NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsAbstract
In the pair of the nematode Caenorhabiditis elegans serotonergic chemosensory neurons ADF, the TRPV channel protein OCR-2 interacts with another TRPV protein, OSM-9, to control the production of the neurotransmitter serotonin. The activity and specificity of OCR-2 in the serotonergic neurons is governed by structural determinants within the channel protein in concert with defined cellular components. The dynamic gating mechanisms, multiple sensory modalities, and functional conservation in diverse organisms make TRPV channels ideal candidates for the long-awaited molecular sensors that underscore the ancient role of the serotonergic system in coupling sensory cues and internal milieu to behavior and physiology.
INTRODUCTION
The serotonergic system is an ancient sensor of diverse stimuli. Serotonin (5-hydroxytryptamine; 5-HT) is a monoamine and functions as a neuromodulator participating in the elaboration of adaptive responses to external stimuli and physiological challenges. The first demonstration of serotonin in regulating sensory response was from studies in Aplysia, where increased serotonin promotes sensitization of the gill-withdrawal reflex in response to repetitive tactile stimulus to the mantle or the edge of the siphon (Brunelli et al., 1976; Bailey et al., 1992). Although a serotonin signal is produced from the interneurons rather than from the sensory neurons in the gill-withdrawal reflex circuitry (Kandel and Schwartz, 1982), this showed that the activity of a specific set of serotonergic neurons is modulated by a specific external cue to influence behavior. Anatomic analysis of the serotonergic system in mammalian CNS revealed their close relationship to blood vessels, leading to the proposal that the raphe serotonergic neurons may directly function as chemoreceptors and mech-anoreceptors to transduce diverse neuronal and nonneuronal signals into discrete behavior and physiological processes (Scheibel et al., 1975; Azmitia, 1999). Consistent with this notion, application of distinct chemical and physical stimuli to whole organisms or brain slices induces up- and downregulation of serotonin synthesis in discrete serotonergic neurons in the CNS (Boadle-Biber, 1993; Leibowitz and Alexander, 1998; Azmitia, 1999; Chaouloff et al., 1999; Adell et al., 2002).
If serotonergic neurons can discriminate ligands or cues that reflect external stimuli and internal milieu, molecular biology predicts that different serotonergic neurons express different receptors. Two such cell-specific regulators have been identified in C. elegans: the TRPV (vanilloid subfamily of transient receptor potential) channel proteins OCR-2 and OSM-9. This chapter highlights the conservation of the serotonergic system in C. elegans, describes the role of OCR-2 and OSM-9 in the serotonergic neurons, explains how these two TRPV channel proteins can be activated by multiple sensory stimuli but maintain specificity to each modality, and, finally, discusses the possibility of serotonin as a conserved readout of TRP channel signaling.
CONSERVATION OF THE SEROTONERGIC SYSTEM IN C. ELEGANS
As in all animals, serotonergic neurons represent a small population in C. elegans. A mature hermaphroditic worm has exactly 302 neurons (White et al., 1986). The nine neurons that can be stained by an antibody raised against serotonin are composed of five neural classes, with four classes consisting of two bilaterally symmetric neurons (Horvitz et al., 1982; Sze et al., 2000). C. elegans does not have a defined CNS; nevertheless, the serotonergic neurons are connected to the central neuronal integration center called the nerve ring (White et al., 1986) and influence sensory behavior, both innate and learned, and diverse physiological processes (for examples, see Weinshenker et al., 1995; Colbert and Bargmann, 1997; Nurrish et al., 1999; Sze et al., 2000; Sawin et al., 2000; Zhang et al., 2005). ADF are the only serotonergic sensory neurons in the animal. The sensory cilia of ADF are exposed to the external environment (White et al., 1986) and sense salts (Bargmann and Horvitz, 1991; Hukema et al., 2006). A recent study showed that serotonin produced from ADF mediates olfactory learning (Zhang et al., 2005). NSM are secretory neurons with their cell bodies located in the pharynx and are likely sensing signals released from food (Sawin et al., 2000). HSN are motor neurons that couple feeding status and the rhythm of vulva muscle contractions to release fertilized eggs (Desai et al., 1988). RIH and AIM are interneurons, bridging between sensory inputs and command outputs (White et al., 1986). The unique, distinguishable function of the serotonergic neurons in C. elegans provides a genetic tractable model to investigate the genes that regulate serotonergic phenotypes in identified neural types.
