proteins. As a consequence, calyculin A treatment forms cortical actin. The implications of establishing independent connections between either PLCs and the actin cytoskeleton, or TRPC4-5 and the actin skeleton, which could interfere with TRPC’s function as an ion channel, are not clear.
However, formation of transient complexes is likely to be involved in the process by which TRPCs are activated. Analysis of TRPC6 activation by Gq-coupled M1 muscarinic receptors in PC12D cells by Kim and Saffen [63] showed the formation of time-sensitive macromolecular complexes involving PKC and phosphorylation of TRPC6 at a conserved PSPK site 23aa downstream of the TRPC EWFKAR motif. These authors found that once PKC was dissociated from the phosphorylated channel, the PS(PO3−)PK motif of TRPC6 recruits the FK506 binding protein FKBP12, which in turn recruits calcineurin (CN) and calmodulin (CaM). CN then dephosphorylates TRPC6, causing dissociation of the complex into its individual components. The complexes did not form if the Ser of the PSPK motif was mutated, when PKC was inhibited, when the immunophilin FKBP12 was blocked with FK506 or rapamycin, or when cells had been treated with cyclosporin. In all these instances, the channel was not dephosphorylated and the M1R stayed associated with the channel.
Activation by Conformational Coupling: The Role for IP3 Receptor as an Activator of TRPC through Protein-Protein Interaction
The conformational coupling concept emerged originally from the elucidation of the role of the skeletal muscle voltage-gated Ca2+ channel in mediating a depolarization-induced contraction. Skeletal muscle fibers have a particularly strong Ca2+ uptake activity performed by SERCA pumps. As a consequence, the Ca2+ released from the sarcoplasmic reticulum (SR) in response to Ca2+-activated Ca2+ release through the ryanodine receptor/Ca2+ release channel is almost quantitatively reabsorbed into the SR with little or no loss through the action of plasma membrane Ca2+ pumps. Isolated skeletal muscle fibers then contract repeatedly in response to repeated depolarizing stimuli. Initially, the Ca2+ that triggers Ca2+-activated Ca2+ release through the RYR was thought to come from the extracellular milieu, admitted through the skeletal muscle voltage-gated Ca2+ channel (CaV1.1). In support, depolarizing in the presence of a CaV1.1 channel blocker, such as a dihydropyridine (DHP), abolished the response to depolarization. It was surprising that contractions that were blocked by DHPs could still be elicited in totally Ca2+-free media. Activation of the RYR did not depend on an initial Ca2+ influx through the channel but did nevertheless depend on the presence of a fully functional voltage and DHP-sensitive complex. The molecular basis for this phenomenon became clear when it was discovered that upon membrane depolarization, the Ca2+ channel changed its conformation and interacted physically with the nearby RYR, triggering its initial opening, initial Ca2+ release, and the ensuing explosive Ca2+-activated Ca2+ release responsible for activation of the actomyosin contractile machinery (reviewed in reference [64]).
The conformational coupling model for TRPC activation is based on the skeletal muscle excitation-coupling model, in which the information flow is from a plasma membrane ion channel (CaV1.1) to a endomembrane Ca2+ release channel (RYR), but with the flow of information (i.e., the sense of the signaling pathway) inverted. The model postulates that IP3, the same stimulus that activates the IP3R to release Ca2+ from the endoplasmic reticulum Ca2+ stores, also activates a TRPC-activating function of the IP3 receptor. Activation of TRPCs would come about by physical binding of a TRPC-binding domain of the IP3R to TRPC with attendant activation of the affected TRPC.
This hypothesis was tested in our laboratory in the late 1990s using a GST pull-down approach [65]. We found that a region on the post-transmembrane C-terminal domain of TRPCs interacts with a region located between (IP3R-3 numbering) amino acids 675 and 800 of the IP3Rs. This lies about 100aa C-terminal to the IP3 binding domain (aa 225–575) distal to the C-terminally located ion channel forming trans-membrane domains (aa 224–2565) of the 2761 aa IP3R-2. More important, transient overexpression of GST-fusion fragments of TRPC-interacting sequences of the IP3R either inhibited or extended the duration of Ca2+ influx through endogenous receptor-or store depletion–activated Ca2+ entry channels [65]. Thus, IP3R sequences identified as TRPC binding sequences affect Ca2+ entry that is postulated to be mediated by TRPC channels. The regions identified on IP3R-2 are conserved in IP3R-1 and IP3R-3, and the sequences identified on TRPC3 as interacting with IP3Rs are preserved in other TRPCs.