Classic mutant screens in conjunction with gene knockout technology have led to the isolation of mutant animals with deficits in serotonin biosynthesis, serotonin release, and serotonin receptors (Duerr et al., 1999; Sze et al., 2000; Ranganathan et al., 2000; Hare and Loer, 2004; Dempsey et al., 2005). Genes used to produce serotonergic phenotypes are highly conserved between worms and humans. Three of those—tph-1, cat-1, and mod-5—are relevant here. The tph-1 gene encodes the tryptophan hydroxylase that catalyzes the rate-limiting first step of serotonin biosynthesis, and the tph-1 deletion mutant has no detectable serotonin (Sze et al., 2000). Like in mammals, signaling by serotonin in C. elegans involves two distinct mechanisms of serotonin transporter. cat-1 encodes the vesicular monoamine transporter (VMAT) on the secretory vesicles that pump newly synthesized serotonin into vesicles for regulated exocytosis (Duerr et al., 1999), and mod-5 encodes the membrane serotonin transporter (SERT) for reuptake of extracellular serotonin (Ranganathan et al., 2001). SERT is the target of two major classes of antidepressants: selective serotonin reuptake inhibitors (SSRIs) and tricyclic drugs. It is generally assumed that these drugs exert therapeutic effects by blocking SERT from reuptaking serotonin, thereby increasing the availability of serotonin at the synapse (Blier and de Montigny, 1998). A recent study in our laboratory found that worms either bearing a deletion in the SERT gene mod-5, or wild-type worms treated with the SSRI fluoxetine, accumulate serotonin only in the ADF, NSM, and HSN neurons (C. Dempsey and J. Sze, unpublished). This indicates that two classes of the serotonergic neurons in the head (AIM and RIH) can only absorb extracellular serotonin via MOD-5/SERT and that fluoxetine blocks MOD-5 activity. Such serotonin-absorbing neurons have also been observed in developing rat embryos throughout sensory pathways from the CNS to sensory neurons (Hansson et al., 1998; Lebrand et al., 1998), and in the hypothalamic dorsomedial nucleus in postnatal life (Hoffman et al., 1998). In worms as well as in mammals, these neurons do not express the complete set of serotonin synthesis enzymes, but they do express VMAT. One possible function for these neurons could be serving as “relay stations” that pass serotonin from originating neuron sources to specific serotonin receptor subtypes on distant targets. The potential effects of the blockage of SERT by SSRIs and tricyclic drugs in the pathways mediated by these neurons are fascinating but beyond the scope of this chapter. What is relevant is the conservation of the mechanisms of serotonin neurotransmission and of the principle action of the SSRIs on SERT in the worm, which gives a more concrete reason to use the C. elegans serotonergic system as a model for understanding the fundamental mechanisms that regulate serotonin signaling.
THE FUNCTION AND MECHANISM OF OCR-2 AND OSM-9 IN SEROTONERGIC NEURONS
A role of TRPV channels in serotonin signaling was identified from an unbiased genetic screen for serotonin synthesis mutants (Zhang et al., 2004). Because tryptophan hydroxylase is the key enzyme for serotonin synthesis, and the expression of GFP tagged the tph-1 gene (tph-1::gfp), which can be unambiguously identified and, to some extent, quantified in specific serotonergic neurons of living worms, a goal of this screen was to ask whether tph-1 expression in different classes of neurons is regulated by different genes. It turned out most mutants retrieved from the screen were missing tph-1::gfp expression specifically in one class of the serotonergic neurons. The first two genes identified that regulate tph-1 expression in the ADF chemosensory neurons are the TRPV genes osm-9 and ocr-2. Null mutations in either osm-9 or ocr-2 dramatically downregulate tph-1::gfp expression in the ADF neurons, but tph-1::gfp expression in the NSM and HSN neurons is unaffected in the mutants. And there is no detectable difference in the intensity of tph-1::gfp expression between double mutants of ocr-2 and osm-9 with each of the single mutants.
Several lines of evidence indicate that both OCR-2 and OSM-9 act in the ADF neurons to regulate serotonin production. OCR-2 and OSM-9 are coexpressed in ADF (Tobin et al., 2002; Zhang et al., 2004). By expressing wild-type ocr-2 or osm-9 coding sequences under cell-specific promoters, it was demonstrated that expression of OCR-2 and OSM-9 in ADF is necessary and sufficient to restore tph-1 expression in respective mutants. These findings are consistent with earlier evidence that OCR-2 and OSM-9 require each other for routing to the sensory cilia of the neurons (Tobin et al., 2002), suggesting an OCR-2 and OSM-9 protein complex at the sensory cilia of ADF that regulate tph-1 expression. For the rest of this chapter, the term OCR-2/OSM-9 will be used when they are considered as one complex.
Does OCR-2/OSM-9 represent a molecular sensor that couples sensory cues to serotonin signaling? To address this question, it is important to consider how specific tph-1 downregulation is in the mutants. Biophysical characterization of OCR-2 and OSM-9 in a heterologous expression system has not been successful, but TRP channels are generally considered as calcium-permeable cation channels (Montell, 2005; Pedersen et al., 2005; Ramsey et al., 2006). Disruption of cellular calcium homeostasis can affect a battery of cellular events, even causing cell death (Berridge et al., 2003). However, ocr-2 and osm-9 mutations do not appear to cause cell-fate transformation. In addition to ADF, OSM-9 and OCR-2 are coexpressed in five pairs of nonserotonergic chemosensory neurons: AWA, ADL, ASH in the head, and PHA and PHB in the tail (Tobin et al., 2002). Mutations in osm-9 and ocr-2 do not cause these neurons to degenerate or to exhibit a morphological change detectable at the level of fluorescence microscopy (Colbert et al., 1997; Zhang et al., 2004). Null mutations in osm-9 and ocr-2 also do not reduce the expression of every gene in ADF. In fact, all the ADF neuronal markers that have been examined showed no distinguishable difference between mutants and wild-type animals, including the expression of the cat-1/VMAT gene and the cat-4 gene that encodes the tryptophan hydroxylase cofactor GTP-cyclohydrolase I (Zhang et al., 2004). These findings suggest that the signaling from OCR-2/OSM-9 selectively, if not specifically, regulates tph-1 expression. An attractive model is that the TRPV channel is regulated by environmental signals to in turn regulate serotonin signaling by transcriptional regulation of the key serotonin synthetic enzyme tryptophan hydroxylase.
Characterization of OCR-2/OSM-9 function in other chemosensory neurons provides some consensus why tph-1 is a selected target of the channel in the ADF neurons. ocr-2 and osm-9 mutants also are defective in olfactory sensation to the odorant diacetyl. Diacetyl is sensed by the G-protein-coupled receptor ODR-10, which is expressed specifically in the AWA neurons (Sengupta et al., 1996). odr-10 expression but not several other AWA markers is reduced in ocr-2 and osm-9 mutants (Colbert et al., 1997; Tobin et al., 2002). It seems that OCR-2/OSM-9 signaling regulates the expression of genes unique for sensory detection and sensory output of the particular cell. Such activity-dependent transcriptional regulation of sensory components may endow the TRPV channel to regulate the sensitivity to sensory stimuli and underscore the mechanisms of serotonin signaling in modulating behavior based on the sensory experience.
SPECIFICITY OF OCR-2 AND OSM-9 SIGNALING PATHWAYS IN DIFFERENT SENSORY NEURONS
OCR-2 and OSM-9 are colocalized in the sensory cilia and plasma membrane of four pairs of chemosensory neurons: ADF, AWA, ASH, and ADL. The sensory cues and activation mechanisms of OCR-2/OSM-9 in ADF are not yet determined. Nevertheless, genetic analyses of OCR-2 and OSM-9 in individual sensory neuron functions and their functional interactions with other components in the sensory signaling pathways have produced some basic ideas for understanding and further deducing how the same channel entity mediates multiple sensory modalities but remains faithful to each sensory function in vivo (Figure 18.1).
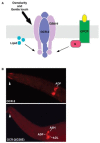
FIGURE 18.1
Polymodality and specificity of OCR-2/OSM-9 in vivo. (A) OCR-2/OSM-9 in different sensory functions are regulated by distinct mechanisms. OCR-2 and OSM-9 likely form a heteromeric channel and are located in the sensory cilia and plasma membrane of four (more...)