Researchers in Sage’s laboratory [66] used a different approach to test the conformational coupling hypothesis. Working with human platelets, they probed for co-immunoprecipitation of endogenous TRPC1 with endogenous IP3R. No IP3R co-immunoprecipitated from control lysates of control platelets, but activation of Ca2+ influx secondary to store depletion, induced by inactivation of SERCA pumps with thapsigargin, resulted in IP3R co-immunoprecipitating with the human TRPC1. As was the case in earlier experiments with A7r5 and DDT1-MF2 smooth muscle cell lines [62], induction of cortical actin in platelets with jasplakinolide blocked the interaction of the IP3R with TRPC1. These experiments are especially relevant because the activation-dependent interaction of IP3R with TRPC was shown in a normal cell with normal complements of the interacting partners. Interactions observed under these conditions do not suffer from the drawbacks of interactions shown only on overexpression of the interacting partners. It remains to be determined how IP3R-mediated activation of TRPC channels interfaces with STIM1 translocation and activation of Icrac.
Activation of TRPCs by Diacylglycerol (DAG) and Inhibition of TRPCs by Protein Kinase C (PKC)
In 1999, Hofmann et al. [67] reported that diacylglycerols, one of the two reaction products resulting from PLC activation, activate TRPC3 and TRPC6, and that they do so independently of PKC activation. That same year, Okada et al. [25] reported the same finding for the newly cloned TRPC7. In both studies the effects of DAGs could be augmented by inhibiting DAG lipase or DAG kinase, thus sparing DAG removal, and were insensitive to PKC inhibitors. The study by Okada et al. [25] also showed that activation of PKC inhibits activation of TRPC7 by subsequently adding DAG and indicating opposite roles for PKC and DAG.
A superficial examination of DAG’s effects on TRPC4 and TRPC5 does not show stimulation by DAG. Yet upon inhibition of PKCs, both TRPCs are robustly activated by DAG [25,55]. Indeed, activation of PKC inactivates all TRPCs so far studied for this effect (TRPC3 through TRPC7), not only for activation by DAG but also by the Gq-coupled and the RTK (receptor tyrosine kinase) signaling pathways [25,55,68]. But, the rates at which DAG and PKC act on the TRPCs differ with TRPC subtype. Thus, for the TRPC3 family of TRPCs (TRPC3, TRPC6, and TRPC7), activation by DAG is faster than phosphorylation by PKC so that activation by DAG is the dominant phenomenon. On the other hand, for TRPC4 and TRPC5, the DAG-induced inhibition by PKC is established before DAG can activate the TRPC channel. It has not been reported whether this differs after induction of cortical actin.
DAG also activates the TRPC2 channel in its native environment, the vomero-nasal sensory neuron [69]. The effect is fast, potentiated by DAG lipase and DAG kinase inhibitors and unaffected by PKC inhibitors. Whether PKC is inhibitory to TRPC2, as it is for TRPC3 through TRPC7, has not been reported. Given that the Ser phosphorylated by PKC is located in a motif that is conserved in all TRPCs [68], it is tempting to suggest that PKC is inhibitory to all. In agreement with the interpretation that the channel being activated by DAG in vomeronasal sensory neurons is indeed TRPC2, genetic ablation of TRPC2 resulted in loss of the DAG-activated current [69].
Activation of Ca2+ Entry by Channel Translocation from Endomembranes to the Plasma Membrane
Insulin stimulates glucose uptake into its target tissues by promoting the translocation of GLUT4-bearing endovesicles to the plasma membrane. The incorporation of AMPA-type glutamate receptors into the postsynaptic membrane of neurons undergoing high-frequency stimulation is the basis for the establishment of early long-term memory (eLTP) in hippocampal CA1 neurons. The TRPV2 channel, also called the growth factor–regulated channel or GRC, transitions from internal membranes to the plasma membrane after myotubes are treated with insulin-like growth factor-1 (IGF-1). Boulay and coworkers [70] tested whether TRPC6 might be under similar control and indeed saw an increase of cell surface TRPC6, as seen by an increase of biotinylated TRPC6 by immunostaining within 30 sec of stimulating cells either with an agonist for a Gq-coupled GPCR (M5R) or with the store-depleting agent thapsigargin. In a different context, Clapham and collaborators showed that activation of Rac1 in hippocampal neurons leads to translocation of TRPC5 from endomembranes to the plasma membrane in a process that involves activation by Rac1 of PIP(5)K (phophatidylinositol-4-phosphate 5-kinase) and synthesis of PIP2 (phosphatidylintositol-4,5-bisphosphate). Rac1 in turn is activated by stimulation of an RTK (e.g., EGFR) activating PI3K with formation of PIP3 (phosphatidyl-inositol-3,4,5-triphosphate), which in turn activates a Rac1 guanine nucleotide exchange factor (Rac-GEF), leading to augmented GTP-Rac1 [71]. This signaling mechanism (RTK to TRPC5 incorporation into plasma membrane) has been implicated in Ca2+-dependent repression of neurite outgrowth, because there is an inverse correlation of PIP(5)K with neurite length.
Physiological Role for TRPC Channels as Electrogenic Devices That Couple GPCR-Gq Activation to Voltage-Gated Ca [2]+ Channel Activation
Activation of nonselective monovalent cation channels leads to a collapse of the membrane potential. Many TRP and TRP-related channels are mostly nonselective cation channels, some with selectivity for monovalent cations over divalents, some nonselective with respect to both monovalent and divalent cations. As such, activation of these types of channels dissipates the transmembrane potential of the cells in which the channels are expressed. This property was highlighted in a report from the Fleig-Penner laboratory in which the channel properties of TRPM5, a Ca2+-activated nonselective monovalent cation channel (i.e., a CAN), was characterized [72]. More recently, Soboloff et al. [73], studying the effect of down-regulating TRPC6 with small interfering RNA (siRNA), found that, in A7r5 smooth muscle cells, TRPC6 fulfills the role of an electrogenic coupling mechanism by coupling a Gq-coupled GPCR to Ca2+ influx through a dihydropyridine-sensitive Ca2+ channel. This became apparent when, upon siRNA treatment, they saw a >90 percent reduction of muscarinic receptor-stimulated TRPC6 channel activity, measured by the patch clamp technique, with essentially no loss of Ca2+ influx, measured with the fluorescent Ca2+ indicator dye, fura2. Muscarinic receptor-stimulated Ca2+ influx into siRNA-treated cells was completely inhibited by a dihydropyridine Ca2+ channel blocker (see reference [73]). Although this is the first demonstration of an electrogenic coupling role for a TRPC channel, it is likely that other examples are soon to follow, especially in natural tissue cells.
A Role for Tyrosine Kinases in Voltage-Gated Ca2+ Channel-Independent Ca2+ Influx
The original observation that tyrosine phosphorylation may be an important regulator participating in activation of receptor- and store-operated Ca2+ entries (ROCE and SOCE) in nonexcitable cells came from studies showing an inhibitory effect of tyrosine kinase inhibitors on these forms of calcium entry in human foreskin fibroblasts [74,75]. This was followed by studies that showed the total loss of bradykinin-stimulated ROCE and partial absence of SOCE in embryonic fibroblasts derived from mice lacking the src tyrosine kinase [76]. Given that evidence has accumulated showing that tyrosine phosphorylation is a common consequence associated with cell stimulation via receptors that signal by using the Gq-PLC-calcium mobilizing pathway [77,78], we became interested in the possibility that tyrosine phosphorylation may be an activating signal for one of the events that leads to receptor- or store-operated calcium entry. Indeed, experiments parallel to ours that tested for a role of tyrosine kinases in the receptor-mediated activation of the type 3 TRPC (TRPC3) revealed that the activation of this TRPC by a PLC-stimulating GPCR in HEK cells is inhibited by inhibitors of tyrosine kinases; when expressed in src kinase–negative cells, the transfected TRPC3 is not activated by a cotransfected Gq-coupled receptor [79]. This recapitulated the earlier findings with an endogenous (bradykinin-activated) GPCR acting via Gq activation on the endogenous complement of the receptor-operated Ca2+ entry pathway [76].
STUDIES ON THE ROLE(S) OF TYROSINE PHOSPHORYLATION IN TRPC FUNCTION
During the last few years and in order to learn more about the possible role of tyrosine kinase(s) in TRPC-mediated events, as well as in SOCE, we used in vitro and in cell protein–protein interaction assays [80]. We found that c-src phosphorylates TRPC3 on tyrosine 226 (Y226) located on the TRPC3 N-terminus and that formation of phospho-Y226 is essential for TRPC3 activation. Surprisingly, this requirement is unique for TRPC3, because (1) mutation of the cognate tyrosines of the closely related TRPC6 and TRPC7 channels had no effect on their function; (2) both TRPC6 and TRPC7 were activated in src-, yes-, and fyn-deficient cells; and (3) src, but not yes or fyn, rescued TRPC3 activation in cells lacking src, yes, and fyn. Yet we found the SH2 domain of c-src to interact not only with TRPC3 but also with either the N-termini or the C-termini of all other TRPCs. This suggests that other tyrosine kinases may play a role in ion fluxes mediated by TRPCs.
A side-by-side comparison of the effects of genistein on endogenous ROCE and SOCE in YF, SYF, HEK, and COS-7 cells showed these influxes to be inhibitable in all three cell types but with differing sensitivities (Figures 1.7 and 1.8). Taken together, these results argue for the channels mediating ROCE and SOCE to be heterogeneous and to differ from tissue to tissue.

FIGURE 1.7
Comparison of the inhibitory effect of genistein on ROCE and SOCE endogenous to HEK cells, COS-7 cells, and mouse embryonic fibroblasts lacking the indicated members of the src-family of tyrosine kinases. In the experiments shown in this and the other (more...)