Sensory Receptors
One mechanism that dictates OCR-2/OSM-9 modality appears to be G-protein signaling. As stated above, the odorant diacetyl is sensed by the G-protein-coupled receptor ODR-10, which is specifically expressed in the AWA neurons. Thus, it is likely that the activity of OCR-2/OSM-9 in AWA is regulated by ODR-10 and receptor-coupled signaling cascades. There is compelling genetic evidence to suggest that OCR-2/OSM-9 directly senses environmental osmotic strength and tactile stimuli at the nose through the ASH neurons (Liedtke et al., 2003). However, the function of OCR-2/OSM-9 in osmotic and mechanical sensation also depends on the G protein ODR-3 (Roayaie et al., 1998). This suggests that the OCR-2/OSM-9 function in osmotic and mechanical sensation is specified by a compound activation of mechanical gating and G-protein signaling. A mammalian TRPV4 can substitute OSM-9 to direct osmotic and mechanical sensation in C. elegans (Liedtke et al., 2003). The TRPV4 operates via mechanical gating as well as chemical activation in heterologous expression systems (Liedtke et al., 2000; Watanabe et al., 2002; Vriens et al., 2004), suggesting conservation of the function and gating mechanism between worms and mammals.
Components in Signaling Cascades
Besides ocr-2 and osm-9, several essential components involved in diacetyl sensation and osmotic sensation have been identified by genetic approaches. Phenotypic analysis of these mutants revealed components that are required for OCR-2/OSM-9 function in AWA for diacetyl sensation and in ASH for osmotic and mechanical sensation, but they are not required for OCR-2/OSM-9 to upregulate tph-1 expression in ADF. The Gα protein ODR-3 is expressed in AWA, ASH, and ADF (Roayaie et al., 1998). odr-3 mutants are severely defective in response to diacetyl, osmolarity, and gentle touch to the nose (Roayaie et al., 1998), and normal ODR-3 activity is required for the rat TRPV4 to direct osmotic and tactile sensation (Liedtke et al., 2003). However, odr-3 mutations or deletion mutations in any other Gα proteins that are expressed in ADF have no detectable effects on tph-1 expression and serotonin synthesis (Zhang et al., 2004). A recent study found that polyunsaturated fatty acids produced by the fat-3 gene regulate OCR-2/OSM-9 function in diacetyl, osmotic, and mechanical sensation (Kahn-Kirby et al., 2004). Again, tph-1 expression is unaffected in fat-3 deletion mutants (I. Sokolchik and J. Sze, unpublished). These findings suggest that OCR-2/OSM-9 is assembled to a distinct signaling pathway to regulate serotonin production.
That OCR-2/OSM-9 drives distinct signaling cascades is supported by studies of the channel functions in the ASH neurons. OCR-2/OSM-9 mediates at least three sensory modalities through the ASH neurons: high osmolarity, gentle touch at the nose, and noxious olfactory stimuli. Sensation of noxious chemicals induces C. elegans worms to aggregate; the function of OCR-2/OSM-9 in this nociceptive sensation, unlike its activity in osmotic and mechanical sensation, is independent of ODR-3 (de Bono et al., 2002). Furthermore, the rat TRPV4 can restore osmotic and mechanical sensation of osm-9 mutants, but it cannot substitute for OSM-9 to transduce noxious odorant stimuli (Liedtke et al., 2003). These results reinforce the notion derived from studies in Drosophila and mammals that TRP channels are assembled to distinct macromolecular signaling complexes to transduce distinct sensory cues (Tsunoda et al., 1997; Ramsey et al., 2006). The selective sensory functions of the rat TRPV4 in the ASH neurons suggest that the ability for a TRP channel to be integrated into sensory signaling complexes is dictated by structural determinants intrinsic to the channel protein, and these determinants may be selectively conserved and segregated through evolution.
Intrinsic Modality Determinant
The role of intrinsic modality elements in OCR-2 was revealed from phenotypic analysis of the ocr-2(yz5) mutation, which is a single nucleotide change that results in a glycine-to-glutamate (G36E) substitution in the cytoplasmic region of the predicted OCR-2 protein (Zhang et al., 2004). The ocr-2(yz5) mutant was isolated from the genetic screen for serotonin synthesis mutants, and the mutant animals exhibited dramatic reduction of tph-1 expression in ADF, equivalent to the mutant carrying a deletion that removes the first five transmembrane segments of OCR-2, or those carrying ocr-2/osm-9 double mutation. The ocr-2(yz5) mutants also show severe defects in osmotic sensation; however, they respond as well as wild-type animals to diacetyl sensation over the range of one-million-fold dilutions (Sokolchik et al., 2005).