FIGURE 1.8
Comparison of the inhibitory effect or lack thereof of genistein on ROCE mediated by the indicated TRPC channels as seen in HEK cells. Adapted from Kawasaki et al. [80]
The finding that TRPC6 is active in SYF and YF cells was unexpected, as it has been shown to be a substrate of fyn [81] and to behave essentially the same as TRPC3 in in vitro and cell expression assays. Thus, TRPC6 is phosphorylated by coexpression with fyn in COS cells, and it associates with fyn in GST pull-down assays by interaction of its N-terminus with the SH2 domain of fyn [81], and TRPC6 activation is inhibited by the tyrosine kinase inhibitor PP2, regardless whether it is activated by a receptor-tyrosine kinase-PLCγ pathway (triggered by EGF) [81] or by the DAG pathway [73]. Further, addition of fyn to inside-out membrane patches from cells expressing TRPC6 increased basal and oleyl-acetyl-glyceride (OAG)–stimulated TRPC6 activity [81]. Yet, TRPC6 ROCE is activated in cells lacking not only fyn but also yes and src (Figures 1.7 and 1.8). Not known at this time is whether this discrepancy is either due to our use of the GPCR-Gq-PLCβ activation pathway instead of a RTK-PLCγ pathway (which might be impaired in SYF cells) or due to the different form of assessing TRPC6 ROCE (fura2 in our case and electrophysiological in inside-out membrane patches in the case of Hisatsune et al.) [81]. If, however, the lack of sensitivity of TRPC6 to genistein in our experiments and the activity of TRPC6 in SYF cells is not due to the use of differing activation pathways or to the method used to assess TRPC6 activation, the data may also indicate that functionally, in addition to src, fyn, and yes, there is at least one more “PP2-sensitive src-family tyrosine kinase” able to regulate TRPC6 and other TRPCs. As mentioned, the fact that TRPC1 through C7 all interacted with src in GST pull-down assays raises the possibility that all TRPC channels depend on tyrosine phosphorylation for their functioning as an effector system for the activation of the GPCR-Gq-PLCβ/RTK-PLCγ pathways.
Table 1.3 summarizes properties of TRPC channels in terms of which signaling pathways have been shown to activate each channel and also possible mechanisms by which each is activated. Two pathways feed into TRPCs. One generates DAG from the action of PLCβ-activated by Gq and Gi-derived Gβγ, and the other generates DAG from the action of PLCγ-activated by tyrosine phosphorylation either directly by the RTK-type receptor or secondarily to non-RTK tyrosine kinase recruitment such as happens with T- and B-cell receptor activation. Activation of TRPCs by DAG may be aided by IP3R acting by protein–protein interaction according to a conformational coupling model and by the formation of a multimolecular signaling complex with or without involvement of a protein acting as a nucleating scaffold.
TABLE 1.3
Regulation of TRPC Channels: Comparison to Endogenous ROCE and SOCE of HEK Cells, yes- and fyn-Deficient Mouse Embryonic Fibroblasts (YF MEFs), COS-7 Cells, and HSWP Human Foreskin Fibroblasts
A direct IP3R–TRPC interaction, especially if facilitated by the action STIM1 [10–12], may also account for activation of TRPC channels by store depletion.
TRPCs and SOCE
In contrast to the rather satisfactory models one can set up for explaining how TRPC channels may be activated (Figure 1.6), there are only scant data that suggest how, if at all, TRPC channels participate in SOCE. The strongest data set was recently published by Villereal studying the natural channels that contribute to SOCE in HEK293 cells testing for interference with siRNA. More than 80 percent downregulation of the naturally expressed TRPC1, TRPC3, and TRPC7 proteins resulted in a ca. 50 percent reduction in thapsigargin-induced SOCE [82]. The 50 percent loss is curious, because it happens to coincide with the similar loss of acetyl choline–induced NO-mediated vascular relaxation of aortic rings seen in mice lacking TRPC4 [83].
The missing information or hypothesis relating TRPCs to SOCE is how nonse-lective cation channels may come together to form a highly Ca2+-selective ion channel. On a purely speculative level there are three possibilities that come to mind. One is that ion selectivity does change in channels that are heteromeric in nature. The other is that ion selectivity is altered by post-translational modification. Dietrich et al. [84] have shown that basal or constitutive activity is affected by glycosylation. Kawasaki et al. [80] found that at least one channel, TRPC3, depends on tyrosine phosphorylation for activity. The effect of compound kinase actions has not been explored. Groschner and collaborators, by showing that in HEK cells TRPC3-mediated Ca2+ influx depends on extracellular Ca2+ and a functional Na-Ca exchanger operating in reverse [85], raised the possibility that the Ca2+ selectivity is the result of a tandem arrangement whereby Na+ entering through a TRPC channel is extruded not only by the Na-K ATPase but also by the Na-Ca exchanger, an exquisitely Ca2+-selective machine.
The third possibility regarding the molecular makeup of ROCE, and especially SOCE channels, is that the field may have been somewhat naive in assuming that there is only one Icrac channel. The fact that inhibition of endogenous SOCE by genistein shows varying degrees of sensitivity may indicate that SOCE channels—presumed to be equivalent to Icrac channels—are heterogenous in nature. If so, TRP-related channels other than TRPCs may form SOCE channels. Participation of various TRPVs, especially TRPV5 and TRPV6, comes to mind. In line with this reasoning, Schindl et al. [86] noted commonalities between TRPV6 (CaT1) and Icrac. The studies reported by Rosker et al. [85], implicating the Na-Ca exchanger in ROCE mediated by TRPC3 in HEK cells, prompted us to test the effectiveness with which the Na-Ca exchanger (NCX) inhibitor KB-R7943 affects ROCEs mediated by several TRPCs in HEK cells and ROCEs mediated by endogenous ROCE channels in HEK and other cells. The picture that emerged is not one to support an obligatory role for Na-Ca exchangers in ROCE or SOCE—for this KB-R7943 is too nonspecific—but one that suggests that endogenous channels mediating ROCE and SOCE are likely to be heterogeneous in their molecular makeup (Figure 1.9).

FIGURE 1.9
Comparison of the effect of the partially selective Na-Ca exchange inhibitor, KB-R7943, on ROCE mediated by TRPCs and on ROCE and SOCE mediated by endogenous channels.
ROCE and SOCE are forms of Ca2+ entry with specific functions in a large list of diverse cellular functions that include smooth muscle contraction, B- and T-cell activation, and vascular permeability and development of the central nervous system, to name a few. Yet, the molecular makeup of the channels mediating ROCE and SOCE is still largely a matter of speculation. New tools are needed to confirm or negate the hypothesis that TRPCs alone or in combination with other members of the TRP superfamily participate in these forms of Ca2+ entry.
ACKNOWLEDGMENTS
Research was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences.
REFERENCES
- 1.
- Pak WL, Grossfield J, Arnold K. Mutant of the visual pathway of Drosophila melanogaster. Nature. 1970;227:518. [PubMed: 5428475]
- 2.
- Hotta Y, Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc. Natl. Acad. Sci. USA. 1970;67:1156. [PMC free article: PMC283331] [PubMed: 5274445]
- 3.
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313. [PubMed: 2516726]
- 4.
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631. [PubMed: 1314616]
- 5.
- Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze D, Schilling WP. Appearance of a novel Ca2+-influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochem. Biophys. Res. Commun. 1994;132:346. [PubMed: 7516156]
- 6.
- Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z. Coupling of photoexcited rhodopsin to inositol phos-pholipid hydrolysis in fly photoreceptors. Proc. Natl. Acad. Sci. USA. 1987;84:6939. [PMC free article: PMC299200] [PubMed: 3116547]
- 7.
- Selinger Z, Minke B. Inositol lipid cascade of vision studied in mutant flies. Cold Spring Harbor Symp. Quant. Biol. 1988;53:333. [PubMed: 3151172]
- 8.
- Hu Y, Schilling WP. Receptor-mediated activation of recombinant trpl expressed in Sf9 insect cells. Biochem. J. 1995;305:605. [PMC free article: PMC1136405] [PubMed: 7832780]
- 9.
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc. Natl. Acad. Sci. USA. 1996;93:15195. [PMC free article: PMC26380] [PubMed: 8986787]
- 10.
- Liou L, Kim ML, Jones JT, Myers JW, Ferrel JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235. [PMC free article: PMC3186072] [PubMed: 16005298]
- 11.
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Vilicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435. [PMC free article: PMC2171946] [PubMed: 15866891]
- 12.
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902. [PMC free article: PMC1618826] [PubMed: 16208375]
- 13.
- Kwan CY, Takemura H, Obie JF, Thastrup O, Putney JW Jr. Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am. J. Physiol. 1990;258:C1006. [PubMed: 2360617]
- 14.
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+ ATPase. Proc. Natl. Acad. Sci. USA. 1990;87:2466. [PMC free article: PMC53710] [PubMed: 2138778]
- 15.
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353. [PubMed: 1309940]
- 16.
- Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1. [PubMed: 2420465]
- 17.
- Putney JW Jr. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611. [PubMed: 1965707]
- 18.
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA. 1993;90:6295. [PMC free article: PMC46915] [PubMed: 8392195]
- 19.
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptor cells. Neuron. 1992;8:643. [PubMed: 1314617]
- 20.
- Hardie RC, Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends in Neurosci. 1993;9:371. [PubMed: 7694408]
- 21.