The OCR-2(G36E) substitution is located about 220 amino acids apart from the first ankryin motif, and this region does not show strong homology to any conserved functional domains. Consistent with its normal function in diacetyl sensation, the OCR-2(G36E) protein appears to be expressed and properly localized in vivo (Figure 18.1). The substitution is unlikely to cause a global change in the assembly of the ankryin motifs, as two osm-9 alleles have different amino acid substitutions in the ankyrin motifs; both alleles affect every sensory function involving OCR-2/OSM-9. A clue to the mechanistic role of this mutation comes from expression of a chimeric channel of OCR-2 and another C. elegans TRPV channel protein OCR-4 in ocr-2 deletion mutants. OCR-4 itself cannot substitute for any OCR-2 function. Replacing the N-terminal segment preceding the first ankyrin motif of OCR-2 for the cognate segment in OCR-4 significantly rescued tph-1 expression and osmotic sensation, but the chimeric protein failed to rescue diacetyl sensation of ocr-2 deletion mutants, suggesting that the primary structure residing in the cytoplasmic N-terminus of OCR-2 governs a subset of sensory modalities of the channel. Because the glycine residue appears to be in a region conserved in OCR-4, and OCR-4 cannot substitute for OCR-2 function, the glycine itself may not be the modality determinant.
One plausible function of this N-terminal region is gating the channel. Recent studies demonstrated that the antiparallel α-helices of ankyrin motifs stack to form a superhelical spiral, acting as a gate spring of TRPN1 channels in hair cells and in Drosophila bristles (Sotomayor et al., 2005; Lee et al., 2006). TRPN1 orthologues have dazzling 17 to 29 ankyrin repeats; the ankyrin spring is thought to directly sense mechanical force from sound waves or a tactile stimulus, thereby coupling the tension in the plane of the membrane bilayer to the gate of the channel (Walker et al., 2000; Sidi et al., 2003; Corey et al., 2004; Shin et al., 2005). However, atomic force microscopy showed that the most efficient staking structure is generated from the proteins with four to six ankryin repeats (Lee et al., 2006), which is a common feature of the members of TRPV channel proteins, including OCR-2 (Tobin et al., 2002). In a hypothetical model, the N-terminal tip region of OCR-2 might fold into a moiety subserving as the door attached to the spring formed by the ankyrin repeats, and the G36E substitution alters the gating property (Figure 18.1). This model resembles the ‘‘ball-and-chain’’ gating used by the Shaker K+ channels, in which the N-terminal residues act as the ‘‘ball’’ to block the channel (Yellen, 2002). Consistent with the lack of sequence conservation among N-termini of TRPV channel proteins, there is little sequence homology between various N-termini capable of producing ‘‘ball’’ blockers in the Shaker K+ channel proteins. However, charges and hydrophobic characters of the “ball” are important for the interaction with the gate of the Shaker K+ channel (Murrell-Lagnado and Aldrich, 1993). Intriguingly, the tyrosine-to-alanine substitution at the N-terminal part of the third transmembrane segment of mouse TRPV4 selectively impairs activation of TRPV4 by heat and phorbol esters but not by cell swelling or arachidonic acid (Vriens et al., 2004), and residues at the cytophasmic phase between the second and third transmembrane segments of mammalian TRPV1 confer the sensitivity to vanilloids (Jordt and Julius, 2002). It is possible that the N-terminal ‘‘ball’’ interacts with specific residues around the cavity of the TRPV channel, or the “ball” may recruit specific intracellular ligands to the gate, thereby coupling sensory information, channel activity, and the signaling cascades.
TRP CHANNELS IN MAMMALIAN SEROTONERGIC SYSTEMS?
Identification of OCR-2/OSM-9 as a regulator of serotonin production in C. elegans raises the question of whether TRP channels also function in mammalian serotonergic systems. There is not yet explicit evidence indicating that any mammalian TRPV protein is expressed in serotonergic neurons. However, there is compelling evidence that TRPV1–V5 are expressed broadly in the CNS, including the hippocampus, amygdala, and hypothalamus (Xu et al., 2002; Smith et al., 2002; Vennekens et al., 2002). These brain areas receive rich serotonergic innervation and are the commanders for sensory-endocrine integration, emotion, and cognitive functions. TRPV1 and V4 act as molecular sensors in vivo, and TRPV1–V4 are each activated by diverse chemical and physical stimuli in heterologous expression systems (Ramsey et al., 2006). Interestingly, intranigral injection of the TRPV1 ligand depleted serotonin level in the nigra (Dawbarn et al., 1981). Also, peripherally administered capsaicin resulted in changes in the electroencephalogram activity in the dorsal raphe, the area enriched with serotonergic nuclei (Rabe et al., 1980), and TRPV4 is a locus associated with bipolar affective disorders (Delany et al., 2001). These studies do not establish the TRPV1 and TRPV4 regulating serotonin signaling but they imply that TRPV channels play a broad role through their functions in the CNS and probably via the serotonergic system.