- Sinkins WG, Vaca L, Hu Y, Kunze DL, Schilling WP. The COOH-terminal domain of Drosophila TRP channels confers thapsigargin sensitivity. J. Biol. Chem. 1996;271:2955. [PubMed: 8621686]
- 22.
- Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193. [PubMed: 7589464]
- 23.
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA. 1995;92:9652. [PMC free article: PMC40860] [PubMed: 7568191]
- 24.
- Zhu X, Jiang M, Peyton MJ, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ influx. Cell. 1996;85:661. [PubMed: 8646775]
- 25.
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse TRP homologue TRP7 that forms a background and receptor-activated Ca2+ permeable cation channel. J. Biol. Chem. 1999;274:27359. [PubMed: 10488066]
- 26.
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Caterina MJ, Clapham DE, Heller S, Julius D, Scharenberg AM, Schultz G, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 2002;9:229. [PubMed: 11864597]
- 27.
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:7461. [PMC free article: PMC124253] [PubMed: 12032305]
- 28.
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of trp1 and trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J. Biol. Chem. 2000;275:27799. [PubMed: 10882720]
- 29.
- Struebing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 2003;278:39014. [PubMed: 12857742]
- 30.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816. [PubMed: 9349813]
- 31.
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436. [PubMed: 10201375]
- 32.
- Kanzaki M, Zhang Y-Q, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factorI. Nature Cell Biol. 1999;1:165. [PubMed: 10559903]
- 33.
- Hunter JJ, Shao J, Smutko JS, Dussault BJ, Nagle DL, Woolf EA, Holmgren LM, Moore KJ, Shyjan AW. Chromosomal localization and genomic characterization of the melastatin gene (Mlsn 1). Genomics. 1998;54:116. [PubMed: 9806836]
- 34.
- Veldhuisen B, Spruit L, Dauwerse HG, Breuning MH, Peters DJM. Genes homologous to the autosomal dominant polycystic kidney disease genes (PKD1 and PKD2). Eur. J. Hum. Genet. 1999;7:860. [PubMed: 10602361]
- 35.
- Birnbaumer L, Yildirim E, Abramowitz J. A comparison of the genes coding for canonical TRP channels and their M, V, and D relatives. Cell Calcium. 2003;33:419. [PubMed: 12765687]
- 36.
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Bove C, Kaneski CR, Nagel J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type 4 is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 2000;9:2471. [PubMed: 11030752]
- 37.
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden HC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819. [PubMed: 12654248]
- 38.
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin S-Y, Vollrath MA, Amalfitano A, Cheung EL-M, Derfler BH, Duggan A, Geleoc GSG, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;342:723. [PubMed: 15483558]
- 39.
- Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem. 1999;274:8375. [PubMed: 10085067]
- 40.
- Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem. 1999;274:22739. [PubMed: 10428857]
- 41.
- Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium release-activated calcium channel. Nature. 2001;410:705. [PubMed: 11287959]
- 42.
- Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol. Sci. 2004;25:633. [PubMed: 15530641]
- 43.
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52. [PubMed: 11882888]
- 44.
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745. [PubMed: 15674243]
- 45.
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600. [PubMed: 15681390]
- 46.
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898. [PubMed: 15758951]
- 47.
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894. [PubMed: 15758952]
- 48.
- Liman ER, Corey DP, Dulac C. TRP2, a candidate transduction channel for mammalian pheromone sensory signaling. Proc. Natl. Acad. Sci. USA. 1999;96:5791. [PMC free article: PMC21939] [PubMed: 10318963]
- 49.
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRPC2. Science. 2002;295:1493. [PubMed: 11823606]
- 50.
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behavior. Nature Rev. Neurosci. 2003;4:551. [PubMed: 12838330]
- 51.
- Zufall F, Ukhanov K, Lucas P, Leinders-Zufall T. TRPC2: from gene to behavior. Pfluegers Arch. Eur. J. Physiol. 2005;451:61. [PubMed: 15971083]
- 52.
- Vannier B, Zhu MX, Brown D, Birnbaumer L. The membrane topology of hTRP3 as inferred from glycosylation scanning mutagenesis and epitope immunocytochemistry. J. Biol. Chem. 1998;273:8675. [PubMed: 9535843]
- 53.
- Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human trp3 stably expressed in human embryonic kidney (HEK) 293 cells. Evidence for a non-capacitative Ca2+ entry. J. Biol. Chem. 1998;273:133. [PubMed: 9417057]
- 54.
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723. [PubMed: 2457447]
- 55.
- Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 2003;278:29031. [PubMed: 12721302]
- 56.
- Shieh B-H, Zhu M-Y. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991. [PubMed: 8630257]
- 57.
- Huber A, Sander P, Gobert A, Baehner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC, and InaD. EMBO J. 1996;15:7036. [PMC free article: PMC452529] [PubMed: 9003779]
- 58.