Multiple sensory modalities, diverse gating mechanisms, and associations to distinct signaling cascades of OCR-2/OSM-9 provide insights into how a single channel entity can be dedicated to multiple sensory functions in vivo. The five mammalian TRPV channel genes expressed in the brain can produce a vast array of distinct channels through splicing variants, heteromeric combinations, and associations with distinct cellular components. These properties endow TRP channels to serve as molecular sensors in billions of years of metazoan evolution. It is tempting to speculate that these ancient molecular sensors and the ancient cellular censors, the serotonergic neurons, co-evolved. Like in C. elegans, TRP channels may function in a small subset of the serotonergic neurons in mammals. Alternatively, myriad diverse channels may underscore distinct serotonergic neurons in a wide spectrum of sensory functions. Even brain serotonergic neurons are not directly exposed to the external environment like the ADF neurons in C. elegans, but they may still sense chemical ligands and physical stimuli from other neuronal and nonneuronal cells and from the vesicular systems (Scheibel et al., 1975), therefore linking the gating information of TRP channels, sensory stimuli, serotonin production, and its downstream signaling.
REFERENCES
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–80. Review. [PubMed: 12423765]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21:33S–45S. Review. [PubMed: 10432487]
- Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–49. [PubMed: 1585177]
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–42. [PubMed: 1660283]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. Review. [PubMed: 12838335]
- Blier P, de Montigny C. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biological Psychiatry. 1998;44:313–23. [PubMed: 9755353]
- Boadle-Biber MC. Regulation of serotonin synthesis. Prog Biophys Mol Biol. 1993;60:1–15. Review. [PubMed: 8480026]
- Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194:1178–81. [PubMed: 186870]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. Review. [PubMed: 10432486]
- Colbert HA, Bargmann CI. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem. 1997;4:179–91. [PubMed: 10456062]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17(21):8259–69. [PMC free article: PMC6573730] [PubMed: 9334401]
- Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–30. [PubMed: 15483558]
- Dawbarn D, Harmar AJ, Pycock CJ. Intranigral injection of capsaicin enhances motor activity and depletes nigral 5-hydroxytryptamine but not substance P. Neuropharmacology. 1981;20:341–46. [PubMed: 6170024]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. [PMC free article: PMC3955269] [PubMed: 12410303]
- Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–74. [PubMed: 11160995]
- Dempsey CM, Mackenzie SM, Gargus A, Blanco G, Sze JY. Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics. 2005;169:1425–36. [PMC free article: PMC1449529] [PubMed: 15654117]
- Desai C, Garriga G, McIntire SL, Horvitz HR. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature. 1988;336:638–46. [PubMed: 3200316]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. [PMC free article: PMC6782383] [PubMed: 9870940]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. [PubMed: 9502257]
- Hare EE, Loer CM. Function and evolution of the serotonin-synthetic bas-1 gene and other aromatic amino acid decarboxylase genes in Caenorhabditis. BMC Evol Biol. 2004;4:24. [PMC free article: PMC514703] [PubMed: 15287963]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Frontiers in Neuroendocrinology. 1998;19:187–231. [PubMed: 9665836]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. 5HT and octo-pamine in the nematode C. elegans. Science. 1982;216:1012–14. [PubMed: 6805073]
- Hukema RK, Rademakers S, Dekkers MP, Burghoorn J, Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–22. [PMC free article: PMC1383522] [PubMed: 16407969]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘‘hot’’ chili peppers. Cell. 2002;108:421–30. [PubMed: 11853675]
- Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119(6):889–900. [PubMed: 15607983]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–43. [PubMed: 6289442]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, et al. Transient developmental expression of monoamine transporters in the rodent forebrain. Journal of Comparative Neurology. 1998;401:506–24. [PubMed: 9826275]
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behaviour of ankyrin repeats. Nature. 2006;440(7081):246–49. [PubMed: 16415852]
- Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol Psychiatry. 1998;44:851–64. Review. [PubMed: 9807640]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. [PMC free article: PMC2211528] [PubMed: 11081638]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100:14531–36. [PMC free article: PMC304114] [PubMed: 14581619]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005 Ref 3. [PubMed: 15728426]
- Murrell-Lagnado RD, Aldrich RW. Interactions of amino terminal domains of Shaker K channels with a pore-blocking site studied with synthetic peptides. J Gen Physiol. 1993;102:949–75. [PMC free article: PMC2229190] [PubMed: 8133245]
- Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–42. [PubMed: 10677040]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–52. Review. [PubMed: 16098585]
- Rabe LS, Buck SH, Moreno L, Burks TF, Dafny N. Neurophysiological and thermoregulatory effects of capsaicin. Brain Res Bull. 1980;5:755–58. [PubMed: 6162532]
- Ramsey IS, Delling M, Clapham DE. An introduction to trp channels. Annu Rev Physiol. 2006;68:619–47. [PubMed: 16460286]
- Ranganathan R, Cannon SC, Horvitz HR. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 2000;408:470–75. [PubMed: 11100728]
- Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci. 2001;21:5871–84. [PMC free article: PMC6763176] [PubMed: 11487610]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. [PubMed: 9459442]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–31. [PubMed: 10896158]
- Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84(6):899–909. [PubMed: 8601313]
- Scheibel ME, Tomiyasu U, Scheibel AB. Do raphe nuclei of the reticular formation have a neurosecretory or vascular sensor function? Exp Neurol. 1975;47:316–29. [PubMed: 806458]
- Shin JB, Adams D, Paukert M, Siba M, Sidi S, Levin M, Gillespie PG, Grunder S. Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc Natl Acad Sci USA. 2005;102(35):12572–77. [PMC free article: PMC1194908] [PubMed: 16116094]
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. [PubMed: 12805553]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–90. [PubMed: 12077606]
- Sokolchik I, Tanabe T, Baldi PF, Sze JY. Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins. J Neurosci. 2005;25:1015–23. [PMC free article: PMC6725639] [PubMed: 15673683]
- Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–82. [PubMed: 15837205]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–64. [PubMed: 10676966]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–18. [PubMed: 12160748]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–49. [PubMed: 9230432]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–64. Review. [PubMed: 12098215]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101:396–401. [PMC free article: PMC314196] [PubMed: 14691263]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. [PubMed: 10744543]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–77. [PubMed: 11827975]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–85. [PMC free article: PMC6577982] [PubMed: 7472454]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;275:327–48. [PubMed: 8806]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–86. [PubMed: 12077604]
- Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2005;419(6902):35–42. Review. [PubMed: 12214225]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–84. [PubMed: 16281027]
- Zhang S, Sokolchik I, Blanco G, Sze JY. Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons. Development. 2004;131:1629–38. [PubMed: 14998926]
- Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins.[J Neurosci. 2005]Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins.Sokolchik I, Tanabe T, Baldi PF, Sze JY. J Neurosci. 2005 Jan 26; 25(4):1015-23.
- Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons.[Development. 2004]Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons.Zhang S, Sokolchik I, Blanco G, Sze JY. Development. 2004 Apr; 131(7):1629-38. Epub 2004 Mar 3.
- Intraflagellar transport/Hedgehog-related signaling components couple sensory cilium morphology and serotonin biosynthesis in Caenorhabditis elegans.[J Neurosci. 2009]Intraflagellar transport/Hedgehog-related signaling components couple sensory cilium morphology and serotonin biosynthesis in Caenorhabditis elegans.Moussaif M, Sze JY. J Neurosci. 2009 Apr 1; 29(13):4065-75.
- Review TRPV channels' role in osmotransduction and mechanotransduction.[Handb Exp Pharmacol. 2007]Review TRPV channels' role in osmotransduction and mechanotransduction.Liedtke W. Handb Exp Pharmacol. 2007; (179):473-87.
- Review TRPV Channels’ Function in Osmo- and Mechanotransduction.[TRP Ion Channel Function in Se...]Review TRPV Channels’ Function in Osmo- and Mechanotransduction.Liedtke WB. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. 2007
- The TRPV Channel in C. elegans Serotonergic Neurons - TRP Ion Channel Function i...The TRPV Channel in C. elegans Serotonergic Neurons - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...