- Xu X-ZS, Choudhury A, Li X, Montell C. Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J. Cell Biol. 1998;142:545. [PMC free article: PMC2133053] [PubMed: 9679151]
- 59.
- Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95. [PubMed: 9010208]
- 60.
- Montell C. Physiology, phylogeny and functions of the TRP superfamily of cation channels. Science’s SKTE. 2001. http://skte
.sciencemag .org/cgi/content/full /OC_sigtrans;2001/90/re1. [PubMed: 11752662] - 61.
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Rameshi V, Zhu MX. Association of mammalian Trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J. Biol. Chem. 2000;275:37559. [PubMed: 10980202]
- 62.
- Patterson RL, van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487. [PubMed: 10481913]
- 63.
- Kim JY, Saffen D. Activation of M1 muscarinic acetylcholine receptors stimulates the formation of a multiprotein complex centered on TRPC6 channels. J. Biol. Chem. 2005;280:32035. [PubMed: 15994335]
- 64.
- Rios E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol. Rev. 1991;71:849. [PubMed: 2057528]
- 65.
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that binds transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc. Natl. Acad. Sci. USA. 1999;96:14955. [PMC free article: PMC24754] [PubMed: 10611319]
- 66.
- Rosado JA, Sage SO. Activation of store-mediated calcium entry by secretion-like coupling between the inositol 1,4,5-trisphosphate receptor type II and human transient receptor potential (hTrp1) channels in human platelets. Biochem. J. 2001;356:191. [PMC free article: PMC1221827] [PubMed: 11336651]
- 67.
- Hofmann T, Obukhov AG, Scharfer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacyglycerol. Nature. 1999;397:259. [PubMed: 9930701]
- 68.
- Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW Jr. Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol. Pharmacol. 2005;67:558. [PubMed: 15533987]
- 69.
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551. [PubMed: 14642279]
- 70.
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J. Biol. Chem. 2004;279:7241. [PubMed: 14662757]
- 71.
- Bezzerides V, Ramsey S, Greka A, Clapham DE. Rapid translocation and insertion of TRPC5 channels. Nature Cell Biol. 2004;6:709. [PubMed: 15258588]
- 72.
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397. [PubMed: 12015988]
- 73.
- Soboloff J, Spassova M, Xu W, He LP, Cuestal N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J. Biol. Chem. 2005;280:39786. [PubMed: 16204251]
- 74.
- Lee K-M, Toscas K, Villereal ML. Inhibition of bradikynin- and thapsigargin-induced calcium entry tyrosine kinase inhibitors. J. Biol. Chem. 1993;268:9945. [PubMed: 7683685]
- 75.
- Lee K-M, Villereal ML. Tyrosine phosphorylation and activation of pp60c-src and pp125FAK in bradikynin-stimulated fibroblasts. Am. J. Physiol. 1996;270:C1430. [PubMed: 8967444]
- 76.
- Babnigg G, Bowersox SR, Villereal ML. Role of pp60c-src in the regulation of calcium via store-operated calcium channels. J. Biol. Chem. 1997;272:29434. [PubMed: 9368000]
- 77.
- Gutkind JS, Robbins KC. Activation of transforming G-protein–coupled receptors induces rapid tyrosine phosphorylation of cellular proteins including p125FAK and p130src substrate. Biochem. Biophys. Res. Commun. 1992;188:155. [PubMed: 1329743]
- 78.
- Igishi T, Gutkind JS. Tyrosine kinases of the src family participate in signaling to MAP kinase from both Gq- and Gi-coupled receptors. Biochem. Biophys. Res. Commun. 1998;244:5. [PubMed: 9514877]
- 79.
- Vazquez G, Wedel BJ, Kawasaki BT, StJohn Bird G, Putney J. Obligatory role for src kinase in the signaling mechanism for TRPC3 cation channels. J. Biol. Chem. 2004;279:40521. [PubMed: 15271991]
- 80.
- Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc. Natl. Acad. Sci. USA. 2006;103:335. [PMC free article: PMC1326167] [PubMed: 16407161]
- 81.
- Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mitsutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 2004;279:18887. [PubMed: 14761972]
- 82.
- Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J. Biol. Chem. 2005;280:29559. [PubMed: 15972814]
- 83.
- Freichel M, Vennekens R, Olausson J, Hoffmann M, Muller C, Stolz S, Scheunemann J, Weissgerber P, Flockerzi V. Functional role of TRPC proteins in vivo: lessons from TRPC4-deficient mouse models. Biochem. Biophys. Res. Commun. 2004;322:1352. [PubMed: 15336983]
- 84.
- Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J. Biol. Chem. 2003;278:47842. [PubMed: 12970363]
- 85.
- Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, Groschner K. Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J. Biol. Chem. 2004;279:13696. [PubMed: 14736881]
- 86.
- Schindl R, Kahr H, Graz I, Groschner K, Romanin C. Store depletion-activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2-aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca2+ release-activated Ca2+ channel) channels. J. Biol. Chem. 2002;277:26950. [PubMed: 12011062]
- 87.
- Zhang Z, Tang T, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of TRP3 by IP3 receptor through displacement of inhibitory calmodulin from a common binding site. Proc. Natl. Acad. Sci. USA. 2001;98:3168. [PMC free article: PMC30625] [PubMed: 11248050]
- 88.
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and IP3 receptors on the carboxyl-termini of TRP channels. J. Biol. Chem. 2001;276:21303. [PMC free article: PMC1847329] [PubMed: 11290752]
- 89.
- Trost C, Bergs C, Himmerkus N, Flockerzi V. The transient receptor potential, TRP4, cation channel is a novel member of the family of calmodulin-binding proteins. Biochem. J. 2001;355:663. [PMC free article: PMC1221781] [PubMed: 11311128]
- 90.
- Yildirim E, Dietrich A, Birnbaumer L. Characterization of mouse TRPC2 transcripts: alternative splicing of the primary transcript and calmodulin binding to the translated N-terminus. Proc. Natl. Acad. Sci. USA. 2003;100:2220. [PMC free article: PMC151321] [PubMed: 12601176]
- 91.
- Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J. Biol. Chem. 2000;275:3403. [PubMed: 10652333]
- 92.
- Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capactitative Ca2+ entry channel. Proc. Natl. Acad. Sci. USA. 1999;96:2060. [PMC free article: PMC26736] [PubMed: 10051594]
- 93.
- Jungnickel MS, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates Ca2+ entry into sperm triggered by egg ZP3. Nature Cell Biol. 2001;3:499. [PubMed: 11331878]
- 94.
- Vazquez G, Wedel BJ, Trebak M, Bird GS, Putney JW Jr. Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J. Biol. Chem. 2003;278:21649. [PubMed: 12686562]
- 95.
- Yildirim E, Kawasaki BT, Birnbaumer L. Molecular cloning of TRPC3a, an N-terminally extended, store-operated variant of the human C3 transient receptor potential channel. Proc. Natl. Acad. Sci. USA. 2005;102:3307. [PMC free article: PMC552946] [PubMed: 15728370]
- 96.
- Philipp S, Cavalie A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996;15:6166. [PMC free article: PMC452437] [PubMed: 8947038]
- 97.
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274. [PMC free article: PMC1170761] [PubMed: 9687496]
- 98.
- Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JD, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J. Biol. Chem. 2002;277:12302. [PubMed: 11805119]
- 99.
- Lievremont JP, Bird GS, Putney JW Jr. Canonical transient receptor potential TRPC7 can function as both a receptor and store-operated channel in HEK-293 cells. Am. J. Physiol. Cell Physiol. 2004;287:C1709. [PubMed: 15342342]
- 100.
- Schaefer M, Plant TD, Stresow N, Albrecht N, Schultz G. Functional differences between TRPC4 splice variants. J. Biol. Chem. 2002;277:3752–3759. [PubMed: 11713258]
- 101.
- Jung S, Muehle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J. Biol. Chem. 2003;278:3562. [PubMed: 12456670]
- 102.
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular 1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001;88:325. [PubMed: 11179201]
- Review TRP Channels in Vision.[Neurobiology of TRP Channels. ...]Review TRP Channels in Vision.Katz B, Payne R, Minke B. Neurobiology of TRP Channels. 2017
- Direct activation of trpl cation channels by G alpha11 subunits.[EMBO J. 1996]Direct activation of trpl cation channels by G alpha11 subunits.Obukhov AG, Harteneck C, Zobel A, Harhammer R, Kalkbrenner F, Leopoldt D, Lückhoff A, Nürnberg B, Schultz G. EMBO J. 1996 Nov 1; 15(21):5833-8.
- Review Photosensitive TRPs.[Handb Exp Pharmacol. 2014]Review Photosensitive TRPs.Hardie RC. Handb Exp Pharmacol. 2014; 223:795-826.
- The Drosophila light-activated conductance is composed of the two channels TRP and TRPL.[Cell. 1996]The Drosophila light-activated conductance is composed of the two channels TRP and TRPL.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. Cell. 1996 May 31; 85(5):651-9.
- Properties of single Drosophila Trpl channels expressed in Sf9 insect cells.[Am J Physiol. 1997]Properties of single Drosophila Trpl channels expressed in Sf9 insect cells.Kunze DL, Sinkins WG, Vaca L, Schilling WP. Am J Physiol. 1997 Jan; 272(1 Pt 1):C27-34.
- The TRPC Family of Ion Channels: Relation to the TRP Superfamily and Role in Rec...The TRPC Family of Ion Channels: Relation to the TRP Superfamily and Role in Receptor- and Store-Operated Calcium Entry - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...